To the editor:
The efficacy rates of vaccines to prevent infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have not been specifically investigated in kidney transplant recipient (KTRs). Preliminary results suggest that among KTRs who received the first injection of an mRNA-based vaccine, the antibody response is weak.1 , 2 This study reports on the immunization rates of KTRs who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine (Moderna).3
All participants had a negative history for coronavirus disease 2019 (COVID-19) and tested negative for anti–SARS-CoV-2 antibodies on the day of first injection. Serologic response was assessed on the day of the second injection and 1 month thereafter using the ARCHITECT IgG II Quant test (Abbott). Titers >50 arbitrary units (AUs)/ml were considered positive (detection range, 6.8–80,000 AUs/ml). This assay is reported to correlate with in vitro virus neutralization.4
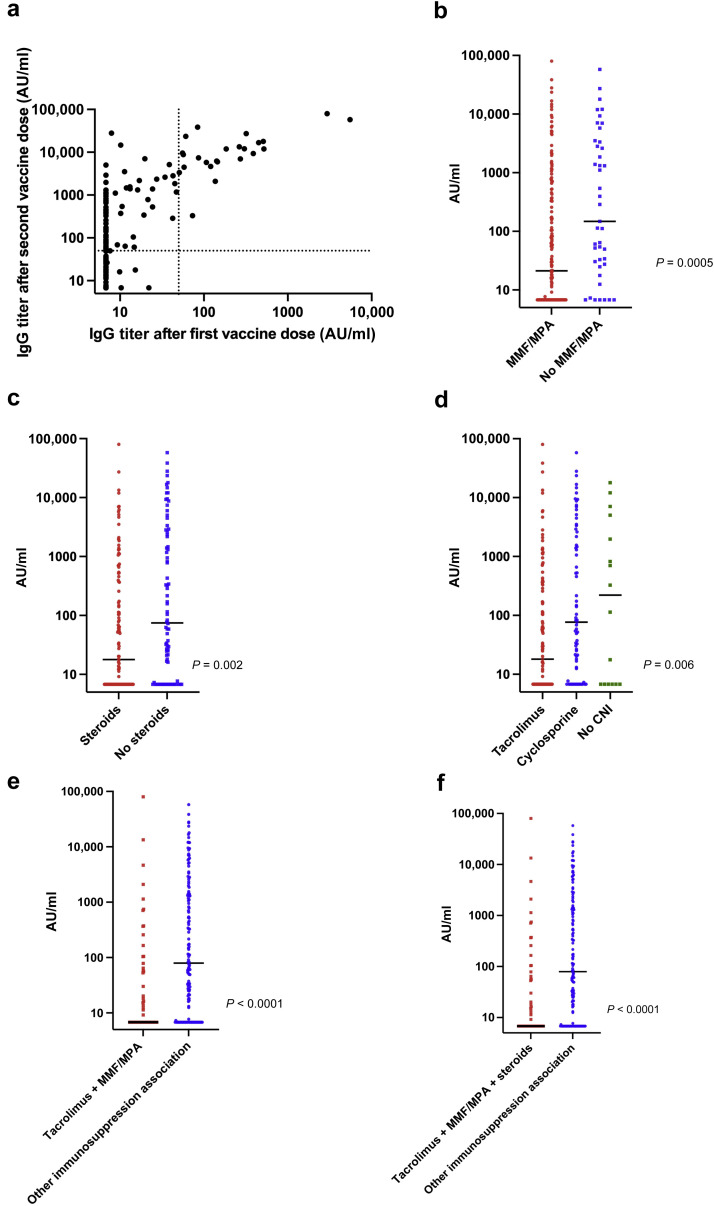
The study sample consisted of 205 KTRs (Table 1 ). Only 98 patients displayed a positive serology 28 days after the second dose. The median antibody titer was 803.2 AUs/ml (interquartile range, 142.6−4609.6 AUs/ml). Compared with patients who did not respond after the first injection, patients with a positive serology after the first dose (n = 24 [11.7%]) displayed a higher antibody titer after the second injection (104 vs. 9415 AUs/ml, respectively; P = 7.3–11). Antibody titers measured 1 month after the first and second injections were significantly correlated to each other (Figure 1 a). Patients with a first kidney transplantation, a longer time from transplantation, better kidney function, and less immunosuppression were more likely to seroconvert (Table 1). Patients treated with calcineurin inhibitors, mycophenolate mofetil, or steroids showed significantly lower anti–SARS-CoV-2 antibody titers (Figure 1b–f). One patient developed a severe form of COVID-19 five days after the second injection.
Table 1.
Characteristics of kidney transplant recipients stratified according to the serologic response after 2 doses of the mRNA-1273 SARS-CoV-2 vaccine
| Characteristics | Entire cohort (n = 204)a | SARS-CoV-2–seronegative patients (n = 106) | SARS-CoV-2–seropositive patients (n = 98) | P | Missing data |
|---|---|---|---|---|---|
| Age, yr | 57.7 (49.4–67.5) | 58 (51–67.7) | 57.3 (46.9–66.2) | 0.45 | 0 |
| Male sex | 130 (63.8) | 66 (62.3) | 64 (65.3) | 0.66 | 0 |
| BMI, kg/m2 | 25.6 (22.4–28.5) | 25.4 (22.3–27.6) | 25.9 (22.6–29.9) | 0.3 | 2 |
| Time from kidney transplantation, yr | 6.2 (3–12.8) | 5.4 (2.4–12) | 7.1 (3.8–14.7) | 0.04 | 1 |
| First transplantation | 170 (83.3) | 80 (75.5) | 90 (91.8) | 0.002 | 0 |
| Deceased donor | 163 (79.9) | 84 (79.3) | 79 (80.6) | 0.86 | 0 |
| ABO group | 0.1 | 2 | |||
| O | 84 (41.6) | 38 (36.5) | 46 (46.9) | ||
| A | 86 (42.6) | 48 (46.2) | 38 (38.8) | ||
| B | 11 (10.9) | 15 (14.4) | 7 (7.1) | ||
| AB | 10 (5) | 3 (2.9) | 7 (7.1) | ||
| Induction treatment | 0.5 | 9 | |||
| Anti-thymocyte globulin | 118 (60.5) | 63 (61.8) | 55 (59.1) | ||
| Anti-CD25 | 70 (35.9) | 37 (36.3) | 33 (35.5) | ||
| No induction | 7 (3.6) | 2 (2) | 5 (5.4) | ||
| CNI | 0.13 | 0 | |||
| Tacrolimus | 115 (56.4) | 67 (63.2) | 48 (49) | ||
| Cyclosporine | 73 (35.8) | 32 (30.2) | 41 (41.8) | ||
| No CNI | 16 (7.8) | 7 (6.6) | 9 (9.2) | ||
| MMF/MPA | 161 (78.9) | 91 (85.9) | 70 (71.4) | 0.02 | 0 |
| Azathioprine | 6 (2.9) | 0 | 6 (6.12) | 0.01 | 0 |
| mTOR inhibitors | 27 (13.2) | 9 (8.5) | 18 (18.4) | 0.04 | 0 |
| Steroids | 122 (59.8) | 69 (65.1) | 53 (54.1) | 0.12 | 0 |
| Tacrolimus + MMF/MPA | 98 (48) | 60 (56.6) | 38 (38.8) | 0.001 | 0 |
| Tacrolimus + MMF/MPA + steroids | 64 (31.3) | 46 (43.4) | 18 (18.4) | 0.0001 | 0 |
| Belatacept | 5 (2.5) | 4 (3.8) | 1 (1) | 0.37 | 0 |
| eGFR, ml/min per 1.73 m2 | 57.1 (42.4–70.6) | 54.4 (38.1–67.5) | 62.5 (47.8–72.5) | 0.004 | 1 |
| Serum creatinine, μmol/L | 120 (100–161) | 137 (109–173) | 110 (96–141) | 0.0003 | 1 |
BMI, body mass index; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Continuous variables are presented as medians (interquartile ranges), whereas categorical variables are given as n (%).
The patient who developed COVID-19 was excluded from the analysis.
Figure 1.
Anti-spike IgG antibody titers (arbitrary unit [AU]/ml) measured after the second injection of the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in 204 kidney transplant recipients without a history of coronavirus disease 2019 (COVID-19). Patients with titers >50 AU/ml were considered as seropositive. Bars represent median values. (a) Scattergram showing a significant positive correlation between anti-spike IgG antibody titers (AU/ml) after the first and second vaccine injections (Spearman ρ = 0.68; P < 0.0001). (b) Kidney transplant recipients being treated with antimetabolites (mycophenolate mofetil [MMF]/mycophenolic acid [MPA]) had lower median antibody titers compared with those who did not receive antimetabolites (21.2 [interquartile range {IQR}, 6.8−401.9] AU/ml vs. 147.4 [IQR, 27.5−3352.5] AU/ml, respectively; P = 0.0005). (c) Kidney transplant recipients being treated with steroids had lower median antibody titers compared with those who did not receive steroids (17.9 [IQR, 6.8−378.9] AU/ml vs. 74.8 [IQR, 7.2−2417.9] AU/ml; P = 0.002). (d) Kidney transplant recipients being treated with calcineurin inhibitors (CNIs) had lower median antibody titers compared with those who did not receive CNIs (18.1 [IQR, 6.8−330] AU/ml for patients under tacrolimus and 76.6 [IQR, 10.1−2392.2] AU/ml for patients under cyclosporine vs. 220 [IQR, 6.8−4269] AU/ml for patients who did not receive CNIs). (e) Kidney transplant recipients being treated with tacrolimus + antimetabolites (MMF/MPA) had lower median antibody titers compared with those did not (9.2 [IQR, 6.8−110.2] AU/ml vs. 90.9 [IQR, 7.7−1976] AU/ml, respectively; P < 0.0001). (f) Kidney transplant recipients being treated with tacrolimus + antimetabolites (MMF/MPA) + steroids had lower median antibody titers compared with those who did not (6.8 [IQR, 6.8−57.4] AU/ml vs. 79.4 [IQR, 6.9−1393.5] AU/ml, respectively; P < 0.0001).
In summary, the immunization rate among KTRs who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine can be as low as 48%. The issue of a third vaccine dose in nonresponsive KTRs is an intriguing one that could be usefully explored in further research.
Footnotes
see commentary on page 1275
References
- 1.Benotmane I., Gautier-Vargas G., Cognard N. Weak anti–SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99:1487–1489. doi: 10.1016/j.kint.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky B.J., Werbel W.A., Avery R.K. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients [e-pub ahead of print]. JAMA. https://doi.org/10.1001/jama.2021.4385 Accessed April 22, 2021. [DOI] [PMC free article] [PubMed]
- 3.Centers for Disease Control and Prevention. Moderna COVID-19 vaccine overview and safety. Updated April 5, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html Available at: Accessed March 10, 2021.
- 4.Prendecki M., Clarke C., Brown J. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]