To the editor:
On December 21, 2020, the European Commission granted conditional marketing approval to the BNT162b2 coronavirus disease 2019 (COVID-19) mRNA vaccine developed by BioNTech.1 , 2 In the general population, the first dose of BNT162b2 was reported to produce a rapid antibody response with 52% efficacy in preventing severe infection, similar to the protection induced by the natural disease.2 , 3 There was great hope that vaccination would protect fragile individuals, and societies of nephrology asked that patients with end-stage kidney disease should be given priority in being vaccinated.4
The situation of in-center hemodialysis patients is a double challenge: their fragility and their proximity to others have made this population particularly vulnerable. In France, for instance, the cumulative incidence of COVID-19 is now >10% in dialysis patients, with a mortality rate of about 15% in those who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
However, the response to SARS-CoV-2 vaccine in dialysis patients is still unknown, as is the need for a second dose and what its timing should be.5 , 6 We therefore wish to report on the antibody response to the first dose of BNT162b2 vaccination in a cohort of high-comorbidity patients on in-center hemodialysis.
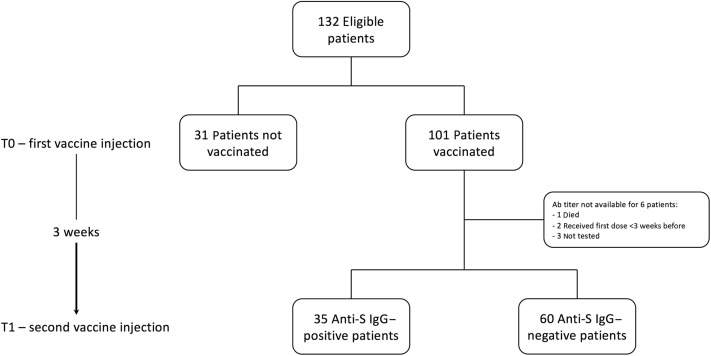
All the chronic hemodialysis patients treated in our center between January 1 and February 28, 2021, were enrolled in this observational analysis. We dosed specific IgG anti-spike protein (anti-S IgG) (Elecsys Anti-SARS-CoV-2 S; Roche Diagnostics) at the time of the first vaccine injection (T0) and at the time of the second one (T1) 3 weeks later (Figure 1 ). Of the 132 eligible patients (mean age, 67.54 ± 15.71 years; 57% males; average dialysis vintage, 2.21 years), 101 (77%) gave their consent to be vaccinated (Table 1 ). At baseline, only 2 patients tested positive (anti-S IgGs, 1200 and 22 U/ml, respectively). Both had previously had symptomatic COVID-19. At T1, only 35 (35%) of the patients had developed neutralizing antibodies, with a median titer of 8.22 U/ml [interquartile range, 1.73–28.70] (Figure 1). The patients who developed neutralizing antibodies were younger and had a lower comorbidity burden (Table 1). However, in this subset of cases, no correlation was found between antibody titer and age, comorbidity, or dialysis vintage.
Figure 1.
Flowchart. Ab, antibody; Anti-S, anti-spike.
Table 1.
Characteristics of our cohort
| Baseline | All | Vaccinated | Not vaccinated | P |
|---|---|---|---|---|
| n (%) | 132 (100) | 101 (77) | 31 (23) | — |
| Age, yr | 67.54 ± 15.71 | 68.89 ± 14.86 | 63.13 ± 17.74 | 0.0739 |
| Male, n (%) | 75 (57) | 60 (59) | 15 (48) | 0.3054 |
| Dialysis vintage, yr | 2.21 [0.80–4.68] | 2.28 [0.81–4.58] | 2.21 [0.41–5.97] | 0.5637 |
| Charlson Comorbidity Index | 8 [5–10] | 8 [5–10] | 8 [6–9] | 0.3649 |
| T1 | Responders | Nonresponders | P |
|---|---|---|---|
| No. | 35 | 60 | — |
| Age, yr | 62.31 ± 16.20 | 73.72 ± 11.18 | 0.0001 |
| Male, n (%) | 20 (57) | 35 (58) | 0.9999 |
| Dialysis vintage, yr | 1.44 [0.67–3.97] | 2.99 [0.96–5.99] | 0.3149 |
| Charlson Comorbidity Index | 6 [4–8] | 9 [7–10] | 0.0006 |
| Anti–SARS-CoV-2 S IgG titer, U/ml | 8.22 [1.73–28.70] | — | — |
S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T1, time of the second vaccine injection.
Age is expressed as mean ± SD; dialysis vintage and Charlson Comorbidity Index are expressed as median [interquartile range].
Bold P values indicate statistically significant variables.
To our knowledge, this is the first report on the initial response to COVID-19 vaccine in patients on hemodialysis. At difference with other recent reports in health care workers,7 our data suggest that only about one-third of hemodialysis patients develop neutralizing antibodies after the first dose of BNT162b2 COVID-19 mRNA vaccine, and that these are at low titers, as could be expected, in a high-comorbidity cohort (median Charlson Comorbidity Index: 8). Younger patients, with lower comorbidity, are more likely to mount an antibody response. Although, because of a shortage of vaccine, some institutions proposed a policy of delayed second-dose administration in the general population,8 , 9 a timely second dose of the BNT162b2 COVID-19 mRNA vaccine seems necessary to ensure protection in hemodialysis patients. In the wait for long-term studies on larger groups, needed to enable us to assess the vaccine-induced immune response and kinetic, we considered that this first report could be of help by suggesting that to best protect dialysis patients, for the time being we cannot let our guard down, even after vaccination.
Footnotes
see commentary on page 1275
References
- 1.Cavaleri M., Enzmann H., Straus S. The European Medicines Agency's EU conditional marketing authorisations for COVID-19 vaccines. Lancet. 2021;397:355–357. doi: 10.1016/S0140-6736(21)00085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manisty C., Otter A.D., Treibel T.A. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis A., Baigent C., Ikizler T.A. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: a call to action. Kidney Int. 2021;99:791–793. doi: 10.1016/j.kint.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glenn D.A., Hegde A., Kotzen E. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int Rep. 2021;6:1407–1410. doi: 10.1016/j.ekir.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Windpessl M., Bruchfeld A., Anders H.J. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17:291–293. doi: 10.1038/s41581-021-00406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley T., Grundberg E., Selvarangan R. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine [e-pub ahead of print]. N Engl J Med. https://doi.org/10.1056/NEJMc2102051 Accessed April 23, 2021. [DOI] [PMC free article] [PubMed]
- 8.Kadire S.R., Wachter R.M., Lurie N. Delayed second dose versus standard regimen for Covid-19 vaccination. N Engl J Med. 2021;384:e28. doi: 10.1056/NEJMclde2101987. [DOI] [PubMed] [Google Scholar]
- 9.Pimenta D., Yates C., Pagel C., Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372:n710. doi: 10.1136/bmj.n710. [DOI] [PubMed] [Google Scholar]