To the editor:
Patients receiving maintenance hemodialysis (MHD) may have altered vaccine responses.1 Although mRNA vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have demonstrated dramatic efficacy in preventing symptomatic forms of coronavirus disease 2019 (COVID-19) in the nondialysis population,2 the characterization of vaccine response in patients receiving MHD remains a major unmet need.
We studied the humoral response after the BNT162b2 mRNA vaccine using anti–spike(S)1 IgG antibody (Beckman Coulter Access; reference range for antibody positivity signal-to-cutoff >1; gray zone, 0.8–1) in a single-center cohort of 69 patients receiving MHD.
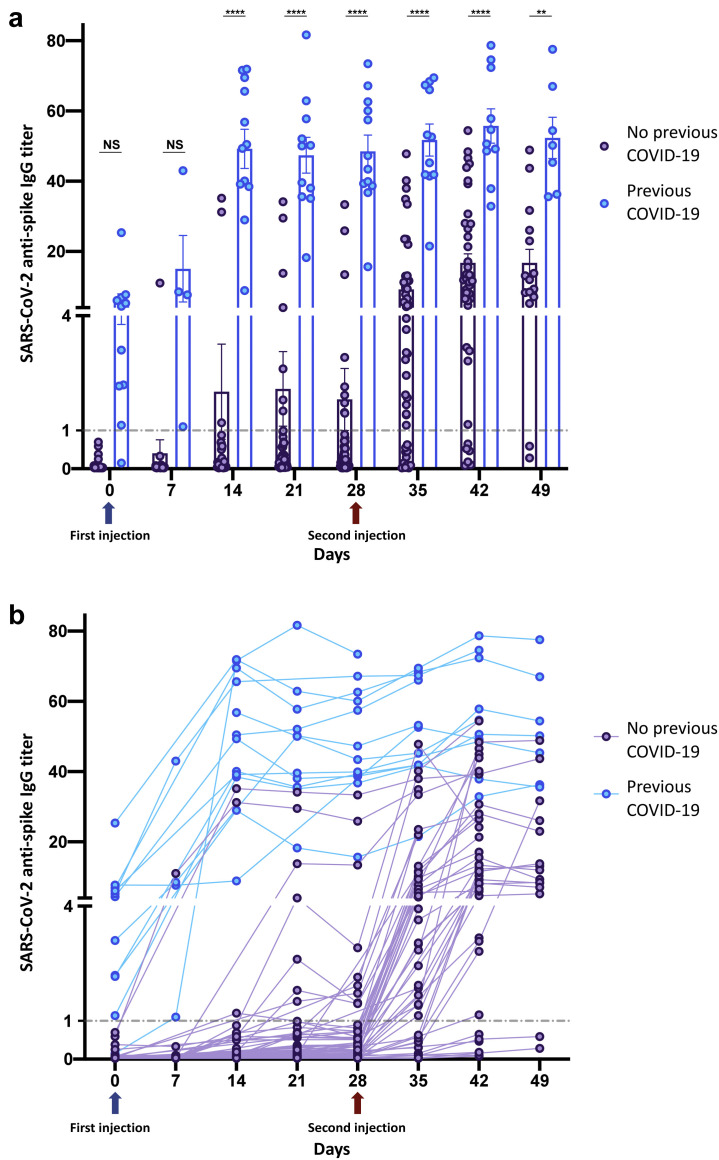
Three hundred seventy-eight samples were analyzed (Figure 1 ). Thirteen patients (19%) had a history of previous COVID-19 or positive baseline serology. Samples until week 6 to 7 were available for 64 patients. Overall seropositivity rate at last follow-up was 55 of 64 (86%) (Supplementary Table S1). Patients aged >70 years were less likely to reach seropositivity at last follow-up (28 of 37 [75%]; P = 0.01; Supplementary Table S1). Conversely, immunocompromised status did not influence the seroconversion rate (7 of 8 [87%] seropositive among immunocompromised patients). The rate of early seropositivity was associated with a history of COVID-19 (Supplementary Table S2). Since week 2, the mean anti-S1 levels of these patients were significantly higher than those of infection-naïve individuals, even after both injections (Supplementary Table S3; Figure 1a). No difference in patient characteristics was observed between both groups (Supplementary Table S3). Among infection-naïve patients, anti–S1 IgG levels progressively increased among time (Figure 1b). The seropositivity rate was 10 of 56 (18%) before the second injection and 43 of 52 (82%) at last follow-up (Supplementary Tables S3–S5). Older age was associated with a reduced late seropositivity rate (Supplementary Table S5). Interestingly, 2 infection-naïve patients developed paucisymptomatic SARS-CoV-2 infection 5 and 6 weeks after first vaccine dose. Anti-S1 titers were 0.5 and 1.4, respectively, in these patients.
Figure 1.
Serologic response after first and second injections of the BNT162b2 mRNA vaccine in patients with or without history of coronavirus disease 2019 (COVID-19). (a) Comparison of the evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti–spike antibodies over time (days) after the first (day 0; blue arrow) and second (day 28; red arrow) BNT162b2 mRNA vaccine between a group with previous COVID-19 (n = 13) and a group with no history of COVID-19 (n = 57). Scatterplot with bar is shown. Means ± SEM are shown. The cutoff for negative serology was defined according to the manufacturer (index signal-to-cutoff [S/CO] <1; dashed line). (b). Kinetics of the evolution of SARS-CoV-2 anti–spike antibodies over time (days) with repeated measures matched for each patient, after the first (day 0; blue arrow) and second (day 28; red arrow) BNT162b2 mRNA vaccine between a group with previous COVID-19 (n = 13) and a group with no history of COVID-19 (n = 57). Spaghetti plot is shown. The cutoff for negative serology was defined according to the manufacturer (index S/CO <1; dashed line). Statistical analysis with mixed-effect analysis with Sidak multiple-comparison test. ∗∗P < 0.01, ∗∗∗∗P < 0.0001. NS, nonsignificant.
In this analysis of postvaccine humoral response, patients receiving MHD have an overall anti-S1 seropositivity rate >80% after 2 doses of the BNT162b2 mRNA vaccine, but only 18% after a single injection. Moreover, patients receiving MHD with a previous SARS-CoV-2 infection have a higher rate of positive anti–S1 IgG (as expected) and higher anti–S1 IgG levels, compared with infection-naïve individuals. Older age is associated with a lower seropositivity rate in our cohort. These data are probably close to those observed in patients not receiving dialysis (as for long-term humoral response post–COVID-193), although seropositivity rates appear to be lower in patients receiving MHD.4 Although anti–S1 IgG titer is reported to correlate with in vitro virus neutralization,4 postvaccine immunity longevity (including cellular immunity not studied in this work) and protection from symptomatic infection remain to be studied in patients receiving MHD.
Overall, this study suggests that most patients receiving MHD given both doses of the BNT162b2 mRNA vaccine are expected to develop an anti–S1 IgG response that may be protective.
Acknowledgements
We thank all the patients involved in this study.
Footnotes
see commentary on page 1275
Supplementary Methods.
Table S1. Characteristics of patients according to late serologic status.
Table S2. Characteristics of patients according to early serologic status.
Table S3. Characteristics of patients according to previous COVID-19 history.
Table S4. Characteristics of infection-naïve patients according to early serologic status.
Table S5. Characteristics of infection-naïve patients according to late serologic status.
Supplementary Material
References
- 1.Kato S., Chmielewski M., Honda H. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakhi H., Dahmane D., Attias P. Kinetics of anti-SARS-CoV-2 IgG antibodies in hemodialysis patients six months after infection [e-pub ahead of print]. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020111618 Accessed February 26, 2021. [DOI] [PMC free article] [PubMed]
- 4.Prendecki M., Clarke C., Brown J. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.