Abstract
Insulin-like growth factor 2 (IGF2) mRNA-binding protein 2 (IGF2BP2) is a secreted protein that can bind to IGF2 and has been reported to promote inflammation. The data from the ENCORI database have predicted that IGF2BP2 can bind caspase 4, which mediates pyroptosis and promotes airway inflammation and lipopolysaccharide (LPS)-induced lung injury. The present study investigated whether IGF2BP2 can regulate LPS-induced lung cell inflammation by targeting caspase 4. Therefore, the non-tumorigenic lung epithelial cell line Beas-2B was transfected with short hairpin RNA (shRNA)-IGF2BP2 and stimulated with LPS. A number of parameters, including cell viability, production of interleukin (IL)-1β and IL-18, activation of gasdermin D (GSDMD) and the expression levels of IGF2BP2, caspase 4 and cleaved-caspase 1, were subsequently assessed using CCK-8, ELISA kits, western blotting and immunofluorescence staining, respectively. RNA pull-down assay was used to probe the possible interaction between IGF2BP2 and caspase 4 RNA. LPS treatment was found to inhibit cell viability, trigger IL-1β and IL-18 production and increase IGF2BP2 expression in a concentration-dependent manner. Compared with cells transfected with shRNA-negative control, cells that were transfected with shRNA-IGF2BP2 exhibited enhanced cell viability, reduced IL-1β and IL-18 concentrations, decreased GSDMD activation in addition to reduced expression levels of caspase 4 and cleaved-caspase 1 following stimulation with 1 µg/ml LPS. Concomitantly, the effects of IGF2BP2 silencing on caspase 4 expression were higher compared with those noted on caspase 1. In addition, binding of IGF2BP2 to caspase 4 RNA was also observed. To conclude, data from the present study suggest that IGF2BP2 knockdown inhibited LPS-induced Beas-2B cell inflammation by targeting caspase 4, thereby inhibiting the non-canonical pyroptosis pathway.
Keywords: insulin-like growth factor 2, inflammation, lung injury, pyroptosis, sepsis
Introduction
Sepsis is primarily caused by dysregulation of host immune response as a result of infection, which can ultimately lead to life-threatening organ dysfunction (1). Imbalances in inflammation and immune response leads to uncontrolled microbial growth, which in turn results in fatal inflammation, tissue damage and organ dysfunction (1). The lung is the organ that is the most susceptible to in sepsis-induced dysfunction, where acute lung injury (ALI) is one of the most serious manifestations of sepsis (2). Therefore, preventing or alleviating ALI caused by sepsis is the key to reducing septic mortality.
Excessive activation of inflammatory cytokines is the main mechanism of organ damage in patients with sepsis, especially in patients with ALI (3). It has been previously reported that inhibition of inflammatory responses can protect lung function from sepsis in rats (3). In particular, pyroptosis is a form of programmed cell death that occurs when host cells are infected by pathogenic microorganisms or are stimulated by endogenous danger signals (4). Pyroptosis is characterized by cell swelling and lysis, followed by the release of a large number of proinflammatory cytokines (4). This process can be divided into canonical and non-canonical pathways. In the non-canonical pathway, exogenous cytotoxins, such as lipopolysaccharide (LPS), can directly induce the activation of caspase 11, caspase 4 and caspase 5, which causes cleavage of the pyroptosis executor gasdermin D (GSDMD), leading to perforation of the cell membrane (5). In addition, the N-terminus of GSDMD can activate nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3 (NLRP3), resulting in the release of interleukin (IL)-1β and IL-18(5). It has also been demonstrated that inflammasome-mediated non-canonical cell pyroptosis can activate a cascade of inflammatory responses, causing or aggravating inflammation-associated diseases, such as lung injury (6).
Insulin-like growth factor 2 (IGF2) mRNA-binding protein 2 (IGF2BP2) is a member of the conserved single-stranded RNA-binding protein family IGF2 that is expressed in a wide range of fetal tissues (7). IGF2BP2 can function as a post-transcriptional regulator of mRNA localization, stability and translation (8). Dysregulation of IGF2BP2 has been frequently associated with human diseases, including diabetes and cancer (8-10). It has been reported that IGF2BP2 can serve a proinflammatory role in non-alcoholic fatty liver disease, promoting inflammation-induced carcinogenesis and subsequent tumor proliferation and invasion (11). Following ENCORI database search, IGF2BP2 was shown to bind to the mRNA of caspase-4, which is a crucial regulator in the non-canonical pathway of cell pyroptosis (4). Caspase-4-mediated pyroptosis can promote a number of inflammatory signaling pathways in the airway, serving a key role in LPS-induced tissue damage (12-15).
Therefore, the present study investigated the potential effects of IGF2BP2 on LPS-induced lung cell inflammation and caspase 4 activity, with emphasis on caspase 4-mediated pyroptosis.
Materials and methods
Cell culture and treatment
Human bronchial epithelial (Beas-2B) cells (American Type Culture Collection) were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Thermo Fisher Scientific, Inc.) and 1% penicillin and streptomycin antibiotics (Thermo Fisher Scientific, Inc.) in an atmosphere containing 5% CO2 at 37˚C. The in vitro simulation of lung injury was achieved by treating the cells with 0.1, 1 and 10 µg/ml LPS (Thermo Fisher Scientific, Inc.) at 37˚C for 12 h.
Cell transfection
Short hairpin RNA (shRNA) against IGF2BP2 and its corresponding scrambled negative control (NC) vector, shRNA-NC, were designed and synthesized by Shanghai GenePharma Co., Ltd. Beas-2B cells at the density of 2x106/ml were transfected in vitro with 2 µg/ml shRNA-IGF2BP2 or shRNA-NC using Lipofectamine 2000® (Invitrogen, Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol, which were described in a previous study (16). At 48 h post-transfection, cells were used for subsequent experiments.
Cell Counting Kit (CCK)-8 assay
CCK-8 assay was used for evaluating cell viability. Briefly, cells (5x103 cells/well) were seeded into 96-well plates, treated with LPS after shRNA transfection before being finally incubated with 10 µl CCK-8 working solution (MedChemExpress) for 2 h under normal cell culture conditions. Absorbance in each well was measured at 450 nm using a microplate reader.
ELISA
The expression levels of IL-1β (cat. no. ab214025) and IL-18 (cat. no. ab215539) in the supernatant of the cell culture medium were detected using ELISA following the manufacturer's protocols (Abcam). The assay was conducted as described previously (17). The results are expressed as optical density at 450 nm.
Western blotting
Beas-2B cells were lysed with RIPA buffer (Beyotime Institute of Biotechnology) on ice for 30 min before the protein concentration was determined using the bicinchoninic acid kit (Thermo Fisher Scientific, Inc.). Equal amounts of protein (18 µg/lane) were separated by 12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. Following blocking with 5% non-fat milk at room temperature for 2 h, the membranes were incubated with the designated primary antibodies overnight at 4˚C. Goat anti rabbit IgG secondary antibodies conjugated with horseradish peroxidase (ProteinTech Group, Inc.; cat. no. SA00001-2; 1:10,000) were added and incubated at room temperature for 2 h. The proteins bands were visualized using enhanced chemiluminescence Prime Western blot detection reagent (Cytiva). The antibodies used (ProteinTech Group, Inc.) included the following: Rabbit polyclonal IGF2BP2 (cat. no. 11601-1-AP; 1:5,000), rabbit polyclonal caspase 4 (cat. no. 11856-1-AP; 1:1,000), rabbit polyclonal caspase 1 (cat. no. 22915-1-AP; 1:2,000), rabbit polyclonal cleaved-caspase 1 (cat. no. 22915-1-AP; 1:1,000) and rabbit polyclonal GAPDH (cat. no. 10494-1-AP; 1:10,000). Densitometry analysis was performed using Image Lab system software (Bio-Rad Laboratories, Inc. Version 1.52)
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from Beas-2B cells using the RNA extraction kit (RNAiso Plus; cat. no. 9108; Takara Bio, Inc.). A total of 5 µg RNA was reverse-transcribed to cDNA (High-Capacity cDNA Reverse Transcription kit; cat. no. 4368813; Applied Biosystems; Thermo Fisher Scientific, Inc.). Next, a SYBR Green PCR kit (Takara Bio, Inc.) was used to determine gene expression on an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR reaction procedure was as follows: 5 min at 95˚C, followed by 40 cycles of 30 sec at 95˚C and 45 sec at 65˚C. The specific primer sequences used were as follows: IGF2BP2 forward, 5'-CGGGGAAGAGACGGATGATG-3' and reverse, 5'-CGCAGCGGGAAATCAATCT-G-3'; caspase 4 forward, 5'-CCTATGGCAGAAGGCAACCA-3' and reverse, 5'-GGCAGTTGCGGTTGTTGAAT-3'; caspase 1 forward, 5'-ACAAGACCTCTGACAGCACG-3' and reverse, 5'-TTCACTTCCTGCCCACAGAC-3' and β-actin forward, 5'-AAATCGTGCGTGACATCAAAGA-3' and reverse, 5'-GGCCATCTCCTGCTCGAA-3'. The results were normalized to those of β-actin expression and the 2-ΔΔCq method (18) was utilized to calculate the relative changes in gene expression.
Immunofluorescence staining
Beas-2B cells were fixed with 4% paraformaldehyde at 4˚C for 15 min and permeabilized with 0.2% Triton X-100 at 37˚C for 20 min. After blocking with 10% Bovine Serum Albumin (Thermo Fisher Scientific, Inc.) for 30 min at room temperature. The cells were then incubated with primary antibodies against cleaved N-terminal GSDMD (cat. no. ab215203; Abcam; 1:100) at 4˚C overnight. The following day, the cells were incubated with 100 µl/well working solution containing Alexa Fluor 488-conjugated goat anti-rabbit secondary antibodies (Abcam; cat. no. ab150081; 1:1,000) at room temperature for 1 h. DAPI was used for nuclear counterstaining. The stained slides were imaged using an inverted fluorescence microscope (magnification, x200; Olympus Corporation).
RNA pull-down assay
The interaction of caspase 4 mRNA and IGF2BP2 protein was examined following lysis of Beas-2B cells using RIPA buffer (Beyotime Institute of Biotechnology) and subsequent incubation with biotin-labeled caspase 4, caspase 1 and IgG (Shanghai GenePharma Co., Ltd.) at 4˚C for 2 h. A total of 50 µl streptavidin magnetic beads (Invitrogen; Thermo Fisher Scientific, Inc.) were added to each sample and the mixtures were incubated at 4˚C for 2 h. The beads were washed with 1x wash buffer included in the kit and elution buffer (Pierce™ Magnetic RNA-Protein Pull-Down kit; Thermo Fisher Scientific, Inc.), centrifuged at 8,000 x g at 4˚C for 15 min and analyzed via western blotting analysis to assess the expression levels of the IGF2BP2 protein in the pull-down products (19). The antibodies used for IGF2BP2 and GAPDH were same as those listed in the western blotting section.
Statistical analysis
The data are presented as mean ± SD from ≥ three independent experiments. Statistical analysis was performed using one-way ANOVA followed by Tukey's test and Student's t-test using GraphPad Prism 8 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
LPS stimulation increases IGF2BP2 expression of Beas-2B cells in a concentration-dependent manner
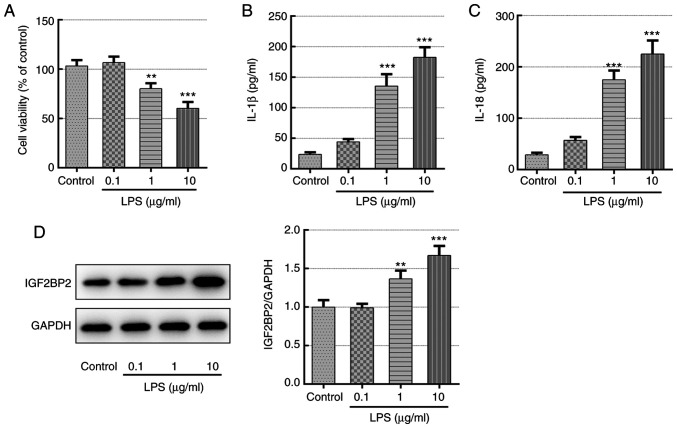
Different concentrations of LPS (0.1, 1 and 10 µg/ml) were first used to treat Beas-2B cells for 12 h before cell viability, inflammatory cytokine production and IGF2BP2 expression were quantified. Compared with those in the control group, LPS was found to significantly reduce cell viability and trigger the generation of inflammatory cytokines IL-1β and IL-18 in a concentration-dependent manner (Fig. 1A-C), demonstrating the successful induction of lung cell inflammation. In addition, IGF2BP2 protein expression was also significantly enhanced by LPS in a concentration-dependent manner compare with that in control (Fig. 1D), suggesting a potential role of IGF2BP2 in LPS-induced lung cell inflammation.
Figure 1.
Detection of cell viability, inflammatory cytokine concentration and IGF2BP2 expression in Beas-2B cells following treatment with LPS. (A) Relative cell viability of Beas-2B cells exposed to different concentrations of LPS. Concentrations of (B) IL-1β and (C) IL-18 in the supernatant of Beas-2B cells following treatment with different concentrations of LPS. (D) IGF2BP2 protein expression in Beas-2B cells following treatment with different concentrations of LPS. **P<0.01 and ***P<0.001 vs. control. IGF2BP2, IGF2 mRNA-binding protein 2; LPS, lipopolysaccharide; IL, Interleukin.
Knockdown of IGF2BP2 enhances cell viability and inhibits the activity levels of pyroptosis-associated inflammatory cytokines, including IL-1β and IL-18, in LPS-treated Beas-2B cells
To explore the specific role of IGF2BP2 and its association with caspase 4-mediated pyroptosis, two shRNA sequences were constructed that targeted IGF2BP2 to knockdown its expression. Scrambled shRNA was used as negative control and shRNA-IGF2BP2-1 was selected for subsequent experiments based on its superior knockdown efficacy (Fig. 2A). Untreated Beas-2B cells and Beas-2B cells transfected with shRNA-IGF2BP2-1 or shRNA-NC were subsequently exposed to 1 µg/ml LPS, which was added to the culture medium for 12 h (20). Beas-2B cells incubated in normal culture medium were used as the control group and Beas-2B cells stimulated with 1 µg/ml LPS (in the absence of transfection) were considered as the model group. Cells in the model group, which was stimulated with LPS, exhibited a significant decrease in cell viability compared with that in cells in the control group (Fig. 2B). However, compared with cells transfected with shRNA-NC, IGF2BP2 knockdown resulted in significantly increased cell viability following stimulation with LPS (Fig. 2B). This suggested that the knockdown of IGF2BP2 can recover the cell viability previously reduced by LPS. In addition, the levels of the inflammatory cytokines IL-1β and IL-18, which are typically released during cell pyroptosis (5), were also measured. Although LPS induced the generation of IL-1β and IL-18, IGF2BP2 knockdown significantly reduced the concentration of these two cytokines compared with those in cells transfected with shRNA-NC in the presence of LPS (Fig. 2C and D). This observation indicated that the knocking down IGF2BP2 expression rescued cell viability and inhibited inflammation in response to LPS stimulation.
Figure 2.
IGF2BP2 knockdown enhances cell viability and inhibits the activity levels of pyroptosis-associated inflammatory cytokines in LPS-treated Beas-2B cells. (A) mRNA levels of IGF2BP2 in LPS-treated Beas-2B cells following IGF2BP2 knockdown. (B) Cell viability in LPS-treated Beas-2B cells following the knockdown of IGF2BP2. Concentrations of (C) IL-1β and (D) IL-18 in the supernatant of LPS-treated Beas-2B cells following the knockdown of IGF2BP2. **P<0.01, ***P<0.001, #P<0.05 and ##P<0.01. IGF2BP2, IGF2 mRNA-binding protein 2; LPS, lipopolysaccharide; IL, interleukin; Sh, short hairpin; NC, negative control.
Knockdown of IGF2BP2 inhibits GSDMD activation and caspase 4/1 expression in LPS-treated Beas-2B cells
Subsequently, the expression level of GSDMD, caspase 4 and caspase 1, specific markers involved in cell pyroptosis (5), were investigated. LPS treatment led to the activation of GSDMD (Fig. 3). However, knockdown of IGF2BP2 prevented the expression of activated GSDMD. Furthermore, in cells transfected with shRNA-IGF2BP2 that were treated with LPS, the significant reductions in IGF2BP2 protein expression levels compared with those in cells transfected with shRNA-NC was observed. This appeared to be concomitant with the finding that shRNA-IGF2BP2 transfection resulted in significant reductions in caspase 4 and cleaved-caspase 1 levels compared with those in cells transfected with shRNA-NC (Fig. 4). These results implicated the inhibitory effects of IGF2BP2 knockdown on GSDMD/caspase-4- or caspase 1-mediated pyroptosis.
Figure 3.
Knocking down IGF2BP2 inhibits GSDMD activation in LPS-treated Beas-2B cells. Expression levels of GSDMD were assessed using immunofluorescence staining against cleaved N-terminal GSDMD (green). DAPI (blue) was used to stain the nucleus. Magnification, x200. IGF2BP2, IGF2 mRNA-binding protein 2; GSDMD, gasdermin D; LPS, lipopolysaccharide; sh, short hairpin; NC, negative control.
Figure 4.
Knocking down IGF2BP2 inhibits LPS-mediated caspase 4/1 activation in Beas-2B cells. The protein expression levels of IGF2BP2, caspase 4, c-caspase 1 and caspase 1 were detected by western blot analysis. *P<0.05, **P<0.01 and ***P<0.001. #P<0.05, ##P<0.01 and ###P<0.001. IGF2BP2, IGF2 mRNA-binding protein 2; LPS, lipopolysaccharide; c-caspase 1, cleaved caspase 1; sh, short hairpin; NC, negative control.
IGF2BP2 functions as an mRNA binding protein and binds to the RNA of caspase 4
The present study next examined whether the effects of IGF2BP2 on LPS-induced lung cell inflammation and pyroptosis were dependent on binding of this protein to caspase 4. The mRNA levels of both caspases 4 and 1 were assessed following IGF2BP2 knockdown. The results indicated that both caspase 4 and 1 mRNA levels were significantly reduced following IGF2BP2 knockdown compared with those in cells transfected with shRNA-NC (Fig. 5A and B). However, knocking down IGF2BP2 expression produced a larger reduction on caspase 4 expression compared with that noted for caspase 1 (Fig. 5A and B). To determine the direct interaction between IGF2BP2 and caspase 4, RNA pull-down assay was performed and the results confirmed the direct interaction between these two proteins (Fig. 5C).
Figure 5.
IGF2BP2 binds to the RNA of caspase 4 and caspase 1. Relative mRNA expression levels of (A) caspase 4 and (B) caspase 1 were assessed in LPS-treated Beas-2B cells following the knockdown of IGF2BP2. (C) The interaction between IGF2BP2 and caspase 4/1 RNA was assessed by the RNA pull-down assay. ***P<0.001, #P<0.05 and ###P<0.001. IGF2BP2, IGF2 mRNA-binding protein 2; LPS, lipopolysaccharide.
Discussion
Aberrant activation of the inflammatory response is one of the major mechanisms underlying lung and airway damage (1). As a novel form of programmed cell death that was discovered relatively recently, cell pyroptosis has been reported to be involved in the generation of inflammatory cytokines and amplification of the inflammatory response (6). In the present study, IGF2BP2 was identified as a novel regulator in LPS-induced inflammation in Beas-2B cells. Subsequently, it was demonstrated that the effects mediated by IGF2BP2 were possibly through caspase 4, which is a key protein involved in pyroptosis.
It has been previously reported that LPS from gram-negative bacteria can activate the immune response and inflammation by activating pyroptosis (6). During non-canonical pyroptosis, intracellular LPS can activate caspase 4/5 in humans, leading to the cleavage of GSDMD and the activation of caspase 1, which causes the release of the inflammatory factors, including IL-1β and IL-18(5). During canonical pyroptosis, LPS can directly activate caspase 1 by binding to NLRP3, thereby leading to generation of inflammatory cytokines such as IL-1β, IL-18 and IL-6(5). Results from the present study demonstrated that LPS treatment inhibited cell viability in addition to triggering IL-1β and IL-18 production in a concentration-dependent manner in Beas-2B cells, which was consistent with previous findings. In addition, LPS was also found to activate GSDMD, caspase 4 and caspase 1, all of which can mediate the non-canonical pyroptosis pathway (21,22). These data suggested that cell pyroptosis is at least part responsible for in LPS-induced Beas-2B cell inflammation, indicating a modulatory role of pyroptosis in lung injury.
The mRNA-binding protein IGF2BP2 has been shown to function as an oncogene by targeting long non-coding (lnc)RNAs and microRNAs (miRs) upstream of promoting cancer cell proliferation, migration and invasion (9,23,24). For example, IGF2BP2 can be stabilized by the lncRNA long intergenic non-coding RNA for IGF2BP2 stability to promote the development of colorectal cancer (8). In addition, IGF2BP2 is downregulated by the cellular communication network factor 6 protein in metaplastic breast carcinomas, causing the inhibition of this tumor (25). However, in the present study, the data demonstrated that IGF2BP2 may be involved in LPS-induced inflammation in Beas-2B cells based on its upregulated expression following LPS stimulation. Knocking down IGF2BP2 expression reversed the LPS-induced reduction in cell viability, in addition to reversing the LPS-induced production of IL-1β and IL-18. Furthermore, IGF2BP2 knockdown also reversed the activation of GSDMD, caspase 4 and caspase 11 in the presence of LPS. All these data suggested the participation of IGF2BP2 in LPS-induced pyroptosis in Beas-2B cells. Subsequently, the role of IGF2BP2 in inflammation was also assessed. A previous study reported that increases in the expression levels of IGFBP2 in the sputum may contribute to the development of idiopathic pulmonary fibrosis (26), suggesting a potential role of the IGF family of proteins in lung-associated diseases. In addition, IGF2 has also reported to be involved in inflammation-associated diseases, such as acute pneumonia (27). For example, lncRNA small nucleolar RNA host gene 16 was found to promote LPS-induced acute pneumonia in A549 cells by targeting the miR-370-3p/IGF2 axis (27). In another study, miR-3941 targeted IGF2 to control LPS-induced acute pneumonia in A549 cells (28).
IGF2BP2 is a secreted protein that can bind to IGF2 and regulate its localization. Therefore, it was predicted that IGF2BP2 could also regulate LPS-induced acute pneumonia or lung cell injury.
To identify the potential targets of IGF2BP2 in LPS-induced Beas-2B cell inflammation, the ENCORI database (http://starbase.sysu.edu.cn/rbpClipRNA.php?source=mRNA&flag=RBP&clade=mammal&genome=human&assembly=hg19&RBP=IGF2BP2&clipNum=&panNum=&target=) was searched, where the RNA of caspase-4 was found to be a potential target that could bind to IGF2BP2. In the present study, expression levels of caspase 4 were increased following LPS stimulation, which was reversed by IGF2BP2 knockdown. In accordance with these findings, the direct interaction between IGFBP2 and caspase 4 mRNA was confirmed by performing a RNA pull down assay. In addition, the expression levels of caspase 1 were also reduced by IGF2BP2 knockdown in the presence of LPS. During non-conical pyroptosis, caspase 1 is one of the downstream proteins of caspase 4(5). Therefore, knocking down IGF2BP2 in LPS-stimulated Beas-2B cells targeted caspase 4, thereby promoting the cleavage of GSDMD and cell membrane rupture in addition to activating of caspase 1. These events ultimately led to the release of inflammatory cytokines, including IL-1β and IL-18. In addition, during pyroptosis cells typically undergo phenotypic changes, including cell swelling and lysis (5). However, immunofluorescence staining against cleaved N-terminal GSDMD did not reflect this phenotypic change in the cells. Therefore, other techniques, such as transmission electron microscopy, should be utilized to further validate these findings from the present study.
Taken together, the present study demonstrated that in LPS-stimulated Beas-2B cells, IGF2BP2 could activate the non-conical pyroptosis pathway by targeting caspase 4. This promoted the release of inflammatory cytokines to aggravate the inflammatory response. Therefore, inhibition of IGF2BP2 expression may provide a therapeutic approach for alleviating LPS-induced lung cell injury and ALI.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ND and JW contributed to study conception and design. JW and XY contributed to acquisition, analysis and interpretation of data. ND drafted the work and revised it critically for important intellectual content. ND and JW have seen and can confirm the authenticity of the raw data. All authors read and approved the final version of the manuscript
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Perner A, Gordon AC, De Backer D, Dimopoulos G, Russell JA, Lipman J, Jensen JU, Myburgh J, Singer M, Bellomo R, Walsh T. Sepsis: Frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 2016;42:1958–1969. doi: 10.1007/s00134-016-4577-z. [DOI] [PubMed] [Google Scholar]
- 2.Mehaffey JH, Charles EJ, Schubert S, Salmon M, Sharma AK, Money D, Stoler MH, Laubach VE, Tribble CG, Roeser ME, Kron IL. In vivo lung perfusion rehabilitates sepsis-induced lung injury. J Thorac Cardiovasc Surg. 2018;155:440–448 e442. doi: 10.1016/j.jtcvs.2017.08.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aji G, Li F, Chen J, Leng F, Hu K, Cheng Z, Luo Y, Xu X, Zhang J, Lu Z. Upregulation of PCP4 in human aldosterone-producing adenomas fosters human adrenocortical tumor cell growth via AKT and AMPK pathway. Int J Clin Exp Pathol. 2018;11:1197–1207. [PMC free article] [PubMed] [Google Scholar]
- 4.Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019;26(101239) doi: 10.1016/j.redox.2019.101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersoug LG, Møller P, Loft S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev. 2018;31:153–163. doi: 10.1017/S0954422417000269. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Zhang H, Guo X, Zhu Z, Cai H, Kong X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J Hematol Oncol. 2018;11(88) doi: 10.1186/s13045-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen YX, Liu J, Luo XJ, Meng Q, Pu HY, et al. lncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18(174) doi: 10.1186/s12943-019-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, Chen ZH, Zeng ZL, Wang F, Zheng J, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(112) doi: 10.1186/s12943-019-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai N. The diverse functions of IMP2/IGF2BP2 in metabolism. Trends Endocrinol Metab. 2020;31:670–679. doi: 10.1016/j.tem.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Simon Y, Kessler SM, Bohle RM, Haybaeck J, Kiemer AK. The insulin-like growth factor 2 (IGF2) mRNA-binding protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut. 2014;63:861–863. doi: 10.1136/gutjnl-2013-305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker PJ, Boucher D, Bierschenk D, Tebartz C, Whitney PG, D'Silva DB, Tanzer MC, Monteleone M, Robertson AAB, Cooper MA, et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45:2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Juarbe N, Bradley KM, Riegler AN, Reyes LF, Brissac T, Park SS, Restrepo MI, Orihuela CJ. Bacterial pore-forming toxins promote the activation of caspases in parallel to necroptosis to enhance alarmin release and inflammation during pneumonia. Sci Rep. 2018;8(5846) doi: 10.1038/s41598-018-24210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaslona Z, Flis E, Wilk MM, Carroll RG, Palsson-McDermott EM, Hughes MM, Diskin C, Banahan K, Ryan DG, Hooftman A, et al. Caspase-11 promotes allergic airway inflammation. Nat Commun. 2020;11(1055) doi: 10.1038/s41467-020-14945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srisaowakarn C, Pudla M, Ponpuak M, Utaisincharoen P. Caspase-4 mediates restriction of burkholderia pseudomallei in human alveolar epithelial cells. Infect Immun. 2020;88(e00868) doi: 10.1128/IAI.00868-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao Z, Chen L, Guo S. Knockdown of SLC34A2 inhibits cell proliferation, metastasis, and elevates chemosensitivity in glioma. J Cell Biochem. 2019;120:10205–10214. doi: 10.1002/jcb.28305. [DOI] [PubMed] [Google Scholar]
- 17.Ko IG, Hwang JJ, Chang BS, Kim HS, Jin JJ, Hwang L, Kim CJ, Choi CW. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-kB signaling pathway in rats. Int Immunopharmacol. 2020;83(106444) doi: 10.1016/j.intimp.2020.106444. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Dou YQ, Kong P, Li CL, Sun HX, Li WW, Yu Y, Nie L, Zhao LL, Miao SB, Li XK, et al. Smooth muscle SIRT1 reprograms endothelial cells to suppress angiogenesis after ischemia. Theranostics. 2020;10:1197–1212. doi: 10.7150/thno.39320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv X, Zhou X, Yan J, Jiang J, Jiang H. Propofol inhibits LPS-induced apoptosis in lung epithelial cell line, BEAS-2B. Biomed Pharmacother. 2017;87:180–187. doi: 10.1016/j.biopha.2016.12.074. [DOI] [PubMed] [Google Scholar]
- 21.Aaltomaa S, Kärjä V, Lipponen P, Isotalo T, Kankkunen JP, Talja M, Mokka R. Reduced alpha- and beta-catenin expression predicts shortened survival in local prostate cancer. Anticancer Res. 2005;25:4707–4712. [PubMed] [Google Scholar]
- 22.He C, Zhao Y, Jiang X, Liang X, Yin L, Yin Z, Geng Y, Zhong Z, Song X, Zou Y, et al. Protective effect of Ketone musk on LPS/ATP-induced pyroptosis in J774A.1 cells through suppressing NLRP3/GSDMD pathway. Int Immunopharmacol. 2019;71:328–335. doi: 10.1016/j.intimp.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Wan BS, Cheng M, Zhang L. Insulin-like growth factor 2 mRNA-binding protein 1 promotes cell proliferation activation of AKT and is directly targeted by microRNA-494 in pancreatic cancer. World J Gastroenterol. 2019;25:6063–6076. doi: 10.3748/wjg.v25.i40.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrenica B, AlShaaban M, Czubryt MP. The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2009;46:674–681. doi: 10.1016/j.yjmcc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 25.McMullen ER, Gonzalez ME, Skala SL, Tran M, Thomas D, Djomehri SI, Burman B, Kidwell KM, Kleer CG. CCN6 regulates IGF2BP2 and HMGA2 signaling in metaplastic carcinomas of the breast. Breast Cancer Res Treat. 2018;172:577–586. doi: 10.1007/s10549-018-4960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guiot J, Henket M, Corhay JL, Moermans C, Louis R. Sputum biomarkers in IPF: Evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS One. 2017;12(e0171344) doi: 10.1371/journal.pone.0171344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhang B, Cao WB, Wang HY, Niu L, Zhang GZ. Study on clinical significance of lncRNA EGOT expression in colon cancer and its effect on autophagy of colon cancer cells. Cancer Manag Res. 2020;12:13501–13512. doi: 10.2147/CMAR.S285254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fei S, Cao L, Pan L. microRNA-3941 targets IGF2 to control LPS-induced acute pneumonia in A549 cells. Mol Med Rep. 2018;17:4019–4026. doi: 10.3892/mmr.2017.8369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.