Abstract
The aim of the present study was to compare the clinical and economic benefits of intra-articular injections of platelet-rich plasma (PRP) and hyaluronic acid (HA) in Chinese patients with knee osteoarthritis (OA). A total of 86 patients (42 treated with PRP and 44 with HA) were treated with three weekly intra-articular injections. The inclusion criteria included patients between 18 and 75 years of age, with chronic knee pain or swelling lasting >3 months and X-ray findings of degenerative joint alterations according to the Kellgren-Lawrence score grade I-III. Clinical examinations were performed before treatment, at 1- and 6-month post-injection intervals. International Knee Documentation Committee subjective, Western Ontario and McMaster Universities and visual analogue scale scores were determined at each examination. Adverse reactions, average cost, treatment time and patient satisfaction were also recorded. Compared with patients injected with HA, PRP was found to be associated with increased and more severe post-injection pain and swelling, where the duration of adverse reactions was greater in the PRP group (P=0.02). During the follow-up evaluations, both groups showed statistically significant improvements in all clinical scores from pre-injection to 1- and 6-month assessments (P<0.05). However, no significant inter-group (PRP vs. HA) differences were observed in the clinical scores between the two follow-up time points. There were also no significant differences in clinical score between the groups with regards to the Kellgren-Lawrence grade I, II or III. The average cost of PRP injections was 22.8X that of HA administration and the average treatment time was 5X that of HA, but there was no significant difference in patient satisfaction. These preliminary results indicate that although PRP injections can significantly improve clinical outcome in patients with knee OA, PRP is not any more effective compared with HA. Furthermore, PRP injections are associated with higher costs and treatment times. Therefore, additional clinical studies are required before PRP injections can be considered as a first-line treatment option for knee OA.
Keywords: platelet-rich plasma, intra-articular injection, hyaluronic acid, osteoarthritis
Introduction
Due to the limited self-healing capacity of the articular cartilage, knee osteoarthritis (OA) has become one of the most common causes of pain and disability in middle-aged and older patients (1). The majority of mild and moderate cases of OA can be successfully managed with conservative interventions, including drugs, such as non-steroidal analgesics, weight control and lifestyle changes (2). Intra-articular injections of hyaluronic acid (HA) are commonly used to treat knee OA (3). The exact mechanism of HA action is unclear. Restoration of the elastoviscous properties of synovial fluid seems to be the most logical explanation of the function of HA, while other mechanisms include possible anti-inflammatory and antinociceptive properties or stimulation of in vivo HA synthesis by the exogenously injected HA (3). Although HA is effective at reducing adverse symptoms, it does not modify the biochemical environment of the joint or promote cartilage healing (4). Therefore, further clinical and laboratory research are focused on developing novel biological methods for repairing damaged cartilage.
In previous years, the biological effects of growth factors (GFs) on cartilage repair have been well documented both in vivo and in vitro (5-8). In particular, platelet-rich plasma (PRP) is a fraction of the plasma containing platelets and GF at concentrations above baseline that can be produced by centrifugal separation of whole blood (9,10). PRP has been indicated to exhibit a positive effect on the local environment, reducing intra-articular synovial hyperplasia and adjusting the synovial fluid concentration of IL-1 receptor antagonist (11). PRP has been used to successfully treat chronic elbow tendinosis and refractory wounds (12-14), such that a number of studies have previously reported its effectiveness for the treatment of knee OA (15-18). However, there remains to be a lack of clinical research into the cost and treatment time of PRP administration and its potential use as a therapeutic option for articular cartilage injuries.
The present study aimed to compare the clinical and economic benefits of PRP intra-articular injections with those of HA intra-articular injections in Chinese patients with mild knee OA. In addition, frequency, proportion, pain score, duration and prognosis of adverse reactions were also recorded.
Patients and methods
Patients
The present study was a retrospective comparative study using HA as the control. The PRP procedure was approved by the Ethics Committee of Ningbo No. 6 Hospital (Ningbo, China) prior to study commencement. Between March 2017 and December 2018, 101 patients with knee OA receiving PRP or HA injections in the Department of Joint Surgery of Ningbo No. 6 Hospital, were recruited. There were 25 female and 17 male patients in the PRP group and 27 female and 17 male patients in the control group. The age of patients ranged from 43 to 72 years, with a mean of 57.57±5.87 in the PRP group and 59.66±5.21 in the control group, with no significant difference (P=0.08) (Table I). All patients with knee OA in the associated study period were screened for inclusion. The inclusion criteria were as follows: i) Patients were aged between 18 and 75 years old; ii) chronic knee pain or swelling lasting >3 months; and iii) X-ray findings of degenerative joint changes [Kellgren-Lawrence score (19), grade I-III]. The exclusion criteria included: i) Kellgren-Lawrence scores of grade 0 or IV; ii) pregnant or lactating women; iii) diabetes; iv) rheumatoid arthritis; v) gout; vi) blood diseases; vii) severe cardiovascular diseases; viii) infections; ix) immunodepression; x) patients receiving anticoagulant therapy; and xi) patients with hemoglobin values >11 g/dl and platelet values >150,000/mm3. Patients who did not complete three injections or 6 months of follow-up were also excluded from the study.
Table I.
Basal characteristics of patients in the two groups.
| Parameter | Platelet-rich plasma | Hyaluronic acid | P-value |
|---|---|---|---|
| No. of patients/knees | 42/42 | 44/47 | |
| Age (years) | 57.57±5.87 | 59.66±5.21 | 0.08 |
| Male sex, n (%) | 17 (40.48%) | 17 (38.64%) | |
| Female sex, n (%) | 25 (59.52%) | 27 (61.36%) | |
| Body mass index (kg/m2) | 23.74±2.87 | 24.31±2.82 | 0.35 |
| Kellgren-Lawrence grade | |||
| I | 15 | 16 | |
| II | 19 | 22 | |
| III | 8 | 9 |
Treatment, preparation and clinical evaluation
For each injection, a 50-ml blood sample was collected from the median elbow vein using a 50-G needle, such that the ratio of blood to anticoagulant reached 9:1. The blood samples collected were then transferred to a separation tube (Weigao New Polymer Materials Co., Ltd.), centrifuged twice to first separate erythrocytes and then to concentrate platelets (378 x g for 10 min each; Fig. 1); this resulted in a unit of 4.5 ml PRP (Fig. 1). All procedures were performed in the same therapeutic office at room temperature (25˚C). A total of 3.5 ml PRP was immediately transported to the injection room whereas the remainder was sent to the laboratory of Ningbo No. 6 Hospital for platelet concentration analysis. The PRP platelet and leukocyte counts were found to be 819.47±136.32x109/l and 32.2±10.42x1012/l, respectively, which were 6.40±1.10 and 6.10±1.93 times greater than those in the preoperative peripheral blood.
Figure 1.
Preparation of PRP. (A) Peripheral blood samples were collected from patients (50 ml each), transferred to separation tubes and then centrifuged twice at 378 x g for 10 min each. (B) Erythrocytes were removed after the first centrifugation step, and (C) the second centrifugation resulted in a middle layer of PRP, (D) which was collected using a syringe. PRP, platelet-rich plasma.
The patients received three PRP or HA intra-articular injections at 1-week intervals. Following iodopovidone-based disinfection, a 21-gauge needle with a 10-ml syringe was used for knee injections, following which the skin was sterilely dressed and excess effusion fluid was extracted before intra-articular injection of 3.5 ml PRP or 2.5 ml HA (ARTZ; Seikagaku Corporation) into the knee joint (Video S1). At the end of the procedure, the injection site was covered with a sterile dressing and patients were asked to bend and extend the knee several times. The patients were then given the following instructions: i) No showering for 3 days; ii) ipsilateral joint activities should be limited over the next 24 h; iii) symptoms such as pain and swelling are normal; iv) and ice on the knee helps to reduce pain and swelling.
Outcome assessments were performed by SCW blinded to the treatment groups. The patients were clinically evaluated 1 h before injection and at 1- and 6-month post-injection intervals. During each evaluation, International Knee Documentation Committee (IKDC) subjective scores (20), Western Ontario and McMaster Universities (WOMAC) scores (21) and the visual analogue scale (VAS) were evaluated (22). These three scales are used to assess joint pain, activity and instability. Joint infection, deep vein thrombosis and hematoma were defined as major adverse events, whilst joint pain and swelling were defined as mild adverse reactions. Frequency, proportion, pain score, start time, end time and duration of these reactions were also observed. Average cost, treatment time and patient satisfaction in the two groups were also recorded. Patient satisfaction referred to whether the patient was satisfied with the treatment, which was divided into satisfaction and dissatisfaction.
Statistical analysis
The data are expressed as the mean ± SD unless otherwise indicated. Two-way ANOVA was performed to assess the differences between groups at different follow-up times. Friedman's test followed by Wilcoxon signed rank test with Bonferroni correction was used to evaluate the data at different time points within a single group. Mann-Whitney U test was used to compare the numerical parameters between HA and PRP groups, including post-injection VAS scores, treatment time and average cost per person. Statistical analyses were performed using SPSS version 23.0 (IBM Corp.), where P<0.05 was considered to indicate a statistically significant difference.
Results
Basal information
Due to incomplete treatment data, 15 of these patients were excluded from the present study. In total, 86 patients (89 knees; 3 patients with OA in both knees) met the study criteria (PRP group, 42 patients and 42 knees; HA group, 44 patients and 47 knees) and completed 6 months of follow-up examinations. No differences in basal characteristics were observed between the two groups (Table I).
Clinical scores
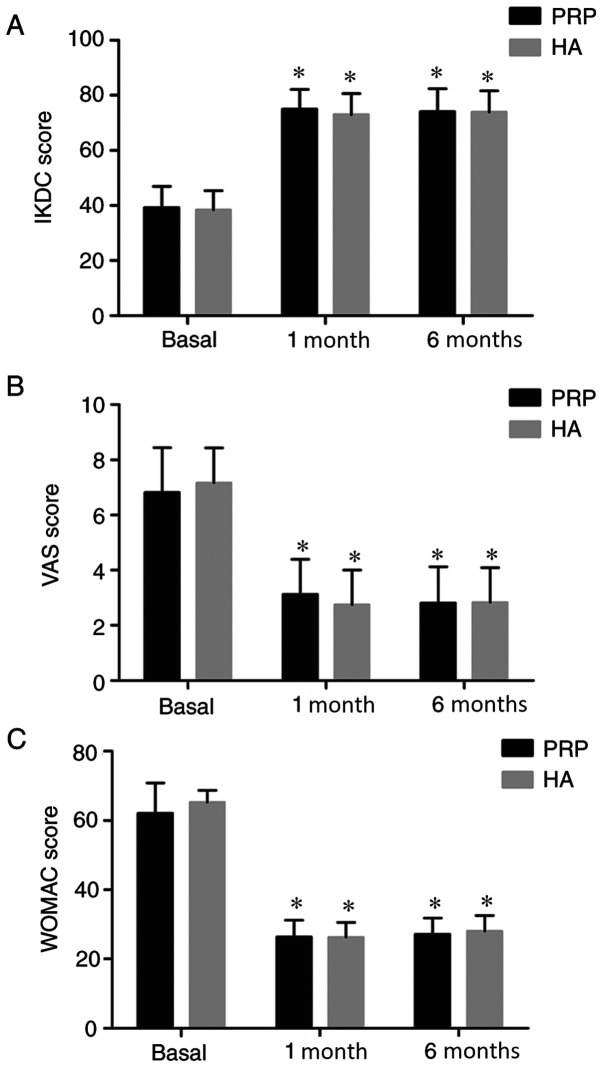
Preliminary analysis of patients in the PRP and HA groups demonstrated a statistically significant improvement in the three clinical scores from pre-injection to the 1- and 6-month follow-up assessments (P<0.05). However, no significant inter-group differences were observed in the clinical scores between the two follow-up time points. In the PRP group, the IKDC score increased from 39.21±7.71 at basal evaluation to 74.95±7.15 after 1 month and 74.00±8.42 after 6 months (Fig. 2A), the VAS score decreased from 6.80±1.62 at basal evaluation to 3.12±1.27 after 1 month and 2.81±1.31 after 6 months (Fig. 2B) and the WOMAC score decreased from 62.09±8.74 at basal evaluation to 26.35±4.90 after 1 month and 27.14±4.66 after 6 months (Fig. 2C). In the HA group, the IKDC score increased from 38.40±6.95 at the basal evaluation to 72.96±7.64 after 1 month and then 73.87±7.76 after 6 months evaluation (Fig. 2A), the VAS score decreased from 7.17±1.26 at basal evaluation to 2.74±1.26 after 1 month and 2.83±1.25 after 6 months (Fig. 2B) and the WOMAC score decreased from 65.28±3.49 at basal evaluation to 26.31±4.27 after 1 month and 28.00±4.55 after 6 months (Fig. 2C).
Figure 2.
Clinical scoring. (A) IKDC score, (B) VAS and (C) WOMAC score in the PRP and HA groups before, and then 1 and 6 months after treatment. *P<0.05 vs. basal. IKDC, International Knee Documentation Committee; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities; PRP, Platelet-rich plasma; HA, hyaluronic acid.
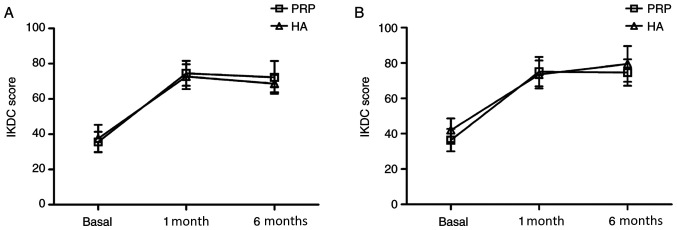
No significant differences in the Kellgren-Lawrence grade I, II or III scores were observed between the PRP and HA groups (P>0.05). However, further analysis revealed slightly different results in patients affected by varying degrees of cartilage degeneration. At the 6-month follow-up assessment, patients in the PRP group who were affected by Kellgren-Lawrence grade I lesions exhibited higher IKDC scores (72.00±9.30) compared with that in the HA group (68.63±4.93) (P=0.20; Fig. 3A). HA appeared to improve the IKDC score (79.55±10.16) of patients with Kellgren-Lawrence grade III degenerative joint changes compared with that in the PRP group (74.60±7.52), though this did not reach statistical significance (P=0.28; Fig. 3B).
Figure 3.
IKDC scores. IKDC scores in patients with Kellgren-Lawrence grades (A) I and (B) III lesions. IKDC, International Knee Documentation Committee; PRP, Platelet-rich plasma; HA, hyaluronic acid.
Complications and adverse reactions
Complications, including infection, deep vein thrombosis, hematoma or other major adverse events were not observed during treatment itself. However, compared with HA, PRP was associated with more post-injection pain and swelling (33.33 vs. 10.64%) and significantly more severe pain (P<0.01; Table II). The start time of adverse reactions in the PRP group was 0.33±0.42 h after injection, compared with 0.10±0.21 h in the HA group, though this difference was not statistically significant (Table II). Furthermore, mild reactions subsided significantly later in the PRP group (45.71±18.41) compared with that in the HA group (31.20±23.04) (P=0.02; Table II), suggesting that the duration time was significantly longer in the PRP group (49.00±18.27) than that in the HA group (31.10±23.11) (P=0.02; Table II). However, all reactions were self-limiting and did not require further treatment.
Table II.
Mild adverse reactions of the two groups.
| Parameter | Platelet-rich plasma | Hyaluronic acid | P-value |
|---|---|---|---|
| Total injections (n) | 126 | 141 | |
| Frequency, n (%) | 42 (33.33) | 15 (10.64) | |
| Pain score (visual analogue scale) | 3.59±2.24 | 1.93±0.80 | <0.01 |
| Start time (h) | 0.33±0.42 | 0.10±0.21 | 0.05 |
| End time (h) | 45.71±18.41 | 31.20±23.04 | 0.02 |
| Duration (h) | 49.00±18.27 | 31.10±23.11 | 0.02 |
Treatment time and preparation cost
The cost of PRP treatment (4,996.00±12.00 renminbi) included the PRP kit and associated preparation costs, which significantly exceeded the cost of HA administration (219.00±5.50 renminbi) (P<0.01; Table III). PRP preparation also included two centrifugation steps, which also markedly prolonged the total treatment time in the PRP group (30.00±5.12 min) than that in the control group (6.00±1.30 min) (P<0.01; Table III). The average cost of PRP treatment per patient was 22.81 times that of HA treatment, whereas the average treatment time was 5 times that of HA (Table III). However, at the 6-month follow-up, patient satisfaction rates reached 71.43 and 70.21% for the PRP and HA groups, respectively (Table III), indicating no significant difference between the two treatment options.
Table III.
The cost and satisfaction rates of the two groups.
| Parameter | Platelet-rich plasma | Hyaluronic acid | P-value |
|---|---|---|---|
| Average cost per person (Renminbi) | 4,996.00±12.00 | 219.00±5.50 | <0.01 |
| Treatment time (min) | 30.00±5.50 | 6.00±1.30 | <0.01 |
| Satisfaction rate, n (%) | 30 (71.43%) | 33 (70.21%) |
Discussion
The positive biological effects of GFs on cartilage repair have been well documented both in vivo and in vitro (5,6). The present study was designed to obtain preliminary data on the short-term clinical efficacy and safety of PRP intra-articular injections. The results indicate that PRP treatment reduces pain and improves patient knee function after 6 months, but the three clinical scores of PRP injections are ultimately not better compared with HA administration. PRP was also associated with higher incidences and more serious post-injection pain and swelling, though these reactions were self-limiting and required no further treatment.
Over the past decade, numerous studies have demonstrated advantages of intra-articular PRP injections (15-18). Since OA is an intra-articular lesion, intra-articular PRP administration is a direct and minimally invasive treatment that is well tolerated by patients (18). PRP is derived from autologous peripheral blood, meaning that it has good biocompatibility, is not associated with immune rejection or disease transmission and potentially confers anti-infection effects (23).
Previous studies have demonstrated the positive effects of PRP on knee OA (15-18). Lisi et al (15) previously reported a phase II randomized controlled trial where 50 patients received three PRP (n=28) or HA (n=22) intra-articular injections at 4-week intervals. Using MRI scanning, PRP was concluded to reduce articular damage in as soon as 6 months after treatment, which was better than that by HA. Sánchez et al (16) treated patients with OA using intra-articular injections of autologous platelet-rich growth factors (PRGFs), with those receiving hyaluronan injections serving as the control group. Each group included 30 patients. After 5 weeks, the success rate for the pain subscale reached 33.4 and 10% for the PRGF and hyaluronan groups, respectively. These results were encouraging, though due to short follow-up times (5 weeks) and small sample sizes, this previous study (16) included the same limitations as the aforementioned study by Lisi et al (15). Furthermore, calcium chloride was used as a PRP activator in both of these previous studies. Following activation by calcium chloride, PRP is transformed into liquid PRGF and solid PRP gels (24,25). PRGF injection alone causes GF loss from the PRP gel. However, applying PRP with calcium chloride results in the rapid formation of the PRP gel in the joint, which is not conducive to the distribution of GF in the damaged cartilage (26). Wang-Saegusa et al (17) published a prospective study of 261 patients with knee OA. The patients received three injections of PRP before clinical evaluations were conducted after 6 months using the WOMAC score, VAS, Lequesne Index and the Short Form 36 Health Survey (SF-36). Statistical analysis revealed significant results with an improvement in all clinical scores, though the study did not include a control group. Furthermore, Kon et al (18) reported that PRP resulted in a better clinical outcome compared with those of low molecular weight HA (LWHA) and high molecular weight HA (HWHA), where 150 patients (50 in each group) were treated with three injections of either PRP, LWHA or HWHA.
However, a number of studies have also reported negative effects of PRP injection therapy (27,28). In 2016, Filardo et al (27) reported the results of a randomized, blinded and controlled trial with 12 months of follow-up, which included 192 patients with knee OA (96 treated with HA and 96 with PRP). The patients received three weekly intra-articular injections and corresponding clinical evaluations. The patients in both the PRP and HA groups exhibited improved IKDC scores, which did not differ at any of the follow-up points. It was therefore concluded that PRP injections were no more beneficial than HA treatment. In addition, a concern was also highlighted in this study. PRP preparation involves harvesting 150 ml peripheral blood followed by two centrifugation steps, which yields 20 ml PRP (27). After the first injection of 4-5 ml, the remaining PRP was frozen for 2 weeks and then thawed for the second and third injections. It was suggested that storing PRP at -20˚ and then thawing for subsequent use may significantly decrease the levels of GFs, including epidermal growth factor, vascular endothelial growth factor, platelet-derived growth factor, insulin-like growth factor 1 and transforming growth factor (29), which may subsequently alter the clinical efficacy of PRP.
Using the results of previous studies, the present study proposes an improved method of PRP administration. To activate PRP more slowly and thus prolong GF activity, calcium chloride was not used before injection. Instead, pure PRP was directly injected to enable even distribution within the joint. To avoid freezing, PRP was produced from fresh peripheral blood prior to each injection, which not only ensured the freshness of the PRP, but also reduced the possibility of exogenous contamination.
Generally speaking, previous studies have reported that treatment with PRP causes more adverse reactions compared with HA (27,28). In the present study, adverse reactions were recorded in more detail, which included frequency, proportion, pain score and duration of these events. More frequent and severe pain reactions were observed following PRP, compared with that after HA injection. The duration of the adverse reactions in the PRP group was also longer compared with that in the HA group, which may be associated with stimulation of the joint synovium by components within the PRP, such as red and white blood cells.
The cost of PRP treatment is primarily determined by the price of the PRP kit in China. There are currently >10 types of PRP preparation kits available at varying prices, though the PRP kit from Weigao New Polymer Materials Co., Ltd. is one of the few currently approved kits for intra-articular injections in China. Therefore, the cost of PRP treatment may decrease as novel PRP kits are approved for clinical use. Additionally, centrifugal preparation of PRP greatly prolongs treatment time. Filardo et al (27) proposed freezing a single PRP sample, which could be thawed for subsequent injections. However, this may reduce the levels of available GFs, thus reducing the clinical benefits of PRP treatment.
There are a number of limitations to the present study, including the non-randomized double-blind design, small sample size and short follow-up time, in addition to a lack of direct evidence of cartilage repair, including data from arthroscopic or radiological examinations. After outlining the different effects of PRP and HA injections, the patients were allowed to select their preferred method of treatment, which may also have introduced bias to the study results.
These preliminary results indicated that although PRP injections could significantly improve clinical outcome after 6 months in patients with knee OA, PRP is not more effective compared with HA. PRP was also associated with higher incidence rates and more serious post-injection pain and swelling compared with HA, although these reactions were self-limiting and required no further treatment. Furthermore, PRP injections were associated with higher costs and treatment times compared with HA. Therefore, additional clinical studies are required before PRP injections can be considered as a first-line treatment option for knee OA.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by grants from the Ningbo Natural Science Foundations (grant no. 2018A610261) and Zhejiang Provincial Key Laboratory of Pathophysiology (grant no. 201911).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HL and ML conceived and designed the study; HL, ML, ZYH and SCW performed the literature search; HL, ML, ZLD and JHZ performed the quality assessment of the screened literature; ZYH and SCW were involved in PRP production and patient follow-up evaluation; ZLD and JHZ were responsible for patient enrollment and PRP administration; HL and ML were responsible for confirming the authenticity of all the raw data, writing the manuscript and performing the final revision. All authors have read and approved the final version of the manuscript, and due care has been taken to ensure the integrity of the work.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Ningbo No. 6 Hospital (Ningbo, China). The participating patients had complete clinical data, and written informed consent was obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Robinson DL, Kersh ME, Walsh NC, Ackland DC, de Steiger RN, Pandy MG. Mechanical properties of normal and osteoarthritic human articular cartilage. J Mech Behav Biomed Mater. 2016;61:96–109. doi: 10.1016/j.jmbbm.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Brzusek D, Petron D. Treating knee osteoarthritis with intra-articular hyaluronans. Curr Med Res Opin. 2008;24:3307–3322. doi: 10.1185/03007990802490124. [DOI] [PubMed] [Google Scholar]
- 4.Maricar N, Callaghan MJ, Felson DT, O'Neill TW. Predictors of response to intra-articular steroid injections in knee osteoarthritis - A systematic review. Rheumatology (Oxford) 2013;52:1022–1032. doi: 10.1093/rheumatology/kes368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah NJ, Geiger BC, Quadir MA, Hyder MN, Krishnan Y, Grodzinsky AJ, Hammond PT. Synthetic nanoscale electrostatic particles as growth factor carriers for cartilage repair. Bioeng Transl Med. 2016;1:347–356. doi: 10.1002/btm2.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medeiros Da Cunha CM, Perugini V, Bernegger P, Centola M, Barbero A, Guildford AL, Santin M, Banfi A, Martin I, Marsano A. Vascular endothelial growth factor sequestration enhances in vivo cartilage formation. Int J Mol Sci. 2017;18(2478) doi: 10.3390/ijms18112478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Zhang J, Jin Q, Li J, He Z, Di Z. Role of platelet-rich plasma in articular cartilage lesions. Chin Med J (Engl) 2014;127:3987–3992. doi: 10.1055/s-0034-1384672. [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas R, Saltzman BM, Fortier LA, Cole BJ. Role of platelet-rich plasma in articular cartilage injury and disease. J Knee Surg. 2015;28:3–10. doi: 10.1055/s-0034-1384672. [DOI] [PubMed] [Google Scholar]
- 9.Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY, Kawase T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF) Int J Implant Dent. 2016;2(19) doi: 10.1186/s40729-016-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016;27:467–471. doi: 10.3109/09537104.2016.1143922. [DOI] [PubMed] [Google Scholar]
- 11.Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290–296. doi: 10.2460/ajvr.68.3.290. [DOI] [PubMed] [Google Scholar]
- 12.Alessio-Mazzola M, Repetto I, Biti B, Trentini R, Formica M, Felli L. Autologous US-guided PRP injection versus US-guided focal extracorporeal shock wave therapy for chronic lateral epicondylitis: A minimum of 2-year follow-up retrospective comparative study. J Orthop Surg (Hong Kong) 2018;26(2309499017749986) doi: 10.1177/2309499017749986. [DOI] [PubMed] [Google Scholar]
- 13.Karaduman M, Okkaoglu MC, Sesen H, Taskesen A, Ozdemir M, Altay M. Platelet-rich plasma versus open surgical release in chronic tennis elbow: A retrospective comparative study. J Orthop. 2016;13:10–14. doi: 10.1016/j.jor.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rainys D, Samulėnas G, Kievišas M, Samulėnienė E, Pilipaitytė L, Rimdeika R. Platelet biology and the rationale of PRP therapy in chronic wounds. Eur J Plast Surg. 2017;40:1–10. [Google Scholar]
- 15.Lisi C, Perotti C, Scudeller L, Sammarchi L, Dametti F, Musella V, Di Natali G. Treatment of knee osteoarthritis: Platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018;32:330–339. doi: 10.1177/0269215517724193. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–913. [PubMed] [Google Scholar]
- 17.Wang-Saegusa A, Cugat R, Ares O, Seijas R, Cuscó X, Garcia-Balletbó M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg. 2011;131:311–317. doi: 10.1007/s00402-010-1167-3. [DOI] [PubMed] [Google Scholar]
- 18.Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agel J, LaPrade RF. Assessment of differences between the modified Cincinnati and International Knee Documentation Committee patient outcome scores: A prospective study. Am J Sports Med. 2009;37:2151–2157. doi: 10.1177/0363546509337698. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy N. WOMAC: A 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473–2476. [PubMed] [Google Scholar]
- 22.Dones I, Messina G, Nazzi V, Franzini A. A modified visual analogue scale for the assessment of chronic pain. Neurol Sci. 2011;32:731–733. doi: 10.1007/s10072-011-0570-z. [DOI] [PubMed] [Google Scholar]
- 23.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Król W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: An in vitro study. J Bone Joint Surg Br. 2007;89:417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: Experimental study in a rabbit model. J Oral Maxillofac Surg. 2007;65:1951–1957. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34:589–597. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Zhang CQ. Clinical application and biomaterial properties of platelet-rich plasma. J Clin Rehabil Tissue Eng Res. 2011;8:1445–1448. (In Chinese) [Google Scholar]
- 27.Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, Marcacci M, Kon E. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A randomized controlled trial. Am J Sports Med. 2015;43:1575–1582. doi: 10.1177/0363546515582027. [DOI] [PubMed] [Google Scholar]
- 28.Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, Fornasari PM, Marcacci M. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13(229) doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosnuter M, Aslan C, Isik D, Caliskan G, Arslan B, Durgun M. Functional assessment of autologous platelet-rich plasma (PRP) after long-term storage at -20 ˚C without any preservation agent. J Plast Surg Hand Surg. 2017;51:235–239. doi: 10.1080/2000656X.2016.1237956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.