Abstract
The Amphibia are considered the most threatened vertebrate class globally, yet in Brazil they are also one of the more diverse and species rich groups. Although, in recent years there has been strong focus on amphibian related research, their parasites have not received the same attention. In Brazil, only a single species of Hepatozoon, namely H. leptodactyli (Lesage, 1908) Pessoa, 1970, has been described from anuran hosts. The present study aimed to describe three new species of Hepatozoon parasitising Leptodactylus labyrinthicus and Leptodactylus latrans from Mato Grosso State, Brazil. From 66 anurans screened for haemogregarines, four belonging to the Leptodactylidae were found positive for species of Hepatozoon. Based on the morphological analysis of peripheral blood gamonts and spleen and liver tissue meronts, three different morphotypes of Hepatozoon spp. were identified. Morphotype 1 (M1) and morphotype 2 (M2) in L. labyrinthicus and morphotype 3 (M3) in L. latrans. Molecular data based on partial 18S rDNA sequences revealed an interspecific divergence, between the species ranging from 0.43% to 1.16%. Phylogenetic analysis recovered isolates from the present study monophyletic with other isolates from Brazilian reptile and anuran hosts, sister to a clade comprising species isolated from African, North American and European reptile and anuran host species. Thus, using morphological and molecular analysis three new species infecting Brazilian Leptodactylidae anurans were identified and described. This study increases the knowledge of Brazilian anurans blood parasites and demonstrates the importance of using integrative approaches for diagnosis of hemoparasites.
Keywords: Hemoparasite, Haemogregarine, Anuran, Phylogeny, 18S rDNA
Graphical abstract
Highlights
-
•
Diversity and phylogenetic relationship of haemogregarines from Brazilian anurans.
-
•
Description of a three new species of Hepatozoon from Brazilian anurans.
-
•
First report of species of Hepatozoon descriptions using both morphological and molecular approaches from Brazilian anurans.
-
•
Merogonic data of Hepatozoon spp. infection on Brazilian anurans.
1. Introduction
Brazil is one of the most species rich areas for amphibians globally (Segalla et al., 2016). However, amphibians are also considered as the most threatened vertebrate group with many species facing extinction (Wake and Vredenburg, 2008). Amphibians also host a variety of endoparasites and ectoparasites, which in some cases influence their fitness, behaviour, feeding, reproduction, and fertility (Barta and Desser, 1984; Leal et al., 2015; Laison et al., 2003).
With regards to anuran haemoparasites, intracellular apicomplexans of the family Hepatozoidae Miller, 1908 (Apicomplexa: Adeleorina) are commonly reported (Davies and Johnston, 2000; Leal et al., 2015; Netherlands et al., 2018). The life cycle of species of Hepatozoon is characterized by the formation of syzygy followed by sporogonic development in a definitive host or vector (invertebrate host) following a blood meal from an infected vertebrate (intermediate) host (Desser et al., 1995; Smith, 1996). The intermediate host contains intra-erythrocytic or intra-leukocytic gamonts with merogonic development occurring mainly in the liver and spleen (Desser et al., 1995; Smith, 1996).
In addition, certain vertebrates may also serve as the first intermediate host for species of Hepatozoon that are transmitted to a second intermediate host following the ingestion of the first (Smith, 1996, Viana et al., 2012). Viana et al. (2012) showed that Leptodactylus chaquensis Cei, 1950 may be the first intermediate host for the crocodilian haemoparasites Hepatozoon caimani Carini, 1909, with the presence of cysts occurring in the liver of the frog after ingesting sporulated oocysts from infected Culex Linnaeus, 1758 mosquitoes.
Several species of Hepatozoon have been described in anurans from Africa, Asia, and Europe (Levine, 1988; Netherlands et al., 2014a, 2014b) and according to Netherlands et al. (2018), there are 45 recognized species of Hepatozoon infecting anurans globally. In Brazil the only species of Hepatozoon described from an anuran hosts is Hepatozoon leptodactyli (Lesage, 1908) Pessoa, 1970 infecting species of Leptodactylus based solely on morphological data (Costa et al., 1973). Costa et al. (1973) screened 90 Leptodactylus latrans (Steffen, 1815) and Leptodactylus pentadactylus (Laurent, 1768) reporting 17 infected with H. leptodactyli. Furthermore, the leech Haememteria lutzi Pinto, 1920 was considered as the possible vector of H. leptodactyli (Costa et al., 1973).
However, although there are a number of reports of haemogregarines infecting Brazilian anurans, none were formally described other than H. leptodactyli and Hemolivia stellata Petit, Landau, Baccam and Lainson, 1990. Since the advances in molecular diagnosis, two studies on species of Hepatozoon in Brazilian anurans were conducted (Leal et al., 2015; Ferreira et al., 2020). Leal et al. (2015) collected 145 L. chaquensis and Leptodactylus podicipinus from Mato Grosso do Sul State, (Cope, 1862) finding a prevalence of 26.47% and 31.17%, respectively. Moreover, although, 18S rDNA fragments were obtained from the Leal et al. (2015) study, sequence data was isolated only from blood samples of L. chaquensis and no species were described. In a recent study using only PCR assays to target a fragment of 18S rDNA, Ferreira et al. (2020) investigated the occurrence of apicomplexan parasites in anurans from south-eastern Brazil, successfully amplifying Hepatozoon spp. isolates from a single L. latrans and Rhinella dypctycha (Cope, 1862).
The present study aimed to describe through morphological, morphometric and molecular diagnosis, three new species of Hepatozoon infecting anurans collected at Mato Grosso State, Brazil. Furthermore, this study aimed to increase the knowledge of Brazilian anurans blood parasites and demonstrates the importance of using both molecular and morphological approaches.
2. Material and methods
2.1. Anuran collection
In August of 2018, 66 anurans were captured for a major project of biodiversity of parasites in amphibians (FAPESP 2018/09623–4; FAPESP 2018/00754–9), belonging to eight species, five genera and three families (Table 1). The anurans were collected in the municipality of Nova Xavantina, Mato Grosso State, Brazil (coordinates 14°31′48.03″ S 51°41′43.88″ W). Animals were physically restrained and the blood was collected by puncture of the cervical paravertebral sinus (Zippel et al., 2001) using sterile syringes and needles. During the containment, specimens were examined for ectoparasites, and were subsequently measured and weighed, as well as the sex (male/female) and age of each specimen determined. After the blood collection, three thin blood smears were made on glass slides and the remaining blood sample was stored in EDTA tubes and frozen at −10 °C for further molecular analysis. All applicable international, national, and/or institutional guidelines for the ethical handling of animals were followed (IBAMA license 60640–1; CEUA-UNESP 1061). The access to the genetic data was authorized by the Brazilian Ministry of Environment (Sisgen A21DF67, A132B2E and A9D77FC).
Table 1.
Data on sample number, positivity, and prevalence of anurans collected in 2018, from Mato Grosso State, Brazil, and surveyed for Hepatozoon species.
| Family | Genus | Species | N (%) | P (%) | |
|---|---|---|---|---|---|
| Anuran hosts | Bufonidae | Rhinella | R. diptycha (Cope, 1862) | 13 (19.70) | 0 |
| R. mirandaribeiroi (Gallardo, 1965) | 1 (1.52) | 0 | |||
| Hylidae | Boana | B. raniceps (Cope, 1862) | 6 (9.09) | 0 | |
| Scinax | S. fuscovarius (Lutz, 1925) | 4 (6.06) | 0 | ||
| Trachycephalus | T. typhonius (Linnaeus, 1758) | 2 (3.03) | 0 | ||
| Leptodactylidae | Leptodactylus | L. labyrinthicus (Spix 1824) | 8 (12.12) | 2 (25.0) | |
| L. latrans (Steffen, 1815) | 31 (46.96) | 2 (6.45) | |||
| L. petersii (Steindachner, 1864) | 1 (1.52) | 0 | |||
| Total | 3 | 5 | 8 | 66 (100) | 4 (6.06) |
For histopathological analysis, the anurans were euthanized using 50 mg/kg Tiopentax®, a commercial anaesthetic administered intracerebrally, following the guidelines of Sebben (2007) and the Animal Ethics Committee of Veterinary Medicine. The liver, spleen, heart, and kidney were fixed in 4% buffered neutral formalin and stained with haematoxylin-eosin (Eisen and Schall, 2000).
2.2. Morphological and morphometric analysis
The blood smears were fixed with absolute methanol and stained with 10% Giemsa Methylene Blue Eosin Merck® diluted in distilled water (pH 7.0 for 50min), according to Eisen and Schall (2000). For morphological analysis of the intra-erythrocytic parasite stages, digital images were captured and measured using a compound microscope at 1000 × magnification with the Leica software application suite LAS V3.8 (Leica Microsystems). Measurements are in micrometres (μm) comprising the parasite's length, width and area, with mean and standard deviation (means ± standard deviation) given. Parasitaemia was calculated per 100 erythrocytes, with ~104 erythrocytes examined per blood smear following Cook et al. (2009).
The effect of the parasite on the erythrocytes was evaluated by the comparison of infected and non-infected parameters using the non-parametric Mann-Whitney Test, with a significance level of 5%.
2.3. Molecular analysis
DNA was extracted from whole blood and tissue samples following the blood protocol of the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA). Two PCR assays were performed targeting two different regions of the 18S rDNA of apicomplexan parasites using the HepF300 and Hep900 pair of primers, which amplifies 600 bp (Ujvari and Marques, 2005) and Hemo1 and Hemo2 pair of primers, which amplifies 900 bp (Perkins and Keller, 2001). PCR amplification reactions were carried out in a final volume of 25 μL, containing 1 μL each of 10 pmol primers, 12.5 μL of Master Mix MyFi™ Mix Bioline®, and 5 μL of extracted DNA, with nuclease-free water accounting for the remaining volume. PCR amplification was performed on a Peltier 200 Thermocycler (MJ Research, Watertown, MA) following the conditions of O'Dwyer et al. (2013).
PCR products were subjected to gel electrophoresis at 80 V in a 1.5% agarose gel, stained with Gel Red, and observed using an ultraviolet transilluminator. The products of interest were purified by adding 2 μL of ExoSAP-IT® (Affymetrix, Santa Clara, CA, USA) to 5 μL of PCR product according to the manufacturer's recommendations. Amplicons were then sequenced using PCR primers on a 3500 Genetic Analyzer capillary sequencer (Applied Biosystems) and after BigDye Terminator Cycle Sequencing Ready Reaction Kit v.3.1 (Applied Biosystems) according to the manufacturer's recommendations. A consensus sequence was created from the forward and reverse assembled electropherograms using Geneious version 7.1.3 (Kearse et al., 2012). For each positive animal, the newly generated sequences from HepF300/Hep900 and Hemo1/Hemo2 contigs were concatenated using Geneious version 7.1.3 (Kearse et al., 2012), forming a long sequence of ~1300 bp.
The sequences from this study were compared with other isolates from haemogregarine parasites available on GenBank. The newly generated sequences of partial 18S rDNA were aligned using Geneious version 7.1.3 (Kearse et al., 2012) with MUSCLE algorithm implemented from within Geneious version 7.1.3 (Biomatters, www.geneious.com) and default settings with related sequences that appeared on Blastn search. Two alignments were made, one with long sequences available on Genbank, with final alignment of 1116 bp, and another with short sequences with 582 bp. The phylogenetic analyses were performed using both alignments using the Bayesian inference (BI) and Maximum Likelihood (ML) methods. For ML method, JModelTest v.2.1.10 (Darriba et al., 2012) was used to identify the best evolutionary model. Based on Akaike information criterion (AIC) the Transitional model (Posada, 2003) with a discrete Gamma distribution (TVM + G) was chosen. Phylogenetic analysis was inferred using PhyML (Guindon and Gascuel, 2003) with 1000 replicate bootstraps (>50%). The BI analysis was carried out using MrBayes implemented from the computational resource CIPRES (Miller et al., 2010), the best BIC score was the General Time Reversible model (GTR + I + Γ) (Tavaré, 1986). The Markov chain Monte Carlo (MCMC) algorithm was run for 10,000,000 generations, sampling one tree every 1000 generations. On the burn-in, the first 25% of generations were discarded, and the consensus trees were estimated using the remaining trees. Bayesian posterior probabilities (BPP) cut off was considered > 50%.
The isolates from adeleorinid parasites (Haemogregarinidae, Hepatozoidae, Karyolysidae, and Dactylosomatidae) available from GenBank were used to construct both trees. The resultants phylogenetic trees for both BI and ML analysis were edited in FigTree v1.4 (Rambaut, 2012). Adelina dimidiata Schneider, 1875, Adelina grylli Butaeva, 1996 [GenBank: DQ096835–DQ096836], Klossia helicina Schneider, 1875 [GenBank: HQ224955] and Klossiella equi [GenBank: MH211602] from the suborder Adeleorina were selected as outgroups following Netherlands et al. (2020). A pair-wise distance (p-distance) matrix was used to compare the interspecific divergence between species of Hepatozoon sequences isolated from snakes and anurans hosts (Table 2).
Table 2.
The shaded matrix (upper) shows the p-distance (pair-wise distance) and the non-shaded matrix (lower) shows the percentage of similarity (%) of the nucleotide sequences among the Hepatozoon sequences from anurans and snakes available at Genbank (582 nt).
3. Results
Of the 66 captured anurans (Table 1), four specimens of Leptodactylidae were found infected with Hepatozoon spp.: one adult female Leptodactylus labyrinthicus (Spix, 1824) (73.88 mm snout-vent length and weight of 29 g); one adult male L. labyrinthicus (66.72 mm snout-vent length and weight of 23 g); one adult male L. latrans (58.23 mm snout-vent length and weight of 13 g); and one adult female L. latrans (52.80 mm snout-vent length and weight of 9 g). The total prevalence found was 6.06% (4/66); the prevalence of L. labyrinthicus and L. latrans were 25% (2/8) and 6.45% (2/31), respectively.
Based on the morphological analysis of peripheral blood gamonts and spleen and liver tissue meronts it was possible to identify three different species of Hepatozoon morphotypes. Morphotype 1 (M1) found in one L. labyrinthicus, morphotype 2 (M2) in one L. labyrinthicus, and morphotype 3 (M3) in two L. latrans.
3.1. Species description
Phylum: Apicomplexa Levine 1970
Class: Conoidasida Levine, 1988.
Subclass: Coccidia Leuckart 1879
Order: Eucoccidiorida Léger 1911
Suborder: Adeleorina Léger 1911
Family Hepatozoidae Wenyon, 1926
Genus Hepatozoon Miller, 1908
3.1.1. Hepatozoon formosus Úngari, Netherlands, Silva and O'Dwyer n. sp.
Type-host: Leptodactylus labyrinthicus (Anura: Leptodactylidae).
Type-locality: Municipality of Nova Xavantina, Mato Grosso State, Brazil (coordinates 14°35′47″ S 51°43′9,59″ W).
Site of infection: Peripheral blood erythrocytes, and liver and spleen fragment tissues.
Vector: Unknown.
Etymology: The morphology of gamonts and meronts have shown richness in details and development stages. Thus, the word “formosus” is the Latin translation of the word “beautiful” in English.
Parasitaemia: The parasitaemia of L. labyrinthicus was 1.17%.
Prevalence: The total prevalence found was 1.51% (1/66) and 12.5% (1/8) among the L. labyrinthicus individuals analysed.
Material deposited: Hapantotypes, one blood smear from L. labyrinthicus, one histological slide of the liver, and one histological slide of the spleen, were deposited in the collection of the National Institute of Amazonian Research (INPA), Manaus, Brazil [INPA20].
Gene sequence: The 18S rDNA sequence obtained with 1384 bp from a pepper frog L. labyrinthicus liver tissue was deposited in GenBank under accession number [MW584356].
3.1.1.1. Description
Gamonts (Fig. 1): only infecting host erythrocytes and varying in the developmental stage (n = 50).
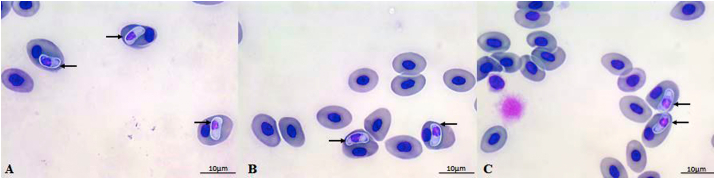
Fig. 1.
(A–F). Different gamonts developmental stages of Hepatozoon formosus n. sp. in the blood smears of Leptodactylus labyrinthicus from Mato Grosso State, Brazil (indicated by the arrows). A-C) Early-stage gamonts; D-E) Immature gamonts; F) Mature gamonts. Scale bar: 10 μm.
Early-stage gamonts (Fig. 1 A - C): Varying from small to slightly larger, tapering towards one end, rounded and robust to the other end, with or without cytoplasmic vacuoles observed in the tapering end, and no capsule evidenced; measurements of 7.83 ± 0.98 μm long (7.29–8.50), 3.05 ± 0.23 μm (2.81–3.33) wide, with an area of 19.08 ± 1.02 μm2 (17.87–20.33). Dense nuclei, stained dark-purple and occupying almost the entire parasite; measurements of 4.09 ± 2.48 μm (2.81–5.70) long, 4.04 ± 2.18 μm (2.9–4.58) wide, with an area of 14.81 ± 1.23 μm2 (11.88–16.76).
Immature gamonts (Fig. 1 D - E): Larger size, with both ends rounded with one end slightly larger, with or without cytoplasmic vacuoles, and no capsule evidenced; measurements of 9.33 ± 0.16 μm (9.17–9.88) long, 3.43 ± 0.11 μm (3.15–3.83) wide, with an area of 21.27 ± 0.74 μm2 (20.33–22.38). Quadrangular, dense, and reduced nuclei, staining dark-purple; measurements of 5.04 ± 1.35 μm (4.79–6.07) long, 3.53 ± 0.56 μm (3.15–3.83) wide, with an area of 16.60 ± 0.19 μm2 (14.15–17.16).
Mature gamonts (Fig. F): The largest of the developmental gamonts stages. Robust and elongated, with both ends rounded, thin parasitophorous capsule (sometimes not evidenced), cytoplasm stained whitish without cytoplasmic vacuoles; measurements of 11.93 ± 0.61 μm (9.82–13.67) long, 4.18 ± 0.24 μm (3.93–4.67) wide, with an area of 40.20 ± 3.01 μm2 (37.76–44.00). Dense and quadrangular nuclei, located centrally of the parasite body, stained dark-purple; measurements of 5.77 ± 0.23 μm (5.57–5.97) long, 3.91 ± 0.23 μm (3.33–3.93) wide, with an area of 17.29 ± 1.29 μm2 (15.26–18.77).
Effects on the host cell: Early-stage gamonts do not cause displacement of the host cell nucleus or any morphological changes to the host cell. Immature gamonts, in some cases, cause a slight displacement of the host cell nucleus. Mature gamonts cause displacement of the host cell nucleus and slight morphological changes to the erythrocyte. Infected erythrocytes measured 16.85 ± 1.04 μm (15.39–18.45) long (U = 692.0; p = 0.601) and 12.43 ± 1.22 μm (10.90–14.89) wide (U = 1121.0; p ≤ 0.001) with an area of 145.59 ± 4.10 μm2 (133.64–164.44) (U = 826.0; p = 0.033) (n = 20). Non-infected erythrocytes measured 16.84 ± 0.37 μm (16.33–17.54) long and 9.94 ± 1.39 μm (8.05–11.59) wide with an area of 136.53 ± 4.32 μm2 (125.42–147.16) (n = 50).
Tissue merogony (Fig. 2, Fig. 3): the liver and spleen from L. labyrinthicus presented a number of meronts with merozoites of Hepatozoon formosus n. sp. The meronts varied in size and morphology, it was observed one macromeront with 4 macromerozoites were lodged in the spleen and micromeronts with 16–35 micromerozoites lodged in the liver and spleen.
Fig. 2.
(A–F). Histological slides of Leptodactylus labyrinthicus liver with Hepatozoon formosus n. sp. tissue merogony. A-C) An overview showing the amount and diversity of tissue merogonic stages infecting the anurans. D-F). Different developmental stages of meronts with merozoites. D) Macromeronts with four macromerozoites indicated by the arrow. E-F) Stages of micromeronts with micromerozoites. Scale bar: 100 μm and 20 μm.
Fig. 3.
(A–F). Histological slides of the Leptodactylus labyrinthicus spleen with Hepatozoon formosus n. sp. micromeronts with micromerozoites in different developmental stages. A, B, C and F) Early stage of micromeronts indicated by the arrow. Scale bar: 20 μm.
Macromeront (Fig. 2D arrow): Rounded cyst with thick cell wall (8.08 μm) containing four large macromerozoites with a granular cytoplasm staining pinkish purple, the merozoite nuclei are dense, rounded, and stained dark-purple. meront measured 27.98 μm long, 28.50 μm wide with an area of 600.93 μm2. Including wall thickness, the macromerozoites measured 15.30 ± 0.83 μm long × 7.05 ± 0.71 μm wide, and area of 99.85 ± 1.23 μm2.
Micromeronts (Fig. 2, Fig. 3): Rounded to ovoid meronts in different developmental stages, with wall thickness measuring 6.03 ± 2.02 μm (4.01–9.15). The meronts measured 37.62 ± 1.34 μm (32.86–41.46) long, 36.84 ± 1.28 μm (31.23–39.96) wide with an area of 937.95 ± 33.69 μm2 (827.02–1212.90) (n = 15) (including wall thickness). It was possible to observe between 16 and 35 micromerozoites in different processes of development, with early stage meronts showing a layer of micromerozoites, stained purplish, closer to the wall and a mass located in the middle of the cyst stained whitish (arrow-head). Micromerozoites are slender and long, measuring 7.49 ± 0.72 μm (5.99–9.64) long, 1.93 ± 0.16 μm (1.44–2.17) wide, with an area of 14.77 ± 0.87 μm2 (10.35–18.54); with a long, rectangular and dense nuclei, stained dark-purple, measuring 4.32 ± 0.82 μm (3.15–5.39) long, 1.82 ± 0.30 μm (1.40–2.79) wide, with an area of 5.85 ± 0.59 μm2 (3.04–8.88) (n = 30).
3.1.1.2. Remarks
Based on morphology and morphometry of peripheral blood and tissue stages from L. labyrinthicus, Hepatozoon formosus n. sp. does not conform morphologically to H. leptodactyli (currently the only recognized species of Hepatozoon from Brazilian anurans); or to any other species of Hepatozoon described from anurans worldwide.
The gamonts of H. leptodactyli occurred both as intracellular and extracellular forms; the immature gamonts are characterized by a crescent or roundish form with no cytoplasmic vacuoles; mature gamonts presented a visible distinct capsule with a curved pole and ranged in size from 5.1 μm long × 2.3 μm wide to 21 μm long × 8.3 μm wide. Furthermore, gamonts of H. leptodactyli often cause hypertrophy to the host cell, displacement of host nucleus, and multiple gamonts have been reported to parasitize a single cell. Whereas for H. formosus n. sp. only erythrocytic gamonts were observed; immature gamonts are smaller, tapering towards one end and rounded to the other end, and in some cases, vacuoles are present; cause no displacement of the host cell nucleus or morphological changes to the host cell; mature gamonts are smaller compared to H. leptodactyli and possess a capsule (not frequently observed) with both ends of the gamont rounded. Mature gamonts also cause hypertrophy of the host cell and slight displacement of the host cell nucleus, but no multiple infections were observed.
Tissue stages of H. leptodactyli were also reported in liver and spleen tissue. The micromeronts measured 13 μm long × 16 μm wide containing between 16 and 32 micromerozoites, with average of 2.5 μm long × 3 μm wide. Macromeronts measured 14 μm long × 14.7 μm wide containing between 2 and 8 macromerozoites measuring an average of 18 μm long × 8 μm wide. Macromeront and micromeronts of Hepatozoon formosus n. sp. from the current study are also reported. Macromeront measured 27.98 μm long × 28.50 μm wide with 4 macromerozoites measuring 15.30 μm long × 7.05 μm wide. Micromeronts measured an average of 37.62 μm long × 36.84 μm wide possessing between 16 and 35 micromerozoites measuring 7.49 μm long × 1.93 μm wide.
Hepatozoon formosus n. sp. most closely resembles the Hepatozoon sp. reported by Leal et al. (2015) in two Brazilian anurans (L. chaquensis and L. podicipinus). As in the present study, these authors observed early stage gamonts with the nuclei occupying almost the entire parasite. However, the authors also reported extraerythrocytic gamonts with varying morphological characteristics, measuring 9.7 μm long × 4.5 μm wide from L. chaquensis and 13.95 μm long × 4.6 μm wide from L. podicipinus, with nuclei occupying the entire host cell or rounded dislocated by one end of the parasite; the nuclear chromatin ranging from fragmented to dense and stained from blue-gray to pink (Leal et al., 2015); all of these characteristics differed from those observed in Hepatozoon formosus n. sp.
3.1.2. Hepatozoon longinucleus Úngari, Netherlands, Silva and O'Dwyer n. sp.
Type-host: Leptodactylus labyrinthicus (Anura: Leptodactylidae).
Type-locality: Municipality of Nova Xavantina, Mato Grosso State, Brazil (coordinates 14°35′47″ S 51°43′9,59″ W).
Site of infection: Peripheral blood erythrocytes.
Vector: Unknown.
Etymology: During morphological analysis of the mature gamonts, a unique characteristic was observed. The gamont nucleus is long, occupying ¾ of the parasite's length. This character has not been observed in other species of Hepatozoon from anurans globally. The word “longinucleus” is a combination of the Latin words “longus” and “nucleus".
Parasitaemia: The parasitaemia of L. labyrinthicus was 0.2%.
Prevalence: The total prevalence found was 1.51% (1/66) and 12.5% (1/8) among the L. labyrinthicus individuals analysed.
Material deposited: Hapantotype, one blood smear from L. labyrinthicus deposited in the collection of the National Institute of Amazonian Research (INPA), Manaus, Brazil [INPA22].
Gene sequence: The 18S rDNA sequence obtained with 1396 bp from a pepper frog L. labyrinthicus blood was deposited in GenBank under accession number [MW584361].
3.1.2.1. Description
Gamonts (Fig. 4): Elongated gamonts only found infecting host erythrocytes with a thin parasitophorous capsule (in some cases not evidenced), both ends rounded with one slightly tapered and more curved than the other, with cytoplasm stained purplish-blue; gamonts measured 12.58 ± 0.62 μm (13.27–11.50) long and 4.17 ± 0.41 μm (4.36–2.40) wide, with an area of 42.24 ± 5.94 μm2 (43.35–31.37). Thin and elongated nucleus with dense chromatin stained purple, slightly displaced to one side of the parasite and occupying ¾ of the parasite body; measuring 9.21 ± 0.32 μm (10.74–7.50) long and 2.18 ± 0.54 μm (2.94–1,71) wide, and area of 19.16 ± 1.04 μm2 (20.98–16.78) (n = 15).
Fig. 4.
(A–C). Gamonts of Hepatozoon longinucleus n. sp. in the blood smears of Leptodactylus labyrinthicus from Mato Grosso State, Brazil (indicated by the arrows). Scale bar: 10 μm.
Effects of host cell: Gamonts cause displacement of the host cell nucleus. In some cases, the parasite causes morphological changes in the host cell (hypertrophy). Infected erythrocytes measured 17.65 ± 2.74 μm (16.16–19.67) long (U = 344.0; p = 0.016) and 12.97 ± 1.40 μm (10.98–13.24) wide (U = 278.0; p ≤ 0.001) with an area of 158.82 ± 3.20 μm2 (142.85–170.97) (U = 168.0; p ≤ 0.001) (n = 15). Non-infected erythrocytes measured 16.93 ± 1.07 μm (18.49–15.43) long and 11.03 ± 0.40 μm (9.58–11.90) wide with an area of 140.42 ± 5.21 μm2 (133.18–151.72) (n = 50).
3.1.2.2. Remarks
Based on gamont morphology and its elongated nucleus observed in the peripheral blood of L. labyrinthicus, Hepatozoon longinucleus n. sp. does not resemble Hepatozoon formosus n. sp., H. leptodactyli or any other currently recognized species of Hepatozoon from anurans globally.
With regards to H. leptodactyli, extracellular gamonts, intracellular early-stage and intracellular mature gamonts were reported with measurements ranging from between 5.1 μm long × 2.3 μm wide to 21 μm long × 8.3 μm wide; the nuclei is located in median or subterminal portion varying from granules and threads to compact oval or rounded; a visible capsule and curved pole were observed, and red staining cytoplasmic inclusions are visible when stained with Giemsa. In comparison to Hepatozoon longinucleus n. sp., mature gamonts are in some cases folded within a capsule, all intracellular measuring 12.58 μm long × 4.17 μm wide, with a relatively long nuclei occupying 2/3 of parasites length, furthermore no red inclusions were observed, the parasite's cytoplasm stained purplish-blue and the nuclei purplish.
The main difference between H. formosus n. sp. and H. longinucleus n. sp. is gamont morphology. Hepatozoon formosus n. sp is characterized by the presence of different gamont stadium of infection, ranging from early to mature stages, as compared to Hepatozoon longinucleus n. sp. of which only mature stages were observed. However, these differences could be related to the recent infection of H. formosus n. sp. in its host, as compared to H. longinucleus n. sp. and its host. Mature gamonts of H. formosus n. sp. are robust and elongated, with both ends rounded, containing a dense and quadrangular nucleus, located centrally to the parasites body. While, H. longinucleus n. sp. is characterized by elongated gamonts with one end slightly tapered and more curved than the other, cytoplasm stained purplish-blue and an elongated nucleus slightly displaced to one side of the parasite and occupying ¾ of the parasite's body.
Compared to other haemogregarines reported in Brazilian frogs, Sousa and Filho (1974) observed a haemogregarine-like parasite that was not identified to genus level, with varying morphology ranging from small gamonts (7.7 μm long × 1.7 μm wide) to larger gamonts (9.8 μm long × 3.4 μm wide). Leal et al. (2015) also reported extraerythrocytic and intraerythrocytic gamonts with a variety of sizes and shapes belonging to species of Hepatozoon. Parasite morphometrics and measurements cited above does not compare to H. longinucleus n. sp., differing in size, shape and structure of mature gamonts, and position and size of the nucleus.
3.1.3. Hepatozoon latrensis Úngari, Netherlands, Silva and O'Dwyer n. sp.
Type-host: two specimens of Leptodactylus latrans (Anura: Leptodactylidae).
Type-locality: Municipality of Nova Xavantina, Mato Grosso State, Brazil (coordinates 14°35′47″ S 51°43′9,59″ W).
Site of infection: Peripheral blood erythrocytes, liver, and spleen fragment tissues.
Vector: Unknown.
Etymology: This new species of Hepatozoon was found in two anuran hosts, belonging to the species L. latrans. Therefore, the name “latrensis” refers to the host species, translated into Latin nomenclature.
Parasitaemia: The parasitaemia found in the anurans was 0.5% and 18.21%.
Prevalence: The total prevalence found was 3.03% (2/66) and 6.45% (2/31) among L. latrans analysed.
Material deposited: Hapantotypes, one blood smear from L. latrans, one histological slide of the liver, and one histological slide of the spleen, were deposited in the collection of the National Institute of Amazonian Research (INPA), Manaus, Brazil [INPA21].
Gene sequence: The 18S rDNA gene sequences obtained, with 1403 bp and 1343 bp, from the two pepper frog L. latrans blood were deposited in GenBank under accession number [MW584362 and MW584365].
3.1.3.1. Description
Gamonts (Fig. 5): Observed infecting host erythrocytes, robust with both ends rounded, parasitophorous capsule evident, cytoplasm staining whitish-purple with cytoplasmic vacuoles visible in some cases; gamonts measuring 9.88 ± 0.56 μm (9.06–10.74) long and 4.35 ± 0.41 μm (3.27–5.02) wide, with an area of 37.46 ± 3.00 μm2 (34.91–43.95). Nuclei rounded to quadrangular in shaped, staining purplish-blue, large in width, almost the same as the parasite, slightly displaced to one of the extremities and occupying 1/3 of the parasite's body; measuring 4.17 ± 0.57 μm (3.13–5.02) long and 3.37 ± 0.60 μm (2.67–4.02) wide, with an area of 8.87 ± 1.54 μm2 (7.02–9.13) (n = 15).
Fig. 5.
(A–C). Gamonts of Hepatozoon latrensis n. sp. in the blood smears of two Leptodactylus latrans from Mato Grosso State, Brazil (indicated by the arrows). Scale bar: 10 μm.
Effects of host cell: The parasite causes displacement of the host cell nucleus to its extremities, morphological changes in the host cell related to the cell structure, and hypertrophy. Infected erythrocytes measured 16.35 ± 0.45 μm long (U = 860.5; p ≤ 0.001) and 10.02 ± 0.31 μm wide (U = 344.0; p = 0.003), with an area of 133.93 ± 0.51 μm2 (U = 915.0; p ≤ 0.001) (n = 15). Non-infected erythrocytes measured 14.10 ± 0.28 μm long and 10.74 ± 1.01 μm wide, with an area of 113.86 ± 0.78 μm2 (n = 50).
Tissue merogony (Fig. 6A–F): Histological sections of liver and spleen revealed the presence of micromeronts with micromerozoites, macromeronts with macromerozoites, and a single dizoic cyst.
Fig. 6.
(A–F). Histological sections of two Leptodactylus latrans anurans livers with Hepatozoon latrensis n. sp. tissue merogony. Histological slides of the liver from one anuran specimen showing macromeronts with macromerozoites (A and B); micromeronts with micromerozoites (C–E) and dizoic cyst with cystozoites (F). D-E) Micromeronts with merozoites of the liver from the second anuran specimen. Macromeronts (Ma), micromeronts (Mi), and dizoic cyst (DC) are indicated by arrows. Scale bar: 100 μm and 20 μm.
Macromeronts (Fig. 6 A and B): Ovoid meronts measuring 23.19 ± 1.23 μm (21.38–26.50) long, 19.66 ± 0.85 μm (15.64–22.11) wide and 347.32 ± 35.78 μm2 (307.01–479.29) area, with an irregular shaped wall measuring 7.95 ± 1.28 μm (5.30–9.87) (n = 2). Each macromeront contained between 3 and 5 visible macromerozoites, with cytoplasm granular and staining purplish-pink, in some cases vacuoles also visible. Macromerozoites measured 18.25 ± 0.15 μm (18.04–18.70) long and 5.70 ± 0.37 μm (5.03–5.84) wide, with an area of 69.74 ± 0.45 μm2 (69.31–69.80). Nuclei are rounded, compact and dense, staining dark-purple, measuring 2.17 ± 0.48 μm (1.98–2.30) long and 2.10 ± 0.15 μm (1.99–2.40) wide, with an area of 4.53 ± 0.84 μm2 (4.35–5.01) (n = 8).
Micromeronts (Fig. 6 C - E): Rounded early stages meronts of development measuring 15.08 ± 0.93 μm (15.23–16.98) long, 14.99 ± 1.53 μm (13.99–17.86) wide, 212.22 ± 28.17 μm2 (195.17–298.15) area, with meronts encased in an irregular wall measuring 4.01 ± 1.20 μm (3.24–7.48) (n = 5). A whitish-purple to pink stained mass, likely from where merozoite formation is initiated (B and E), also visible. Micromeronts were only observed in early stages of development and could not be measured or counted, however, possible early stages nuclei formation visible, with chromatin forming dense rounded structures staining dark-purple.
Dizoic cyst (C): Ovoid cyst with thick wall (7.09 μm) containing two slender and long cystozoites with cytoplasm staining purplish-pink. Cystozoite nuclei rounded, compact and staining dark-purple. Cyst measured 17.17 μm long and 23.11 μm wide, with an area of 297.07 μm2 (measurements including wall); respective cystozoites measured 11.25 and 12.07 μm long, and 2.26 and 3.01 μm wide, with an area of 27.08 and 30.07 μm2 respectively; cystozoites nuclei measured 2.04 and 2.19 μm long and 1.21 and 1.33 μm wide, with an area of 3.08 and 3.71 μm2.
3.1.3.2. Remarks
Based on gamont and meront morphology and morphometrics observed in the peripheral blood and tissue of L. labyrinthicus, Hepatozoon latrensis n. sp. does not conform to H. formosus n. sp., H. longinucleus n. sp., H. leptodactyli or any of other species of Hepatozoon from anurans worldwide.
Hepatozoon leptodactyli share certain characteristics with Hepatozoon latrensis n. sp., such as nucleus size and the presence of a visible capsule. However, H. latrensis n. sp. differs from H. leptodactyli in average size of mature gamonts stage measuring 9.88 μm long × 4.35 μm wide, the absence of red inclusions in the cytoplasm, both ends of the parasite are equal and rounded, with a round shaped nuclei positioned towards one end of the gamont occupying in some the entire width of the parasite's body and staining purplish-blue.
With regards to the tissue merogony of H. leptodactyli high parasitaemia was reported in the lung, intestine, heart, and brain; the micromeronts average size was 13 μm wide × 16 μm long, with between 16 and 32 micromerozoites visible measuring an average of 3 μm long × 2.5 μm wide; rounded macromeronts of H. leptodactyli measured an average of 14 μm long × 14.7 μm wide with 2–8 macromerozoites measuring 18 μm long × 8 μm wide. Moreover, the meronts from H. latrensis n. sp. were observed in different site of infection (spleen and liver); macromeronts of H. latrensis n. sp. are larger and oval (not rounded as in H. leptodactyli), measuring 23.19 μm long × 19.66 μm wide with 3–5 macromerozoites measuring 18.25 μm long and 5.70 μm wide.
Hepatozoon formosus n. sp. and H. longinucleus n. sp. differ in comparison to H. latrensis n. sp. Mature gamonts of the later species being smaller in size (9.88 ± 0.56 μm long × 4.35 ± 0.41 μm wide with 37.46 ± 3.00 μm2 area). Hepatozoon formosus n. sp. measures 11.93 ± 0.61 μm long, 4.18 ± 0.24 μm wide with an area of 40.20 ± 3.01 μm2 and H. longinucleus n. sp. measures 12.58 ± 0.62 μm long × 4.17 ± 0.41 μm wide with an area of 42.24 ± 5.94 μm2. Moreover, H. formosus n. sp. have a square-shaped nucleus extending the width of the parasite and H. longinucleus n. sp. contains a long-tapered nucleus, compared to the centrally placed rounded nucleus of H. latrensis n. sp.
Regarding the Hepatozoon from Brazilian snakes, Hepatozoon musa described from the snake Philodriyas nattereri Steindachner, 1870 from Ceará State, north-eastern Brazil have mature gamonts, measuring 16.568 μm long × 3.095 μm wide and area of 59.710 μm2, characterized by considerable curvature at both ends and nuclei typically placed centrally, differing from the mature gamonts of H. latrensis n. sp., which are smaller, nucleus is displaced to one end and both ends of the parasite are rounded and not curved.
3.2. Molecular and phylogenetic analysis
From the four positive specimens, four newly generated sequences were obtained. Amplicons of 1384 and 1396 bp derived from H. formosus n. sp. [MW584356] and H. longinucleus n. sp. [MW584361], respectively, isolated from the blood of L. labyrinthicus, and two sequence fragments of 1403 and 1343 bp sharing 100% identity were derived from H. latrensis n. sp. isolated from the blood L. latrans [MW584362/MW584365].
From the two alignments provided, 582 bp and 1116 bp, the phylogenetic analyses using BI and ML methods from each one alignment resulted in identical tree topologies (Fig. 7, Fig. 8). In the present study's phylogenetic analyses from both alignments, species of Dactylosoma isolated from anurans were recovered as the earliest diverging clade of the ingroup, sister to the monophyletic clade of Haemogregarina from chelonids and all other haemogregarines (Karyolysidae and Hepatozoidae) analysed. Isolates from Hemolivia clustered together; separate from monophyletic Karyolysus spp. and polyphyletic Hepatozoon spp. Isolates from Hepatozoon isolated from large mammals formed a monophyly sister to the Karyolysidae Clade comprising species of Karyolysus.
Fig. 7.
Consensus phylogram of haemogregarines based on 18S rDNA sequences (582 nt). The topology trees were inferred by Bayesian (BI) and Maximum Likelihood (ML) methods (represented by ML tree). The isolates Adelina dimidiata (DQ096835), Adelina grylli (DQ096836), Klossia helicina (HQ224955), and Klossiela equi (MH211602) were used as an out-group.
Fig. 8.
Consensus phylogram of haemogregarines based on 18S rDNA sequences (1116 nt). The topology trees were inferred by Bayesian (BI) and Maximum Likelihood (ML) methods (represented by ML tree). The isolates Adelina dimidiata (DQ096835), Adelina grylli (DQ096836), Klossia helicina (HQ224955), and Klossiela equi (MH211602) were used as an out-group.
With regards to the phylogenetic analysis based on the alignment comprising shorter sequence fragments (Fig. 7), the species of Hepatozoon from the present study H. formosus n. sp. [MW584356], H. longinucleus n. sp. [MW584361], and H. latrensis n. sp. [MW584362/MW584365] grouped within other Hepatozoon isolates from Brazilian anurans [MK503646, MK503647, MK503645, MK503648 and MK503643]. The isolates from Brazilian anurans formed a sister group to a clade comprising species of Hepatozoon from Brazilian snakes, Hepatozoon musa Borges-Nojosa et al., (2017) [MF497767], Hepatozoon sp. [MF497768], and Hepatozoon cuestensis O'Dwyer et al., (2013) [MF497077].
Concerning the phylogenetic analysis with longer sequences (Fig. 8), the species of Hepatozoon from the present study grouped in the same clade but different branches with four isolates from snakes: Hepatozoon domerguei Maia et al. 2014 reported in two Madagascar snakes [KM234646 and KM234648]; Hepatozoon ayorgbor Sloboda et al. 2007 reported in a snake from Gana [EF157822]; Hepatozoon chinensis Wu et al. 2013 reported in a snake from China [KF939620] and Hepatozoon sp. Boiga from Australia [AF297085]. Furthermore, in the both phylogenetic trees, species of Hepatozoon isolated from Brazilian anurans were recovered as distantly related to other species of Hepatozoon from North American, European and African anurans.
With regards to gene similarity and pair-wise distance data from the tree (Table 2), the three new species from the present study have shown an interspecific divergence ranging from 0.43% to 1.16%. Hepatozoon formosus n. sp. reported an interspecific divergence of between 0.93 and 8.01% and H. longinucleus n. sp. showed a divergence of between 0.43%–8.39% in comparison with other species of Hepatozoon from reptile and anuran hosts. Hepatozoon latrensis n. sp., showed 100% of gene similarity with four Hepatozoon sp. isolates [MK503646, MK503647, MK503645, and MK503648] reported previously reported from Brazilian anurans (Ferreira et al., 2020).
4. Discussion
Currently, 7166 anuran species have been described around the world, with 1039 species reported from Brazil (Frost, 2020). In addition, anurans are known to harbour a great number of haemoparasites, with haemogregarines being the most commonly reported (Barta and Desser, 1984; Smith, 1996; Telford, 2009). In the present study anurans of Bufonidae, Hylidae, and Leptodactylidae were screened for intracellular blood parasites, however, only species from Leptodactylidae were positive for species of Hepatozoon. Barta and Desser (1984) evaluated the prevalence of haemoparasites in blood smears from Canadian frogs and found higher prevalence among aquatic lifestyle frogs when compared to terrestrial ones. Another study conducted in South Africa analysed the diversity of haemoparasites from anurans with different lifestyles (aquatic, semi-aquatic, semi-terrestrial, and terrestrial), and also reported a higher prevalence of species of Hepatozoon among semi-aquatic and semi-terrestrial anurans (Netherlands et al., 2015). These authors suggest that the number of potential vectors may be higher surrounding aquatic habitats, as compared to terrestrial habitats (Netherlands et al., 2015). Furthermore, a recent study in Brazil by Ferreira et al. (2020) reported higher prevalence for haemogregarine parasites in the semi-aquatic nocturnal L. latrans (100%) as compared to terrestrial nocturnal R. diptycha. Therefore, the present study corroborates with those described above, since the Leptodactylidae includes species with a semi-aquatic lifestyle. Moreover, although there are only a few reports on Hepatozoon prevalence among Brazilian frogs, individuals of Leptodactylidae are commonly reported to be parasitised by species of Hepatozoon (Cunha and Muniz, 1927; Da Costa, et al. 1973; Leal et al., 2015; Ferreira et al., 2020).
Cunha and Muniz (1927) reported in detail the schizogony in the liver, lung, and intestine of a haemogregarine infecting Leptodactylus latrans from Brazil. Another study, by Da Costa et al. (1973) used morphological and morphometric data, to formally described H. leptodactyli, infecting individuals of Leptodactylidae. From 90 Leptodactylus pentadactylus and L. latrans analysed, only 17 (17.17%) frogs were positive for H. leptodactyli. In this study, from 40 Leptodactylidae frogs collected, only four (10%) were positive. Moreover, using microscopy screening, Leal et al. (2015) reported on the presence of species of Hepatozoon in blood smears of eight (11.76%) L. chaquensis and two (2.60%) L. podicipinus from Mato Grosso do Sul State.
With regards to other Brazilian anurans, Sousa and Filho (1974) reported a 1% prevalence of species of Haemogregarina (this was most likely an incorrect identification of species of Hepatozoon) from 100 anurans screened. Intra-erythrocytic gamonts infecting the blood smears of one Rhinella crucifer (Wied-Neuwied, 1821) (syn. Bufo crucifer) from Rio de Janeiro State, Brazil, were found with a parasitaemia of 0.5%. In another study by Kattar (1986), from the 100 Brazilian anurans analysed, eight (8%) were positive for haemogregarine parasites infecting blood smears of Rhinella diptycha (Cope, 1862) (syn. Bufo paracnemis) collected at the municipality of João Pessoa, Paraíba State, Brazil. However, gametocyte morphology conforms rather to species of Hemolivia as compared to species of Hepatozoon.
In relation to Leal et al. (2009), the authors reported a 10% prevalence of haemogregarines in three Brazilian frog species, Leptodactylus chaquensis Cei, 1950, L. podicipinus Cope, 1862, and Pithecopus hypochondrialis (Daubin, 1800), from Mato Grosso do Sul State and São Paulo State. The parasitaemia found in this study was 1.17% for H. formosus n. sp. 0.2% for H. longinucleus n. sp., and 0.5%, and 18.21% for H. latrensis n. sp. These findings may be related to the physiological and immunological patterns of the host and also, the parasite pathogenicity. Lower parasitaemia can be associated with a chronic state of infection, resulting in a cyclic merogonic phase, to maintain the infection for a long time (Tomé et al., 2012). Besides, Tender et al. (2000) suggest a relationship between species of Hepatozoon and Toxoplasma gondii infections, which can persist throughout the hosts life.
There are only a few recognized species of Hepatozoon from anurans globally that have been characterized using morphological, morphometric, and molecular tools. Namely two species of Hepatozoon from North America, Hepatozoon catesbianae (Stebbins, 1904) and Hepatozoon clamatae (Stebbins, 1905). From South Africa, Hepatozoon tenuis Netherlands, Cook and Smit, 2017; Hepatozoon involucrum Netherlands, Cook and Smit, 2017; Hepatozoon thori Netherlands, Cook and Smit, 2017; Hepatozoon theileri (Laveran, 1905) and Hepatozoon ixoxo Netherlands, Cook and Smit, 2014. Lastly from Europe, there is only one species with morphological, morphometric, and molecular data available namely Hepatozoon magna (Grassi and Feletti, 1891) (see Barta et al., 2012; Netherlands et al., 2014a; Netherlands et al., 2014b; Netherlands et al., 2018). However, these species of Hepatozoon from anurans differ in morphology compared to H. formosus n. sp., H. longinucleus n. sp. or H. latrensis n. sp., and through molecular diagnosis reported interspecific divergences ranging from 8.01% to 4.97%, between isolates from this study and those mentioned above.
Furthermore, the phylogenetic analysis with small base pairs sequences, recovered species of Hepatozoon isolated from Brazilian anurans and snakes as monophyletic and sister to species isolated anurans and snakes isolates from South Africa, Europe, and North America. These findings are in contrast to the findings of Netherlands et al. (2018), who suggested that species of Hepatozoon from anurans are monophyletic, providing new insights to the phylogenetic relationships and evolution of species of Hepatozoon form anurans. This divergence in clades could be explained by the geographical distribution and isolation between Brazil (South America) and Africa, Europe and North America.
Moreover, four Hepatozoon sp. isolates from Brazilian anurans, Rhinella diptycha [MK503646, MK503647and MK503648] and Leptodactylus latrans [MK503645], reported by Ferreira et al. (2020) showed 100% of gene similarity with one species from this study, Hepatozoon latrensis n. sp. Although Ferreira et al. (2020) only provided molecular data, being unable to compare the developmental stages among them, the identical gene similarity observed suggest that these sequences belong to the same species, H. latrensis n. sp.
The comparison among the three species from the present study and Brazilian snakes revealed a lower interspecific divergence, ranging from 0.62%–5.98%, with H. musa (MF497767) presenting 99.38% identity to H. latrensis n. sp. Hepatozoon musa described from the snake Philodriyas nattereri Steindachner, 1870 from Ceará State, north-eastern Brazil, belong to a different group of animals and differ in lifestyle behaviour also, comparing with the anuran host of H. latrensis n. sp. The P. nattereri is a diurnal arboreal and terrestrial species, compared to the nocturnal semi-aquatic life-style observed in L. latrans (Uetanabaro et al., 2008; Pimenta et al., 2014; Harrington et al., 2018). Moreover, the morphological data revealed significant differences between both species.
The phylogenetic proximity between Hepatozoon from anurans and snakes has been reported in the literature (Netherlands et al., 2014a, 2014b; Netherlands et al., 2018; Ferreira et al., 2020). It is known that anurans can play an important role as intermediate host for some reptile-associated Hepatozoon spp. (Smith et al., 1994; Laison et al., 2003). Laison et al. (2003) observed an infection in Caiman crocodilus (Linnaeus, 1758) specimens that had been fed with infected Leptodactylus fuscus (Schneider, 1799) and Lithobates cartesbeianus (Schaw, 1802). In another study, Smith et al. (1994) reported a snake Nerodia sipedon (Linnaeus, 1758) infected with Hepatozoon sipedon through an ingestion of a positive Lithobates pipiens (Schreber, 1782) frog. Therefore, the closeness of anuran-reptile Hepatozoon may be related with the predation through the parasite life-cycle, jumping from one vertebrate host to another and leading to adaptation to a new host, resulting in speciation (Ferreira et al., 2020).
Although this study has provided longer sequences, most available sequence isolates of species of Hepatozoon from Brazilian reptiles and amphibians are short fragments (<500 bp). Therefore, future studies should include obtaining longer sequences, as well as sequence data from faster evolving markers (mitochondrial), for further comparison of the phylogenetic position of species of Hepatozoon from anurans.
4.1. Conclusion
The present study used morphological and molecular data to characterise and describe three new species of Hepatozoon parasitising the Brazilians frogs, L. labirinthycus and L. latrans, from the municipality of Nova Xavantina, Mato Grosso State, Brazil. This data shows the value of using a more holistic approach to determine the diversity of Hepatozoon species from anurans in this biodiversity hotspot. Although, in the present study the diversity of known species of Hepatozoon described from Brazilian anurans has significantly increased, there are likely still several more species to be described from this area. Future work should include screening more anuran species from Brazil across the different families.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements and compliance with ethical standards
We thank the team of the Laboratory for Teaching and Research in Wild Animals (LAPAS) and Non-governmental organization for the preservation of wild animals in Brazil (ONG PAS).
All applicable international, national, and institutional guidelines for the care and use of animals were followed (IBAMA license 60640–1; CEUA-UNESP 1061). R.J.S. is supported by FAPESP (2016/50377–1); CNPq (309125/2017–0); CNPq-PROTAX (440496/2015–2). L.P.U. is supported by FAPESP (2018/00754–9; 2018/09623–4). L.H.O. is supported by FAPESP (2018/09623–4).
The financial assistance of the National Research Foundation (NRF) of South Africa, towards ECN, who is supported by DSI/NRF Innovation Postdoctoral Fellowship (Grant UID: 129669), is also hereby acknowledged. Opinions expressed and conclusion arrived at, are those of the authors and are not necessarily to be attributed to the funding bodies.
References
- Barta J.R., Ogedengbe J.D., Martin D.S., Smith T.G. Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Eukaryot. Microbiol. 2012;59(2):171–180. doi: 10.1111/j.1550-7408.2011.00607.x. [DOI] [PubMed] [Google Scholar]
- Barta J.R., Desser S.S. Blood parasites of amphibians from Algonquin Park, Ontario. J. Wildl. Dis. 1984;20:180–189. doi: 10.7589/0090-3558-20.3.180. [DOI] [PubMed] [Google Scholar]
- Borges-Nojosa D.M., Borges-Leite M.J., Maia J.P., Zanchi-Silva D., Braga R.R., Harris D.J. A new species of Hepatozoon Miller, 1908 (Apicomplexa: Adeleina) from the snake Philodryas nattereri Steindacher (Squamata: Diapsadidae) in Northeastern Brazil. Syst. Parasitol. 2017;94:65–72. doi: 10.1007/s11230-016-9676-2. [DOI] [PubMed] [Google Scholar]
- Cook C.A., Smit N.J., Davies A.J. A redescription of Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) from Southern African tortoises (Cyptodira: Testudinidae) with new host data and distribution records. Folia Parasitol. 2009;56:173–179. doi: 10.14411/fp.2009.021. [DOI] [PubMed] [Google Scholar]
- Cunha A.M., Muniz J. Sobre o cyclo endógeno da Haemogregarina peltodactyli Lésage, 1908 (Karyolysus?) Mem. Inst. Oswaldo Cruz. 1927;20(2):307–313. [Google Scholar]
- Da Costa S.C.G., Pessoa S.B., Pereira N.M., Colombo T. The life history of Hepatozoon leptodactyli (Lesage, 1908) Pessoa, 1970 – a parasite of the common laboratory animal – the frog of the genus Leptodactylus. Mem. Inst. Oswaldo Cruz. 1973;71(1):1–18. [Google Scholar]
- Darriba D., Toboada G.L., Doallo R., Posada D. JModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A.J., Johnston M.R.L. The biology of some intraerythrocytic parasites of fishes, amphibian and reptiles. Adv. Parasitol. 2000;45:1–107. doi: 10.1016/s0065-308x(00)45003-7. [DOI] [PubMed] [Google Scholar]
- Desser S.S., Hong H., Martin D.S. The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbianae and the mosquito, Culex territans in Algoquin Park, Ontario. J. Parasitol. 1995;81:212–222. [PubMed] [Google Scholar]
- Eisen R.J., Schall J.J. Life history of malaria parasite (Plasmodium mexicanum): independent traits and basis for variation. Proc. Biol. Sci. 2000;267:793–799. doi: 10.1098/rspb.2000.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D.A.R., Perles L., Machado R.Z., Prado C.P.A., André M.R. Molecular detection of Apicomplexan hemoparasites in anurans from Brazil. Parasitol. Res. 2020;119(10):3469–3471. doi: 10.1007/s00436-020-06835-9. [DOI] [PubMed] [Google Scholar]
- Frost D.R. American Museum of Natural History; New York, USA: 2020. Amphibian Species of the World: an Online Reference.http://research.amnh.org/herpetology/amphibia/index.html (Access September 2020). Electronic Database accessible at. [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Harrington S.M., Haan J.M., Shapiro L., Ruane S. Habits and characteristics of arboreal snakes worldwide: arboreality constrains body size but does not affect lineage diversification. Biol. J. Linn. Soc. 2018;125(1):61–71. [Google Scholar]
- Kattar M.R. vol. 10. Bolm Univ S Paulo; 1986. pp. 189–196. (Ocorrência de uma haemogregarina (Protozoa, Apicomplexa) em Bufo paracnemis Lutz, 1925). [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laison R., Paperna I., Naiff R.D. Development of Hepatozoon caimani (Carini, 1909) Pêssoa, de Biasi and de Souza, 1972 in the Caiman Caiman c. crocodilus, the frog Rana catesbiana and the mosquito Culex fatigans. Mem. Inst. Oswaldo Cruz. 2003;98:103–113. doi: 10.1590/s0074-02762003000100014. [DOI] [PubMed] [Google Scholar]
- Leal D.D.M., Dreyer C.S., Da Silva R.J., Ribolla P.E.M., Paduan K.S., Blanchi I., O’Dwyer L.H. Characterization of Hepatozoon spp. in Leptodactylus chaquensis and Leptodactylus podicipinus from two regions of the Pantanal, state of Mato Grosso do Sul, Brazil. Parasitol. Res. 2015;114:1541–1549. doi: 10.1007/s00436-015-4338-x. [DOI] [PubMed] [Google Scholar]
- Leal D.D.M., O’Dwyer L.H., Ribeiro V.C., Silva R.J., Ferreira V.L., Rodrigues R.B. Hemoparasites of the genus Trypanosoma (Kitenoplastida: Trypanosomatidae) and hemogregarines in Anurans of the São Paulo and Mato Grosso do Sul states – Brazil. An. Acad. Bras. Cienc. 2009;81(2):199–206. doi: 10.1590/s0001-37652009000200006. [DOI] [PubMed] [Google Scholar]
- Levine N.D. vol. 2. CRC Press; Boca Raton: 1988. p. 154. (The Protozoan Phylum Apicomplexa). [Google Scholar]
- Miller M.A., Pfiffer W., Schwartz T. 2010. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. 2010 Gateway Computing Environments Workshop. [Google Scholar]
- Netherlands E.C., Cook C.A., Du Preez L.H., Vanhove M.P.M., Brendonck L., Smit N.J. An overview of the Dactylosomatidae (Apicomplexa: Adeleorina: Dactylosomatidae), with the description of Dactylosoma kermiti n. sp. parasiting Ptychadena anchietae and Sclerophrys gutturalis from South Africa. Int. J. Parasitol.: Parasites Wildl. 2020;11:246–260. doi: 10.1016/j.ijppaw.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherlands E.C., Cook C.A., Du Preez L.H. Monophyly of the species of Hepatozoon (Adeleorina: Hepatozoidae) parasiting (African) anurans, with the description of three new species from hyperoliid frogs in South Africa. Parasitology. 2018;145:1039–1050. doi: 10.1017/S003118201700213X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherlands E.C., Cook C.A., Du Kruger D.J.D., Du Preez L.H., Smit N.J. Biodiversity of frog haemoparasites from sub-tropical northern KwaZulu-Natal, South Africa. Int. J. Parasitol.: Parasites Wildl. 2015;4:135–141. doi: 10.1016/j.ijppaw.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherlands E.C., Cook C.A., Smit N.J. Hepatozoon species (Adeleorina: Hepatozoidae) of African bufonids, with morphological description and molecular diagnosis of Hepatozoon ixoxo sp. nov. parasiting three Amietophrynus species (Anura: Bufonidae) Parasites Vectors. 2014;7:552–563. doi: 10.1186/s13071-014-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherlands E.C., Cook C.A., Smit N.J., Du Preez L.H. Redescription and molecular diagnosis of Hepatozoon theileri (Laveran, 1905) (Apicomplexa: Adeleorina: Hepatozoidae), infecting Amietia quecketti (Anura: Pyxicephalidae) Folia Parasitol. 2014;61:239–300. [PubMed] [Google Scholar]
- O’Dwyer L.H., Moço T.C., Paduan Kdos S., Spenassatto C., da Silva R.J., Ribolla P.E. Description of three new species of Hepatozoon (Apicomplexa, Hepatozoidae) from Rattlesnakes (Crotalus durissus terrificus) based on molecular, morphometric and morphologic characters. Exp. Parasitol. 2013;135(2):200–207. doi: 10.1016/j.exppara.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Perkins S.L., Keller A.K. Phylogeny of nuclear small subunit rRNA genes of hemogregarines amplified with specific oligonucleotides. J. Parasitol. 2001;87:870–876. doi: 10.1645/0022-3395(2001)087[0870:PONSSR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pimenta B., Costa D., Murta-Fonseca R., Pezzuti T.L. first ed. Belo Horizonte; Bicho do Mato: 2014. Anfíbios: Alvorada de Minas, Conceição do MATO Dentro, Dom Joaquim, Minas gerais; p. 196. [Google Scholar]
- Posada D. Using MODELTEST and PAUP* to select a model of nucleotide substitution. Curr. Protoc. Bioinf. 2003;6 doi: 10.1002/0471250953.bi0605s00. 6.5.1 – 6.5.14. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2012. FigTree v1.4. Molecular Evolution, Phylogenetics and Epidemiology. [Google Scholar]
- Sebben A. Microdissecação fisiológica a fresco: uma nova visão sobre a anatomia de anfíbios e répteis. In: Nascimento L.B., Oliveira M.E., editors. Herpetologia no Brasil II. first ed. Belo Horizonte - MG: Sociedade Brasileira de Herpetologia; 2007. pp. 311–325. [Google Scholar]
- Segalla M.V., Caramaschi U., Cruz C.A.G., Garcia P.C.A., Grant T., Haddad C.F.B., Langone J. Brazilian amphibians – list of species. 2016. http://www.sbherpetologia.org.br
- Smith T.G. The genus Hepatozoon (Apicomplexa: Adeleorina) J. Parasitol. 1996;82(4):565. [PubMed] [Google Scholar]
- Smith T.G., Dresser S.S., Martin D.S. The development of Hepatozoon sipedon sp. nov. (Apicomplexa: Adeleina: Hepatozoidae) in its natural host, the Northern water snake (Nerodia sipedon sipedon), in the culicine vectors Culex pipiens and C. territans, and in an intermediate host, the Northern leopard frog (Rana pipiens) Parasitol. Res. 1994;80:559–568. doi: 10.1007/BF00933003. [DOI] [PubMed] [Google Scholar]
- Sousa M.A., Filho A.B. Uma nova hemogregarina no sangue de Bufo crucifer Wied, 1821 do Brasil. Mem. Inst. Oswaldo Cruz. 1974;72(3/4):275–282. [Google Scholar]
- Tavaré D. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986;17:57–86. [Google Scholar]
- Telford S.R., Jr. CRC Press; Boca Raton: 2009. Hemoparasite of the Reptilian: Color Atlas and Text. [Google Scholar]
- Tender A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomé B., Maia J.P.M.C., Harris D.J. Hepatozoon infection prevalence in four snakes genera: influence of diet, prey parasitemia levels, or parasite type? J. Parasitol. 2012;98(5):913–917. doi: 10.1645/GE-3111.1. [DOI] [PubMed] [Google Scholar]
- Uetanabaro M., Prado C.P.A., Rodrigues D.J., Gordo Z.C.M. Mato Grosso do Sul; Brazil: 2008. Field Guide to the Anurans of the Pantanal and Surrounding Cerrados. [Google Scholar]
- Ujvari B., Marques E.J. High prevalence of Hepatozoon spp. (Apicomplexa: Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 2005;90:670–672. doi: 10.1645/GE-204R. [DOI] [PubMed] [Google Scholar]
- Viana L.A., Soares P., Silva J.E., Paiva F., Coutinho M.E. Anurans as paratenic hosts in the transmission of Hepatozoon caimani to caimans Caiman yacare and Caiman latirostris. Parasitol. Res. 2012;110:883–886. doi: 10.1007/s00436-011-2570-6. [DOI] [PubMed] [Google Scholar]
- Wake D., Vredenburg V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippel K.C., Lillywhite H.B., Mladinich C.R. New vascular system in reptiles: anatomy and postural hemodynamics of the vertebral venous plexus in snakes. J. Morphol. 2001;250:173–184. doi: 10.1002/jmor.1063. [DOI] [PubMed] [Google Scholar]