Abstract
Chonopeltis lisikili Van As and Van As, 1996 was original described from the Eastern Caprivi (Namibia) and the Okavango System (Botswana), collected from five Synodontis Cuvier, 1816 (Mochokidae) species. This fish genus is endemic to Africa, with 130 valid species, making it one of the most species-rich and widely distributed mochokid catfish family. During parasitological surveys conducted in the Phongolo River (South Africa), a Chonopeltis Thiele, 1900 species was collected from Synodontis zambezensis Peters, 1852. In total, 21 adult females, four adult males, as well as representatives of the larval developmental stages were found. After morphological comparison, this branchiuran was identified as C. lisikili. This paper provides additional information on body measurements and morphological information of all the life stages of the Phongolo material. Information on Chonopeltis material collected from the Okavango River, Botswana, not yet published is also included as well as the first molecular characterisation of a Chonopeltis species using COI and 18 S rRNA partial sequencing. The endemicity of the host and fish lice necessitates a discussion on the host origin and distribution in more than one river system, particularly referring to host specificity for Chonopeltis species.
Keywords: Fish parasites, Synodontis zambezensis, Argulidae, Branchiura, Okavango
Graphical abstract
Highlights
-
•
First COI sequence for genus Chonopeltis.
-
•
First phylogenetic analysis of Branchiurans using 18 S rRNA.
-
•
New host and locality record for Chonopeltis lisikili.
-
•
First illustrations of the majority of C. lisikili life stages, in fact for any of the known Chonopeltis species.
1. Introduction
Since their discovery more than a century ago, species of the branchiuran genus Chonopeltis Thiele, 1900 have intrigued fish parasitologists. The main reason being that in contrast to other branchiuran genera, such as Argulus Müller, 1785 and Dolops Audouin, 1837, all 12 currently accepted species of Chonopeltis are endemic to Africa (Scholz et al., 2018). Furthermore, all these species are host specific, either to a host genus or a specific fish species, and they are mainly restricted to a single river system or drainage basin (Van As et al., 2017). One species that shows both these characteristics is Chonopeltis lisikili Van As and Van As (1996) that has only been reported from five species of Synodontis Cuvier (1861) (Mochokidae) and to date only collected from different sites in the Okavango River system (Van As and Van As, 1996). Current known hosts for C. lisikili are the leopard squeaker, Synodontis leopardinus Pellegrin, 1914; the largespot squeaker, Synodontis macrostigma Boulenger, 1911; the spotted squeaker, Synodontis nigromaculatus Boulenger, 1905; the bubblebard squeaker, Synodontis thamalakanensis Fowler, 1935; and the finetooth squeaker, Synodontis vanderwaali Skelton and White, 1900 (see Van As and Van As, 2015).
During surveys conducted in the lower Phongolo River, South Africa, material of a Chonopeltis species was collected from the plain squeaker, Synodontis zambezensis Peters, 1852. The plain squeaker (also known as the brown squeaker) is a widespread species that has been reported from the upper Congo River basin (Luangwe) and is known to occur from the middle and lower Zambezi system, south to the Phongolo system (Skelton, 2001; Froese and Pauly, 2020).
The Chonopeltis material collected from Phongolo included different larval stages (both male and female) as well as 21 adult females and four adult males. Comparison of the morphological features and measurements of the new material correspond to the original description of C. lisikili, highlighting slight variations observed in the populations. Therefore, this paper reports on a new host record for C. lisikili and extends its distribution record to that of a different river system and drainage basis. It also provides new morphological and morphometrical information on life stages not previously studied and presents the first molecular characterisation of a Chonopeltis species using both mitochondrial cytochrome c oxidase subunit I (COI) and nuclear 18 S rRNA (nu 18 S) partial sequences.
2. Materials and methods
2.1. Host and parasite sampling
During December 2018 in the Phongolo River, Ndumo Game Reserve, north-eastern KwaZulu-Natal, South Africa (−26.925833; 32.325556), 12 specimens of Synodontis zambezensis were collected. Fish collection, dissection and parasitological screening followed Schaeffner et al. (2020), under the research collection permit (reference number OP 1582/2018) and North-West University ethical clearance (reference number NWU-00156-18-A5). During parasitological screening, all attached Chonopeltis specimens were carefully removed with a small brush and fixed in 70% ethanol. In the laboratory, all material was studied by light microscopy and measurements (in mm unless otherwise stated) were made from microscope projection drawings. Drawings were digital inked using Adobe Illustrator and a Wacom Intuos Pro drawing tablet. Specimens were measured using a ZEISS Stemi 305 dissection microscope and ZEISS Labscope.
2.2. DNA extraction, amplification, and sequencing
Chonopeltis genomic DNA was extracted from whole specimens following the manufacturer's protocol of the PCRBIO Rapid Extract PCR Kit (PCR Biosystems Inc., London, UK). DNA amplifications of partial cytochrome c oxidase subunit 1 (cox1) and 18 S rRNA genes were performed using universal COI invertebrate primers, LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HC02198 (5′ TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al., 1994), and 18 S-E (5′-CCGAATTCGTCGACAACCTGGTTGATCCTGCCAGT-3′) (Littlewood and Olson, 2001) and NEM18SR (5′-GGGCGGTATCTGATCGCC-3′) (Floyd et al., 2005). Each polymerase chain reaction (PCR) reaction was performed at a total volume of 25 μl, using 12,5 μl of DreamTaq PCR Master Mix (ThermoFischer Scientific, South Africa), 1.25 μl of 10 μM of each primer, 3 μl of DNA product and 7 μl of double distilled water. PCR conditions for COI were as follows: initial denaturation at 94 °C for 5 min; followed by 35 cycles of a 94 °C denaturation for 30 s, annealing at 47 °C for 50 s, with an end extension at 72 °C for 2 min; and a final extension of 72 °C for 10 min. PCR conditions for 18 S rRNA followed the protocol from Neethling and Avenant-Oldewage (2020). PCR amplicons were purified and sequenced in both directions by a commercial sequencing company, Inqaba Biotechnical Industries (Pty) Ltd, Pretoria, South Africa. Contiguous sequences were assembled and edited using the bioinformatics software platform, Geneious R7.1.3 (Biomatters, Auckland, New Zealand; Kearse et al., 2012).
2.3. Alignment and phylogenetic analyses
Two sequences of COI gene (662 and 697 bp long) and two of 18 S rRNA gene (1038 and 1039 bp long) were newly generated for C. lisikili. One sequence of the 18 S was aligned with five other 18 S sequences of Branchiura available in GenBank (MT274324 Chonopeltis australis, Boxshall, 1976; KM597744 Argulus siamensis Wilson, 1926; KM597746, A. siamensis; KF583878 Argulus bengalensis Ramakrishna, 1952; and M27187 Argulus nobilis Thiele, 1904) that are associated with peer-reviewed publications, and MT367686 Linguatula serrata Frölich, 1789 (Pentastomida: Linguatulidae) that was used as outgroup. Sequences were aligned following MUSCLE default parameters implemented in Geneious v. 7.1.3 software (Kearse et al., 2012). The extremes of the alignment were trimmed, resulting in 1032 bp. Phylogenetic analyses were run under maximum likelihood (ML) and Bayesian inference (BI) criteria, applying the model of nucleotide evolution K2+ G + I, selected by MEGA7. RAxML was used to generate the ML tree (Guindon and Gascuel 2003). Model parameters and bootstrap support values (1000 repetitions) were estimated using RAxML. MrBayes was used to generate the BI tree (Ronquist et al., 2012), running two independent Markov Chain Monte Carlo (MCMC) runs of four chains for 10 million generations and sampling tree topologies every 1000 generations. Burn-in periods were set to the first 25,000 generations. ML and BI analyses were carried out using the computational resource CIPRES (Miller et al., 2010). Nucleotide genetic divergence (p-distance and number of differences) were calculated in MEGA 7. Phylogenetic trees were edited in FigTree v1.4.4 (Rambaut, 2009).
3. Results
The 12 Synodontis zambezensis collected had a mean total length (TL) of 155 mm ± 20.2 (range 125–180) and were all parasitised by various life stages of a Chonopeltis species. Individuals were found on the body surface as well as on the fins, with a slight preference for pectoral fins. Infestation intensity ranged from 1 to 19 (5 ± 5.1) per fish.
3.1. Description
Superfamily: Arguloidea Yamaguti, 1963
Family: Argulidae Leach, 1819
Genus: Chonopeltis Thiele, 1900
Chonopeltis lisikili Van As and Van As (1996).
Chonopeltis lisikili Van As and Van As (1996): 69–77.—Van As and Van As (2015): 9–20.
Holotype: 1 adult female in the collection of the National Museum, Bloemfontein (NMB), South Africa (NMBP 106).
Type-host: Synodontis leopardinus Pellegrin, 1914
Type-locality: Thamalakane River, Okavango Delta (19°451 S, 23°301 E).
Material examined: 21 adult females, 18 sub-adult females, 4 males, 10 sub-adult males and a total of 57 larval stages (both sexes) collected from the body surface of S. zambezensis from the Phongolo River, South Africa (−26.925833; 32.325556); Coll: L. de Necker. Eleven adult females, 30 neotenic females (stage 8), 1 sub-adult female, 4 adult males and additional 248 larvae stages (both sexes) collected from the body surface of S. leopardinus, S. macrostigma, S. nigromaculatus S. thamalakanensis and S. vanderwaali in the Okavango Delta, Botswana; Coll: JG and LL van As. Some of the Botswana Chonopeltis specimens were retrieved from preserved Synodontis hosts, in the fish collection of the South African Institute of Aquatic Biodiversity (see Van As and Van As, 1996, 2015).
Measurements: See Table 1 (females) and Table 2 (males) (first and second larval stages included in Table 1 as sexes cannot be defined at these stages).
Table 1.
Chonopeltis lisikiliVan As and Van As (1996) females and larval stages collected from Synodontis Cuvier, 1816 species from the Okavango Delta, Botswana (Van As and Van As, 1996, 2015) and Synodontis zambezensis Peters, 1852 from the Phongolo River, South Africa (present study). OD = Okavango Delta; PR = Phongolo River.
| Developmental stage | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | Sub-adult | Adult | ||||||||||
| Locality | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR |
| Number measured | 19 | 4 | 35 | 4 | 24 | 3 | 0 | 2 | 18 | 0 | 8 | 0 | 24 | 5 | 30 | 15 | 1 | 18 | 11 | 21 |
| Total Length (TL) | 0.7 | 0.8 | 1.1 | 1 | 1.3 | 1.3 | – | 1.5 | 1.8 | – | 1.9 | – | 1.9 | 2.1 | 2.3 | 2.7 | 2.3 | 3.3 | 4.3 | 4.5 |
| Carapace Length (CL) | 0.4 | 0.4 | 0.6 | 0.5 | 0.7 | 0.7 | – | 0.8 | 1 | – | 1.2 | – | 1.1 | 1.1 | 1.2 | 1.4 | 1.5 | 1.7 | 2.3 | 2.3 |
| CL % of TL | 52 | 54 | 56 | 53 | 56 | 56 | – | 52 | 57 | – | 60 | – | 57 | 51 | 52 | 50 | 63 | 51 | 53 | 51 |
| Length of anterior lobe (LAC) | 0.02 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | – | 0.2 | 0.2 | – | 0.3 | – | 0.3 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 1.0 | 0.9 |
| LAC % of CL | 6 | 31 | 24 | 34 | 29 | 30 | – | 30 | 23 | – | 27 | – | 30 | 29 | 33 | 39 | 49 | 39 | 43 | 41 |
| Carapace Width (CW) | 0.5 | 0.1 | 0.8 | 0.8 | 0.9 | 1 | – | 1.1 | 1.2 | – | 1.3 | – | 1.3 | 1.4 | 1.5 | 1.7 | 1.5 | 2 | 2.5 | 2.6 |
| Width of anterior lobe (WAC) | 0.1 | 0.4 | 0.3 | 0.5 | 0.5 | 0.6 | – | 0.7 | 0.5 | – | 0.7 | – | 0.6 | 0.8 | 0.8 | 1 | 0.9 | 1.1 | 1.4 | 1.5 |
| WAC % of CW | 19 | 71 | 41 | 68 | 55 | 52 | – | 63 | 43 | – | 52 | – | 48 | 58 | 51 | 57 | 60 | 58 | 56 | 57 |
| Sucker diameter (SD) | – | – | – | – | 0.2 | 0.2 | – | 0.2 | 0.3 | – | 0.4 | – | 0.3 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.6 | 0.5 |
| SD % of TL | – | – | – | – | 15 | 15 | – | 13 | 17 | – | 21 | – | 16 | 15 | 17 | 17 | 17 | 15 | 14 | 12 |
| Abdomen Length (AL) | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | – | 0.4 | 0.5 | – | 0.6 | – | 0.6 | 0.6 | 0.6 | 0.8 | 0.7 | 0.9 | 1.1 | 1.1 |
| AL % of TL | 21 | 30 | 25 | 22 | 24 | 21 | – | 24 | 30 | – | 29 | – | 29 | 29 | 27 | 27 | 29 | 26 | 26 | 26 |
| Length of fused part (LF) | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | – | 0.3 | 0.3 | – | 0.3 | – | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.5 | 0.6 | 0.6 |
| LF % of AL | 71 | 77 | 69 | 77 | 67 | 66 | – | 79 | 60 | – | 60 | – | 62 | 53 | 63 | 57 | 57 | 59 | 55 | 52 |
| Length of cleft (LC) | 0.04 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | – | 0.1 | 0.2 | – | 0.2 | – | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 0.4 | 0.5 | 0.5 |
| LC % of AL | 29 | 23 | 31 | 23 | 33 | 33 | – | 33 | 40 | – | 40 | – | 38 | 58 | 37 | 42 | 43 | 43 | 45 | 46 |
| Abdomen Width | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | – | 0.3 | 0.4 | – | 0.4 | – | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.6 | 0.6 |
Table 2.
Chonopeltis lisikiliVan As and Van As (1996) males and larval stages collected from Synodontis Cuvier, 1816 species from the Okavango Delta, Botswana (Van As and Van As, 1996, 2015) and Synodontis zambezensis Peters, 1852 from the Phongolo River, South Africa (present study). OD = Okavango Delta; PR = Phongolo River. * Young male.
| Developmental stage | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | Sub-adult | Adult | ||||||||||
| Locality | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | PR | OD | OD | PR |
| Number measured | 19 | 4 | 0 | 2 | 28 | 0 | 41 | 0 | 29 | 8 | 0 | 10 | 3 | 0 | 0 | 10 | *1 | 3 | 4 |
| Total Length (TL) | 1.2 | 1.3 | – | 1.4 | 1.5 | – | 1.8 | – | 1.9 | 1.9 | – | 2.2 | 2.4 | – | – | 2.6 | 2.5 | 2.4 | 2.9 |
| Carapace Length (CL) | 0.6 | 0.6 | – | 0.7 | 0.8 | – | 0.9 | – | 1 | 1 | – | 1.1 | 1.3 | – | – | 1.4 | 1.3 | 1.5 | 1.6 |
| CL % of TL | 53 | 47 | – | 46 | 56 | – | 53 | – | 52 | 51 | – | 50 | 54 | – | – | 54 | 52 | 63 | 57 |
| Length of anterior lobe (LAC) | 0.2 | 0.3 | – | 0.3 | 0.2 | – | 0.2 | – | 0.3 | 0.3 | – | 0.4 | 0.5 | – | – | 0.6 | 0.3 | 0.6 | 0.7 |
| LAC % of CL | 27 | 42 | – | 45 | 21 | – | 26 | – | 28 | 32 | – | 37 | 39 | – | – | 40 | 24 | 40 | 43 |
| Carapace Width (CW) | 0.8 | 0.8 | – | 0.9 | 1 | – | 1.1 | – | 1.3 | 1.2 | – | 1.3 | 1.4 | – | – | 1.6 | 1.5 | 1.4 | 1.8 |
| Width of anterior lobe (WAC) | 0.4 | 0.6 | – | 0.6 | 0.5 | – | 0.6 | – | 0.6 | 0.7 | – | 0.8 | 1 | – | – | 0.9 | 0.8 | 0.9 | 1 |
| WAC % of CW | 53 | 72 | – | 64 | 48 | – | 51 | – | 50 | 60 | – | 57 | 71 | – | – | 56 | 53 | 64 | 60 |
| Sucker diameter (SD) | 0.2 | – | – | 0.2 | 0.2 | – | 0.3 | – | 0.4 | 0.3 | – | 0.3 | 0.4 | – | – | 0.4 | 0.4 | 0.4 | 0.4 |
| SD % of TL | 17 | – | – | 14 | 13 | – | 17 | – | 21 | 16 | – | 15 | 17 | – | – | 15 | 16 | 16 | 15 |
| Abdomen Length (AL) | 0.3 | 0.3 | – | 0.4 | 0.5 | – | 0.6 | – | 0.7 | 0.7 | – | 0.7 | 0.8 | – | – | 0.8 | 0.8 | 0.8 | 1 |
| AL % of TL | 24 | 25 | – | 30 | 35 | – | 34 | – | 34 | 35 | – | 34 | 33 | – | – | 31 | 31 | 33 | 34 |
| Length of fused part (LF) | 0.2 | 0.3 | – | 0.3 | 0.4 | – | 0.4 | – | 0.5 | 0.5 | – | 0.5 | 0.5 | – | – | 0.5 | 0.5 | 0.6 | 0.7 |
| LF % of AL | 76 | 82 | – | 76 | 74 | – | 73 | – | 73 | 73 | – | 69 | 67 | – | – | 65 | 63 | 75 | 77 |
| Length of cleft (LC) | 0.1 | 0.1 | – | 0.1 | 0.1 | – | 0.2 | – | 0.2 | 0.2 | – | 0.2 | 0.3 | – | – | 0.3 | 0.3 | 0.2 | 0.3 |
| LC % of AL | 24 | 20 | – | 23 | 26 | – | 27 | – | 27 | 27 | – | 31 | 33 | – | – | 36 | 37 | 25 | 27 |
| Abdomen Width | 0.3 | 0.3 | – | 0.4 | 0.4 | – | 0.4 | – | 0.4 | 0.4 | – | 0.5 | 0.5 | – | – | 0.5 | 0.5 | 0.4 | 0.5 |
Representative DNA sequences: The sequence data of C. lisikili here morphologically described have been submitted to GenBank and are as follow: Mitochondrial cytochrome c oxidase subunit I (COI) partial sequence: MW679679, MW679715; nuclear 18 S rRNA (nu 18 S) partial sequences: MW678625, MW678626.
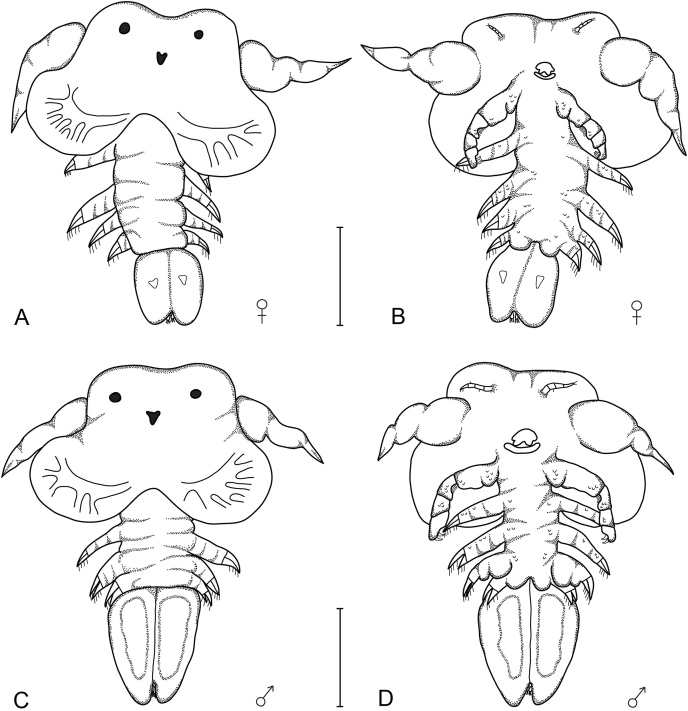
First and second larval stage (Fig. 1)
Fig. 1.
Dorsal and ventral view of the first (A&B) and second (C&D) larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier, 1816 hosts. Scale bars: 0.4 mm.
Both stages are characterised by undifferentiated thorax appendages and an undivided carapace. In both of these stages it is not possible to distinguish between the different sexes, however, some development can be observed in the abdomen of slightly larger individuals. Of the eight specimens that were collected from S. zambezensis, the body length ranged from 0.8 to 1.0 for the two stages, the carapace comprised more than 54% (1st) and 53% (2nd) of the total length and the abdomen comprised 30% (1st) and 22% (2nd) of the total body length, respectively.
Remarks: The morphology of the first larval stage of Chonopeltis brevis Fryer (1961) and Chonopeltis inermis Thiele, 1900, as described by Fryer (1956; 1961), is basically the same. The first and second stages differ mostly in size (Van As and Van As, 1996). Fryer (1956) and Van Niekerk (1984) observed a group of setae at the base of the antenna on C. inermis and C. australis respectively, whilst the setae were not observed in C. brevis (Fryer, 1961). Fryer (1961) suggest that these setae, if present, are only ornamentations and not rudiments of the antennule. Van As and Van As (1996) found 19 and 35 specimens, respectively, belonging to the first and second larval stage of C. lisikili from the Okavango System. All the morphological features of the Phongolo material correspond with what was observed by previous authors.
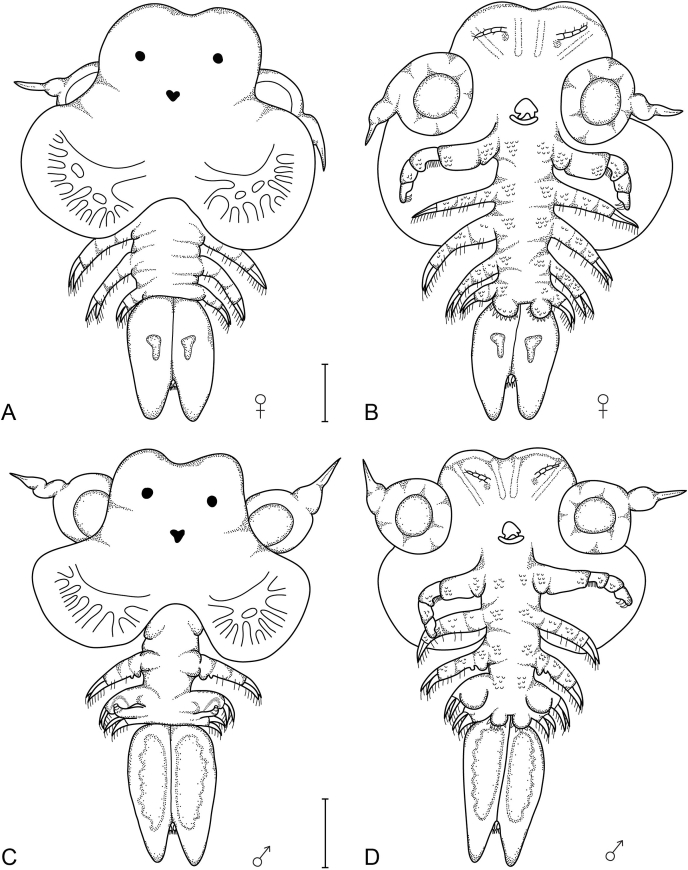
Third larval stage (both sexes) (Fig. 2)
Fig. 2.
Dorsal and ventral view of the female (A&B) and male (C&D) third larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier, 1816 hosts. Scale bars: 0.4 mm.
From this stage onwards, there is a clear morphological differentiation of the sexes, evident in the body and appendages. A trilobed carapace can now be distinguished. The cephalic and thoracic appendages are clearly differentiated and all podomeres are distinguishable. On the maxillulae, the second podomere is swollen and the terminal hook is still very prominent and functional. The bristle seta found on the third podomere of the maxilla, that is already present from the first larval stage, is now even longer and possibly already fully functional. The material collected from S. zambezensis had an average body length of 1.3, the carapace covers 47–56% of the total body length and the abdomen comprises 21–25% of the total body length for both sexes.
Remarks: The third and fourth legs of the male begin to differentiate to form the copulatory structures (Van As and Van As, 1996). The only other species with information of the third stage is C. australis in an unpublished thesis (Van Niekerk, 1984). Van As and Van As (1996) found males (19) and females (24) belonging to the third larval stages of C. lisikili from different Synodontis species from the Okavango system. The newly collected Phongolo specimens illustrated the same morphological development.
Fourth larval stage (both sexes) (Fig. 3)
Fig. 3.
Dorsal and ventral view of the female (A&B) and male (C&D) fourth larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier, 1816 hosts. Scale bars: 0.5 mm.
In the original species description of C. lisikili, this stage was not observed by Van As and Van As (1996). Although the thoracic appendages are larger, the first two legs are not significantly more differentiated. Legs three and four in males are clearly differentiated, so that the peg and socket structure can be recognised, and the natatory lobes can also be distinguished. Judging by the size and development of the legs, two female (1.5) and two male (1.4) specimens collected from S. zambezensis fit this profile. The carapace covers 52% and 46% of the total body length and the abdomen comprises 24% and 30% of the total body length, for the females and males, respectively.
Remarks: This is the first description and illustration of this larval stage for C. lisikili. After comparing the description of Fryer (1961) and Van Niekerk (1984), it was apparent that certain species characters could already be distinguished at this stage of development, allowing comparisons to other species such as C. australis and C. brevis. According to Fryer (1961), there are more setae at the base of the antenna in this stage compared to the previous stage. In the case of C. brevis, the bristle seta that is found on the maxilla is observed for the first time in this stage, whilst in the other species it is already present from earlier stages. In the case of C. brevis, the sucker edge is not yet noticeable, however Van Niekerk (1984) illustrated in his thesis that the sucker edge in the case of C. australis can be distinguished. This is also the case in C. lisikili.
Stage five-eight (both sexes) (Fig. 4, Fig. 5, Fig. 6, Fig. 7)
Fig. 4.
Dorsal and ventral view of the female (A&B) and male (C&D) fifth larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier. 1816 hosts. Scale bars: 0.2 mm.
Fig. 5.
Dorsal and ventral view of the female (A&B) and male (C&D) sixth larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier, 1816 hosts. Scale bars: 0.25 mm.
Fig. 6.
Dorsal and ventral view of the female (A&B) and male (C&D) seventh larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier, 1816 hosts. Scale bars: 0.3 mm.
Fig. 7.
Dorsal and ventral view of the female (A&B) and male (C&D) eighth larval stages of Chonopeltis lisikiliVan As and Van As, 1996 collected from Synodontis Cuvier, 1816 hosts. Scale bars: 0.5 mm.
A general increase in size is prominent and further differentiation of the copulatory structures is taking place as well as initial development of the testis and spermathecae. Judging by the body length and morphological development of the material collected from S. zambezensis, stages five to six were not present in the Phongolo samples. Five female and eight male seventh larval stage specimens were collected with an average body length of 2.1 for the females and 1.9 for the males, respectively. The carapace covers 51% of the total body length for both sexes, while the abdomen comprises 29% and 35% of the total body length of females and males, respectively (see Table 1, Table 2). Fifteen female and 10 male eighth larval stage specimens were collected with an average body length of 2.7 for the females and 2.2 for the males, respectively. The carapace covers 50% of the total body length for both sexes, while the abdomen comprises 27% and 34% of the total body length, for females and males, respectively (see Table 1, Table 2).
Remarks: From stage five onwards, the chitinous rods develop in the anterior carapace, the sclerites of the sucker start to develop on the second podomere, and the terminal hook is still functional (Van As and Van As, 1996). There are more setae and scales on the body of the larvae than in the previous stage. According to Van As and Van As (1996), the body lengths for stages five to eight range from 1.8 to 1.9 for females and 1.5–2.4 for males; the carapace covers on average 54% and 58% of the body length of females and males; and the abdomen comprises 29% and 34% of the total body length for the separate sexes (see Table 1, Table 2). In comparing the female drawing provided by Van As and Van As (1996) with Fig. 7A and B, it is clear that the eighth stage of development of the Phongolo specimens is the same as in the Botswana material that was labelled as sub-adult (stage 8) in the original description. The Phongolo material and the specimens collected later by Van As and Van As (2015), confirm that C. lisikili has up to ten stages of development.
Sub-adult stages (both sexes)
Van As and Van As (1996) note that the ontogeny of the maxillulae follows a similar pattern of development to the sub-adult stage, where the hook rudiment is still present at the maxillulae. The sub-adult females that were collected from S. zambezensis are slightly larger (Table 1, Table 2), compared to the material that was collected from the Okavango system (see Van As and Van As, 1996; 2015), but the overall morphological features are the same. No sub-adult males were collected in the Okavango and even the young male (with a fully developed sucker) that was collected from S. leopardinus, was slightly smaller than the sub-adult males that were sampled from S. zambezensis.
Remarks: With the original species description, 30 neotenic females (stage 8), with eggs already present in the uteri were found from the Okavango, but hook rudiments were still present at the maxillulae (Van As and Van As, 1996). Amongst the Phongolo specimens, 18 females and 10 males were found, which still had small hook rudiments attached to the developed sucker (sub-adults) indicating that indeed there is another moult before the adult stage. Van As and Van As (2015) collected one sub-adult female (see Table 1).
Adult stages (both sexes)
Although the fully developed females and males of the Phongolo were slightly larger than the material collected from the Okavango (see Table 1, Table 2), the rest of the morphological characteristics are the same.
Remarks: In the original description, Van As and Van As (1996) did not find any females that were fully developed and only one young male of C. lisikili that was collected from S. leopardinus and S. macrostigma. Later, Van As and Van As (2015) found 11 female and three male specimens in the Okavango system that were fully developed, without any hook rudiments on the suckers or bristle setae on the maxillae. These specimens were also found on three additional hosts, i.e., S. nigromaculatus, S. thamalakanensis and S. vanderwaali. Of greater importance are the 21 fully developed females and additional four adult males that were found in the Phongolo River during the 2018 survey, from a sixth host, i.e., S. zambezensis, bringing the total number of collected adult females of C. lisikili to 32 and seven males including the different developmental stages.
3.2. Molecular analysis
The mitochondrial cytochrome c oxidase subunit I (COI) partial sequence reported here for the Phongolo C. lisikili is the first for any species of Chonopeltis. Using BLASTn (Basic Local Alignment Search Tool; http://www.ncbi.nlm.nih.gov/blast), the obtained sequences were verified as belonging to the Branchiura. Due to the lack of COI sequences for Chonopeltis spp., no alignment was performed. For the 18 S rRNA partial sequences, ML and BI analyses yielded similar tree topology (Fig. 8). The 18 S tree depicted two main clades: one with a subclade clustering C. lisikili and C. australis as sister taxa with strong support, and another subclade clustering together two A. siamensis with strong support; the second main clade clustered Argulus nobilis and A. benegalensis together (Fig. 8). Nucleotide divergence (p-distance and number of differences in bp) among all taxa used in the phylogenetic analyses are present in Table 3. The sister taxa C. lisikili and C. australis diverged 3% with 30 different nucleotides based on the alignment performed herein.
Fig. 8.
Maximum likelihood phylogram based on partial sequences of the 18 S rRNA gene of selected species of Branchiura. GenBank accession number precedes species name. Newly generated sequence is in bold. Posterior probability followed by bootstrap support values above the branches (posterior probability < 0.90 and bootstrap < 60 not shown). Liguatula serrata Frölich, 1789 (Pentastomida: Liguatulidae) was used as outgroup. Branch length scale bar indicates the number of substitution per site.
Table 3.
Nucleotide genetic divergence among branchiuran 18 S sequences used in the phylogenetic analyses. Values below the diagonal are expressed in percentage (p-distance) while values above the diagonal represent number of differences in nucleotides.
4. Discussion
4.1. Distribution of Chonopeltis lisikili
It was originally thought that species of Chonopeltis were restricted (endemic) to a single river system. With the increase in parasitological surveys throughout specifically southern Africa (see Van As, 2015), we gained a better understanding of the distribution of species of Chonopeltis. For example, current distribution records show that C. inermis occurs in both Lake Malawi (Malawi) as well as in the Limpopo River system (South Africa) (see Van As and Van As, 1993). Another widely distributed species is Chonopeltis meridionalis Fryer, 1964 that, following its taxonomic revision by Van As et al. (2017), has locality records in the Zambezi River system (Eastern Caprivi, Namibia) and Limpopo River system (Nuanetsi River, Zimbabwe and Olifants River, South Africa). That a species can extend across the Zambezi, Okavango and Limpopo systems is not completely surprising, especially when considering the drainage evolution of Africa.
A study on the drainage evolution of Central Africa by Stankiewicz and De Wit (2006) stated that North Africa was mostly below sea level pending the end of the Cretaceous period (65 million years ago). Hereafter, an intricate sequence of uplifts and stream captures created the African river basins we recognise today. The drainage evolution in the Paleocene period started when the Okavango, Kalahari and Zimbabwe axis beheaded the Limpopo River, in turn transforming the Okavango, Cuando and Upper Zambezi into a landlocked system (Stankiewicz and De Wit, 2006). According to Moore et al. (2007) a number of important tributaries form part of the Zambezi System. The Upper Zambezi was probably linked to the Limpopo via the Shashe River. This river is more than 1 km wide near the confluence of the Upper Zambezi and the Limpopo Rivers, which is an indication of the overfit of the ephemeral modern flow regime. The major Cretaceous Zambezi-Limpopo river system entered the Mozambique coastal plain via a line of crustal weakness. Over geological times, the Limpopo River drainage areas have decreased. Up until the late Pleistocene most of the upper Zambezi River drained into the Limpopo River. Head water erosion diverts the flow of the Upper Zambezi from the endoreic Kalahari Basin into the Middle Zambezi, re-establishing the link to the Indian Ocean. This led to a lowering of the base level of the upper Zambezi, and a dramatic increase in the flow to the middle and lower sections of the river (Moore et al., 2007).
According to Moore et al. (2007) long-term changes in the flow regime of the Zambezi are broadly mirrored in the Okavango River. The outflow from the Okavango into Linyanti and into the Zambezi occurs from time to time via the Selinda spillway. Distribution patterns of certain fish species in the modern Zambezi system testify to this. Similarly, a high proportion (41%) of fish species are also common between the Middle Zambezi and the Limpopo rivers, indicating a link at some stage.
The present discovery of C. lisikili in the Phongolo River confirms that the Zambezi, Okavango and Limpopo systems must also have had historic linkages. The Phongolo River originates in South Africa's Mpumalanga Province from where it flows first eastwards into South Africa's northern KwaZulu-Natal, before turning north towards Mozambique through the Ubombo mountain ranges (Acosta et al., 2020). On the border with Mozambique, it joins the Usutu River to form the Maputo River (Rio del Maputo) that continues north until flowing into the sea in the southern section of Maputo Bay (Fig. 9). With the Limpopo River reaching the coast just north of Maputo Bay, it is most likely that the linkage with the Phongolo/Maputo River occurred during previous glacial maximums when sea-levels at Maputo Bay were 130 m below present (De Lecea et al., 2017). This historic linkage is also further supported by the distribution of C. lisikili's fish host (S. zambezensis) in the Phongolo.
Fig. 9.
Map of Lower Phongolo River in South Africa (Mpumalanga Province) as it turns north towards Mozambique. The Usutu River joins the Phongolo River in Ndumo Game Reserve where it becomes the Maputo River before flowing into the Indian ocean in the southern section of Maputo Bay.
4.2. Species of Synodontis as hosts of C. lisikili
The genus Synodontis (Mochokidae) is endemic to Africa and has been found in river systems ranging from Lake Rukwa in Tanzania to the Phongolo River in South Africa. Squeakers rank amongst one of the most species-rich genera of African fish, with 130 valid species (Froese and Pauly, 2020). The majority of Synodontis diversity is within fluviatile habitats (riverine), reaching peak diversity in the Congo drainage basin (Poll, 1971; Koblmüller et al., 2006; Day et al., 2009, 2013).
According to Van Der Waal (1997), the Shase Dam, which was constructed in the early seventies, was stocked with species from the Okavango Delta and was already posing a threat to the Limpopo fish community just twenty years later. Amongst these introductions was the brown squeaker, S. zambezensis.
Of the Synodontis species that were found to be hosts for C. lisikili, three of them have a wider distribution beyond just the Okavango system. Synodontis macrostigma occurs in the Cunene, Okavango, Upper Zambesi and Kafue systems, whilst S. leopardinus has been recorded from the same areas except the Kafue system. Synodontis nigromaculatus has the widest distribution and has been found in the Congo basin, upper Lualaba, Luapula drainage, lakes Mweru and Bangwelo, in the upper Zambezi, Okavango, Cunene and Limpopo systems. Synodontis woosnami occurs in the Okavango basin, Cunene river as well as upper Zambezi, but no branchiurans has been collected from it yet.
4.3. The branchiurans
Literature on Branchiura larval development is not as comprehensive compared to other Crustacea groups (Marin et al., 2014; Hadfield, 2019). Some of the earliest descriptions were on Argulus catostomi Dana and Herric, 1837 and A. foliaceus (Linnaeus, 1758) (see Dana and Herric, 1837; Claus, 1875; Wilson, 1904; 1907). Martin (1932) provided additional information on A. foliaceus, whilst Tokioka (1936) described the development of A. japonicas Thiele, 1900. This was followed more than two decades later with the larval development of A. coregoni Thorell, 1865 (see Shimura, 1981), a more detailed description of A. foliaceus by Rushton-Mellor and Boxshall (1994), as well as additional information on A. japonicus by Lutsch and Avenant-Oldewage (1995). In the case of Dolops, Fryer (1964) and Avenant et al. (1989) provided information on the hatching stages of Dolops ranarum (Stuhlmann, 1892). The most recent information on the hatching of a Dolops larva was by Møller and Olesen (2012) on D. carvalhoi Lemos de Castro, 1949. Larval development for four Chonopeltis species was described by Fryer (1956; 1961), in Van Niekerk (1984) and Van As and Van As (1996, 2019). According to Marin et al. (2014), the morphological diversity of Branchiura larvae is low and emphasises that it is only in the case of Chonopeltis species that the morphology of the larvae differs markedly.
Marin et al. (2014) concluded that there is no evidence to support major gender-based size differences in branchiuran larvae. There also remains some uncertainty on the exact number of larval stages in Chonopeltis development. Fryer (1961) suggested eight to nine larval stages for C. brevis, whilst Van As and Van As (1996) believe there may be 10 stages in the male of C. lisikili before the adult stage is reached. Following Van As and Van As (1996), is seems as if the females develop through nine stages to become mature adults. According to Marin et al. (2014), the general development in Chonopeltis seems to be more gradual compared to the other Branchiura genera. We are of the opinion that it is unnecessary to divide the development in exact seventh, eighth, ninth and tenth stages, but it is more important to focus on specific morphological features that change as the organisms develop.
4.4. Phylogenetic analysis
The present study is the first to provide sequences of a mitochondrial gene (COI) for Chonopeltis, and the second to provide sequences of the nuclear gene 18 S rRNA. Prior to this study, Neethling and Avenant-Oldewage (2020) successfully sequenced the 18 S gene for C. australis also from South Africa, which clustered together with C. lisikili from this study (Fig. 8). Most of the available 18 S sequences for Branchiura, mainly Argulus, are not associated with peer-reviewed publications, therefore these were not included in the phylogenetic analyses of the present study due to the possibility of doubtful taxa identification. As a result, there were not many 18 S sequences of Branchiura left to compare with the new sequence of C. lisikili. Moreover, the only 18 S sequence available in GenBank for another branchiuran genus, Dolops, is Dolops ranarum (DQ813453) collected from Clarias gariepinus (Burchell, 1822) from Lake Victoria, Tanzania, but it is also not associated with a peer-reviewed publication. Even though this is the only Dolops species distributed in Africa, thus no doubtful identification, the sequence is too short (529 bp), which precluded a suitable alignment with the longer sequences. The present study showed that the newly generated 18 S sequence of C. lisikili clusters together with C. australis as sister taxa, confirming the phylogenetic affinities between southern African species. However, more molecular studies of Branchiura species are necessary to better understand the relationships at genera and family levels, as well as patterns of species distribution.
4.5. Concluding remarks
A dozen valid Chonopeltis species have been described from fishes belonging to four families, i.e., Clariidae, Cyprinidae, Mormyridae and Mochokidae. Of these, at least six species are specific to a single host genus (see Neethling and Avenant-Oldewage, 2016), with C. elongatus Fryer, 1974 and C. lisikili parasitising Synodontis (Mochokidae) species (Fryer 1974; Van As and Van As, 1996).
As seen from this study, it appears that Chonopeltis might not be restricted to a certain river system as was originally proposed by Fryer (1968), but they are host specific to either a genus or family. There is also a very close link in the fish host distribution and that of this branchiuran. This can be seen with C. inermis, C. brevis and C. meridionalis to name a few (Fryer, 1961, 1964; Van As and Van As, 1996; Van As et al., 2017).
The historical African waterways were once interconnected, permitting the wide dispersal of ancestral fauna (Greenwood, 1983). The fish fauna of the Okavango River and Delta are now, for all practical purposes, isolated (Ramberg et al., 2006). The link between the eastern part of the Delta and the Zambezi, via the Magwegqana River, also known as the Selinda spillway, no longer receives water permanently. However, this river was flowing to the Zambezi during recent wetter periods (2009–2015). Synodontis zambezensis is one of only two Synodontis species that have been found in Lake Kariba (Sanyanga, 1996), out of the nine species that inhabit the Zambezi system (Skelton, 2001) and the more than 130 species recorded from Africa, that occurs in the Phongolo system as well.
The water links and the distribution of S. zambezensis and S. nigromaculatus, which are both hosts to C. lisikili, are further evidence of the historical link that used to be between the Okavango and Phongolo systems, via the main water ways.
Molecularly, no clear conclusions can be drawn from the work presented here due to the lack of available COI and 18 S sequences of specifically Chonopeltis as well as the other branchiuran genera. Therefore, future studies on these unique fish parasites should include high quality sequences of both COI and 18 S genes in order to develop a better understanding of the phylogenetic relationships between species and genera.
Declaration of competing interest
A summary declaration of interest statement in the title page file (if double-blind) or the manuscript file (if single-blind).
Acknowledgements
We are grateful to Ezemvelo KZN Wildlife for providing the permit OP 1582/2018 for sampling in KwaZulu-Natal. We also extend our sincere thanks to Dr Aline Acosta, North-West University–Water Research Group (NWU-WRG) for assistance with the molecular analysis; Dr Wynand Malherbe (NWU-WRG) for the construction of the map; and Dr Lizaan de Necker (NWU-WRG) for collection of the Phongolo specimens. This work is based on research supported in part by the National Research Foundation (NRF) of South Africa [NRF project CPRR160429163437 grant 105979, NJ Smit, PI; NRF Project Grant Nos: 120240 and 120403, KA Hadfield, PI]. Opinions, findings, conclusions and recommendations expressed in this publication are that of the authors, and the NRF accepts no liability in this regard. This is contribution number 531 from the North-West University–Water Research Group.
References
- Acosta A.A., Netherlands E.C., Retief F., de Necker L., du Preez L., Truter M., Alberts R., Gerber R., Wepener V., Malherbe W., Smit N.J. Conserving freshwater biodiversity in an african subtropical wetland: South Africa's lower Phongolo River and floodplain. In: Kideghesho J., editor. Managing Wildlife in a Changing World. IntechOpen; London: 2020. pp. 1–36. [Google Scholar]
- Avenant A., Van As J.G., Loots G.C. On the hatching and morphology of Dolops ranarum (Crustacea: Branchiura) J. Zool. (London) 1989;217:511–519. [Google Scholar]
- Claus C. Über die entwicklung, organisation und systematische Stelling der Arguliden. Z. Wiss. Zool. 1875;25:1–68. [Google Scholar]
- Dana J.D., Herric k E.C. Description of Argulus catostomi a new parasitic crustaceous animal. Am. J. Sci. 1837;31:297–308. [Google Scholar]
- Day J.J., Bills R., Friel J.P. Lacustrine radiations in African Synodontis catfish. J. Evol. Biol. 2009;22:805–817. doi: 10.1111/j.1420-9101.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- Day J.J., Peart C.R., Brown K.J., Friel J.P., Bills R., Moritz T. Continental diversification of an African catfish radiation (Mochokidae: Synodontis) Syst. Biol. 2013;62:351–365. doi: 10.1093/sysbio/syt001. [DOI] [PubMed] [Google Scholar]
- De Lecea A.M., Green A.N., Strachan K.L., Cooper J.A.G., Wiles E.A. Stepped Holocene sea-level rise and its influence on sedimentation in a large marine embayment: Maputo Bay, Mozambique. Estuar. Coast Shelf Sci. 2017;193:25–36. [Google Scholar]
- Floyd R.M., Rogers A.D., Lambshead P.J.D., Smith C.R. Nematode-specific PCR primers for the 18S small subunit rRNA gene. Mol. Ecol. Notes. 2005;5:611–612. [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Froese R., Pauly D. 2020. FishBase. World Wide Web Electronic Publication. www.fishbase.org (version 12/2019) [dated sited March 2020] [Google Scholar]
- Fryer G. A report of the parasitic copepoda and Branchiura of the fishes of lake nyasa. Proc. Zool. Soc. Lond. 1956;127:293–344. [Google Scholar]
- Fryer G. Larval development in the genus Chonopeltis (Crustacea: Branchiura) Proc. Zool. Soc. Lond. 1961;137:61–79. [Google Scholar]
- Fryer G. Further studies on the parasitic Crustacea of the African freshwater fishes. Proc. Zool. Soc. Lond. 1964;143:79–102. [Google Scholar]
- Fryer G. The parasitic Crustacea of African freshwater fishes, their biology and distribution. J. Zool. (London) 1968;156:45–95. [Google Scholar]
- Fryer G. Un nouvelle espéce de Chonopeltis (Crustacea: Branchiura), parasite d’un Poisson congolais. Rev. de Zool. Afr. 1974;88:437–440. [Google Scholar]
- Greenwood P.H. The zoogeography of African freshwater fishes: bioaccountancy or biogeography? In: Sims R.W., Price J.H., Whalley P.E.S., editors. Evolution, Time and Space: the Emergence of the Biosphere. Academic Press; London: 1983. pp. 179–199. [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hadfield K.A. Chapter 2. History of discovery of parasitic Crustacea. In: Smit N.J., Bruce N.L., Hadfield K.A., editors. Springer Nature; Switzerland: 2019. pp. 7–71. (Parasitic Crustacea: State of Knowledge and Future Trends. Zoological Monographs 3). [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblmüller S., Sturmbauer C., Verheyen E., Meyer A., Slazburger W. Mitochondrial phylogeny and phylogeography of east African squeaker catfishes (Siluriformes: Synodontis) BMC Evol. Biol. 2006;6:49. doi: 10.1186/1471-2148-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood D., Olson P.D. Chapter 25. Small subunit rDNA and the Platyhelminthes: signal, Noise, conflict and compromise. In: Littlewood D., Bray R.A., editors. Interrelationships of the Platyhelminthes. Taylor and Francis; London: 2001. pp. 262–278. [Google Scholar]
- Lutsch E., Avenant-Oldewage A. The ultrastructure of the new hatched Argulus japonicus Thiele, 1900 Larvae (Branchiura) Crustaceana. 1995;68:329–340. [Google Scholar]
- Marin J.W., Olesen J., Høeg J.T. John Hopkins University Press; Baltimore: 2014. Atlas of Crustacean Larvae. [Google Scholar]
- Martin M. On the morphology and classification of Argulus (Crustacea) Proc. Zool. Soc. Lond. 1932;102:771–806. [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. SC10Workshop on Gateway Computing Environments (GCE10), New Orleans, Louisiana. 2010:1–8. 14 November 2010. [Google Scholar]
- Møller O.S., Olesen J. First description of larva stage 1 from a non-African fish parasites Dolops (Branchiura) J. Crustac Biol. 2012;32:231–238. [Google Scholar]
- Moore A.E., Cotterill F.P.D., Main M.P.L., Williams H. John Wiley & Sons, Ltd; Chichester: 2007. Large Rivers: Geomorphology and Management: the Zambezi River. (Chapter 15), 311–331. [Google Scholar]
- Neethling L.A.M., Avenant-Oldewage A. Branchiura- A compendium of the geographical distribution and a summary of their biology. Crustaceana. 2016;89:1243–1446. [Google Scholar]
- Neethling L.A.M., Avenant-Oldewage A. Host selection and notes on the distribution of Chonopeltis australis Boxshall, 1976 in southern Africa. Afr. J. Aquat. Sci. 2020;45:500–508. [Google Scholar]
- Poll M. Révision des Synodontis Africanis (Familie Mochokidae) Ann. Mus. R. Afr. Cent. Sci. Zool. Ser 8. 1971;191:1–497. [Google Scholar]
- Rambaut A. WorldWideWeb electronic publication; 2009. Molecular Evolution, Phylogenetics and Epidemiology: Fig-Tree. [Google Scholar]
- Ramberg L., Van Aarde R., Ferreira S., Hancock P., Lindholm M., Meyer T., Ringrose S., Sliva J., Van As J.G., Van der Post C. Species diversity of the Okavango Delta, Botswana. Aquat. Sci. 2006;1–151 [Google Scholar]
- Ronquist F., Teslenko F.M., VanDerMark P., Ayres D.L., Darling A., Höhna S., Larget S.B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton-Mellor S.K., Boxshall G.A. The developmental sequences of Argulus foliaceus. J. Nat. Hist. 1994;28:763–785. [Google Scholar]
- Sanyanga R.A. Variation and abundance of Synodontis zambezensis (pisces: mochokidae) Peters, 1852 in the inshore fishery of Lake Kariba. Fish. Res. 1996;26:171–186. [Google Scholar]
- Schaeffner B.C., van Rooyen D., Gerber R., Scholz T., Smit N.J. Wenyonia gracilis sp. n. (Cestoda: caryophyllidea) from Synodontis zambezensis (Siluriformes: mochokidae): the first native caryophyllidean tapeworm from southern Africa. Folia Parasitol. 2020;67:35. doi: 10.14411/fp.2020.035. [DOI] [PubMed] [Google Scholar]
- Scholz T., Vanhove M.P.M., Smit N.J., Jayasundera Z., Gelnar M. ABCTaxa; Brussels, Belgium: 2018. A Guide to the Parasites of African Freshwater Fishes; p. 424. [Google Scholar]
- Shimura S. The larval development of Argulus coregoni Thorell (Crustacea: Branchiura) J. Nat. Hist. 1981;15:331–348. [Google Scholar]
- Skelton P.H. Struik Publishers; Cape Town: 2001. A Complete Guide to the Freshwater Fishes of Southern Africa. [Google Scholar]
- Stankiewicz J., De Wit M.J. Proposed drainage evolution model for Central Africa - did the Congo flow east? J. Afr. Earth Sci. 2006;44:75–84. [Google Scholar]
- Tokioka T. Larval development and metamorphosis of Argulus japonicus. Mem. Coll. Sci. Kyoto Univ. Ser. B. 1936;12:93–114. [Google Scholar]
- Van As J.G. A brief history of freshwater fish parasitology in southern Africa. Afr. Zool. 2015;50:93–107. [Google Scholar]
- Van As J.G., Van As L.L. Chapter 4. Adaptations and types of crustacean symbiotic associations. In: Smit N.J., Bruce N.L., Hadfield K.A., editors. Parasitic Crustacea: State of Knowledge and Future Trends. Zoological Monographs 3. Springer nature; Switzerland: 2019. pp. 135–178. [Google Scholar]
- Van As L.L., Van As J.G. First record of Chonopeltis inermis Thiele, 1900 (Crustacea: Branchiura) in the Limpopo river system with notes on its morphology. Syst. Parasitol. 1993;24:229–236. [Google Scholar]
- Van As L.L., Van As J.G. A new species of Chonopeltis (Crustacea: Branchiura) from the southern Rift valley, with notes on larval development. Syst. Parasitol. 1996;35:69–77. [Google Scholar]
- Van As L.L., Van As J.G. Branchiuran parasites (Crustacea: Branchiura) from fishes in the Okavango (Botswana) and Zambezi (Namibia) systems. Afr. J. Aquat. Sci. 2015;40:9–20. [Google Scholar]
- Van As L.L., Smit N.J., Van As J.G. Re-discovery of Chonopeltis meridionalis fryer, 1964 (Crustacea: Branchiura) from Labeo rosae steindachner, Olifants River, Mpumalanga and the taxonomic status of C. victori avenant-oldewage, 1991 and C. koki van as, 1992. Syst. Parasitol. 2017;94:797–807. doi: 10.1007/s11230-017-9737-1. [DOI] [PubMed] [Google Scholar]
- Van Niekerk J.P. Unpublished PhD thesis; UFS: 1984. The Biology of the Genus Chonopeltis Thiele, 1900 (Crustacea: Branchiura) from the Orange-Vaal Catchment Area; p. 171. [Google Scholar]
- Van Der Waal B.C.W. Some observations on the fish life in a seasonal sand river. Afr. J. Aquat. Sci. 1997;23:95–102. [Google Scholar]
- Wilson C.B. The fish parasites of the genus Argulus found in the Woods Hole region. Bull. Bur. of Fish. 1904;24:115–131. [Google Scholar]
- Wilson C.B. Additional notes on the development of the Argulidae, with description of a new species. Proc. U. S. Natl. Mus. 1907;32:411–424. [Google Scholar]