Abstract
The purpose of this study is to use Health Technology Assessment (HTA) through the Six Sigma (SS) and DMAIC (Define, Measure, Analyse, Improve, Control) problem-solving strategies for comparing cemented and uncemented prostheses in terms of the costs incurred for Total hip arthroplasty (THA) and the length of hospital stay (LOS). Multinomial logistic regression analysis for modelling the data was also performed. Quantitative parameters extracted from gait analysis, electromyography and computed tomography images were used to compare the approaches, but the analysis did not show statistical significance. The variables regarding costs were studied with the Mann-Whitney and Kruskal-Wallis tests. No statistically significant difference between cemented and uncemented prosthesis for the total cost of LOS was found, but the cost of the surgeon had an influence on the overall expenses, affecting the cemented prosthetic approach. The material costs of surgery for the uncemented prosthesis and the cost of theatre of surgery for the cemented prosthesis were the most influential. Multinomial logistic regression identified the Vastus Lateralis variable as statistically significant. The overall accuracy of the model is 93.0%. The use of SS and DMAIC cycle as tools of HTA proved that the cemented and uncemented approaches for THA have similar costs and LOSy.
Key Words: health technology assessment, six sigma, cost analysis, multinomial logistic regression, total hip arthroplasty
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Total hip arthroplasty (THA) is one of the most frequent and successful surgical procedures that aims to make patients' lives better through prosthetic replacement of diseased acetabular and femoral heads.1 In recent years, THA is considered a safe procedure and postoperative morbidity and mortality has decreased.2 Changes in living conditions have led to an increase in age of the elderly population and orthopaedic surgeons are increasingly finding themselves treating a greater number of elderly patients who need to undergo THA. For THA, bone quality, assessed from conventional radiograms, is one of the variables, including the patient’s age, sex, and lifestyle, together with the efficiency and assessments of the surgeon, which can influence the choice of the type of implant and the most appropriate method of fixation for the surgery.3-6
There are different approaches to THA, but those analysed in this study include the use of acrylic bone cement (cemented prosthesis) and a pressure fitting against the bone (uncemented prosthesis). However, the debate concerning the choice of cemented or uncemented fixation occurs frequently. The advantages of the uncemented procedure include a lower risk of cardiovascular and thromboembolic complications linked to the cement, the possibility of biological fixation, the reduction of stress of the proximal femur and extended implant survival potential and easier late revision. Bone fusion is the key to supporting the use of uncemented components.7,8 The upper bone-implant interface, created from components coated with hydroxyapatite, produces a constantly remodelling seal between living bone and inert metal, providing excellent fixation.9 In contrast, the choice of the cemented approach provides an immediate post-operative advantage in terms of a better integration between bone, cement and the prosthesis, which allows an early relief of pain.10 The success of the implant after THA is the most frequent study object and even if studies have shown the success of the procedure, failures can always happen. In order to prevent this, doctors recommend routine follow-up visits. Obviously, these reviews are expensive for both the patient and the health system given the growing number of THA procedures performed. Thus, it would be useful to identify an economic model for the management of patients after surgery.11,12 Therefore, orthopaedic surgeons increasingly need sound economic evidence to understand the full value of the operation, to make decisions about available procedures and to justify their practice beyond the traditional clinical preference. Literature shows that managing resources is an important test by trying to evaluate the cost of replenishing the implant compared to the cost of the entire procedure and patient's path. By comparing the two cemented and uncemented approaches uncemented implants seem more expensive.13 From studies that have taken into consideration the cost of the implant, the length of hospital stay(LOS), the probability of reoperations, the duration in the operating room, it emerges that the cementless procedures can be slightly faster but there are tests that indicate that cemented arthroplasties are cheaper in the long run.14 Various approaches and innovative methodologies have been introduced to reduce waste in healthcare, in particular by acting on the waiting times in the hospital.15,16 Advanced analytical techniques have also been introduced to healthcare to help physicians and clinicians with the diagnosis,17-19 and investigation of disease prognosis.20-22 The result is a difference between what is technologically possible and what can be sustained economically.23 Therefore, the purpose of this work will be to use the Health Technology Assessment (HTA) through the Six Sigma (SS) methodology for the comparison between cemented and uncemented prostheses as regards the costs [with euro/Iceland Krona (ISK) currency of 136,8 per day 29/01/2020] and the LOS by using the SS methodology and finally a multinomial logistic regression analysis for modelling the data. This article can serve as a guide on how to apply the SS approach to improve the quality of healthcare. Obviously not to provide answers to precise questions, but to understand the various factors in determining costs of THA.
Materials and Methods
Literature Review
Over the past decades, several countries have faced the challenge of controlling rising health costs and spending. Although the presentation of new technologies in healthcare has been understood as a significant cost factor, at the same time the new technologies have been producing important qualitative improvements. One of the widespread mechanisms is the HTA evaluation.24 HTA is a multidisciplinary process that summarizes information on clinical, economic, social and ethical issues related to the use of health technology, in a systematic, transparent, impartial and solid way. Its goal is to contribute to the identification of safe, effective, patient centred health policies aimed at achieving the best value. The HTA process is based on scientific evidence from studies; indeed, this approach was applied to examine the problem of surgical infections in patients with oral cavity cancer by comparing two drugs in terms of shorter duration of LOS,25 and maximizing health gains through efficient use of resources in Saudi Arabia.24
Therefore, the quality of healthcare is a matter of great concern and finding ways to improve quality and reduce costs is one of the most important issues. To tackle them, a management tool that has emerged in healthcare is the SS methodology.26 SS and its philosophy have found development in many manufacturing industries and are currently widely used in healthcare; the method of improvement and resolution of problems is described by the DMAIC (Define, Measure, Analyse, Improve, Control) approach which develops in five phases. The DMAIC roadmap is applied in healthcare in various fields such as first aid,17 orthopaedics,27,28 and reduction of hospital infections,29 The SS methodology is often associated with Lean Thinking with the aim of increasing positive healthcare outcomes, patient satisfaction by eliminating waste, and reducing costs.24,30-32 Application of these methodologies has led to great successes in healthcare, in fact many studies show improvements in processes such as increased productivity,33 increased quality by eliminating waste in hospital operating costs,34 and improved efficiency in the process of hip replacement surgery in Finland.35 The methodology was applied for the introduction of a diagnostic-therapeutic-assistance path to reduce LOS and costs in the management of elderly patients with femur fracture,36 and to analyse a clinical path through fast track surgery to improve the quality and reduce the costs associated with prosthetic hip and knee replacement surgery.37,38 The SS methodology was also combined with agile manufacturing to manage waiting lists and absenteeism of patients in order to avoid wasting time and resources.39,40 This work focused on the application of the SS methodology for reducing hospital costs related to THA by comparing the cemented and uncemented approaches.
The Clinical Study
The study was conducted at the Landspítali University Hospital in Reykjavik, Iceland. This is the leading hospital in Iceland, the largest workplace for employees in healthcare and has a capacity of 700 beds, performing about 300-400 surgical procedures a year. The data were extracted from a database at the hospital. The initial study included 70 patients who underwent THA for the first time to try to improve their quality of life. 34 received a cemented hip prosthesis and 36 received an uncemented prosthesis. The prostheses used were manufactured by Zimmer. Patient pre- and post-operative assessment was approved by the Icelandic Bioethics committee (Application number: 13-127-S1—Study type and level of evidence: ‘‘Level 2 prospective cohort study’’). All patients underwent a Computed Tomography (CT) scan (64 CT Philips Brilliance) before and immediately after the operation and 52 weeks after surgery and also a biomechanical analysis at the Landspítali rehabilitation clinic 1–2 days before and 1 year after surgery. Moreover, the gait parameters were obtained together with electromyographic (EMG) signals, used as a tool to define muscle activation in experimental studies on human walking.37 They were recorded on the patients' quadriceps [Rectus femoris (RF), Vastus lateralis (VL) and Vastus medialis (VM)] with a frequency of 1600Hz using the Kine Pro system.1 Furthermore, the CT data were optimized for post-processing segmentation for the creation of 3D models of bone and muscle to simulate and calculate bone mineral density (BMD),38 useful for information on the structural integrity of the bone as the loss of BMD could make the bone weaker and more susceptible to fractures, negatively affecting the mechanical stability of an implanted prosthesis.3 For each patient the following variables were collected: gender, age, gait parameters, BMD, EMG analysis, allergies, body mass index (BMI), comorbidities and costs associated to each patient. The costs for both approaches (cemented and uncemented) will be analysed.
They are divided into the following categories:
-
• Cost of hospital stay:
o Surgeon cost
o Other costs (i.e. costs related to hospital stay)
o Administration costs.
-
• Surgical cost:
o Material cost of surgery
o Surgical theatre cost of surgery
o Anaesthesia
o Awakening from anaesthesia
Development of the methodology (The define phase)
In this phase, the purpose of the cycle is to define a multidisciplinary work team and divide the tasks of analysis. The team is made up of biomedical engineers, a surgeon and a biologist with an experience in health management. The team was responsible for collecting and analysing the data of hospital costs, breaking down hospital and surgical costs during the stay in the hospital according to some variables. The champion and the leader supervised and coordinated the study and gave conceptual help in interpreting the data. In SS projects, the champion is the person in a company translating the mission, vision, and values into a SS deployment strategy while the leader coordinates a SS project under the direction of the champion.
Initially, a project diagram was created to define the problem to solve:
• Project title: HTA through SS methodology to assess cemented and uncemented protheses in THA.
• Question: comparison of cemented and uncemented prosthesis.
• Critical to quality: Costs [€] and LOS [days].
• Target: Finding the prosthesis allowing lower costs and shorter LOS.
• In scope:
o Reykjavík University, Institute for Biomedical and Neural Engineering and Landspítali Hospital, Orthopaedic Clinic, Reykjavík, Iceland.
o THA.
• Out of scope: All the other structures and interventions.
• Financial: No funding was provided to reach the target.
• Business need: Identifying the cost that most affects the prosthesis.
Finally, a SIPOC (Suppliers-Inputs-Process-Outputs-Customers) scheme was a good tool to clarify the following main process characteristics:
• Supplier:
o Reykjavík University, Institute for Biomedical and Neural Engineering Iceland;
o Landspítali Hospital, Orthopaedic Clinic, Reykjavík, Iceland;
o Clinical staff.
• Input:
o Needs of patients;
o THA;
o Orthopaedic surgery.
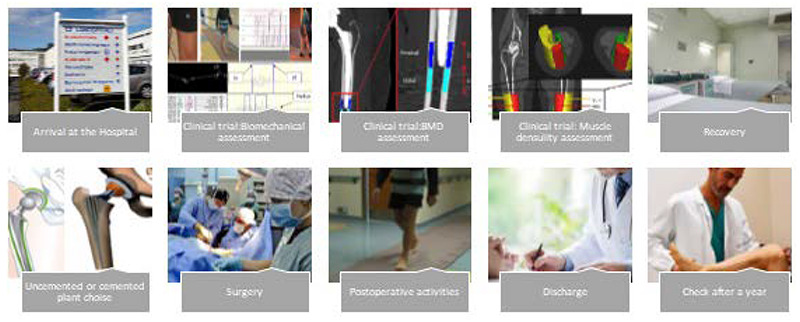
• Process:
o Arrival at the hospital;
o Clinical trials: gait analysis, BMD measurements, x-ray muscles absorption, EMG analysis;
o Recovery;
o Uncemented or cemented plant choice;
o Surgery;
o Postoperative activities;
o Discharge;
o Check after a year.
• Output:
o Better recovery;
o Improved outcome for patients;
o Lower number of complications;
o Reduction of hospital costs during stay.
• Customers:
o Patients;
o Landspítali Hospital, Orthopaedic Clinic, Reykjavík, Iceland.
Dataset description (The measure phase)
Physiological and biometric measures were considered to compare the LOS of the two different surgical approaches in the field of orthopaedic surgery. The following variables were collected for each patient: gender, age, walking parameters, BMD, EMG analysis, allergies, BMI, comorbidities. In particular, the gait parameters include step length, stride length, base support, single support, double support, swing, stance, toe in/out, velocity. The BMD was measured for: proximal, the part above the tip of the femoral stem, and distal, the part under the tip of the femoral stem. Finally, the parameters recorded on the quadriceps of the patients are the activation times of the VM, VL and RF. These variables were studied through a univariate statistical analysis. In particular, from the Shapiro-Wilk test the data emerged as not normally distributed. Therefore, the Mann-Whitney non-parametric test for independent samples was applied. Considering the costs for the cemented and uncemented approaches, described in the collection of data, the dataset is composed of patients who underwent THA for the first time and included all patients with a complete clinical history. After identifying the purpose of the study, the dataset and methodology, the costs were analysed. Initially, the Shapiro-Wilk test was applied (significance level α = 0.05), necessary for the application of statistical tests. The total costs of hospital stay compared to surgical costs are those that have the greatest impact on both cemented and uncemented prostheses.
Fig 1.
Surgical path of the patients
Statistical analysis (The analysis phase)
Figure 1 shows the surgical path of the patients, from arrival in hospital to discharge. Once in the hospital, the patients are subjected to various tests to determine the above-mentioned parameters. Then, they underwent surgery. After surgery, the patient undergoes post-operation measurements and is evaluated for any potential post-surgical complication. Finally, when the patients are recovered sufficiently, they can be discharged and 6 weeks later they will return to the hospital for a check-up. In order to statistically evaluate the costs for the group of patients based on the collected variables, Kruskal-Wallis tests were performed on non-dichotomous categories since the Shapiro-Wilk test showed a non-normal distribution of data. In this analysis, an alpha value of 0.05 is used as the cut off value for significance.
Table 1.
Univariate analysis conducted through Kruskal Wallis to investigate variables influencing costs
| Category | Breakdown of categories | Cemented | Uncemented | |||
|---|---|---|---|---|---|---|
| Mean ± Dev. Std. | p-value | Mean ± Dev. Std. | p-value | |||
| Total cost of hospital stay | Surgeon cost | 3884,33±961,47 | <0,001 | 4366,88±1935,18 | <0,001 | |
| Other cost | 2253,61±1058,39 | 1981,24±771,25 | ||||
| Administration cost | 360,43±141,90 | 326,28±120,60 | ||||
| Surgical cost | Material cost of surgery | 1587,66±701,29 | <0,001 | 2354,95±1615,60 | <0,001 | |
| Surgical theatre cost of surgery | 1299,12±537,55 | 1059,82±442,96 | ||||
| Anaesthesia | 782,85±248,90 | 741,81±307,87 | ||||
| Awakening from anaesthesia | 214,69±98,26 | 210,30±72,90 | ||||
Table 2.
Statistical analysis of LOS related to each variable and category
| Source | Variable | Category | LOS cemented [Mean±Dev. Std.] | LOS uncemented [Mean±Dev. Std.] | P-value |
|---|---|---|---|---|---|
| Overall | 4,86±1,15 | 4,50±0,66 | 0,125 | ||
| Gender | Males | 5,20±1,79 | 4,29±0,61 | 0,391 | |
| Females | 4,75±0,93 | 4,80±0,63 | 0,856 | ||
| Baseline | Age | Age<68 | 4,73±1,01 | 4,46±0,52 | 0,494 |
| Age>69 | 5,00±1,33 | 4,55±0,82 | 0,223 | ||
| BMI | BMI < 30 | 5,15±1,07 | 4,54±0,66 | 0,091 | |
| BMI ≥ 30 | 4,37±1,19 | 4,45±0,69 | 1,000 | ||
| Allergies | No | 4,83±1,40 | 4,55±0,61 | 0,307 | |
| Yes | 4,89±0,78 | 4,25±0,96 | 0,330 | ||
| Step Length | I | 4,58±1,24 | 4,54±0,78 | 0,769 | |
| II | 5,22±0,97 | 4,45±0,52 | 0,080 | ||
| Gait Analysis | Stride Length | I | 4,73±1,19 | 4,64±0,67 | 0,606 |
| II | 5,00±1,15 | 4,38±0,65 | 0,166 | ||
| Base Support | I | 4,90±1,10 | 4,50±0,65 | 0,321 | |
| II | 4,82±1,25 | 4,50±0,71 | 0,426 | ||
| Single Support | I | 4,67±1,32 | 4,50±0,71 | 0,720 | |
| II | 5,00±1,04 | 4,50±0,65 | 0,160 | ||
| Double Support | I | 4,77±0,83 | 4,53±0,64 | 0,363 | |
| II | 5,00±1,60 | 4,44±0,73 | 0,321 | ||
| Swing | I | 5,27±1,00 | 4,33±0,71 | 0,056 | |
| II | 4,40±1,17 | 4,60±0,63 | 1,000 | ||
| Stance | I | 4,40±1,17 | 4,60±0,63 | 1,000 | |
| II | 5,27±1,00 | 4,33±0,71 | 0,056 | ||
| Toe In/Out | I | 4,73±1,14 | 4,42±0,52 | 0,379 | |
| II | 5,00±0,81 | 4,58±0,79 | 0,381 | ||
| Velocity | I | 5,33±0,58 | 4,60±0,52 | 0,161 | |
| [m/s] | II | 4,78±1,21 | 4,43±0,76 | 0,283 | |
| CT | Proximal | I | 4,69±0,95 | 4,63±0,92 | 0,750 |
| II | 5,13±1,46 | 4,44±0,51 | 0,291 | ||
| Distal | I | 4,69±0,95 | 4,58±0,79 | 0,538 | |
| II | 5,13±1,46 | 4,42±0,52 | 0,305 | ||
| RF | I | 4,82±1,25 | 4,25±0,62 | 0,104 | |
| II | 4,90±1,10 | 4,75±0,62 | 0,771 | ||
| EMG | VL | I | 4,82±1,25 | 4,50±0,80 | 0,347 |
| II | 4,90±1,10 | 4,50±0,52 | 0,346 | ||
| VM | I | 4,56±1,13 | 4,44±0,73 | 0,605 | |
| II | 5,08±1,16 | 4,53±0,64 | 0,167 |
Finally, Table 1 shows the results of the analyses indicating that both categories for the cemented and uncemented approaches statistically influence the costs (p-values<0.001).
Comparing the prosthesis (The “Improve”)
The hip is made up of a sphere and an orbit formed by the femur and a section of the pelvis, called the acetabulum. In a normal hip, the cartilage separates the sphere and the handle, thus allowing the ball to slide easily into the handle to cushion the joint. The hip can wear out at various points during life, so THA aims to improve patients' quality of life by providing a joint that functions as normally as possible, is resistant to dislocation, while trying to save as much bone as possible. There are several approaches to choose the type of implant in THA, but the one used in this study involves the use of acrylic bone cement (cemented prosthesis) and a pressure fitting against the bone (uncemented prosthesis). The selection of the type of implant and the most appropriate method of fixation depend on the preferences of the surgeon (age, effectiveness and bone quality).41,42 The prosthesis with cemented fixation, allows almost immediate ambulation, thus allowing fast rehabilitation. This solution is taken into consideration by patients over the age of 60 or those who have poor bone quality or severe forms of arthritis, although it is not very suitable for overweight or particularly active patients, as cement break-down could occur.43 The prosthesis with uncemented fixation, on the other hand, adheres directly to the bone without the use of cement thanks to the conformation of the surface of the prosthesis itself, which is able to facilitate the neoformation of bone tissue around the point of contact. The uncemented stems have different shapes (wedged, tapered, modular and anatomical) to guarantee the initial stability and bone contact. Since the stabilization between the bone and the prosthesis takes place over a longer period, it is necessary to walk with crutches for 6-12 months after surgery. This type of intervention is recommended for younger, more active patients with better bone quality.41
Table 3.
Statistical analysis of L of costs (in €) related to each category
| Category | Breakdown of categories |
Cemented [Mean±Dev. Std.] |
Uncemented [Mean±Dev. Std.] |
p-value |
|---|---|---|---|---|
| Total cost of hospital stay | Overall | 6751,38±1536,81 | 6879,59±2236,70 | 0,755 |
| Surgeon cost | 253,01±104,19 | 205,18±97,69 | 0,010 | |
| Other cost | 2253,61±1058,39 | 1981,24±771,25 | 0,152 | |
| Administration cost | 360,43±141,90 | 326,28±120,60 | 0,218 | |
| Surgical cost | Overall | 3884,33±961,47 | 4366,88±1935,18 | 0,344 |
| Material cost of surgery | 1587,66±701,29 | 2354,95±1615,60 | 0,013 | |
| Surgical theatre cost | 1299,12±537,55 | 1059,82±442,96 | 0,006 | |
| Anaesthesia | 782,85±248,90 | 741,81±307,87 | 0,332 | |
| Awakening from anaesthesia |
214,69±98,26 | 210,30±72,90 | 0,769 |
Control
After carrying out the normality test of Shapiro-Wilk (with a level of uncertainty or an alpha equal to 0,05) which showed the data to not be normally distributed, some statistical tests with an alpha of 0,05 were applied to the subgroups in order to highlight statistically significant differences. The Mann-Whitney test was applied to compare costs and LOS between the two groups of patients.
Multinomial logistic regression
Multinomial logistic regression aims to study and quantify the relationships between one or more independent quantitative variables and a dichotomous dependent variable. In this study this model used the type of cemented and uncemented prosthesis as a dependent variable and all the other quantitative parameters as independent variables.
The variables can be defined by the following equation (1):
| y=β0+β1χ1+β2χ2+β3χ3+ ... +βNχN | (1) |
where y is the value of the expected type of prosthesis, β0 is the intercept value, xi are independent variables and βi estimated regression coefficients of the respective variables.
Before building the model, it was necessary to make the following assumptions:
Independent variables affected by multicollinearity must be removed. This is verified with a Pearson’s Bivariate Correlation test, correlations greater than 0.8 are influential.
Cook's Distance is used in regression analysis to find influential outliers in a set of predictive variables. It is a way of identifying points that negatively affect the regression model. This distance measures how much the residuals of all cases would change if a particular case were excluded from the calculation of the regression coefficients. A high coefficient indicates that, excluding the case from the analysis, the coefficients would have changed substantially. The centre leverage value indicates the data points that have the potential to shift a linear regression line.
Multinomial logistic regression and model diagnostics were performed with IBM SPSS.
Results
Comparing cemented and ubcemented prostyesis in terms of LOS
The average of the LOS for cemented and uncemented prosthesis is shown in Table 2. The data did not show statistical significance based on the categories analysed. The reference cut-off considered for each variable corresponds to the arithmetic mean of the collected values. However, the physiological and biometric measurements that appear to influence LOS are the percentage of gait cycle between last contact of the current footfall to the first contact of the next footfall (Swing) with a p-value of 0.056 and the percentage of gait cycle between two consecutive heel contacts and toe events on the same foot (Stance) with a p-value of 0.056.
Table 4.
Multinomial logistic regression results to distinguish cemented and uncemented prosthesis.
| Variables | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Age | 0.582 (0.386 – 0.879) | 0.010 |
| RF | 0.789 (0.482 – 1.293) | 0.347 |
| VL | 1.956 (0.954 – 4.009) | 0.067 |
| BMI | 1.571 0.952 – 2.5692 | 0.077 |
Odds ratio (95% confidence interval) and p-value for each variable considered in the model. *significance at 0.05, **significance at 0.01
Cost analysis
Each category, both the total costs of hospital stay and the surgical costs, was analysed with a non-parametric statistical test, the Mann-Whitney test, because the Shapiro-Wilk test for the normality of the data showed a p value lower than 0.01, meaning a non-normal distribution of data. From the tests reported in Table 3, it emerges that in the total cost of hospital stay category, there is not a statistically significant difference between cemented and uncemented in terms of cost but what most influences them is the surgeon cost (p-value = 0.010); in particular, the cemented prosthesis approach is affected more than the uncemented. For the surgical cost category there is no statistically significant difference between the two surgical approaches, but the material costs of surgery are the most influential ones (p-value = 0.013) for the uncemented prosthesis and the surgical theatre cost of surgery (p-value = 0.006) for the cemented prosthesis.
Modelling
The multinomial logistic regression attempted to distinguish between the cemented and uncemented prosthesis, the results are shown in Table 4. Two outliers were eliminated from the model. The four odds ratios of the variables included in the model are: age, RF, VL and BMI. The variables that gave significance show that the model reflects the parameters considered by the surgeon in the choice of the prosthesis to be implanted. The VL variable turns out to be almost statistically significant and therefore the muscular component could also be taken into consideration in the choice of the surgeon. The overall accuracy of the model is 93.0 %, the ability to detect cemented group is 90.0 % while the capacity to detect the uncemented group is 95.7 %. The Hosmer-Lemeshow goodness of fit test proved the good overall quality of the model: p-value = 0.979
Discussion
The decision-making process relating to the type of prosthesis has been much discussed in the literature,41,44-47 but different opinions on the subject remain.. The past objectives were to find differences between biomechanical parameters extracted from patients undergoing cemented and uncemented prosthesis THA.1 The aim of this article was to use the HTA through the SS methodology for the comparison between cemented and uncemented prostheses, to investigate the economic differences between two groups receiving different types of prosthesis and finally a multinomial logistic regression analysis to model the data. The study shows how the SS methodology through the DMAIC cycle turns out to be a well-structured problem-solving strategy to address economic issues as well, as already demonstrated by other studies.31,32 In this case, it was used to compare the costs of hip replacement with the cemented and uncemented surgical approaches that patients underwent for the first time.
The results obtained from the comparison between the two prostheses do not show statistical significance based on the categories analysed. However, the physiological and biometric measurements that appear to influence LOS are the percentage of the gait cycle between the last contact of the current gait and the first contact of the next step (Swing) and the percentage of the gait cycle between two consecutive heel contacts, and toe events on the same foot (Stance). A factor that could influence space-time parameters and gait kinematics is pain in walking ability after THA and therefore this could lengthen the LOS in hospital.48
As for the results obtained under the guidance of the DMAIC through statistical tests and graphic representations, they show how in the total cost of the hospitalization category there is no statistically significant difference between cemented and uncemented, but what most influences the overall costs is the cost of the surgeon and in particular it has a greater influence on the cemented prosthetic approach. Similarly, for the surgical costs category there is no statistically significant difference between the two surgical approaches, but the material cost of surgery for an uncemented prosthesis and the cost of theatre surgery for a cemented prosthesis are the most influential.
Finally, the modelling of the data shows how the parameters are considered in the choice of prosthesis; other researchers have tried recently to model through machine learning analysis the choice of the surgeon in the type of prosthesis and demonstrated the importance of the EMG in this choice,49 specifically ML techniques were applied on quantitative biomechanical and bone quality data extracted from CT, EMG and gait analysis, aiming to help clinicians by using patient-specific biomarkers from diagnostic exams in the prosthetic decision-making process. The skeletal muscle parameters such as the start and stop of muscle contraction from EMG signals was particularly important for identifying the best prosthesis for patients. The novelty of this document is the use of the SS methodology with the DMAIC cycle to manage a health problem related to the costs incurred by the orthopaedic department. Obviously, SS cannot replace health technology assessment appropriately, but it could be a valuable analytical tool. A limitation in this study could be the exclusive use of the Zimmer prosthesis that was implemented in all patients; thus, it is not known whether a change in the prosthesis would affect the other costs. Moreover, all patients were Icelandic, and the National Health System may have had an influence on the results, but these factors may also be seen to strengthen the results as they are without any external corruption due to the implanted biomedical technology.
Practical inplications
Healthcare cost in hospitals is a very important topic and is recognized worldwide as a real public health problem, as evidenced by previous studies.28,37 Choosing the best prosthesis for each patient and, simultaneously, reducing hospital costs would be valuable both for the hospital and patients as it would eliminate waste, simplify processes and, consequently, improve the quality of healthcare and patient satisfaction.
Acronyms
- BMD
Bone mineral density
- BMI
Body mass index
- CT
Computed tomography
- DMAIC
Define, Measure, Analyse, Improve, Control
- EMG
Electromyographic signals
- HTA
Health Technology Assessment
- ISK
Iceland Krona
- LOS
Length of Hospital Stay
- LSH
Landspítali Hospital Reykjavík Iceland
- RF
Rectus femoris
- SS
Six Sigma.
- THA
Total hip arthroplasty
- VL
Vastus lateralis
- VM
Vastus medialis
Funding Statement
Funding: This research was supported jointly by the University of Reykjavik and the Icelandic National Hospital (Landspítali Scientific Fund, PI: Paolo Gargiulo, Title: Bone modeling inpatients undergoing THA, Project Number: A-2014-072) with additional funding support from Rannís (Rannís Icelandic Research Fund (Rannsóknasjodur), PI: Paolo Gargiulo, Title: Clinical evaluation score for Total Hip Arthroplasty planning and postoperative assessment, Project Number: 152368-051.
Contributor Information
Imma Latessa, Email: immalatessa@gmail.com.
Carlo Ricciardi, Email: carloricciardi.93@gmail.com.
Deborah Jacob, Email: dcrjacob@gmail.com.
Halldór Jónsson Jr, Email: halldor@landspitali.is.
Monica Gambacorta, Email: m.gambacorta@aslsalerno.it.
Giovanni Improta, Email: ing.improta@gmail.com.
References
- 1.Gargiulo P, Edmunds K J, Gíslason M K, Latour C, Hermannsson Þ, Esposito L, Bifulco P, Cesarelli M, Fraldi M, Cristofolini L, Jónsson H. Patient-specific mobility assessment to monitor recovery after total hip arthroplasty. Proc Inst Mech Eng H. 2018. Oct;232(10):1048-1059. doi: 10.1177/0954 411918797971. Epub 2018. Sep 7. [DOI] [PubMed] [Google Scholar]
- 2.Hunt L P, Ben-Shlomo Y, Clark E M, Dieppe P, Judge A, Mac Gregor A J, Tobias J H, Vernon K, Blom AW. 90-day mortality after 409 096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet. 2013. Sep 28;382(9898):1097-104. doi: 10.1016/S0140-6736(13)61749-3. [DOI] [PubMed] [Google Scholar]
- 3.Gislason M K, Lupidio F, Jónsson Jr H, Cristofolini L, Esposito L, Bifulco P, Gargiulo P. Three dimensional bone mineral density changes in the femur over 1 year in primary total hip arthroplasty patients. Clin Biomech. (Bristol, Avon) 2020. Aug;78:105092. doi: 10.1016/j.clinbiomech.2020.105092. Epub 2020. Jun 11. [DOI] [PubMed] [Google Scholar]
- 4.Esposito L, Minutolo V, Gargiulo P, Jonsson H, Gislason M K, Fraldi M. Towards an app to estimate patient‐specific perioperative femur fracture risk. Applied Sciences (Switzerland). Appl Sci. 2020; 10, 6409. doi: 10.3390/app10186409. [Google Scholar]
- 5.Esposito L, Bifulco P, Gargiulo P, Edmunds K, Cutolo A, Cesarelli M, Iuppariello L, Jónsson H, Fraldi M. Towards a patient-specific estimation of intra-operative femoral fracture risk. Comput Methods Biomech Biomed Engin. 2018. Sep;21(12): 663-672. doi:10.1080/10255842.2018.1508570. [DOI] [PubMed] [Google Scholar]
- 6.Esposito L, Bifulco P, Gargiulo P, Fraldi M. Singularity-free finite element model of bone through automated voxel-based reconstruction. Comput Methods Biomech Biomed Engin. 2016. Feb;19(3): 257-262. doi: 10.1080/10255842.2015.1014347. [DOI] [PubMed] [Google Scholar]
- 7.Pakvis D, Biemond L, van Hellemondt G, Spruit M. A cementless elastic monoblock socket in young patients: a ten to 18-year clinical and radiological follow-up. Int Orthop. 2011. Oct;35(10):1445-51.. doi: 10.1007/s00264-010-1120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallala R, Anderson P, Morris S, Haddad FS. The cost analysis of cemented versus cementless total hip replacement operations on the NHS. Bone Joint J. 2013. Jul;95-B(7):874-6. doi: 10.1302/0301-620X.95B7.26931. [DOI] [PubMed] [Google Scholar]
- 9.Campbell D, Graham M, Nilsson K G, Wells Vet, Field JR, Callary SA. Early migration characteristics of a hydroxyapatite-coated femoral stem: an RSA study. Int Orthop. 2011. Apr;35(4):483-8. doi: 10.1007/s00264-009-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdulkarim A, Ellanti P, Motterlini N, Fahey T, O’Byrne. Cemented versus uncemented fixation in total hip replacement: a systematic review and meta-analysis of randomized controlled trials. Orthop Rev (Pavia). 2013. Mar 15;5(1):e8. doi: 10.4081/or.2013.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X D, Tian M, He Y, Chen H, Chen Y, Mishra R, Liu W, Huang W. Short to midterm follow-up of periprosthetic bone mineral density after total hip arthroplasty with the Ribbed anatomic stem. Biomed Res Int. 2019. Jun 27;2019:3085258. doi: 10.1155 2019/3085258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendricks TJ, Chong AC, Cusick RP. The cost of routine follow-up in total joint arthroplasty and the influence of these visits on treatment plans. Kans J Med. 2018. Aug 30;11(3):59-66. eCollection 2018. Aug. [PMC free article] [PubMed] [Google Scholar]
- 13.Konan S, Abdel MP, Haddad FS. Cemented versus uncemented hip implant fixation: Should there be age thresholds? Bone Joint Res. 2020. Jan 8;8(12):604-607. doi: 10.1302/2046-3758.812.BJR-2019-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blythe R, O’Gorman P, Crawford R, Feenan R, Hatton A, Whitehouse S, Graves N. Fixation method for hip arthroplasty stem following hip fracture: A population-level cost-effectiveness analysis. J Arthroplasty. 2020. Jun;35(6):1614-1621. doi: 10.10 16/j.arth.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Biondi M, Crispino M, Improta G, Triassi M. The condroprotector role in the osteoarthritis of the knee. Giornale Italiano di Ortopedia e Traumatologia. 2013; 39:44–47.8. [Google Scholar]
- 16.Improta G, Romano M, Di Cicco MV, Ferraro A, Borrelli A, Verdoliva C, TriassiM CesarelliM. Lean thinking to improve emergency department throughput at AORN Cardarelli hospital. BMC Health Serv Res. 2018. Dec 3;18(1):914. doi: 10.1186/s12913-018-3654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Addio G, Ricciardi C, Improta G, Bifulco P, Cesarelli M. Feasibility of Machine Learning in Predicting Features Related to Congenital Nystagmus. Henriques J, Neves N, de Carvalho P. (eds) XV Mediterranean Conference on Medical and Biological Engineering and Computing –MEDICON 2019. MEDICON 2019. IFMBE Proceedings, vol 76. Springer, Cham. 2020. doi: 10.1007/978-3-030-31635-8_110. [Google Scholar]
- 18.Ricciardi C, Cantoni V, Green R, Improta G, Cesarelli M. Is It Possible to Predict Cardiac Death? Henriques J, Neves N, de Carvalho P. (eds) XV Mediterranean Conference on Medical and Biological Engineering and Computing –MEDICON 2019. MEDICON 2019. IFMBE Proceedings, vol 76. Springer, Cham. 2020. doi:10.1007/978-3-030-31635-8-101. [Google Scholar]
- 19.Stanzione A, Ricciardi C, Cuocolo R, Romeo V, Petrone J, Sarnataro M, Mainenti PP, Improta G, De Rosa F, Insabato L, Brunetti A, Maurea S. MRI Radiomics for the Prediction of Fuhrman Grade in Clear Cell Renal Cell Carcinoma: a Machine Learning Exploratory Study. J Digit Imaging. J Digit Imaging. 2020. Aug;33(4):879-887. doi: 10.1007/ s10278-020-00336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recenti M, Ricciardi C, Gìslason M, Edmunds K, Carraro U, Gargiulo P. Machine Learning Algorithms Predict Body Mass Index Using Nonlinear Trimodal Regression Analysis from Computed Tomography Scans. Henriques J., Neves N., de Carvalho P. (eds) XV Mediterranean Conference on Medical and Biological Engineering and Computing –MEDICON 2019. MEDICON 2019. IFMBE Proceedings, vol 76. Springer, Cham. 2020. doi: 10.1007/978-3-030-31635-8_100. [Google Scholar]
- 21.Ricciardi C, Edmunds K J, Recenti M, et al. Assessing cardiovascular risks from a mid-thigh CT image: a tree-based machine learning approach using radiodensitometric distributions. Sci Rep. 2020. Feb 18;10(1):2863. doi: 10.1038/s41598-020-59873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Improta G, Ricciardi C, Amato F, D’Addio G, Cesarelli M, Romano M. Efficacy of Machine Learning in Predicting the Kind of Delivery by Cardiotocography. Henriques J, Neves N, de Carvalho P. (eds) XV Mediterranean Conference on Medical and Biological Engineering and Computing – MEDICON 2019. MEDICON 2019. IFMBE Proceedings, vol 76. Springer, Cham. 2020. doi: 10.1007/978-3-030-31635-8_95. [Google Scholar]
- 23.Cutti A G, Lettieri E, Verni G. Health Technology Assessment as Theoretical Framework to Assess Lower-Limb Prosthetics—Issues and Opportunities from an International Perspective. In JPO: Journal of Prosthetics and Orthotics (Vol. 31, No. 1S, pp. P55-P73). LWW. 2019. doi: 10.1097/JPO.0000000000 000235. [Google Scholar]
- 24.Al-Omar HA Attuwaijri AA Aljuffali IA.. Pharmaceutical companies’ views on a health technology assessment (HTA) entity in Saudi Arabia. Saudi Pharm J. 2020. Jun;28(6):662-668. doi: 10.1016 /j.jsps.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricciardi C, Sorrentino A, Improta G, Abbate V, Latessa I, Perrone A, Dell’Aversana Orabona GA. health technology assessment between two pharmacological therapies through Six Sigma: the case study of bone cancer. The TQM Journal. 2020. doi: 10.1108/TQM-01-2020-0013. [Google Scholar]
- 26.Antony J, Palsuk P, Gupta S, Mishra D, Barach P. Six Sigma in healthcare: a systematic review of the literature. International Journal of Quality & Reliability Management. 2018; 35(5), 1075-1092. doi: 10.1108/IJQRM-02-2017-0027 [Google Scholar]
- 27.Improta G, Balato G, Romano M, Carpentieri F, Bifulco P, Alessandro Russo M, Rosa D, Triassi M, Cesarelli M. Lean Six Sigma: a new approach to the management of patients undergoing prosthetic hip replacement surgery. J Eval Clin Pract. 2015. Aug;21(4):662-72. doi: org/10.1111 /jep.12361 [DOI] [PubMed] [Google Scholar]
- 28.Improta G, Balato G, Romano M, Ponsiglione AM, Raiola E, Russo MA, Cuccaro P, Santillo LC, Cesarelli M. Improving performances of the knee replacement surgery process by applying DMAIC principles. J Eval Clin Pract. 2017. Dec;23(6):1401-1407. doi: 10.1111 /jep.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Kuwaiti A, Subbarayalu AV. Reducing hospital-acquired infection rate using the Six Sigma DMAIC approach. Saudi J Med Med Sci. Sep-Dec 2017;5(3):260-266. doi: 10.4103/sjmms.sjmms _98_16. Epub 2017. Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Improta G, Cesarelli M, Montuori P, Santillo LC, Triassi M.. Reducing the risk of healthcare associated infections through Lean Six Sigma: The case of the medicine areas at the Federico II University Hospital in Naples (Italy). J Eval Clin Pract. 2018. Apr;24(2):338-346. doi: 10.1111/jep.12844. Epub 2017. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Improta G, Ricciardi C, Borrelli A, D’alessandro A, Verdoliva C, Cesarelli M. The application of six sigma to reduce the pre-operative length of hospital stay at the hospital Antonio Cardarelli. International Journal of Lean Six Sigma. 2019; 11(3): 555-76. doi:10.1108/IJLSS-02-2019-0014 [Google Scholar]
- 32.Does RJ, van den Heuvel J, De Mast J, Niemeijer G C. Improving quality in healthcare while reducing costs. QualityManagement Forum. 2010; 36(3), 12-15. [Google Scholar]
- 33.D’Andrea MA, Ianni L, Lega F, Sargiacomo M. Lean in healthcare: a comprehensive review. Health policy. 2015. Sept; 119(9), 1197-209. doi: 10.1016/j.healthpol.2015.02.002. Epub 2015. Feb 11. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Li X, Wilck J, Berg T. Cost reduction in healthcare via Lean Six Sigma. IIE Annual Conference. Proceedings (p. 1). Institute of Industrial and Systems Engineers (IISE). 2012. [Google Scholar]
- 35.Peltokorpi A, Kujala J. Time-based analysis of total cost of patient episodes: a case study of hip replacement. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2006;19(2-3):136-45. doi: 10.1108/09526860610651672. [DOI] [PubMed] [Google Scholar]
- 36.Ricciardi C, Fiorillo A, Valente AS, Borrelli A, Verdoliva C, Triassi M, Improta G. Lean Six Sigma approach to reduce LOS through a diagnostic-therapeutic-assistance path at A.O.R.N. A. Cardarelli", The TQM Journal. 2019; Vol. 31 No. 5, pp. 657-672. doi:10.1108/TQM-02-2019-0065 [Google Scholar]
- 37.Improta G, Balato G, Ricciardi C, et al. Lean Six Sigma in healthcare: Fast track surgery for patients undergoing prosthetic hip replacement surgery. The TQM Journal. 2019; Vol. 31 No. 4, pp. 526-540. doi:10.1108/TQM-10-2018-0142. [Google Scholar]
- 38.Ricciardi C, Balato G, Romano M, Santalucia I, Cesarelli M, Improta G. Fast track surgery for knee replacement surgery: a lean six sigma approach. The TQM Journal. 2019; Vol. ahead-of-print No. ahead-of-print. doi:10.1108/TQM-06-2019-0159. [Google Scholar]
- 39.Improta G, Guizzi G, Ricciardi C, Giordano V, Ponsiglione A M, Converso G, Triassi M. Agile Six Sigma in Healthcare: Case Study at Santobono Pediatric Hospital. Int J Environ Res Public Health. 2020; 17, 1052. doi:10.3390/ijerph17031052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heintz S, Gutierrez-Farewik EM. Static optimization of muscle forces during gait in comparison to EMG-to-force processing approach. Gait Posture. 2007. Jul;26(2):279-88. doi: 10.1016/j.gaitpost.2006.09.074. [DOI] [PubMed] [Google Scholar]
- 41.Pétursson Þ, Magnússon B, Helgason B, Magnúsdóttir G, Halldórsson G, Tribel J, Jónsson H, Gargiulo P. Bone and muscle assessment in patients undergoing total hip arthroplasty using HU based analysis. Eur J Transl Myol. 2015. Mar 11;25(2):4913. doi: 10.4081/ejtm.2012.1797. eCollection 2015. Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Çelik T, Mutlu İ, Özkan A, Kişioğlu Y. The effect of cement on hip stem fixation: a biomechanical study. Australas Phys Eng Sci Med. 2017. Jun;40(2):349-357. doi: 10.1007/s13246-017-0539-1. Epub 2017. Mar 20. [DOI] [PubMed] [Google Scholar]
- 43.Junnila M, Laaksonen I, Eskelinen A, Pulkkinen P, Ivar Havelin L, Furnes O, Garellick G. Implant survival of the most common cemented total hip devices from the Nordic Arthroplasty Register Association database. Acta Orthop. 2016. Dec;87(6): 546-553. doi: 10.1080/17453674.2016.1222804. Epub 2016. Aug 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maggs J, Wilson M. The relative merits of cemented and uncemented prosthesis in total hip arthroplasty. Indian J Orthop. Jul-Aug 2017;51(4):377-385. doi: 10.4103/ortho.IJOrtho_405_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meding JB, Ritter MA, Davis KE, Hillery. Cemented and uncemented total hip arthroplasty using the same femoral component. Hip Int. Jan-Feb 2016;26(1):62-6. doi: 10.5301/hipint.5000296. Epub 2015. Oct 6. [DOI] [PubMed] [Google Scholar]
- 46.Rolfson O, Donahue GS, Hallsten M, Garellick G, Kärrholm J, Nemes S. Patient-reported outcomes in cemented and uncemented total hip replacements. Hip Int. 2016. Sep 29;26(5):451-457. doi: 10.5301/hipint.5000371. Epub 2016. May 26. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Han X, Wang J, Yuan Z, Wang T, Zhao M, Han G. Cemented versus uncemented femoral component total hip arthroplasty in elderly patients with primary osteoporosis: retrospective analysis with 5-year follow-up. J Int Med Res. 2019. Apr;47(4):1610-1619. doi: 10.1177/0300060518825 428. Epub 2019. Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Temporiti F, Zanotti G, Furone R, Molinari S, Zago M, Loppini M, Galli M, Grappiolo G, Gatti R. Gait analysis in patients after bilateral versus unilateral total hip arthroplasty. Gait Posture. 2019. Jul;72:46-50. doi: 10.1016/j.gaitpost. 2019.05.026. Epub 2019. May 23. [DOI] [PubMed] [Google Scholar]
- 49.Ricciardi C, Jónsson H, Jacob D, Improta G, Recenti M, Gíslason MK, Cesarelli G, Esposito L, Minutolo V, Bifulco P, Gargiulo P. Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty. Diagnostics (Basel). 2020. Oct 14;10(10):815. doi: 10.3390/diagnostics10100815. [DOI] [PMC free article] [PubMed] [Google Scholar]


