Abstract
Objective
Children with amplified musculoskeletal pain (AMPS) experience significant functional disability, with impairment in their ability to participate in age-appropriate activities of daily living. Parental factors play an important role in a child’s pain symptoms and treatment outcomes, with parental pain catastrophizing and protective behaviors linked to several maladaptive outcomes for children. Aims of the current study were to examine how parental pain catastrophizing, child pain catastrophizing, and parental protective behaviors longitudinally impacted functional disability for children with AMPS.
Methods
Archival data were examined from parent-child dyads presenting to a tertiary pain clinic for treatment of AMPS. Over 1 year, parents completed measures assessing the level of pain catastrophizing, common behavioral responses to child pain, and child functional disability. Children completed measures of pain catastrophizing and functional disability. Measures were collected at initial evaluation, 6-months, and 12-months. Latent growth models (LGM) were conducted to examine how to study variables longitudinally impacted the rate of change in child functional disability.
Results
Examining a comprehensive LGM of study variables, parental catastrophizing emerged as the sole contributing factor to slower improvement in functional disability.
Conclusions
The strong influence of parental pain catastrophizing on functional disability may relate to parents limiting behaviors that promote adaptive coping in children with pain. As such, parents who catastrophize may benefit from specific interventions to increase their use of adaptive behavioral responses, such as redirecting children to complete functional activities and encouraging the use of positive coping skills for pain-related distress.
Keywords: acute pain, chronic and recurrent pain, evidence-based practice, family functioning, longitudinal research, parents, parent psychosocial functioning
Introduction
Over 30 million children in the United States sought pain treatment between 2009 and 2012 (Baldridge et al., 2018). Noninflammatory musculoskeletal pain is one of the most frequent reasons for children to seek pain treatment (Weiss & Stinson, 2018), with amplified musculoskeletal pain syndrome (AMPS) being one of the most common forms (Weiss & Stinson, 2018). AMPS encompasses a spectrum of chronic and recurrent idiopathic pain disorders, which occur due to an over-arousal of the autonomic nervous system (Sherry, 2015). AMPS may develop in response to psychosocial and/or physical stressors (e.g., illness, injury, surgery); however, the amplified pain signal itself is not associated with continued physiological damage (Weiss & Stinson, 2018). Common AMPS subtypes include fibromyalgia and complex regional pain syndrome, and all subtypes share features of an intensified pain signal and increased functional disability, an impairment in the ability to engage in activities of daily living (Kashikar-Zuck et al., 2001; Weiss & Stinson, 2018). In children with AMPS, functional disability frequently impacts their engagement in developmentally appropriate activities, such as completing daily hygiene tasks, walking, attending school, and socializing (Pielech et al., 2018; Weiss & Stinson, 2018). Evidence-based treatment for AMPS involves a multidisciplinary approach, including individual psychotherapy, intensive physical and/or occupational therapy, and consistent functional activity to reset the nerve pathway and improve engagement in activities of daily living (Gmuca & Sherry, 2017; Sherry, 2015; Weiss & Stinson, 2018). Due to the extent of functional impairment children with AMPS experience, improving functionality is generally the primary treatment target (Sherry, 2015).
Parental Influence on Child Pain
The family environment plays an important role in a child’s pain experience, including their ability to engage in functional activities, follow medical recommendations, and cope with pain (Logan et al., 2012). Through the process of social learning (Bandura, 1977), children observe parent responses and learn to respond similarly when experiencing pain themselves (van Tilburg, 2018). As such, substantial research has investigated the role of parental factors in children’s pain experience, particularly parental pain catastrophizing (Caes et al., 2012; Goubert et al., 2006; Pielech et al., 2014; Wilson et al., 2014) and protective behavioral responses (Achiam-Montal & Lipsitz, 2014; Caes et al., 2012; Claar et al., 2010; Guite et al., 2011; Simons et al., 2008; Van Slyke & Walker, 2006).
Pain catastrophizing involves a magnified negative perception regarding an anticipated or current painful experience (Sullivan et al., 2001). It is a particular form of worry related to the experience of pain, and as such is highly related to temperamental factors such as fear/anxiety and perceptual sensitivity (Muris et al., 2007). For children with chronic pain, both parent and child pain catastrophizing have been associated with adverse psychosocial outcomes, such as increased depressive and anxious symptoms (Pielech et al., 2014), and greater functional disability (Goubert et al., 2006; Logan et al., 2012). In a community-based study, greater parental pain catastrophizing was associated with greater pain severity in children, but only among children who scored higher in pain catastrophizing themselves (Birnie et al., 2016). This suggests that children with higher temperamental anxiety may be particularly susceptible to develop catastrophic perceptions of pain, putting them at greater risk for adverse outcomes (Birnie et al., 2016).
Catastrophizing may prompt parents to engage in protective behaviors to ameliorate their child’s pain, which is associated with greater functional disability, longer pain duration, and depressive symptoms (Claar et al., 2010; Claar et al., 2008; Peterson & Palermo, 2004) in children. As such, parental pain catastrophizing has been associated with a greater desire to intervene in order to stop activity associated with their child’s pain (Caes et al., 2012). Interestingly, the relationship between parental pain catastrophizing and protective behaviors remains significant even when the child’s functional abilities are considered (Guite et al., 2011), suggesting that engagement in over-protective behaviors occurs regardless of the child’s level of disability. For children with AMPS, parental protective behaviors may impede treatment progress as it limits children’s ability to engage in functional activities.
Complex relationships exist among parent/child catastrophizing and protective responses, though the underlying mechanisms are poorly understood. Among families presenting to a multidisciplinary pediatric pain clinic (Simons et al., 2015) parental pain catastrophizing was indirectly related to greater functional disability through the pathways of child pain catastrophizing, parental behavioral responses, and child avoidance behaviors. Despite research linking parent/child pain catastrophizing and protective behaviors to maladaptive outcomes for children with pain, little is known about their long-term associations and development over time.
Longitudinal Research
Longitudinal research in this area is limited; however, several studies have investigated the long-term impact of parental factors for children with pain. A study of parents and pediatric surgical patients found that parental pain catastrophizing scores several days postsurgery predicted greater child pain intensity at 12 months (Page et al., 2013). Examination of parent–child dyads in pediatric chronic pain clinics has revealed that baseline levels of parental pain catastrophizing predicted child pain-related fear and catastrophizing, avoidance of activities, anxiety, and school attendance 4-months following their initial evaluation (Chow et al., 2016) and child pain catastrophizing at baseline predicted caregiver pain catastrophizing one month later, though this relationship was not reciprocal (Parker et al., 2020). Change in child and parent pain catastrophizing additionally predicted the individual’s ratings of pain-related interference in functioning (Parker et al., 2020). Further longitudinal research among parent–child dyads presenting for treatment of pediatric chronic pain indicated that child pain catastrophizing mediated the relation between parental protective behaviors and functional disability after 2 months (Welkom et al., 2013) and greater parental distress prior to treatment predicted less improvement in child disability after 1 year, though a bidirectional relationship was not observed among parent and child variables (Law et al., 2017). Taken together, these findings suggest that parent/child pain catastrophizing and protective behaviors indeed influence long-term outcomes in pediatric pain, though further research is needed to examine the development of these variables and elucidate mechanisms that may underlie their associations.
Purpose of the Current Study
The literature provides support for a complex model of parent and child variables that predict functional disability for children with pain. Parental catastrophizing and protective behavioral responses are linked to numerous maladaptive outcomes in pediatric pain research. However, there remains a scarcity of research regarding the long-term associations and natural development of these variables across time. The current study adds to pediatric pain research by examining how parental factors influence child functional disability over a 1-year period through the use of Latent Growth Modeling (LGM), which estimates linear and non-linear growth functions, or rates of change (Keith, 2015). Parental pain catastrophizing, child pain catastrophizing, and parental protective behaviors were examined for their potential impact on the initial level and rate of change (i.e., improvement) in functional disability for children with AMPS at three time points (baseline, 6-months, 12-months). We additionally sought to expand on the investigations of Welkom et al. (2013) and Simons et al. (2015) to investigate whether child catastrophizing and parental protective behaviors would mediate the relation between parental catastrophizing and functional disability over 1-year duration. Based on the findings of prior research reviewed above, it was anticipated that parental protective behaviors and child pain catastrophizing would directly relate to slower improvement in functional disability, mediating the relation between parental pain catastrophizing and functional disability.
Methods
Participants and Procedures
The current study utilized archival data gathered as part of a larger study at a Center for Amplified Musculoskeletal Pain Syndromes (CAMPS) in a large children’s hospital in the northeast U.S. Participants in the initial CAMPS study included parent–child dyads who completed measures of psychological functioning, family functioning, and pain characteristics at three time points. For the initial study, all families underwent an initial multidisciplinary evaluation at CAMPS as part of their medical care. For those who consented to the CAMPS study, baseline data were collected within a 2-week period following the child’s initial evaluation and follow up surveys were completed 6 and 12 months after the completion date of baseline measures, within a 2-week grace period. The CAMPS study was approved by the hospital Institutional Review Board. The current study analyzing this archival data was approved by the PI’s academic institution for a data-sharing agreement.
Children ages 8–17 years were eligible to participate in the initial CAMPS study if they received an AMPS diagnosis at their initial evaluation and were accompanied by at least one parent willing to complete study measures. Parents were eligible to participate if they were able to read and understand English at an 8th grade reading level or above. Exclusion criteria for both child and parent participants included a diagnosis of a cognitive or intellectual disability.
The final sample consisted of 155 children and 158 parents who completed at least one baseline study measure. For the 6-month follow up survey, 79 children and 90 parents completed at least one study measure. For the 12-month survey, 72 children and 75 parents completed at least one study measure.
Recruitment
Eligible participants for the initial CAMPS study were identified through review of CAMPS patient schedule and were contacted by a research assistant, either by phone two weeks prior to their evaluation or in person during their initial evaluation. Those who agreed to participate provided verbal consent/assent and were emailed a link to complete study measures through Redcap, a secure online application for building, capturing, and managing electronic data. Children who turned 18 during the study period were contacted via phone to provide verbal consent for their continued participation.
Measures
Demographic and Medical Information
Electronic medical record reviews were conducted to gather demographic and medical data. For the present study age, sex, race, ethnicity, diagnoses, pain duration, pain severity, and treatment modalities were examined.
Pain Catastrophizing—Child and Parent Measures
The Pain Catastrophizing Scales—Child (PCS-C; Crombez et al., 2003) and Parent versions (PCS-P; Goubert et al., 2006) are 13-item measures representing distinct thoughts in response to pain. The PCS-C assesses pain catastrophizing in children ages 8–17 (Crombez et al., 2003), and the PCS-P assesses parental catastrophizing of their child’s pain regardless of child age (Goubert et al., 2006). Participants indicate how frequently they experience each thought in the preceding 2 weeks on a 5-point Likert scale from 0 (not at all) to 4 (all the time). The sum of the 13 items yields a total score, with higher scores indicating greater catastrophizing (Crombez et al., 2003; Goubert et al., 2006). The PCS-C has significant predictive validity for pain severity (p < .05) and disability (p < .05) in a sample of patients with pediatric pain (Crombez et al., 2003). The PCS-P has significant predictive validity for parental stress related to child illness (p < .0005), parent depressive symptoms (p < .05), anxiety (p < .005), and parent-reported child functional disability (p < .0005; Goubert et al., 2006). The PCS-P evidenced good internal consistency among parents of children with chronic pain [Cronbach’s coefficient alpha (α) = .93]. Updated analysis of PCS-C/P factor validity, however, did not find support for the three-factor structure (Durand et al., 2017; Pielech et al., 2014), instead proposing the removal of items 7 and 8 and using the measure as a unitary construct. Based on the results of the updated factor analyses, the total score of the revised 11-item PCS-C/P was used as a unitary construct in the present study analyses. Internal consistency was assessed using Cronbach’s coefficient alpha (ɑ). In our sample, internal consistency for the PCS-P was 0.939, and for the PCS-C was 0.942.
Parental Protective Behavioral Responses to Pain
The Adult Responses to Children’s Symptoms (ARCS) is a 29-item self-report measure assessing the frequency of parental responses to child pain across three subscales: Protectiveness, Minimization, Encourage/Monitor (Van Slyke & Walker, 2006). Items are rated on a 5-point Likert scale ranging from 0 (never) to 4 (always). The mean for each subscale is calculated, with higher scores indicating greater frequency of a response (Van Slyke & Walker, 2006). Given research supporting the maladaptive nature of protective parental behaviors in pediatric pain outcomes, the current study specifically utilized the Protective subscale of the ARCS, independent of the overall measure, to focus on the unique impact of protective responses. Doing so is in line with similar pediatric pain studies which examined the unique role of protective responses on children’s pain-related outcomes (Langer et al., 2009; Law et al., 2017; Logan et al., 2012). The Protective subscale has adequate internal consistency (α = .86) and has been validated with parent tracking of protective behaviors (p < .05; Walker et al., 2006). It has shown predictive validity for functional disability and child depressive symptoms (p < .05; Claar et al., 2010). Internal consistency for our sample was ɑ = 0.871.
Functional Disability, Parent and Child Versions
The Functional Disability Inventory (FDI) is a 15-item self-report measure assessing the degree to which pain interferes with children’s physical and psychosocial functioning over a 2-week period (Claar & Walker, 2006; Kashikar-Zuck et al., 2011; Solé et al., 2019.). Item responses are rated on a 5-point Likert scale ranging from 0 (no trouble) to 4 (impossible) performing an activity. Higher scores indicate greater functional disability, and scores range from 0 to 60 with three clinical reference points: no/minimal disability (0 –12), moderate disability (13–28), and severe disability (29–60; Kashikar-Zuck et al., 2011). In a large sample of pediatric chronic pain patients, the measure evidenced good internal consistency (α = .77; Kashikar-Zuck et al., 2011). For parent-report of child functional disability, parent-report FDI has high internal consistency (α = .90–.94; Claar & Walker, 2006). As AMPS treatment is focused on increasing activity and function (Weiss & Stinson, 2018) child- and parent-reported total FDI scores were used as the study outcome variables. Internal consistency for our sample FDI parent was ɑ = 0.957 and for FDI child was ɑ = 0.932.
Results
Data Analysis Plan
A series of LGMs were conducted to examine the longitudinal development for the parallel-processes of parent and child pain catastrophizing (PCS-P/C), parental responses to pain (ARC), and child functional disability across 1 year. LGM estimates linear and non-linear growth functions (Keith, 2015) using latent variables (factors) to represent initial level and rate of change, and can include time-invariant (e.g., gender, race, diagnosis) and time-varying covariates (e.g., repeated measures) as predictors and outcomes of growth functions. Additional strengths of LGM include the ability to examine temporal precedence and infer causation by specifying that one variable preceded another in development (Duncan et al., 1999). LGM includes two stages. In the first step, a growth curve is estimated without covariates in the model (i.e., a measurement model). This allowed for testing linear versus non-linear growth (e.g., quadratic). Next, covariates are added to estimate their effects on initial level (baseline) and rate of change (linear or quadratic trend). To assess the fit of the model to the data, there are several commonly employed fit indices. These include chi square (χ2), root mean square error of approximation (RMSEA), confirmatory fit index (CFI), and standardized root mean squared residual (SRMR) analyses. Good fit to the data is observed when χ2 is not significant, indicating that the observed covariance matrix does not significantly differ from the model-implied covariance matrix. RMSEA values of 0.08 or lower, CFI of 0.95 or higher, and SRMR values of 0.05 or lower are considered indicative of good model fit (Cangur & Ercan, 2015; MacCallum et al., 1996).
Preliminary Analyses and Descriptive Statistics
Mplus version 8.3 was used to analyze the LGM. Data were examined in SPSS version 24 for presence of outliers, and means were plotted to determine estimated rate of change for each measure at the three time points. Mean level change was examined to determine whether a linear or quadratic estimation should be used for LGM. Demographics, measure means, and standard deviations are summarized in Table I. Children were predominantly female (80.1%) and Caucasian (82.7%), with a mean baseline age of 13.95 years (SD = 2.39). Baseline mean for pain duration was 26.95 months (SD = 29.02) and usual pain severity was 58.42 out of 100 (SD = 19.26). Mean FDI at baseline was 22.82 (SD = 11.71) for child-report and 21.86 (SD = 11.66) for parent-report, with 26.8% of children participating in an intensive multidisciplinary pain treatment program.
Table I.
Participant Demographics
| Variable | Frequency (%) |
||
|---|---|---|---|
| T1 | T2 | T3 | |
| Sex | |||
| Female | 181 (80.4%) | 70 (88.6%) | 62 (82.4%) |
| Male | 44 (19.6%) | 9 (11.4%) | 13 (17.6%) |
| Race | |||
| White | 177 (82.7%) | 68 (86.1%) | 62 (84.9%) |
| Black/African American | 21 (9.8%) | 8 (10.1%) | 7 (9.6%) |
| Other | 9 (4%) | 0 | 1 (1.4%) |
| Asian | 3 (1.3%) | 2 (2.5%) | 2 (2.7%) |
| Multiple racial identities | 3 (1.3%) | 1 (1.3%) | 1 (1.4%) |
| East Indian | 1 (.4%) | 0 | 0 |
| Ethnicity | |||
| Hispanic | 19 (9%) | 3 (3.9%) | 4 (5.6%) |
| Attended intensive treatment | 60 (26.8%) | ||
| Mean (SD) | |||
| Child age (years) | 13.95 (2.39) | 14.32 (2.02) | 14.37 (2.04) |
| Usual pain severity | 58.42 (19.26) | 42.66 (27.72) | 36.26 (31.27) |
| Pain duration T1 (months) | 26.95 (29.02) | ||
| Time 1 variables | |||
| Parent FDI T1 | 21.86 (11.66) | ||
| Child FDI T1 | 22.82 (11.71) | ||
| Parent ARCS T1 | 1.57 (.71) | ||
| Parent PCS T1 | 29.54 (10.33) | ||
| Child PCS T1 | 29.56 (11.05) | ||
| Time 2 variables | |||
| Parent FDI T2 | 10.83 (12.44) | ||
| Child FDI T2 | 13.30 (11.70) | ||
| Parent ARCS T2 | 1.04 (0.64) | ||
| Parent PCS T2 | 23.46 (11.64) | ||
| Child PCS T2 | 20.69 (12.11) | ||
| Time 3 variables | |||
| Parent FDI T3 | 10.01 (11.71) | ||
| Child FDI T3 | 12.83 (13.10) | ||
| Parent ARCS T3 | 2.13 (8.81) | ||
| Parent PCS T3 | 23.77 (12.56) | ||
| Child PCS T3 | 20.13 (14.73) | ||
Table II presents the final correlation matrices used to inform the mediation LGM analysis (Keith, 2015). Significant associations were observed among parent FDI, PCS, and ARCS scores at baseline (T1) and 6 (T2) months, but not 1-year (T3) follow-up (see Table II). T3 parent FDI was significantly related to child PCS at T2 and T3 but not T1. Pain variables in initial correlational analyses included pain duration, severity, a first-degree relative (parent, sibling) with diagnosis of a pain condition, and treatment through an intensive rehabilitation program. As these covariates were not significantly related to most study measures, they were excluded from LGM analyses.
Table II.
Correlations Among Demographic and Pain Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | — | −.149* | −.098 | −.089 | .239* | .068 | −.031 | .134 | −.096 | .051 | −.194 | .039 | .003 | −.187 | −.182 | .242** |
| 2. Race | — | .607** | .090 | .110 | −.004 | .077 | .154 | .149 | .095 | .093 | .079 | .155 | −.007 | −.034 | −.119 | |
| 3. Ethnicity | — | .003 | .380** | .033 | −.002 | .165* | .226* | .247* | .122 | .180 | .187* | .287* | −.019 | −.082 | ||
| 4. FDI P T1 | — | .289* | −.026 | .282** | .234** | .027 | .217 | .086 | .179 | .267** | .240* | .029 | .025 | |||
| 5. FDI P T2 | — | −.147 | .251* | .361** | .344** | .403** | .145 | .335* | .297** | .389** | .027 | .350** | ||||
| 6. FDI P T3 | — | .134 | −.086 | .012 | −.111 | −.128 | −.089 | −.107 | .009 | −.010 | −.086 | |||||
| 7. C/PCS T1 | — | .360** | .463** | .396** | .409** | .225 | .107 | .046 | −.018 | .010 | ||||||
| 8. P/PCS T1 | — | .233* | .559** | .301* | .609** | .402** | .217 | .108 | −.040 | |||||||
| 9. C/PCS T2 | — | .373** | .683** | .164 | −.078 | .038 | −.066 | −.071 | ||||||||
| 10. P/PCS T2 | — | .380** | .616** | .199 | .401** | .168 | .223* | |||||||||
| 11. C/PCS T3 | — | .345* | .132 | .151 | .241 | .069 | ||||||||||
| 12. P/PCS T3 | — | .332** | .494** | −.019 | .067 | |||||||||||
| 13. ARCS T1 | — | .712** | .047 | −.055 | ||||||||||||
| 14. ARCS T2 | — | .833** | −.042 | |||||||||||||
| 15. ARCS T3 | — | −.130 | ||||||||||||||
| 16. Duration | — |
Significant at .05, two-tailed; **Significant at .01, two-tailed.
Latent Growth Modeling
Parent PCS and ARCS
To examine whether higher levels of parent catastrophizing at baseline would increase protective behavioral responses to pain, a quadratic model fit the data best, χ2(2) = 2.66, p = .264; RMSEA = 0.047; CFI = 0.993; SRMR = 0.046, suggesting good fit of the model to the data. Significant effects were observed from baseline parent catastrophizing to the intercept (b = 6.06, z = 5.5, p < .001), indicating that higher levels of parental catastrophizing led to significantly greater engagement in protective behaviors, which remained stable thereafter. Further, age was a significant predictor in the model at intercept (b = 0.83, z = 2.326, p = .02), revealing that parents engage in greater catastrophizing for older children, and this engagement remained stable thereafter. As child age was positively correlated with pain duration (p < .0001), a longer duration may indirectly lead to increased parental pain catastrophizing and protective behaviors, as parents become increasingly distressed the longer their child experiences pain.
Parent ARCS and FDI
To examine whether greater engagement in protective behavioral responses at baseline would impact rate of change in parent-rated FDI a linear trend fit the data best (i.e., no quadratic trend), χ2(5) = 5.928, p = .313; RMSEA = 0.036; CFI = 0.986; SRMR = 0.137, suggesting good fit of the model to the data. Significant effects were observed for ARCS on the intercept (b = 0.016, z = 3.31, p = .001) indicating that the effects observed at baseline remain stable across time. A linear trend (b = −0.008, z = −1.668, p = .095) revealed decreased acceleration in the rate of change for Parent-reported FDI, indicating that greater engagement in protective behaviors leads to slower improvement in the child’s functional disability.
Parent and Child PCS
An additional LGM was run to examine whether parent catastrophizing at baseline would lead to slower rate of change for child pain catastrophizing. A growth curve model with a quadratic trend best fit the data, χ2(2) = 2.986, p = .225; RMSEA = 0.058; CFI = 0.989; SRMR = 0.059. Rate of change for Child PCS was significantly affected by Parent PCS (b = 0.342, z = 4.606, p < .001), indicating that higher levels of parental catastrophizing lead to higher levels of child catastrophizing. Age was additionally revealed as a significant covariate (b = 0.764, z = 2.081, p = .037), meaning older children experience more pain-related catastrophizing over time.
Child Catastrophizing and FDI
To examine the impact of child pain catastrophizing at baseline on rate of change in Child-reported FDI, a quadratic trend best fit the data, χ2(2) = 0.602, p = .74; RMSEA = 0.000; CFI = 1.0; SRMR = 0.028. Child pain catastrophizing had a stable effect on rate of change in FDI (b = 0.275, z = 3.843, p < .0001), and age was a significant covariate at the intercept (b = 0.722, z = 1.908, p = .056), indicating older children experience faster improvement in functional disability, which remains stable over 1 year.
Mediation Model
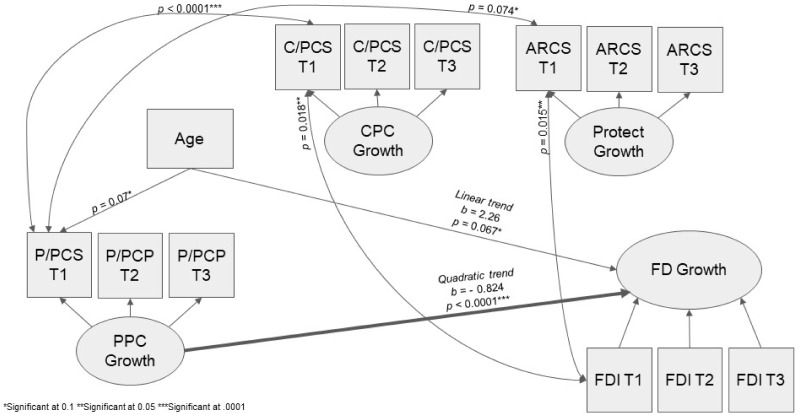
The study hypothesis anticipated that parental pain catastrophizing would indirectly affect the rate of change in Parent-reported FDI through its influence on protective behaviors and child pain catastrophizing. Fit indices for this model indicated good model fit [χ2(2) = 17.61 p = .128; RMSEA = 0.051; CFI = 0.958; SRMR = 0.059], and a quadratic trend best fit the data for both the parent PCS and FDI. Figure 1 displays the model results. Parent PCS had a significant effect on the quadratic trend for FDI (b = −0.824, z = −3.85, p < .0001), indicating that higher levels of parent catastrophizing led to slower rate of change in FDI over 1 year. Age was a significant covariate on the linear trend for FDI (b = 2.26, z = 1.83, p < .067), indicating older children experience faster improvement in functional disability. Neither child PCS nor ARCS predicted rate of change in parent FDI or PCS, indicating they are not mediators between parental pain catastrophizing and functional disability.
Figure 1.
Mediation latent growth model figure. * Significant at .1 ** Significant at .05 *** Significant at .0001.
Discussion
Children with AMPS experience significant functional disability with persistent impairment in their ability to participate in age-appropriate daily activities (Kashikar-Zuck et al., 2001; Gmuca & Sherry, 2017; Pielech et al., 2018; Sherry, 2015; Weiss & Stinson, 2018). Parental factors play an important role in influencing a child’s experience of pain and treatment outcomes (Logan et al., 2012), with parental pain catastrophizing and engagement in protective behavioral responses linked to increased pain severity (Birnie et al., 2016; Caes et al., 2012; Page et al., 2013; Palermo et al., 2014), and functional disability (Caes et al., 2012; Chow et al., 2016; Guite et al., 2011; Lynch-Jordan et al., 2018; Pielech et al., 2014) in children. Understanding how parent and child factors progress and impact a child’s pain experience is especially important to inform the development of interventions to improve treatment outcomes. A series of LGMs were conducted to examine how parental pain catastrophizing, child pain catastrophizing, and parental protective behaviors longitudinally impacted functional disability for children with AMPS at baseline, 6-months, and 12-months. It was hypothesized that child pain catastrophizing and protective behaviors would mediate the relation between parental pain catastrophizing and functional disability.
Higher parental pain catastrophizing was associated with greater engagement in protective behaviors over the course of the year. This may expand on findings of prior research establishing the cross-sectional connection between parental pain catastrophizing and protective behaviors (Caes et al., 2012; Guite et al., 2011), which remains stable even when controlling for the child’s level of functional disability (Guite et al., 2011). Conceptually this link can be understood through the cognitive model (Beck, 1995), which asserts that cognitions and interpretations influence one’s behavioral and emotional responses to a situation. Thus, parents who experience more catastrophic cognitions when their child is in pain tend to engage in a greater number of protective behaviors, despite receiving psychoeducation that AMPS pain is not physiologically harmful and that treatment requires consistent engagement in functional activities. Further, slower improvement in functional disability was observed among dyads with higher parental pain catastrophizing, which may build upon the findings of prior cross-sectional (Goubert et al., 2006; Logan et al., 2012) and longitudinal research (Chow et al., 2016). Higher engagement in protective behaviors also led to slower improvement in functional disability, as found in prior research (Claar et al., 2010). These results confirm that parental pain catastrophizing and protective behaviors independently contribute to slower improvement in child functional disability. As a result, the child may lose opportunities to push themselves to engage in physical activities despite pain, a necessary step in resetting the abnormal nerve pathway in AMPS, leading to slower functional disability improvement.
Similar to the findings of Birnie et al. (2016), higher parental pain catastrophizing led to slower rate of change in child pain catastrophizing. In turn, higher child pain catastrophizing was associated with slower rate of change in functional disability, similar to the results of Tran et al. (2015). Through social learning (Bandura, 1977), children whose parents exhibit higher catastrophizing likely learn through observation to interpret their pain with catastrophic cognitions, perhaps increasing anxious avoidance which impedes the child’s ability to fully engage in physical activities despite pain. Our study findings may additionally be interpreted through the Interpersonal Fear Avoidance Model (Goubert and Simons, 2013) which asserts that when a child experiences pain they may perceive the pain as threatening, leading to experiential avoidance and distress. A child’s expression of perceived threat is then observed and interpreted by the paren and reciprocally interacts with a parent's catastrophic perception of the child’s pain. This leads to a pattern of hypervigilance and fear in the dyad. Heightened fear in the child increases avoidance of activities that may exacerbate pain, which is reinforced and encouraged by the parent.
In addition, age was a significant covariate in several of our growth models, indicating that parents catastrophize more for older children and older children catastrophize more themselves, despite experiencing faster improvement in functional disability. Possibly, parents and children may respond to the sensation and duration of pain with catastrophic interpretations even though disability is improving through treatment. Differences in cognitive and social development may further explain the significance of age, as older children have the ability to interpret or reflect on their pain, which may increase catastrophizing. Social engagement and peer comparison increase through adolescence as well. Thus, older children may compare their abilities to that of their peers, which may contribute to catastrophizing. The increased independence among older children may contribute to parents’ catastrophizing as they are less informed about their child’s pain and functioning.
Examining a comprehensive model, parental pain catastrophizing emerged as the sole contributing factor to slower rate of change in functional disability. Contrary to our hypotheses, neither child pain catastrophizing nor protective behaviors mediated the relation between parental pain catastrophizing and functional disability. These findings differ from prior cross-sectional research noting protective behaviors (Logan et al., 2012; Simons et al., 2015) and child pain catastrophizing (Pielech et al., 2014; Simons et al., 2015; Welkom et al., 2013; Wilson et al., 2014) to be significant mediators. As the majority of this research has been cross-sectional, with only one study conducted over a 2-month duration (Welkom et al., 2013), it is possible that the mediational relationships do not remain significant over extended time periods. Parental pain catastrophizing may remain consistent, whereas parental engagement in protective behaviors may fluctuate, exerting less influence on functional disability when the variables are considered together over time. In this study, parental pain catastrophizing decreased from T1 to T2, but remained stable from T2 to T3. In comparison, protective behaviors decreased from T1 to T2, then increased notably from T2 to T3, exhibiting fluctuation in the use of protective behaviors. Similar to parental pain catastrophizing, child pain catastrophizing decreased from T1 to T2, however remained stable from T2 to T3. As child pain catastrophizing did not significantly impact functional disability in the mediation model, the effects of child pain catastrophizing may be best accounted for by parental pain catastrophizing. The strong and consistent influence of parental pain catastrophizing on functional disability may perhaps be explained by parents limiting behaviors that promote adaptive coping in children with pain, rather than their use of protective behaviors to ameliorate it. Parents who catastrophize may spend less time redirecting children to complete activities despite their pain or less time encouraging them to use positive coping skills (e.g., mindfulness, distress tolerance) for pain-related distress.
Limitations
It is important to note that a major limitation of the study is small sample size and high rates of attrition, which may have failed to capture the mediation effects observed in cross-sectional research. Another limitation lies in the generalizability of results, as our sample was predominantly Caucasian and female. Though these demographics are consistent with the patient characteristics in other pediatric pain clinics (Chow et al., 2016; Kashikar-Zuck et al., 2010; Tran et al., 2015) and AMPS diagnosis is noted to be far more common among females (Sherry, 2015) limited diversity may fail to capture gender, racial, or ethnic differences in the manifestation of AMPS symptoms, parent and child pain catastrophizing, protective behaviors, and functional disability. Further, our study utilized a clinical convenience sample representative of the wide age range seen through clinical care. Doing so limits the ability to examine developmental differences in parenting and parental response to pain, as noted by prior studies (Simons & Kaczynski, 2012). As our study additionally found age to be a significant covariate, and there is evidence that the link between fear of pain and avoidance of activities is significantly stronger for adolescents than young children , future research should be conducted to examine developmental differences in response to and experience pain, as well as how parental response to pain may differ based on a child’s developmental stage.
Clinical Implications and Future Directions
Despite these limitations, this study adds important information to the literature on pediatric pain by being the first to examine the long-term associations among parent/child catastrophizing, protective responses, and functional disability in pediatric AMPS. Our study results highlight the impact of parental pain catastrophizing in treating children with AMPS. When parents endorsed higher catastrophizing, children evidenced slower improvement in their ability to engage in age-appropriate daily activities. Pediatric psychologists and pain specialists may benefit from assessing parental pain catastrophizing at initial evaluation and providing psychoeducation regarding the role of catastrophic pain-related cognitions in impeding treatment progress. Targeted interventions for parents may further reduce maladaptive catastrophizing and instead promote more adaptive responses to a child’s pain, such as encouraging the child to engage in physical activity (e.g., stretching, walking) or using coping skills (e.g., mindfulness, breathing, distraction). This is supported by research indicating a group intervention for parents of children in an intensive pain program improved parental responses and child functional disability, the findings of which were sustained one year later (Pielech et al., 2018). Given research demonstrating the interconnected nature of parent and child factors in pediatric pain (Achiam-Montal & Lipsitz, 2014; Claar et al., 2008; Peterson & Palermo, 2004; Simons et al., 2008), future longitudinal research should investigate long-term dyadic interactions and transactions to develop targeted interventions for parents and children. Longitudinal research in pediatric pain with higher sample sizes is needed to improve study power and capture more diversity, which will increase the generalizability of results and allow for examination of potential cultural differences in parenting as they may influence the efficacy and expediency of their child’s pain treatment.
Funding
Dr. Sherry is funded by the Snider Family.
Conflicts of interest: None declared.
References
- Achiam-Montal M., Lipsitz J. D. (2014). Does parental response to children’s pain moderate the association between pain severity and functional disability? An examination of noncardiac chest pain. Journal of Pediatric Psychology, 39(1), 35–44. [DOI] [PubMed] [Google Scholar]
- Baldridge S., Wallace L., Kadakia A. (2018). The epidemiology of outpatient pain treatment in pediatrics. Journal of Pain Research, 11, 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. (1977). Social learning theory. Prentice Hall. [Google Scholar]
- Birnie K. A., Chambers C. T., Chorney J., Fernandez C. V., McGrath P. J. (2016). Dyadic analysis of child and parent trait and state pain catastrophizing in the process of children's pain communication. Pain, 157(4), 938–948. [DOI] [PubMed] [Google Scholar]
- Caes L., Vervoort T., Trost Z., Goubert L. (2012). Impact of parental catastrophizing and contextual threat on parents’ emotional and behavioral responses to their child’s pain. Pain, 153, 687–695. [DOI] [PubMed] [Google Scholar]
- Cangur S., Ercan I. (2015). Comparison of model fit indices used in structural equation modeling under multivariate normality. Journal of Modern Applied Statistical Methods, 14(1), 152–167. [Google Scholar]
- Chow E. T., Otis J. D., Simons L. E. (2016). The longitudinal impact of parent distress and behavior on functional outcomes among youth with chronic pain. The Journal of Pain, 17(6), 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claar R. L., Guite J. W., Kaczynski K. J., Logan D. E. (2010). Factor structure of the adult responses to children’s symptoms: Validation in children and adolescents with diverse chronic pain conditions. Clinical Journal of Pain, 26, 410–417. [DOI] [PubMed] [Google Scholar]
- Claar R. L., Simons L. E., Logan D. E. (2008). Parental response to children’s pain: The moderating impact of children’s emotional distress on symptoms and disability. Pain, 138, 172–179. [DOI] [PubMed] [Google Scholar]
- Claar R. L., Walker L. S. (2006). Functional assessment of pediatric pain patients: Psychometric properties of the functional disability inventory. Pain, 121(1-2), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombez G., Bijttebier P., Eccleston C., Mascagni T., Mertens G., Goubert L., Verstraeten K. (2003). The child version of the pain catastrophizing scale (PCS-C): A preliminary validation. Pain, 104(3), 639–646. [DOI] [PubMed] [Google Scholar]
- Duncan T. E., Duncan S. C., Strycker L. A., Li F., Alpert A. (1999). An introduction to latent variable growth curve modeling: Concepts, issues, and applications. Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Durand H., Birnie K. A., Noel M., Vervoort T., Goubert L., Boerner K. E., Chambers C. T., Caes L. (2017). State versus trait: Validating state assessment of child and parental catastrophic thinking about children’s acute pain. The Journal of Pain, 18(4), 385–395. [DOI] [PubMed] [Google Scholar]
- Gmuca S., Sherry D. D. (2017). Fibromyalgia: Treating pain in the juvenile patient. Pediatric Drugs, 19(4), 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert L., Eccleston C., Vervoort T., Jordan A., Crombez G. (2006). Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): A preliminary validation. Pain, 123, 254–263. [DOI] [PubMed] [Google Scholar]
- Goubert, L., & Simons, L. E. (2013). Cognitive styles and processes in paediatric pain. In Oxford textbook of paediatric pain. Oxford: Oxford University Press. [Google Scholar]
- Guite J. W., McCue R. L., Sherker J. L., Sherry D. D., Rose J. B. (2011). Relationships among pain, protective parental responses, and disability for adolescents with chronic musculoskeletal pain: The mediating role of pain catastrophizing. The Clinical Journal of Pain, 27(9), 775–781. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck S., Flowers S. R., Claar R. L., Guite J. W., Logan D. E., Lynch-Jordan A. M., Palermo T. M., Wilson A. C. (2011). Clinical utility and validity of the functional disability inventory among a multicenter sample of youth with chronic pain. Pain, 152(7), 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashikar-Zuck S., Goldschneider K. R., Powers S. W., Vaught M. H., Hershey A. D. (2001). Depression and functional disability in chronic pediatric pain. Clinical Journal of Pain, 17(4), 341–349. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck S., Parkins I. S., Ting T.V., Verkamp E., Lynch-Jordan A., Passo M., Graham T. B. (2010). Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology, 49(11), 2204–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith T. Z. (2015). Multiple regression and beyond: An introduction to multiple regression and structural equation modeling. Routledge. [Google Scholar]
- Langer S. L., Romano J. M., Levy R. L., Walker L. S., Whitehead W. E. (2009). Catastrophizing and parental response to child symptom complaints. Children's Health Care, 38(3), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law E. F., Fisher E., Howard W. J., Levy R., Ritterband L., Palermo T. M. (2017). Longitudinal change in parent and child functioning after internet-delivered cognitive-behavioral therapy for chronic pain. Pain, 158(10), 1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D. E., Engle L. B., Feinstein A. B., Sieberg C. B., Sparling P., Cohen L. L., Conroy C., Driesman D., Masuda A. (2012). Ecological system influences in the treatment of pediatric chronic pain. Pain Research & Management, 17(6), 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D. E., Simons L. E., Carpino E. A. (2012). Too sick for school? Parent influences on school functioning among children with chronic pain. Pain, 153(2), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch-Jordan A. M., Peugh J., Cunningham N. R., Trygier J. R., Kashikar-Zuck S. (2018). Maternal protective parenting accounts for the relationship between pain behaviors and functional disability in adolescents. The Clinical Journal of Pain, 34(12), 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum R. C., Browne M. W., Sugawara H. M. (1996). Power analysis and determination of sample size for covariance structure modeling. Psychological Methods, 1(2), 130–149. [Google Scholar]
- Muris P., Meesters C., van den Hout A., Wessels S., Franken I., Rassin E. (2007). Personality and temperament correlates of pain catastrophizing in young adolescents. Child Psychiatry and Human Development, 38(3), 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo T. M., Valrie C. R., Karlson C. W. (2014). Family and parent influences on pediatric chronic pain: A developmental perspective. American Psychologist, 69(2), 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. G., Campbell F., Isaac L., Stinson J., Katz J. (2013). Parental risk factors for the development of pediatric acute and chronic postsurgical pain: A longitudinal study. Journal of Pain Research, 6, 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. M., Birnie K. A., Yoon I. A., Bhandari R. P. (2020). Interpersonal dyadic influences of pain catastrophizing between caregivers and children with chronic pain. The Clinical Journal of Pain, 36(2), 61–67. [DOI] [PubMed] [Google Scholar]
- Peterson C. C., Palermo T. M. (2004). Parental reinforcement of recurrent pain: The moderating impact of child depression and anxiety on functional disability. Journal of Pediatric Psychology, 29(5), 331–341. [DOI] [PubMed] [Google Scholar]
- Pielech M., Ryan M., Logan D., Kaczynski K., White M. T., Simons L. E. (2014). Pain catastrophizing in children with chronic pain and their parents: Proposed clinical reference points and reexamination of the PCS measure. Pain, 155(11), 2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielech M., Wallace D. P., Fitzgerald M., Hoffart C. M. (2018). Parent responses to child pain during intensive interdisciplinary pain treatment and 1-year follow-up. The Journal of Pain, 11, 1275–1284. [DOI] [PubMed] [Google Scholar]
- Sherry D. D. (2015). Pain amplification syndromes. In Petty R. E., Laxer R. M., Lindsley C. B. (Eds), Textbook of Pediatric Rheumatology (7th edn. pp. 681–692). Elsevier Saunders. [Google Scholar]
- Sieberg C. B., Williams S., Simons L. E. (2011). Do parent protective responses mediate the relation between parent distress and child functional disability among children with chronic pain? Journal of Pediatric Psychology, 36(9), 1043–1051. [DOI] [PubMed] [Google Scholar]
- Simons L. E., Claar R. L., Logan D. L. (2008). Chronic pain in adolescence: Parental responses, adolescent coping, and their impact on adolescent’s pain behaviors. Journal of Pediatric Psychology, 33(8), 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L. E., Kaczynski K. J. (2012). The fear avoidance model of chronic pain: Examination for pediatric application. The Journal of Pain, 13(9), 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L. E., Smith A., Kaczynski K., Basch M. (2015). Living in fear of your child’s pain: The parent fear of pain questionnaire. PAIN, 156(4), 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé E., Galán S., de la Vega R., Castarlenas E., Sánchez-Rodríguez E., Jensen M. P., Miró J. (2019). Psychometric properties of the Functional Disability Inventory for assessing pain-related disability in children from the community. Disability and Rehabilitation, 41(20), 2451–2458. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J. L., Thorn B., Haythornthwaite J. A., Keefe F., Martin M., Bradley L. A., Lefebvre J. C. (2001). Theoretical perspectives on the relation between catastrophizing and pain. The Clinical Journal of Pain, 17(1), 52–64. [DOI] [PubMed] [Google Scholar]
- Tran S. T., Mano K. E. J., Hainsworth K. R., Medrano G. R., Khan K. A., Weisman S. J., Davies W. H. (2015). Distinct influences of anxiety and pain catastrophizing on functional outcomes in children and adolescents with chronic pain. Journal of Pediatric Psychology, 40(8), 744–755. [DOI] [PubMed] [Google Scholar]
- Van Slyke D. A., Walker L. S. (2006). Mothers' responses to children's pain. The Clinical Journal of Pain, 22(4), 387–391. [DOI] [PubMed] [Google Scholar]
- van Tilburg M. A. L. (2018). Parents and childhood functional abdominal pain: A narrative review of the literature. Psihologijske Teme, 27(1), 115–124. [Google Scholar]
- Walker L. S., Levy R. L., Whitehead W. E. (2006). Validation of a measure of protective parent responses to children’s pain. The Clinical Journal of Pain, 22(8), 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. E., Stinson J. N. (2018). Pediatric pain syndromes and noninflammatory musculoskeletal pain. Pediatric Clinics of North America, 65(4), 801–826. [DOI] [PubMed] [Google Scholar]
- Welkom J. S., Hwang W. T., Guite J. W. (2013). Adolescent pain catastrophizing mediates the relationship between protective parental responses to pain and disability over time. Journal of Pediatric Psychology, 38(5), 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Moss A., Palermo T. M., Fales J. L. (2014). Parent pain and catastrophizing are associated with pain, somatic symptoms, and pain-related disability among early adolescents. Journal of Pediatric Psychology, 39(4), 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]