Abstract
Purpose
To report a case of a 46-year-old patient affected by polypoidal choroidal vasculopathy (PCV) in large colloid drusen (LCD) and to show how switching to intravitreal injection of aflibercept could be considered as a useful treatment of PCV not responsive to other anti-vascular endothelial growth factor (VEGF) injections.
Observations
A 46-year-old woman was referred to our department with diagnosis of early-onset retinal drusen. Multimodal imaging confirmed the diagnosis of LCD in both eyes, complicated by suggestive PVC in the left eye. Due to the absence of anatomical improvement after 6 intravitreal injections of ranibizumab, the patient was switched and treated by a single injection of aflibercept, showing a complete anatomical and functional recovery.
Conclusions and Importance
This case suggests progressive development of PCV as a possible late evolution of degenerating LCD. In case of exudative complication, intravitreal aflibercept injection could be considered as a useful treatment, especially in patients who are not responsive to others anti-VEGF injections.
Keywords: Cuticular drusen, Early onset drusen, Large colloid drusen, Optical coherence tomography angiography, Polypoidal choroidal vasculopathy
1. Introduction
Drusen are extracellular deposits between the basal lamina of the retinal pigment epithelium (RPE) and the inner collagenous layer of Bruch's membrane.1 Although drusen occur more frequently in the patient above 50 years, early onset drusen (EOD) are identified in young patients. Large colloid drusen (LCD) are a subgroup of EOD that are considered a benign condition.2,3 LCD are large (200–300 μm), bilateral and yellowish lesion, located in the macula and peripheral retina.4 In the intermediate and late phases of indocyanine green angiography (ICGA), LCD core shows a hypofluorescent center surrounded by a hyperfluorescent halo. This halo is bordered with a peripheral thin hypofluorescent ring, harboring a ‘donut shape’.5 On structural spectral domain optical coherence tomography (SD-OCT), most LCD appear convex with medium and homogeneous internal reflectivity.5 SD-OCT suggests that the smallest LCD have no effect on the ellipsoid zone, and the larger LCD erode into the photoreceptors.2
Here, we report a case of a 46-year-old patient affected by polypoidal choroidal vasculopathy (PCV) in LCD and showed how switching to intravitreal injection of aflibercept could be considered as a useful treatment of polypoidal lesion not responsive to other anti-VEGF injections.
2. Case report
A 46-year old woman was referred to our department with diagnosis of LCD that was done 8 years before based on multimodal imaging examinations (Fig. 1, Fig. 2, Fig. 3). The last fluorescein angiography, performed in 2012, showed hyperfluorescent spots compatible with large colloid drusen (Fig. 2). One year before, the patient reported central vision loss because of PCV in the left eyes (LE) (Fig. 1A). The patient was treated with six intravitreal 0.5 mg/0.05 ml ranibizumab injections (Lucentis, Novartis, Basel, Switzerland) in the left eye (the last performed one month before the examination in our department).
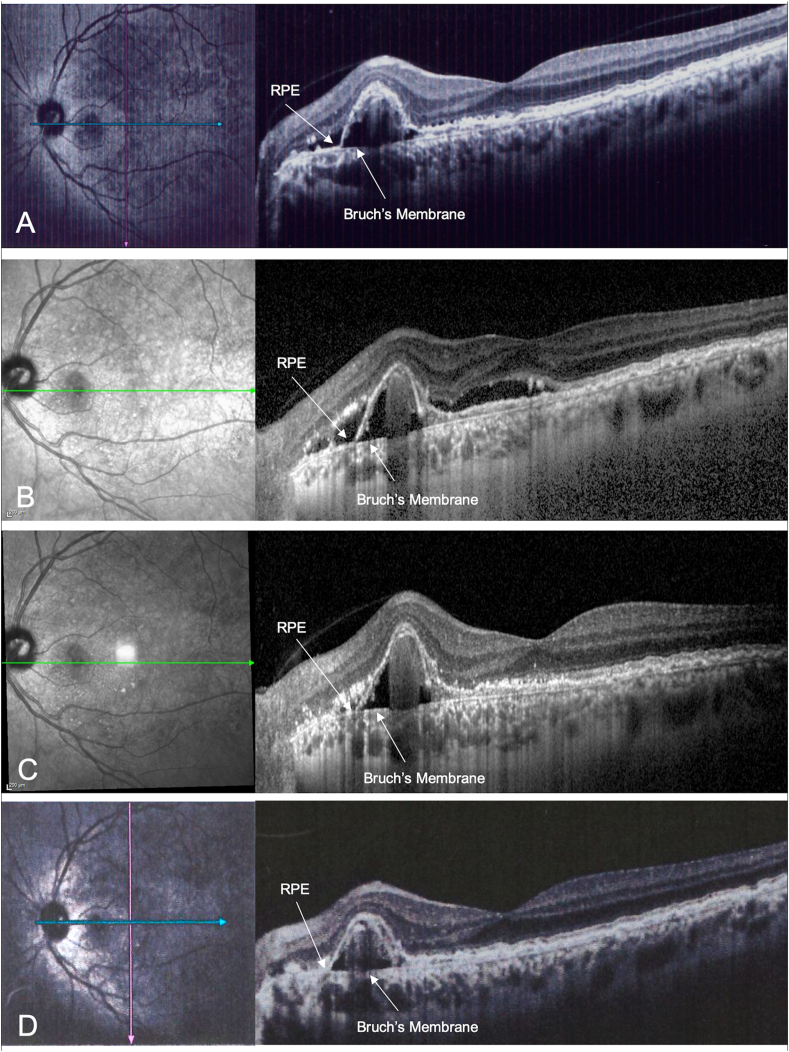
Fig. 1.
Horizontal structural optical coherence tomography (OCT) scans passing through the polypoidal choroidal vasculopathy (PCV) of the left eye at baseline and at all follow-up examinations. A) Combined infrared reflectance imaging and structural OCT at the baseline with a pigment epithelium detachment nasally to the fovea matching PCV associated with subretinal hyporeflective exudation. B) After 6 intravitreal injections of ranibizumab, structural OCT showed the persistence of subretinal fluid, involving the foveal area. C) One month after the intravitreal injection of aflibercept, structural OCT showed a great improvement of subretinal fluid, that persists only nasally to the PED. D) Two months after the intravitreal injection of aflibercept, structural OCT showed the complete resolution of the subretinal fluid. RPE: retinal pigment epithelium.
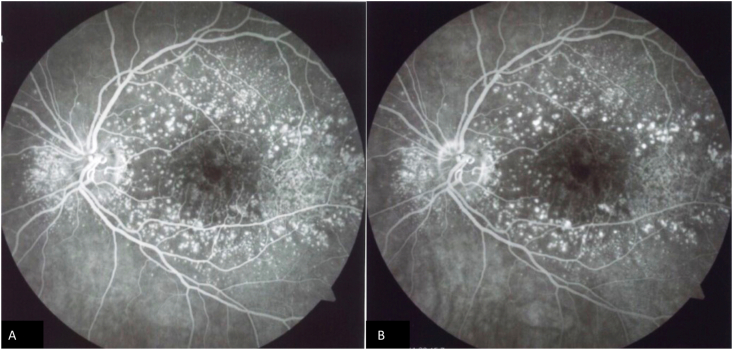
Fig. 2.
Fluorescein angiography (FA) of the left eye of the patient at the time of large colloid drusen diagnosis. Early phases (A) and late phases of FA showing the presence of hyperfluorescent spots compatible with large colloid drusen.
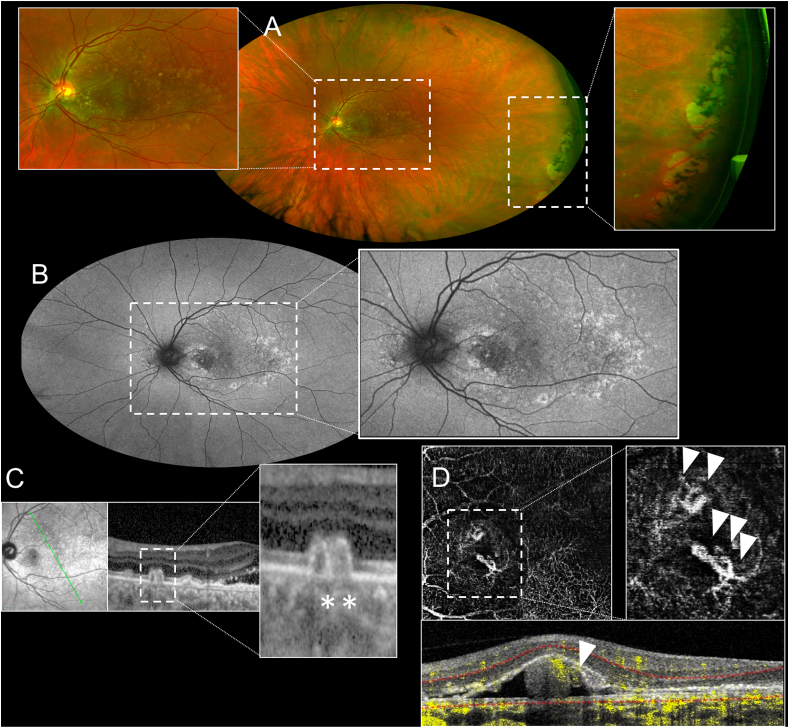
Fig. 3.
Multimodal imaging evaluation of the left eye of the patient. A) Ultra-wide field (UWF) pseudocolor examination showing the presence of large colloid drusen involving the posterior pole (magnification) and the presence of a paving-stone degeneration in the temporal periphery of the retina (magnification). B) UWF fundus autofluorescence showing the presence of hyperautofluorescence areas matching to the large drusen and an inhomogeneous hypoautofluorescence at the posterior pole. C) Combined infrared reflectance imaging and structural optical coherence tomography (OCT) passing through the fovea showing the presence of large colloid drusen (magnification, asterisks). D) OCT-angiography showing a type 1 branching neovascular network (white triangles of en-face image), associated with the polypoidal lesion (white triangle of the B-scan image).
At the presentation, best-corrected visual acuity (BCVA) was 20/40 in the right eye (RE) (amblyopic eye) and 20/25 in the left eye (LE). Intraocular pressure was 16 mmHg in both eyes. Anterior segment examination revealed the result of photorefractive keratectomy in the RE and of radial keratotomy treatment in the LE. Fundus examination revealed the presence of large round drusen in correspondence of the vascular arcades and the mid-periphery in both eyes, with paving-stone degeneration in both eyes (Fig. 3, Fig. 4). Fundus autofluorescence showed hyperautofluorescence from the large drusen and an inhomogeneous hypoautofluorescence at the posterior pole in both eyes (Fig. 3, Fig. 4B). Fundus photography and structural SD-OCT (Spectralis HRA + SD-OCT; Heidelberg Engineering, Heidelberg, Germany) revealed a myopic staphyloma in the RE (Fig. 4A) and the presence of large colloid and cuticular drusen with various inner reflectivity in both eyes (Fig. 3, Fig. 4C). In the LE, structural SD-OCT showed the presence of pigment epithelial detachment (PED) associated with the persistence of subretinal fluid (Fig. 1B), despite the last intravitreal injection of ranibizumab was performed one month before. Swept Source OCT angiography (OCT-A; PlexElite 9000 SS-OCT, Carl Zeiss Meditec, Inc., Dublin, USA) revealed a type 1 branching neovascular network associated with polypoidal lesion (Fig. 3D). So, based on the multimodal imaging evaluation (including structural OCT and OCT-A), but due to the lack of ICGA, a suggestive diagnosis of PCV was performed.
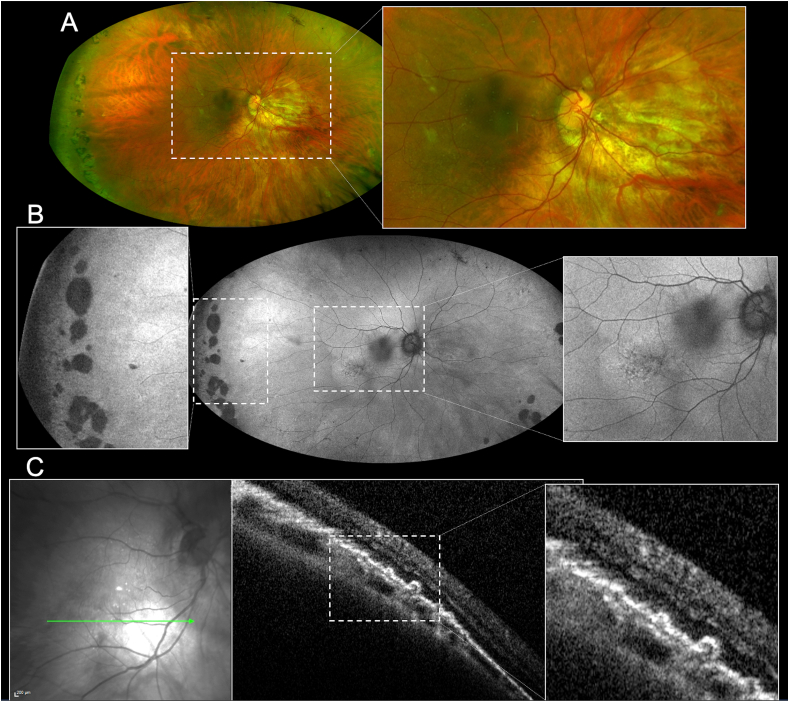
Fig. 4.
Multimodal imaging evaluation of the right eye of the patient. A) Ultra-wide field (UWF) pseudocolor examination showing the presence of myopic staphyloma and some colloid drusen involving the posterior pole (magnification) and the presence of a paving-stone degeneration in the temporal periphery of the retina. B) UWF fundus autofluorescence showing the presence of inhomogeneous hypoautofluorescence and hyperautofluorescence areas matching to the drusen at the posterior pole (magnification on the right) and atrophic areas matching to paving-stone degeneration in the temporal periphery of the retina (magnification on the left). C) Combined infrared reflectance imaging and structural optical coherence tomography (OCT) showing the presence of colloid drusen (magnification).
Due to the absence of anatomical improvement after the last intravitreal injections of ranibizumab, the patient was switched and treated by a single injection of intravitreal 2.0 mg/0.05 ml aflibercept (Eylea, Bayer, Berlin, Germany) in the LE. One month later, BCVA improved to 20/20 with a significant improvement of subretinal fluid (Fig. 1C), and, two months after the injection, structural OCT showed the complete resolution of the subretinal fluid (Fig. 1D).
3. Discussion
Using structural SD-OCT and OCT-A, we described a case of a 46-year-old patient with a suggestive diagnosis of polypoidal choroidal vasculopathy in LCD involving the left eye, and a possible association between LCD and paving-stone degeneration. Although ICGA is considered the most reliable means for the diagnosis of PCV, it is not performed routinely in many hospitals. Several studies demonstrated that the diagnosis of PCV could be suggested using only structural OCT and/or OCT-A signs, as in the presented case.6, 7, 8, 9, 10, 11 For this reason, the diagnosis of our patient was a suggestive diagnosis because of the lack of ICGA.
LCD belong to the EOD, and they have been considered as a benign condition since the original description.2 However, in the last years, our group and others reported LCD cases complicated by choroidal neovascularization (CNV).12, 13, 14 Mathis et al.12 reported the association between LCD and polypoidal choroidal vasculopathy. In addition, our group reported a case of LCD complicated by extensive quiescent CNV in both eyes13 and a case in which this condition was complicated by geographic atrophy in the RE and a CNV in the LE.14 However, in the present study, we reported how the switch to intravitreal injection of aflibercept could be considered as a useful treatment in case of LCD complicated by polypoidal lesion not responsive to other anti-VEGF injections.
Interestingly, the review of examinations performed 8 years before along with multimodal imaging evaluation showed the presence of numerous LCD without CNV. Only during the last year before referral, the patient developed signs of CNV, suggesting the development of polypoidal lesion as a possible late complication of degenerating LCD. Moreover, after six intravitreal injections of ranibizumab, BCVA did not show improvement and SD-OCT revealed the presence of a PED associated with subretinal fluid, completely resolved after only one injection of aflibercept.
Compared to other forms of neovascularization secondary to AMD, patients with PCV are younger and eyes with PCV often manifest as serosanguinous maculopathy or hemorrhagic PED.15 Furthermore, PCV differs in responses to photodynamic therapy and anti-VEGF agents in comparison to other forms of neovascularization.13 The utility of OCT-A in different ocular conditions has been extensively described in the literature.16, 17, 18, 19, 20 Indeed, OCTA is a useful technique for the noninvasive detection of branching choroidal vascular networks including visualization of details such as cluster-like structures and flow.8, 9, 10, 11
Intravitreal aflibercept injection could be considered as a useful treatment in case complicated by exudative polypoidal lesion. The Aflibercept in Polypoidal Choroidal Vasculopathy (PLANET) study aimed to evaluate the efficacy, safety, and tolerability of monotherapy with intravitreal aflibercept injection (IAI) vs IAI plus rescue PDT in the treatment of PCV.21 Improvement in visual and functional outcome was achieved in more than 85% of patients treated with aflibercept in monotherapy. At 1 year, aflibercept monotherapy was non-inferior to combination therapy, with similar results in BCVA and anatomical improvement. Moreover, in a study by Cho et al.22 aflibercept appeared to be more effective than ranibizumab to cause polyp regression. Aflibercept has also been found to be effective in polypoidal lesion non responsive to ranibizumab.23 Previous studies have shown that switching over to aflibercept from ranibizumab may be helpful in refractory cases. Saito et al.,24 in a series of 43 eyes, have reported that aflibercept therapy led to significant improvement in functional and anatomical features, showing no exudation at 3 months follow-up.24 The possible explanation may be the high-affinity binding of aflibercept to VEGF-A along with VEGF-B and placental growth factor compared with ranibizumab or bevacizumab. Moreover, recent advances in retinal imaging, have revealed that the anatomical findings in the choroid are significantly associated with the pathogenesis and the treatment efficacy for PCV.25 In detail, PCV eyes with greater baseline luminal area and smaller baseline stromal area show a better response to aflibercept injections, suggesting that choroidal structure could be a new biomarker for potential BCVA outcome after intravitreal aflibercept injections in PCV.25 Our data supported the efficacy of aflibercept injection also in LCD complicated by exudative PCV. These deposits are probably associated with retinal pigment epithelium dysfunction and could thus be considered, with a choroidal structure, as a new biomarker for potential visual outcomes after intravitreal aflibercept therapy for PCV.
Interestingly, here we reported a possible association between a peripheral paving-stone degeneration and macular LCD, even if we cannot exclude that this association was accidental because paving-stone peripheral degeneration of the retina is a relatively common feature. Such association was previously reported in cases of extensive macular atrophy with pseudodrusen-like appearance (EMAP)26, 27, 28 which is also characterized by early onset but in cases showing reticular pseudodrusen rather than LCD. On the other hand, Vatavuk et al.29 reported angiographic changes in the peripheral retina in aged patients with AMD: they showed that reticular pigmentary changes and paving-stone degeneration occurred more frequently in the AMD group than in healthy controls. Probably, this could happen also in patients affected by EOD.
In conclusion, we reported a case of LCD complicated by a suggestive diagnosis of PCV. Although in the original description LCD has been considered as a benign condition, this report demonstrates that, at least in some cases, LCD may present complications commonly observed in other chorioretinal diseases. Finally, the OCT-A may be considered as a useful tool in the diagnosis, follow-up and treatment decisions in patients affected by LCD. Furthermore, in case of exudative complication, intravitreal aflibercept injection could be considered as a useful treatment, especially in patients who are not responsive to others anti-VEGF injections. However, a large series including different “benign” subtype of early-onset drusen, including LCD, should be investigated in order to understand more about this field.
Patient consent
The patient provided written informed consent for publication of this case report and any accompanying images.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Giovanna Vella: none.
Riccardo Sacconi: consultant for Zeiss (Dublin, USA).
Enrico Borrelli: consultant for Zeiss (Dublin, USA).
Francesco Bandello consultant for: Alcon (Fort Worth, Texas,USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA).
Giuseppe Querques consultant for: Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Bayer Shering-Pharma (Berlin, Germany), Heidelberg (Germany), Novartis (Basel, Switzerland), Sandoz (Berlin, Germany), Zeiss (Dublin, USA).
References
- 1.Ach T., Huisingh C., McGwin G., Jr. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55(8):4832–4841. doi: 10.1167/iovs.14-14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querques G., Massamba N., Guigui B. In vivo evaluation of photoreceptor mosaic in early onset large colloid drusen using adaptive optics. Acta Ophthalmol. 2012;90:327–328. doi: 10.1111/j.1755-3768.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 3.Gass J.D. The CV Mosby Co; St Louis: 1977. Stereoscopic Atlas of Macular Diseases. [Google Scholar]
- 4.Guigui B., Leveziel N., Martinet V. Angiography features of early onset drusen. Br J Ophthalmol. 2011;95:238–244. doi: 10.1136/bjo.2009.178400. [DOI] [PubMed] [Google Scholar]
- 5.Guigui B., Querques G., Leveziel N. Spectral-domain optical coherence tomography of early onset large colloid drusen. Retina. 2013;33:1346–1350. doi: 10.1097/IAE.0b013e318283127d. [DOI] [PubMed] [Google Scholar]
- 6.De Salvo G., Vaz-Pereira S., Keane P.A. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014;158:1228–1238. doi: 10.1016/j.ajo.2014.08.025. e1221. [DOI] [PubMed] [Google Scholar]
- 7.Liu R., Li J., Li Z. Distinguishing polypoidal choroidal vasculopathy from typical neovascular age-related macular degeneration based on spectral domain optical coherence tomography. Retina. 2016;36:778–786. doi: 10.1097/IAE.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 8.Srour M., Querques G., Souied E.H. Optical coherence tomography angiography of idiopathic polypoidal choroidal vasculopathy. Dev Ophthalmol. 2016;56:71–76. doi: 10.1159/000442781. [DOI] [PubMed] [Google Scholar]
- 9.Peiretti E., Iovino C., Sacconi R., Caminiti G., Querques G. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy secondary to chronic central serous chorioretinopathy. Retina. 2019;39(9):1693–1700. doi: 10.1097/IAE.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 10.Srour M., Querques G., Semoun O. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2016;100(11):1489–1493. doi: 10.1136/bjophthalmol-2015-307892. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sheikh M., Iafe N.A., Phasukkijwatana N., Sadda S.R., Sarraf D. Biomarkers of neovascular activity in age-related macular degeneration using oct angiography. Retina. 2018;38(2):220–230. doi: 10.1097/IAE.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 12.Mathis T., Kodjikian L., Mauget-Faÿsse M., Feldman A. Polypoidal choroidal vasculopathy occurring in the context of large colloid drusen. Ophthal Surg Lasers Imag Retina. 2016;47:1154–1156. doi: 10.3928/23258160-20161130-12. [DOI] [PubMed] [Google Scholar]
- 13.Carnevali A., Sacconi R., Querques L. Abnormal quiescent neovascularization in a patient with large colloid drusen visualized by optical coherence tomography angiography. Retin Cases Brief Rep. 2018;12(Suppl 1):S41–S45. doi: 10.1097/ICB.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 14.Carnevali A., Querques G. Choroidal neovascularization and geographic atrophy are potential complications of early-onset large colloid drusen. Ophthal Surg Lasers Imag Retina. 2017;48:586–590. doi: 10.3928/23258160-20170630-11. [DOI] [PubMed] [Google Scholar]
- 15.Laude A., Cackett P.D., Vithana E.N. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19–29. doi: 10.1016/j.preteyeres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Sacconi R., Borrelli E., Corbelli E. Quantitative changes in the ageing choriocapillaris as measured by swept source optical coherence tomography angiography. Br J Ophthalmol. 2019;103(9):1320–1326. doi: 10.1136/bjophthalmol-2018-313004. [DOI] [PubMed] [Google Scholar]
- 17.Sacconi R., Corbelli E., Carnevali A. Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol. 2018;102(12):1684–1690. doi: 10.1136/bjophthalmol-2017-311240. [DOI] [PubMed] [Google Scholar]
- 18.Sacconi R., Freund K.B., Yannuzzi L.A. The expanded spectrum of perifoveal exudative vascular anomalous complex. Am J Ophthalmol. 2017;184:137–146. doi: 10.1016/j.ajo.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Corbelli E., Sacconi R., Rabiolo A. Optical coherence tomography angiography in the evaluation of geographic atrophy area extension. Invest Ophthalmol Vis Sci. 2017;58(12):5201–5208. doi: 10.1167/iovs.17-22508. [DOI] [PubMed] [Google Scholar]
- 20.Rabiolo A., Gelormini F., Sacconi R. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PloS One. 2018;13(10) doi: 10.1371/journal.pone.0205773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee W.K., Iida T., Ogura Y. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136(7):786–793. doi: 10.1001/jamaophthalmol.2018.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho H.J., Kim H.M., Han J.I. Intravitreal aflibercept and ranibizumab inkection for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;165:1–6. doi: 10.1016/j.ajo.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Azuma K., Obata R., Nomura Y., Tan X., Takahashi H., Yanagi Y. Angiographic findings of ranibizumab-resistant polypoidal choroidal vasculopathy after switching to a treat-and-extend regimen with intravitreal aflibercept. Retina. 2016;36:2158–2165. doi: 10.1097/IAE.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 24.Saito M., Kano M., Itagaki K., Oguchi Y., Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014;34(11):2192–2201. doi: 10.1097/IAE.0000000000000236. 2014. [DOI] [PubMed] [Google Scholar]
- 25.Asano S., Azuma K., Shimizu K. Choroidal structure as a biomarker for visual acuity in intravitreal aflibercept therapy for polypoidal choroidal vasculopathy. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0197042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamel C.P., Meunier I., Arndt C. Extensive macular atrophy with pseudodrusen-like appearance: a new clinical entity. Am J Ophthalmol. 2009;147:609–620. doi: 10.1016/j.ajo.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Querques G., Blanco R., Puche N., Massamba N., Souied E.H. Extensive macular atrophy with pseudodrusen-like appearance. Ophthalmology. 2013;120:429. doi: 10.1016/j.ophtha.2012.09.027. e1–429.e4292. [DOI] [PubMed] [Google Scholar]
- 28.Kamami-Levy C., Querques G., Rostaqui O., Blanco-Garavito R., Souied E.H. Choroidal neovascularization associated with extensive macular atrophy with pseudodrusen-like appearance. J Fr Ophtalmol. 2014;37:780–786. doi: 10.1016/j.jfo.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Vatavuk Z., Andrijević Derk B., Knežević T., Belak M., Milošević M., Friberg T.R. Morphological and angiographic peripheral retinal changes in patients with age-related macular degeneration. Ophthalmol Retina. 2018;2(3):201–208. doi: 10.1016/j.oret.2017.06.013. [DOI] [PubMed] [Google Scholar]