Abstract
Background & Aims
Previous studies have identified physical activity as an important lifestyle factor in the pathogenesis of chronic liver diseases (CLD). However, most studies were short in follow-up, and based on self-reported activity. Moreover, it is unknown whether physical activity affects the risk of liver disease development in the general population. Herein, we aimed to clarify the association between physical activity and CLD by examining the risk of liver disease and progression in relation to accelerometer-based physical activity in a large subset of prospectively recruited participants in the UK Biobank.
Methods
We analysed data from 96,688 participants that recorded their physical activity through the use of a wrist accelerometer. Relative risks for development of liver diseases were calculated using multivariable-adjusted Cox regression models. In a subgroup of participants without any previously diagnosed liver disease (n = 95,974), a total of 374 liver disease cases were diagnosed during follow-up (mean = 5.5 years).
Results
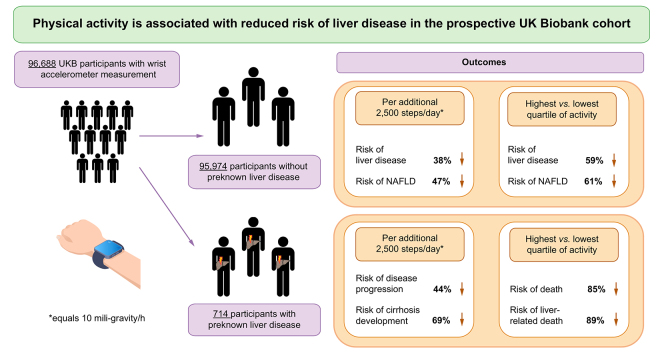
Participants in the top compared with the bottom quartile of physical activity had a reduced risk of both overall CLD (hazard ratio [HR]: 0.41 [0.29–0.59]) and NAFLD (HR: 0.39 [0.21–0.70]). An activity increase of an additional 2,500 steps per day, was associated with a 38% reduction in CLD and a 47% reduction in NAFLD development, independent of adiposity. In the subgroup of participants with previously diagnosed liver disease (n = 714), participants in the top compared with the bottom quartile of physical activity had a striking 89% risk reduction in liver-related death (HR: 0.11 [0.02–0.86]), and 85% risk reduction in all-cause mortality (adjusted HR: 0.15 [0.05–0.44]). Walking an additional 2,500 steps per day was associated with 44% reduction in liver disease progression.
Conclusions
Greater physical activity is associated with a dose-dependent reduction in liver disease, which appears to be independent of adiposity.
Lay summary
In this study, we aimed to clarify the association between accelerometer-measured physical activity and chronic liver disease by examining risk of overall and specific liver diseases and their progression in relation to accelerometer-based physical activity in 96,688 participants in the UK Biobank. Our results show a clear, dose-dependent protective association between accelerometer-measured physical activity and liver disease development and progression. The linkage of device-measured activity could therefore create a framework for using wearables for personalised prevention of liver diseases.
Keywords: Accelerometer, NAFLD, Survival, Liver-related death, Liver disease progression
Abbreviations: CLD, chronic liver disease; DM, diabetes mellitus; HR, hazard ratio; ICD-10, International Classification of Diseases 10th edition; MET, metabolic equivalent of task; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio
Graphical abstract
Highlights
-
•
Physical activity is associated with a dose-dependent reduction in liver disease that appears to be independent of adiposity.
-
•
An increase of 2,500 steps per day was associated with a 38% reduction in liver disease and a 47% reduction in NAFLD.
-
•
In patients with liver disease, activity was associated with a reduction in liver disease progression and cirrhosis development.
-
•
In patients with liver disease, increased physical activity significantly reduced the risk of liver-related death.
Introduction
For most of the past, humans have lived in an environment in which physical activity and fitness were central for survival. Although our genetic constitution was selected for the environment of the Stone-Age hunter-gatherers, our level of physical activity and energy expenditure has decreased significantly.
A large number of epidemiological studies have shown that an inactive lifestyle is closely associated with an increased risk of diseases such as obesity,1 diabetes mellitus (DM)2 or hypertension.3 Similarly, there has been a dramatic rise in chronic liver diseases (CLD).4 Non-alcoholic fatty liver disease (NAFLD) is already the most common liver disease worldwide accounting for an increase in liver-related morbidity and mortality.4 Recent work in the field of NAFLD has identified physical activity as a disease modifier independent of diet and obesity.5,6 However, current studies of exercise interventions mostly rely on self-reported activity, and suffer from either relatively small sample sizes, short follow-up duration, or insufficient power to detect clinically meaningful benefits. Moreover, data on the role of physical activity in CLD other than NAFLD and its role in liver disease progression remain scarce. Finally, it remains unknown whether physical activity is associated with decreased occurrence of CLD in the general population.
The purpose of this study was to assess the associations between physical activity and liver disease in participants who measured their physical activity using a wrist accelerometer. We analysed data from 96,688 participants in the UK Biobank who recorded their physical activity for 1 week between 2013 and 2015 with a mean follow-up of 5.5 years, which is the largest study of accelerometer-measured physical activity to date. We divided this cohort into patients with (n = 714) and without previously diagnosed liver disease (n = 95,714) and investigated whether device-measured physical activity was associated with the occurrence of liver disease, liver disease progression, or liver-related and all-cause mortality.
Our study highlights physical activity as an important actionable risk factor for CLD, which is not limited to NAFLD and independent of other well-described risk factors such as age, diet, obesity, and diabetes mellitus.
Participants and methods
Data were obtained from the UK Biobank (reference number 59657).
The UK Biobank is a population-based cohort study that was conducted in the UK from 2006 to 2010, which recruited 502,505 volunteers aged 37–73 years at baseline. All participants were registered with the UK National Health Service and were encouraged by post to attend an assessment centre for an initial examination, which is followed by a long-term follow-up through various health data systems. Our study population comprises the baseline assessment (2006–2010) and the first follow-up, in which the participants provided demographic and clinical data. On both visits, after informed consent was given, blood samples were taken and physical measures, which included impedance measurement, were performed. At the baseline interview, height and weight were measured, and the Tanita BC-418MA (Tanita, Tokio, Japan) body composition analyser was used to measure body fat mass using bioimpedance. BMI was calculated by dividing weight (kg) by the square of standing height (m2). All participants gave informed consent for data linkage to medical reports. Covariate data from the interview undertaken closest to the accelerometry were used, the accelerometry time point being the analytical baseline for this present analysis.
A web-based 24-h dietary assessment was e-mailed out once every 3–4 months for a total of 5 times between 2011 and 2012. Between February 2013 and December 2015, 236,519 participants, all of whom had provided a valid e-mail address, were invited to participate in a 7-day accelerometer study (on average, ~5.5 years after recruitment). Beginning in June 2013, participants were sent wrist-worn triaxial accelerometers (Axivity AX3, Newcastle upon Tyne, UK) that were programmed to capture 3-dimensional acceleration data at 100 Hz with a dynamic range of ± 8 g. Participants were provided with instructions to wear the accelerometer on their dominant wrist continuously for 7 days and then to return the device to the coordinating centre using the prepaid envelope provided. Researchers affiliated with UK Biobank extracted physical activity information from raw 100-Hz triaxial acceleration data after calibrating the data, removing gravity and sensor noise, and identifying wear and non-wear episodes. In the present analyses, the ‘non-wear-time adjusted acceleration average’ variable was used (data field 90087), which is the average vector magnitude of acceleration corrected for the non-wear time. Accelerometer data were not used from participants with poor wear time (n = 6,995) or poor data calibration (n = 4) because of insufficient data. Alcohol consumption was measured as a continuous variable and was assessed using a questionnaire on the frequency of alcohol consumption in all participants and the weekly consumption of a standardised amount of ‘beer or cider’, ‘champagne or white wine’, red wine, and spirits or other. We estimated the alcohol consumption per day. After a mean time of three years after accelerometry, liver magnetic resonance imaging (MRI) was performed for a subset of participants and the proton density fat fraction was extracted as a measurement of liver steatosis.
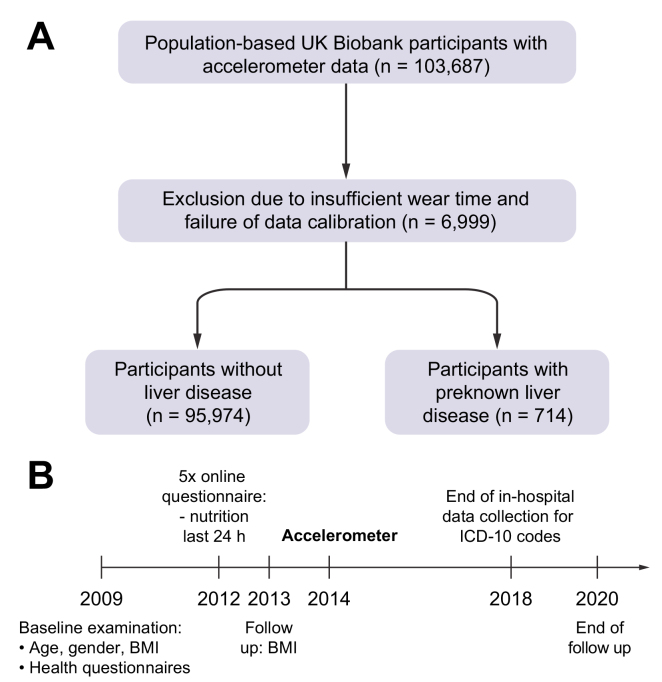
In this project, we analysed a total of 96,688 accelerometer datasets in the UK Biobank and divided this group of participants into 2 subgroups: individuals with and without previously diagnosed liver disease (Fig. 1A). Previously diagnosed liver disease was defined as a first diagnosis of liver disease (K70–K76), chronic viral hepatitis (B18) or malignant liver diseases (C22) before accelerometry. Therefore, ongoing inpatient hospital records beginning in 1996 were used to identify diagnoses according to International Classification of Diseases 10th edition (ICD-10) codes. All reported ICD-10 codes were connected with the date of their first diagnosis. For follow-up, hospital inpatient data, national cancer registries, or death registration were used. End of follow-up was defined as death or end of hospital inpatient data collection in March 2018, whichever occurred first. The following primary ICD-10 codes were evaluated, which occurred after the accelerometer was worn: overall liver disease (C22, B18, and K70–K77), NAFLD (K76.0) and fibrosis and cirrhosis (K74). As potential cofactors we evaluated hypertension (I10) and dyslipidaemia (E78). The UK Biobank receives death notifications (age at death and primary ICD diagnosis that led to death) through linkage to national death registries. Specific causes of death included liver cancer (C22), chronic hepatitis (B18), and liver diseases (K70–K77).
Fig. 1.
Overview of the analysed cohorts.
(A) UK Biobank participants of European ancestry aged 37–73 years. (B) Timeline of data acquisition. ICD-10, International Classification of Diseases 10th edition.
Statistics
All continuous variables were analysed by unpaired, 2-tailed t tests or by an appropriate multivariable model. The results are presented as mean ± SD. All categorical variables were displayed as relative (%) frequencies and the corresponding contingency tables were analysed using the Chi-square test. Odds/hazard ratios (ORs/HRs) were presented with their corresponding 95% CIs given in brackets. Multivariable-adjusted Cox regression with attained age as the underlying time variable was used to estimate HRs for the associations between physical activity and liver disease risk.
Assessment of interaction terms between each exposure of interest and the underlying time variable did not suggest significant deviation from proportional hazards. Variables that were associated with both the exposure of interest and causally associated with the outcome were included in regression models as possible confounders. Multivariable Cox regression was performed to test for independent associations. All multivariable analyses were adjusted for age (years), sex, BMI (kg/m2), and daily alcohol consumption (g/day). Given the low level of missing data, we excluded participants with specific missing data from each analysis as needed.
Normality was tested using the Kolmogorov-Smirnov test and homoscedasticity was tested using the Goldfeld-Quandt test. Differences were considered to be statistically significant when p <0.05. The data were analysed using R version 4.0.2 (R Foundation for Statistical Computing; Vienna, Austria), SPSS Statistics version 26 (IBM; Armonk, NY, USA) and Prism version 8 (GraphPad, La Jolla, CA, USA).
Results
Physical activity is associated with reduced liver disease development
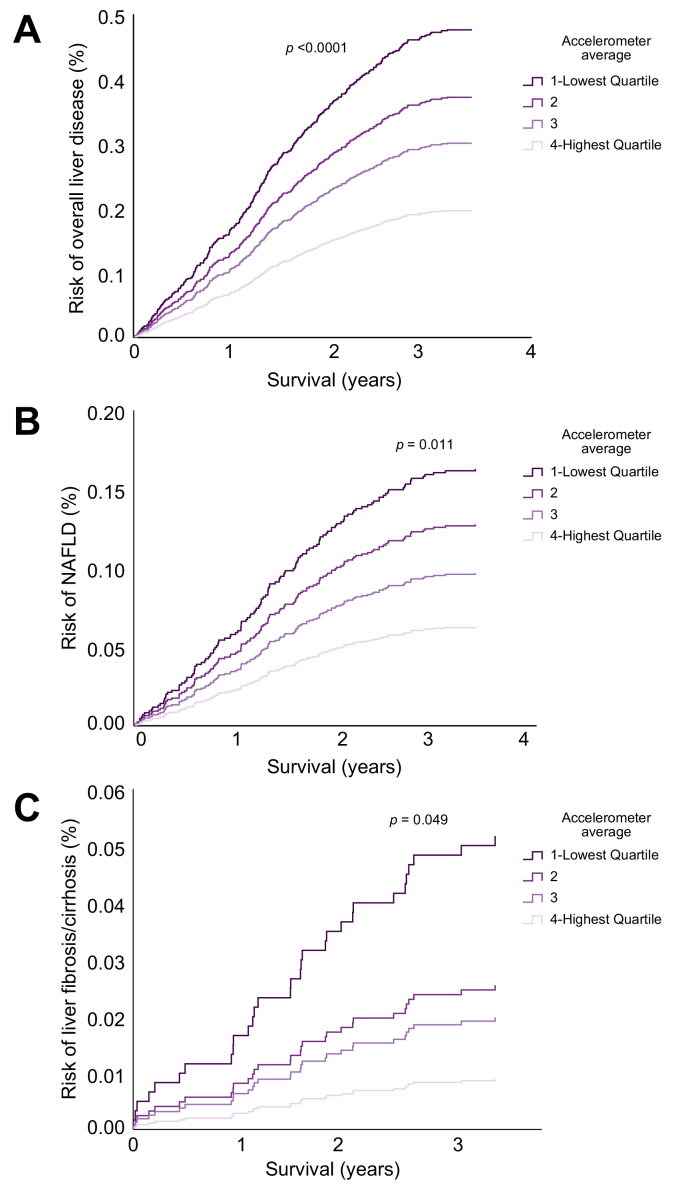
First, we aimed to analyse the association of physical activity and liver disease development. We analysed a total of 95,574 participants without previously diagnosed liver disease (Fig. 1A). During a mean follow-up of 5.5 years, a total of 374 (0.4%) participants were diagnosed with liver disease. Of these, 137 cases were NAFLD (K76.0) and 31 were fibrosis/cirrhosis (K74, Table 1). Physical activity was associated with an overall reduced risk of liver disease development (Fig. 2A), which was most pronounced for physician-diagnosed NAFLD and fibrosis/cirrhosis (Fig. 2B and C). Strikingly, participants in the top quartile of physical activity had a 59% decreased risk of liver disease and 61% lower risk of NAFLD development compared with those in the bottom quartile (Fig. 2). The relationship between activity and CLD development could be modelled with a linear function (p = 0.005, Fig. S1A). The same linear association was observed for NAFLD (p = 0.009, Fig. S1B). To further corroborate the lower risk of liver disease in participants with the highest physical activity, we constructed multivariable Cox regression models accounting for several potential confounders (Table S1). In all models, participants with the highest physical activity had significantly lower odds for overall liver disease and NAFLD development (Table S1). As we observed a clear dose-dependent effect of physical activity on risk of liver disease development (Fig. S1), we constructed a linear risk reduction model (Table 2). In this model an increase of 10 mG/h of accelerometer average was associated with a 38% (HR 0.62; 95% CI 0.53–0.73) reduction in liver disease development and a 47% (HR 0.53; 95% CI 0.41–0.70) reduction in NAFLD development (Table 2). Moreover, there was a negative correlation between physical activity and MRI-measured liver fat three years after accelerometry (Fig. S2). Thus, higher physical activity is strongly associated with a clear dose-dependent reduction in overall liver disease development, especially for NAFLD.
Table 1.
Descriptive characteristics of the samples by accelerometer average quartiles in participants without pre-known liver disease.
| Quartile 1, n = 23,998 | Quartile 2, n = 23,983 | Quartile 3, n = 23,992 | Quartile 4, n = 24,001 | |
|---|---|---|---|---|
| Acceleration average (mG/h), mean (SD) | 19 (3) | 25 (1) | 30 (2) | 39 (7) |
| Days of accelerometer wearing (days), mean (SD) | 7(1) | 7(1) | 7(1) | 7(1) |
| Month of accelerometry, mean (SD) | 7 (4) | 7 (3) | 7 (3) | 7 (3) |
| Deaths, n (%) | 1,096 (4.6) | 522 (2.2) | 387 (1.6) | 285 (1.2) |
| Death caused by liver diseases, n (%) | 32 (0.1) | 10 (0.0) | 14 (0.1) | 8 (0.0) |
| Death secondary caused by liver diseases, n (%) | 24 (0.1) | 8 (0.0) | 4 (0.0) | 3 (0.0) |
| Survival (years), mean (SD) | 5.5 (0.9) | 5.6 (0.7) | 5.6 (0.7) | 5.6 (0.7) |
| New diagnosis of liver diseases during follow-up | ||||
| Overall liver disease, n (%) | 159 (0.7) | 101 (0.4) | 72 (0.3) | 42 (0.2) |
| Fibrosis/cirrhosis, n (%) | 17 (0.1) | 7 (0.0) | 5 (0.0) | 2 (0.0) |
| NAFLD, n (%) | 60 (0.3) | 37 (0.2) | 26 (0.1) | 15 (0.1) |
| Characteristics | ||||
| Age at accelerometry (years), mean (SD) | 64.6 (7.3) | 62.6 (7.6) | 61.1 (7.7) | 59.0 (7.7) |
| Female sex, n (%) | 11,881 (50) | 13,636 (57) | 14,212 (59) | 14,342 (60) |
| White ethnicity, n (%) | 23,307 (97.1) | 23,211 (96.8) | 23,161 (96.5) | 23,003 (95.8) |
| Missing | 102 (0.4) | 80 (0.3) | 83 (03) | 71 (0.3) |
| Smoking, n (%) | ||||
| Never, n (%) | 12,681 (52.8) | 13,679 (57.0) | 14,031 (58.5) | 14,344 (59.8) |
| Previous, n (%) | 9,143 (38.1) | 8,621 (35.9) | 8,437 (35.2) | 8,183 (34.1) |
| Current, n (%) | 2,102 (8.8) | 1,616 (6.7) | 1,463 (6.1) | 1,418 (5.9) |
| Missing, n (%) | 72 (0.3) | 67 (0.2) | 61 (0.2) | 56 (0.2) |
| Alcohol intake (g/day) mean (SD) | 9.3 (11.3) | 9.7 (10.7) | 9.7 (10.6) | 10.0 (10.6) |
| Alcohol consumption, n (%) | ||||
| Never, n (%) | 814 (3.4) | 648 (2.7) | 643 (2.7) | 674 (2.8) |
| Previous drinker, n (%) | 820 (3.4) | 640 (2.7) | 569 (2.4) | 574 (2.4) |
| Current drinker, n (%) | 22,343 (93.1) | 22,669 (94.5) | 22,764 (94.9) | 22,725 (94.7) |
| Missing, n (%) | 21 (0.1) | 26 (0.1) | 16 (0.0) | 26 (0.1) |
| Nutrition | ||||
| Mean energy intake (kJ/day), mean (SD) | 8,611 (2,472) | 8,797 (2,433) | 8,928 (2,492) | 9,236 (2,681) |
| Missing, n (%) | 6,639 (28) | 6,382 (27) | 6,327 (26) | 6,594 (27) |
| Carbohydrates (g/day) mean (SD) | 247 (84) | 251 (82) | 258 (84) | 268 (90) |
| Missing, n (%) | 6,639 (28) | 6,382 (27) | 6,327 (26) | 6,594 (27) |
| Fat intake (g/day), mean (SD) | 76 (28) | 78 (28) | 79 (28) | 82 (31) |
| Missing, n (%) | 6,639 (28) | 6,382 (27) | 6,327 (26) | 6,594 (27) |
| Protein intake (g/day), mean | 81 (23) | 82 (23) | 83 (24) | 84 (25) |
| Missing, n (%) | 6,639 (28) | 6,382 (27) | 6,327 (26) | 6,594 (27) |
| Sugar (g/day), mean (SD) | 116 (48) | 119 (47) | 122 (48) | 129 (51) |
| Missing, n (%) | 6,639 (28) | 6,382 (27) | 6,327 (26) | 6,594 (27) |
| Sleep (h), mean (SD) | 7(1) | 7(1) | 7(1) | 7(1) |
| Missing, n (%) | 10 (0) | 10 (0) | 10 (0) | 13 (0) |
| BMI, mean (SD) | 28.4(5.1) | 26.9 (4.4) | 26.2 (4.1) | 25.2 (3.7) |
| Obese ≥30 kg∗m−2, n (%) | 9,295 (38.7) | 6,370 (26.6) | 5,004 (20.9) | 3,317 (13.8) |
| Missing, n (%) | 97 (0.4) | 48 (0.2) | 38 (0.2) | 29 (0.1) |
| Fat mass of trunk (kg), mean (SD) | 15.3 (5.4) | 13.6 (4.8) | 12.6 (4.5) | 11.1 (4.3) |
| Missing, n (%) | 467 (1.9) | 345 (1.4) | 292 (1.2) | 289 (1.2) |
| Proton density fat fraction, mean (SD) | 5.3 (5.8) | 3.9 (4.6) | 3.5 (4.1) | 2.9 (3.5) |
| Missing, n (%) | 23,352 (97.3) | 23,333 (97.3) | 23,333 (97.3) | 23,358 (97.3) |
| Diagnosis of diabetes, n (%) | 1,664 (6.9) | 717 (3.0) | 554 (2.3) | 332 (1.4) |
| Missing, n (%) | 64 (0.3) | 44 (0.2) | 43 (0.2) | 34 (0.1) |
| Waist circumference (cm), mean (SD) | 94 (14) | 89 (13) | 87 (12) | 84 (12) |
| Missing, n (%) | 57 (0) | 34 (0) | 23 (0) | 20 (0) |
| Dyslipidaemia, n (%) | 3,231 (14) | 1,937 (8) | 1,485 (6) | 921 (4) |
| Missing, n (%) | 4,061 (17) | 5,042 (21) | 5,394 (23) | 5,898 (25) |
| Hypertension, n (%) | 6,683 (28) | 4,370 (18) | 3,317 (14) | 2,232 (9) |
| Missing, n (%) | 4,061 (17) | 5,042 (21) | 5,394 (23) | 5,898 (25) |
| Number of falls, n (%) | 1.2 (0.6) | 1.2 (0.5) | 1.2 (0.5) | 1.2 (0.5) |
| Missing, n (%) | 10 (0) | 10 (0) | 10 (0) | 14 (0) |
| Overall health rating, n (%) | 2 (1) | 2 (1) | 2 (1) | 2 (1) |
| Missing, n (%) | 10 (0) | 10 (0) | 10 (0) | 14 (0) |
Fig. 2.
Prospective liver-disease risk as a function of physical activity.
(A) Risk of overall liver disease as a function of physical activity. Participants were followed prospectively from the time of wearing the accelerometer until death or end of follow-up. (B) Risk of NAFLD development as a function of physical activity. (C) Risk of liver fibrosis/cirrhosis as a function of physical activity. Hazard ratios were calculated by Cox regression, adjusted for sex, age, BMI, and alcohol consumption. The p values given in the figure are overall p values comparing all 4 groups. (A) Overall liver disease: quartile 4 vs. 1: HR: 0.41 [0.29–0.59], quartile 3 vs. 1: HR: 0.63 [0.47–0.84], quartile 2 vs. 1: HR: 0.78 [0.61–0.99; (B) NAFLD: quartile 4 vs. 1: HR: 0.39 [0.21–0.70], quartile 3 vs. 1: HR: 0.59 [0.37–0.96]; (C) fibrosis/cirrhosis: quartile 4 vs. 1: HR: 0.17 [0.04–0.79]. NAFLD, non-alcoholic fatty liver disease.
Table 2.
Linear trend estimation of decrease in risk of liver diseases per 10 milligravity (mG) increase in physical activity in participants without prior liver disease; adjusted for age, sex, BMI, and alcohol consumption.
| Number of cases | Risk of liver diseases per 10 mG/h increase in physical activity |
||
|---|---|---|---|
| Hazard ratio [95% CI] | p value | ||
| Overall liver disease | 374 | 0.62 [0.53–0.73] | <0.0001 |
| Alcoholic liver disease (K70) | 19 | 0.32 [0.15–0.68] | 0.003 |
| Liver failure (K72) | 19 | 0.19 [0.08–0.46] | <0.0001 |
| Fibrosis/cirrhosis (K74) | 31 | 0.58 [0.33–1.02] | 0.056 |
| Inflammatory liver diseases (K75) | 45 | 0.60 [0.38–0.95] | 0.030 |
| Other liver diseases (K76) | 277 | 0.59 [0.49–0.71] | <0.0001 |
| NAFLD (K76.0) | 137 | 0.53 [0.41–0.70] | <0.0001 |
| Chronic hepatitis (B18) | 29 | 1.28 [0.93–1.72] | 0.56 |
All liver diseases with at least 10 cases are displayed. Bold font indicates significant hazard ratio. Forty-six participants developed more than 1 liver disease. Hazard ratios were calculated by Cox regression, adjusted for sex, age, BMI, and alcohol consumption. For a fictive 80-kg male participant an increase in 10 mG/h is estimated to equal 45 min of additional walking per day. NAFLD, non-alcoholic fatty liver disease.
Physical activity reduces risk of liver disease especially in patients with risk factors
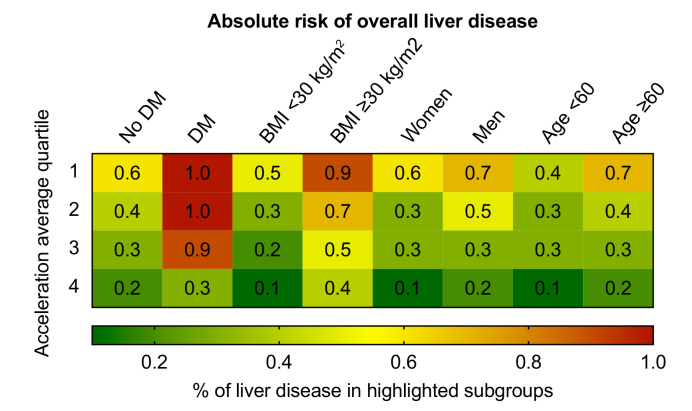
Next, we determined the frequency of newly developed liver disease during follow-up distributed by quartile of physical activity in clinically relevant subpopulations (Fig. 3). Among the analysed parameters, the association of physical activity with reduced liver disease development was more pronounced in the presence of DM and obesity (Fig. 3). Interestingly, in participants with DM only those individuals in the highest quartile of physical activity had reduced risk of liver disease, whereas in obese participants a reduction of liver disease was observed in all quartiles except the quartile with the lowest physical activity. We also determined the association between physical activity and liver disease in clinically relevant subpopulations of participants such as those who were overweight or obese, over 60 years of age, or stated in their interview that they consumed alcohol regularly (Fig. S3). The protective association of physical activity on liver disease development was more pronounced in the presence of BMI >25, age over 60 years, or regular alcohol consumption (Fig. S3). For participants in the highest quartile of physical activity compared with the bottom quartile, the risk of liver disease development in older participants, regular alcohol consumers, or overweight and obese individuals was reduced by up to 62%. Together, these data demonstrate that the association of physical activity with reduced liver disease development was more pronounced in those participants who were at the highest risk of liver disease development.
Fig. 3.
Risk of overall liver disease among the highlighted subpopulations stratified by average acceleration quartile.
Physical activity measured by accelerometer was grouped into quartiles (1 lowest activity – 4 highest activity). Relative frequencies (%) are shown and visualised by a colour coding (right panel). DM, diabetes mellitus.
Physical activity reduces risk of death in participants with liver diseases
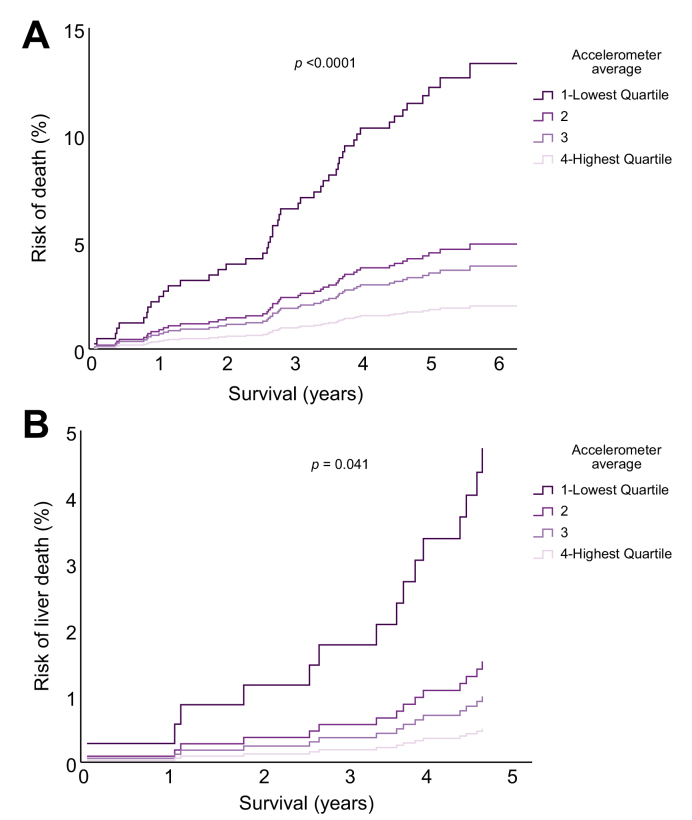
After analysing the association between physical activity and liver disease development, we asked whether physical activity may also correlate with liver disease progression and outcomes in patients with known liver disease (Table S3). Here, physical activity was associated with reduction in overall death (Fig. 4A) and especially in liver-related death (Fig. 4B). Participants in the top quartile of physical activity, had an 85% decreased risk of death (aHR 0.15; 95% CI 0.05–0.44) and a striking 89% decrease in liver-related death (aHR 0.11; 95% CI 0.02–0.86) compared with those in the bottom quartile (Fig. 4). For linear trends according to accelerometer-based physical activity, an increase of 10 mG/h of accelerometer average was associated with a 44% reduction in overall liver disease progression, defined as diagnosis of a new liver related ICD-10 code during follow-up and a 69% reduction in new physician-diagnosed cirrhosis (ICD-10: K74.6) (Table 3). In NAFLD patients a 10 mG/h increase in physical activity was associated with a striking 86% reduction in death (Table 3).
Fig. 4.
Prospective risk of overall or liver-related death as a function of physical activity in participants with previously diagnosed liver diseases.
(A) Risk of all-cause mortality as a function of physical activity. Participants were followed prospectively from the time of wearing the accelerometer until death or end of follow-up. (B) Risk of liver-related death as a function of physical activity. The p values given in the figure are overall p values comparing all 4 groups. Hazard ratios were calculated by Cox regression, adjusted for sex, age, BMI, and alcohol consumption. (A) Overall death: quartile 4 vs. 1: HR: 0.15 [0.05–0.44], quartile 3 vs. 1: HR: 0.29 [0.13–0.64], quartile 2 vs. 1: HR: 0.36 [0.18–0.75]; (B) liver-related death: quartile 4 vs. 1: HR: 0.11 [0.02–0.86], Quartile 3 vs. 1: HR: 0.21 [0.05–0.98].
Table 3.
Linear trend estimation of risk decrease for liver disease progression/liver death per 10 milligravity/h increase in physical activity in participants with previously diagnosed liver disease, adjusted for age, sex, BMI, and alcohol consumption.
| Number of cases | Risk of liver disease progression per 10 mG/h increase in physical activity |
||
|---|---|---|---|
| Hazard ratio (HR) [95% CI] | p value | ||
| In all (n = 714) | |||
| Overall liver disease progression | 86 | 0.58 [0.43–0.79] | 0.009 |
| Fibrosis/cirrhosis (K74) | 19 | 0.37 [0.18–0.75] | 0.006 |
| Cirrhosis (K74.6) | 17 | 0.31 [0.14–0.68] | 0.003 |
| Varices (I85) | 47 | 0.35 [0.22–0.56] | <0.0001 |
| Ascites (R18) | 37 | 0.41 [0.25–0.70] | 0.001 |
| Portal hypertension (K76.6) | 12 | 0.27 [0.10–0.74] | 0.011 |
| Death | 50 | 0.37 [0.23–0.57] | <0.0001 |
| Liver death | 15 | 0.32 [0.14–0.74] | 0.008 |
| Secondary liver death | 13 | 0.47 [0.21–1.01] | 0.065 |
| In NAFLD (n = 246) | |||
| Overall liver disease progression | 22 | 0.76 [0.47–0.99] | 0.049 |
| Death | 8 | 0.14 [0.03–0.56] | 0.006 |
Liver disease progression was defined as any new liver-related ICD code diagnosis after the accelerometer was worn. Only subgroups with at least 8 cases were analysed. Bold font indicates significant hazard ratio. Hazard ratios were calculated by Cox regression, adjusted for sex, age, BMI, and alcohol consumption. For a fictive 80-kg male participant an increase in 10 mG/h is estimated to equal 2,500 steps per day.
Liver disease risk reduction by physical activity is independent from BMI and visceral fat
It is often assumed that the reduction in BMI as a result of physical activity might be the cause of its positive effects on health. Therefore, after establishing a strong connection between physical activity and risk of liver disease and its progression, we analysed the effect of physical activity on BMI. In participants both with and without prior liver disease, the correlation between BMI and accelerometer average was significant, but interestingly not strong (r = -0.2, not shown). We included high physical activity, BMI, and their interaction term (activity × BMI) in a Cox proportional hazard model on liver disease development. Although both high physical activity and BMI made significant contributions in the main effects model, additionally including the interaction term yielded a non-significant testing result (p = 0.32, Table S4). This indicated independent and additive contributions by physical activity and obesity. However, BMI is an imperfect measurement of obesity, and visceral fat is a better predictor of liver-related events than BMI.7 Therefore, we included physical activity, objectively measured fat mass of the trunk via impedance measurement, and their interaction term (activity × trunk fat) in a Cox proportional hazard model on liver disease development. Although both high physical activity and trunk fat made significant contributions in the main effects model (both p <0.05, Table S5), additionally including the interaction term yielded a non-significant testing result (p = 0.14, Table S5). This indicated independent contributions by physical activity and trunk fat.
Implications for patient counselling
To clarify the implication of the physical activity quartiles, if confirmed by prospective studies, for patient consultation, we calculated a rough estimate of various forms of movement that correlate with the mean of activity recorded by the accelerometer per hour for a fictive 80-kg male participant with (Table S6) and without previously diagnosed liver disease (Table 4). A participant without prior liver disease is classified as quartile 1 if overall activity per day is around 88 min of walking. The same participant could increase his activity by an additional walk of 39 min per day to reach the mean of quartile 2 (Table 4). This increase in physical activity could be associated, based on our study, with an absolute risk reduction of liver disease development of 22% (HR of quartile 1 vs. quartile 2, Fig. 2A).
Table 4.
Estimated minutes of activity equalling the accelerometer average/h per quartile group for a fictive 80-kg male participant without liver disease.
| Activity | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| Accelerometer average (mG) | 19/h | 25/h | 30/h | 39/h |
| Acceleration (estimation of 17 h activity/day) | 323/day | 425/day | 510/day | 663/day |
| Equals | ||||
| Number of steps/day | 6,500 | 8,000 | 9,250 | 12,000 |
| Minutes of very slow walking (1.5 km/h) | 212 | 280 | 336 | 437 |
| Minutes of walking (4 km/h) | 88 | 127 | 139 | 180 |
| Minutes of fast running (10 km/h) | 19 | 26 | 31 | 40 |
| Minutes of slow cycling (10 km/h) | 46 | 62 | 74 | 96 |
For estimated minutes of activity, the acceleration average per hour was multiplicated with the estimated waking hours (24 h – 7 h sleeping). Acceleration was then converted to the metabolic equivalent of task (MET). The following estimations were calculated based on previously published work;34,35 very slow walking = 3 METs = 91 mG; walking = 4.5 METs = 220 mG; fast running = 11 METs = 1,000 mG; cycling = 6 MET = 414 mG; 1,000 steps per kilometre of walking was estimated for number of steps. Example: A fictive 80-kg male participant with current physical activity in Quartile 1, can increase his activity by walking 40 min more per day to reach Quartile 2 and may decrease his risk of liver disease by these measures.
Discussion
Driven by fundamental changes in lifestyle factors, societies have experienced a dramatic surge of chronic liver diseases.4 To meet this global challenge, a better understanding of how modifiable lifestyle-related factors shape liver disease susceptibility and drive its progression is urgently needed. An increasing body of evidence highlights physical activity as an important driver for the development of various metabolic and cardiovascular diseases. However, data on its role in development of CLD, especially in CLD other than NAFLD, remain scarce.8 In this large prospective study, we explored the beneficial effects of accelerometer-based physical activity on the risk of liver disease development and its progression. To this end, we analysed data of 96,688 UK Biobank participants who measured their physical activity using a wrist accelerometer. Our study unveils a clear dose-dependent protective association between physical activity and liver disease development and progression, which appears to be independent of BMI.
Our data are consistent with several studies focusing on the effect of physical activity and liver disease progression, showing increased general health9,10 and decreased hepatic venous pressure.11,12 However, until now, its impact on survival was unknown. In our study, the subgroup of participants with previously diagnosed liver disease in the top compared with the bottom quartile of physical activity had a remarkable risk reduction of 89% of liver-related death. An increase of 10 mG/h, equalling 2,500 steps per day, was associated with a 44% reduction in liver disease progression and a 69% reduction in liver disease progression to cirrhosis. It is known that patients with advanced liver disease have a reduced tolerance for exercise.[13], [14], [15] Particularly in advanced stages, cirrhosis patients may suffer from fatigue, deconditioning, sarcopaenia, and pulmonary or cardiac complications, which all pose considerable limitations to exercise ability. Although our current study design does not allow direct causal inference, it clearly points towards a protective effect of physical activity in participants with known liver diseases. Hence, patients with liver diseases should be motivated to include steady daily physical activity at their own pace, for example walking 3 additional hours per week as no adverse effects have been linked to moderate exercise even in advanced disease stages.[9], [10], [11], [12]
Prior studies have not assessed overall liver disease development in the general population, nor have they included prospectively updated exposures, or accounted for key clinical and lifestyle factors, including alcohol use, diet, sleep, and smoking, which are essential to accurately estimate the long-term effects of physical activity on hepatic outcomes. Our study demonstrates that physical activity is not only associated with reduced liver disease progression, but also linked to reduced liver disease development. Several studies have focused on the role of physical activity in NAFLD, whereas data on other liver diseases are limited.16 Our findings suggest an increase of 10 mG/h, which equals 2,500 additional steps per day, was associated with 47% reduction in NAFLD development. These findings are in agreement with a cross-sectional study demonstrating that each additional 30 min of moderate activity per day reduced the risk of computed-tomography-measured steatosis by 38%.17 This dose-dependent negative association of physical activity with CLD in our study is also consistent with data reporting a dose-response association between physical activity and NAFLD.18 Based on our data, an additional walk of 40 min per day (e.g. change from quartile 1 to quartile 2) could decrease the risk of liver disease development by 22%.
The protective effect of physical activity was most pronounced among those prone to liver disease development or progression. In obese individuals, elderly participants or regular alcohol consumers, walking up to 12,000 steps per day (equals quartile 4) reduced the risk of liver disease development by up to 62%. Our data suggested a linear effect of physical activity on liver-related outcomes. Interestingly, this linear effect was much less pronounced in patients with DM, who appeared to benefit meaningfully only from high levels of activity. These findings are in line with a meta-analysis which found that individuals with type 2 diabetes who met the recommendation for physical activity,19 which equals our quartile 3 or 4, had improved overall health. It was often assumed that the reduction of adiposity as a result of physical activity might be the cause of its positive effects on health. Therefore, after establishing a strong connection between physical activity and risk of liver disease and progression, we studied the relation of objectively measured trunk fat, physical activity, and liver disease and found an independent association of physical activity on liver disease development. In addition, interventional studies in NAFLD or obese patients have shown that exercise has a beneficial effect independent of adiposity reduction.[20], [21], [22] Our results are in line with these data, and extend this association to liver diseases other than NAFLD.
The mechanism underlying the association between physical activity and liver disease reduction remains unclear, but may involve pathways such as increased fat oxidation, reduced chronic inflammation,23 and enhanced immune function.24,25 Exercise improves adipocyte insulin sensitivity, reducing the flow of fatty acids to the liver irrespective of BMI, which might be a mechanism that affects NAFLD development in physically active participants.26 Moreover, exercise was able to reverse endothelial dysfunction in patients with NAFLD27 and increased cardiorespiratory fitness directly correlated with reductions in intrahepatic triacylglycerol and serum liver enzymes.21 Further studies are needed to explore whether the mechanisms that were mostly established for NAFLD and physical activity are also involved in reduction of other liver diseases in physically active participants.
In the UK Biobank cohort, the diagnosis of liver disease is based on UK hospital admission codes (ICD-10). Outcomes based on ICD codes are likely to suffer from some degree of misclassification or underdiagnosis and are thereby likely only to capture the most severe events. In particular, we found the numbers of NAFLD classified participants in the UK Biobank to be quite low compared with other cohorts.28,29 Therefore, for subgroups with few outcomes (e.g. liver failure) this analysis can be underpowered and larger samples are needed to verify the associations.
A major limitation of this cohort study is its design which precludes causal relationships. Moreover, the UK Biobank is not the perfect representative example of the general population, given self-referral bias and predominantly European and Caucasian ancestry. Furthermore, participants needed to provide an e-mail address to participate in the accelerometer study and 44.8% of invited participants participated in the accelerometer study. All of these factors may lead to selection bias and healthy volunteer bias resulting in a healthier cohort with low levels of chronic disease. This is highlighted by the low frequency of events seen in this study, and therefore, the external validity of these findings needs to be confirmed in future studies. Together, these limitations might lead to an underestimation of the effect of physical activity on liver disease development in the UK Biobank. Despite this, we were able to show a robust association between physical activity and protection from liver disease and its progression. Notably, multiple well-performed studies used the same approach with the UK Biobank[30], [31], [32], [33] and saw a similar performance as other case-control studies.
An advantage of the UK Biobank cohort is its community-based setting. The accelerometry cohort contains over 500,000 person-years of data on objectively measured accelerometer-based physical activity, objective data on BMI, trunk fat, and MRI data. Additional strengths of our study include the prospective design, large sample size, and the availability of data on a wide range of potential confounders.
We controlled for a wide range of important confounders, including age, comorbidities, and reported alcohol use; however, based on the study design, residual confounding cannot be excluded entirely. There are likely other parameters that are not captured in our analyses and associated with liver-related events, for example alcohol consumption is seldom correctly reported. Moreover, individuals with a high physical activity are likely to be in a good general health condition and lead a healthy lifestyle. Therefore, further studies are needed to corroborate our claim that physical activity is more than a marker of good health.
In conclusion, our results show a clear dose-dependent protective association between accelerometer-measured physical activity, liver disease development, and its progression. We observed a pronounced inverse association between physical activity and liver disease risk in all groups, which remained highly significant even after adjusting for BMI or visceral fat. These data suggest that beneficial effects of physical activity are mediated by non-adiposity-related factors.
Given the lack of published data or clinical guidelines regarding the optimal physical activity level required to improve major hepatic outcomes, our study might help to provide a framework for patient recommendations on physical activity. Future research should aim to provide a definite causal relationship and further clarify the mechanisms underlying the observed reduction in liver disease risk associated with physical activity.
Financial support
This research has been conducted using the UK Biobank Resource under Application Number 59657. CVS is supported by Walter-Benjamin Fellowship from the German Research Foundation (SCHN 1640/1-1). KMS is supported by the German Research Foundation (DFG) consortium (SCHN 1626/1-1). IZ is supported by the National Institutes of Health T32 Training Grant (5T32DK007066-45).
Conflict of interest
None.
Authors’ contributions
Acquisition of data: CVS
Analysis and interpretation of data: CVS, KMS
Drafting of the manuscript: CVS, KMS
Critical revision of the manuscript for important intellectual content: CVS, IZ, CAT, KMS
Figures and tables: CVS, KMS
Statistical analysis: CVS, KMS
Obtained funding: CAT, KMS
Study supervision: KMS
Full access to all of the data and approved the final version of this manuscript: all authors
Responsibility for the integrity of the data and the accuracy of the data analysis: all authors
Data availability statement
Details of the rationale, design, and survey methods for UK Biobank have been described fully elsewhere, and information on data availability and access procedures is on the study website (http://www.ukbiobank.ac.uk).
Conflict of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100263.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Chin S.-H., Kahathuduwa C.N., Binks M. Physical activity and obesity: what we know and what we need to know. Obes Rev Off J Int Assoc Study Obes. 2016;17:1226–1244. doi: 10.1111/obr.12460. [DOI] [PubMed] [Google Scholar]
- 2.Hu F.B., Leitzmann M.F., Stampfer M.J., Colditz G.A., Willett W.C., Rimm E.B. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 3.Shiroma E.J., Lee I.-M. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743–752. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Gerber L., Otgonsuren M., Mishra A., Escheik C., Birerdinc A., Stepanova M. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772–781. doi: 10.1111/apt.12038. [DOI] [PubMed] [Google Scholar]
- 6.van der Windt D.J., Sud V., Zhang H., Tsung A., Huang H. The effects of physical exercise on fatty liver disease. Gene Expr. 2018;18:89–101. doi: 10.3727/105221617X15124844266408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasson A., Carlsson A.C., Önnerhag K., Hagström H. Predictive capacity for mortality and severe liver disease of the relative fat mass algorithm. Clin Gastroenterol Hepatol. 2019;17:2619–2620. doi: 10.1016/j.cgh.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Berzigotti A., Saran U., Dufour J.-F. Physical activity and liver diseases. Hepatology. 2016;63:1026–1040. doi: 10.1002/hep.28132. [DOI] [PubMed] [Google Scholar]
- 9.Román E., Torrades M.T., Nadal M.J., Cárdenas G., Nieto J.C., Vidal S. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59:1966–1975. doi: 10.1007/s10620-014-3086-6. [DOI] [PubMed] [Google Scholar]
- 10.Zenith L., Meena N., Ramadi A., Yavari M., Harvey A., Carbonneau M. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–1926. doi: 10.1016/j.cgh.2014.04.016. e2. [DOI] [PubMed] [Google Scholar]
- 11.Berzigotti A., Albillos A., Villanueva C., Genescá J., Ardevol A., Augustín S. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 12.Macías-Rodríguez R.U., Ilarraza-Lomelí H., Ruiz-Margáin A., Ponce-de-León-Rosales S., Vargas-Vorácková F., García-Flores O. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7 doi: 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn M.A., Josbeno D.A., Schmotzer A.R., Tevar A.D., DiMartini A.F., Landsittel D.P. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transplant. 2016;22:1324–1332. doi: 10.1002/lt.24506. [DOI] [PubMed] [Google Scholar]
- 14.Peng S., Plank L.D., McCall J.L., Gillanders L.K., McIlroy K., Gane E.J. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 15.Dharancy S., Lemyze M., Boleslawski E., Neviere R., Declerck N., Canva V. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86:1077–1083. doi: 10.1097/TP.0b013e318187758b. [DOI] [PubMed] [Google Scholar]
- 16.Simon T.G., Kim M.N., Luo X., Yang W., Ma Y., Chong D.Q. Physical activity compared to adiposity and risk of liver-related mortality: results from two prospective, nationwide cohorts. J Hepatol. 2020;72:1062–1069. doi: 10.1016/j.jhep.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long M.T., Pedley A., Massaro J.M., Hoffmann U., Esliger D.W., Vasan R.S. Hepatic steatosis is associated with lower levels of physical activity measured via accelerometry. Obesity (Silver Spring) 2015;23:1259–1266. doi: 10.1002/oby.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., He F., He Y., Pan X., Wu Y., Hu Z. Dose-response association between physical activity and non-alcoholic fatty liver disease: a case-control study in a Chinese population. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiel D.M., Al Sayah F., Vallance J.K., Johnson S.T., Johnson J.A. Association between physical activity and health-related quality of life in adults with type 2 diabetes. Can J Diabetes. 2017;41:58–63. doi: 10.1016/j.jcjd.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Kantartzis K., Thamer C., Peter A., Machann J., Schick F., Schraml C. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. doi: 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]
- 21.Keating S.E., Hackett D.A., George J., Johnson N.A. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Lee S., Kuk J.L., Davidson L.E., Hudson R., Kilpatrick K., Graham T.E. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol. 2005;99:1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 23.Farzanegi P., Dana A., Ebrahimpoor Z., Asadi M., Azarbayjani M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. 2019;19:994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 24.Johnson N.A., Sachinwalla T., Walton D.W., Smith K., Armstrong A., Thompson M.W. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 25.Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Zvibel I., Goldiner I. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 26.DiPietro L., Dziura J., Yeckel C.W., Neufer P.D. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–149. doi: 10.1152/japplphysiol.00474.2005. [DOI] [PubMed] [Google Scholar]
- 27.Pugh C.J.A., Spring V.S., Kemp G.J., Richardson P., Shojaee-Moradie F., Umpleby A.M. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298–H1306. doi: 10.1152/ajpheart.00306.2014. [DOI] [PubMed] [Google Scholar]
- 28.Dunn W., Xu R., Wingard D.L., Rogers C., Angulo P., Younossi Z.M. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleveland E.R., Ning H., Vos M.B., Lewis C.E., Rinella M.E., Carr J.J. Low awareness of nonalcoholic fatty liver disease in a population-based cohort sample: the CARDIA Study. J Gen Intern Med. 2019;34:2772–2778. doi: 10.1007/s11606-019-05340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty A., Jackson D., Hammerla N., Plötz T., Olivier P., Granat M.H. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tin Tin S., Reeves G.K., Key T.J. Body size and composition, physical activity and sedentary time in relation to endogenous hormones in premenopausal and postmenopausal women: findings from the UK Biobank. Int J Cancer. 2020;147:2101–2115. [Google Scholar]
- 32.Pearce M., Strain T., Kim Y., Sharp S.J., Westgate K., Wijndaele K. Estimating physical activity from self-reported behaviours in large-scale population studies using network harmonisation: findings from UK Biobank and associations with disease outcomes. Int J Behav Nutr Phys Act. 2020;17:40. doi: 10.1186/s12966-020-00937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strain T., Wijndaele K., Dempsey P.C., Sharp S.J., Pearce M., Jeon J. Wearable-device-measured physical activity and future health risk. Nat Med. 2020;26:1385–1391. doi: 10.1038/s41591-020-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vähä-Ypyä H., Vasankari T., Husu P., Mänttäri A., Vuorimaa T., Suni J. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based mean amplitude deviation (MAD) PLoS One. 2015;10 doi: 10.1371/journal.pone.0134813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vähä-Ypyä H., Husu P., Suni J., Vasankari T., Sievänen H. Reliable recognition of lying, sitting, and standing with a hip-worn accelerometer. Scand J Med Sci Sports. 2018;28:1092–1102. doi: 10.1111/sms.13017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Details of the rationale, design, and survey methods for UK Biobank have been described fully elsewhere, and information on data availability and access procedures is on the study website (http://www.ukbiobank.ac.uk).