Key Points
Question
What clinical features are associated with intravenous rehydration and hospitalization in children with acute gastroenteritis?
Findings
In this secondary analysis of 2 randomized clinical trials with 1846 children, independent variables associated with intravenous rehydration included a higher clinical dehydration score, care in the US relative to Canada, greater frequency and duration of vomiting, prior intravenous rehydration, and lack of oral ondansetron. A higher clinical dehydration score, care in the US, greater frequency of vomiting, and lack of oral ondansetron were associated with hospitalization.
Meaning
These findings suggest that oral ondansetron may support oral rehydration therapy to reduce intravenous rehydration and the hospitalization of children with gastroenteritis.
This secondary analysis of 2 randomized clinical trials investigates which variables are associated with intravenous rehydration and hospitalization in children with acute gastroenteritis.
Abstract
Importance
Despite guidelines endorsing oral rehydration therapy, intravenous fluids are commonly administered to children with acute gastroenteritis in high-income countries.
Objective
To identify factors associated with intravenous fluid administration and hospitalization in children with acute gastroenteritis.
Design, Setting, and Participants
This study is a planned secondary analysis of the Pediatric Emergency Research Canada (PERC) and Pediatric Emergency Care Applied Research Network (PECARN) probiotic trials. Participants include children aged 3 to 48 months with 3 or more watery stools in 24 hours between November 5, 2013, and April 7, 2017, for the PERC study and July 8, 2014, and June 23, 2017, for the PECARN Study. Children were from 16 pediatric emergency departments throughout Canada (6) and the US (10). Data were analyzed from November 2, 2018, to March 16, 2021.
Exposures
Sex, age, preceding health care visit, distance between home and hospital, country (US vs Canada), frequency and duration of vomiting and diarrhea, presence of fever, Clinical Dehydration Scale score, oral ondansetron followed by oral rehydration therapy, and infectious agent.
Main Outcomes and Measures
Intravenous fluid administration and hospitalization.
Results
This secondary analysis of 2 randomized clinical trials included 1846 children (mean [SD] age, 19.1 [11.4] months; 1007 boys [54.6%]), of whom 534 of 1846 (28.9%) received oral ondansetron, 240 of 1846 (13.0%) received intravenous rehydration, and 67 of 1846 (3.6%) were hospitalized. The following were independently associated with intravenous rehydration: higher Clinical Dehydration Scale score (mild to moderate vs none, odds ratio [OR], 8.73; 95% CI, 5.81-13.13; and severe vs none, OR, 34.15; 95% CI, 13.45-86.73); country (US vs Canada, OR, 6.76; 95% CI, 3.15-14.49); prior health care visit with intravenous fluids (OR, 4.55; 95% CI, 1.32-15.72); and frequency of vomiting (per 5 episodes, OR, 1.66; 95% CI, 1.39-1.99). The following were independently associated with hospitalization: higher Clinical Dehydration Scale score (mild to moderate vs none, OR, 11.10; 95% CI, 5.05-24.38; and severe vs none, OR, 23.55; 95% CI, 7.09-78.25) and country (US vs Canada, OR, 3.37; 95% CI, 1.36-8.40). Oral ondansetron was associated with reduced odds of intravenous rehydration (OR, 0.21; 95% CI, 0.13-0.32) and hospitalization (OR, 0.44; 95% CI, 0.21-0.89).
Conclusions and Relevance
Intravenous rehydration and hospitalization were associated with clinical evidence of dehydration and lack of an oral ondansetron-supported oral rehydration period. Strategies focusing on oral ondansetron administration followed by oral rehydration therapy in children with dehydration may reduce the reliance on intravenous rehydration and hospitalization.
Trial Registration
ClinicalTrials.gov Identifiers: NCT01853124 (PERC) and NCT01773967 (PECARN)
Introduction
Acute gastroenteritis (AGE) accounts for nearly 500 000 deaths in children younger than 5 years annually.1 Although AGE is generally a mild, self-limited condition in high-income countries, it accounts for almost 1.7 million emergency department (ED) visits2 and 60 000 hospitalizations annually in the US.3 Guidelines uniformly support oral rehydration therapy (ORT), reserving intravenous rehydration for children with severe dehydration.4,5,6 Unfortunately, clinical dehydration scales have variable accuracy,7 and most overestimate dehydration severity in high-income countries.8 These challenges, combined with the presence of vomiting, the need to minimize ED length of stay, and caregiver expectations,9 often lead to intravenous rehydration use.10
Intravenous rehydration has potentially deleterious effects. Children rate intravenous insertion as one of the most painful aspects of hospital care,11 influencing future reactions to painful events.12 Compared with ORT, intravenous rehydration is associated with phlebitis, longer hospital stays, and major adverse events,13,14 and is one of the risk factors most strongly associated with ED revisits, presumably because it reinforces the decision to seek ED care and reduces the educational focus on ORT.15 Although quality improvement initiatives have been able to reduce intravenous rehydration rates, in many institutions, use remains frequent.14,16 Thus, a better understanding of the factors associated with intravenous rehydration is needed to identify approaches to mitigate use.
Reducing unnecessary hospitalizations is also a priority given cost considerations. In 2010, the Agency for Healthcare Research and Quality estimated that the cost of preventable pediatric hospitalizations for AGE in the US was nearly $150 million dollars.17 Although rotavirus vaccination has reduced AGE hospitalizations by 36% globally,18 this enormous burden continues.19 Furthermore, there is considerable variation in hospitalization rates for AGE, and nonobjective measures of dehydration may be an important driver.20
To address these issues, we conducted a secondary analysis of 2 large simultaneously collected data sets. Our objective was to explore factors associated with intravenous rehydration and hospitalization in children with AGE in the US and Canada.
Methods
Design
This study was a planned secondary analysis of the Pediatric Emergency Research Canada (PERC) Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment (PROGUT) (trial protocol available in Supplement 1)21,22 and Pediatric Emergency Care Applied Research Network (PECARN) (trial protocol available in Supplement 2)23,24 randomized clinical trials of probiotics in children with AGE-associated diarrhea. Research assistants at each site obtained written informed consent from the children’s parents. Participants were enrolled between November 5, 2013, and June 23, 2017, in 1 of 16 EDs, 6 in the PERC trial and 10 in the PECARN trial. Research ethics board approval was obtained at each site. The manuscript analysis plan for the present study is available in Supplement 3. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants
Eligible children were aged 3 to 48 months and had 3 or more watery stools reported in the preceding 24 hours. Exclusion criteria were prior enrollment in the study, hematochezia, bilious emesis, chronic gastrointestinal disease, structural heart disease, indwelling vascular access line, immunotherapy or history of immunodeficiency, inability to be contacted for daily follow-up while symptomatic, supplemental probiotic use in the preceding 14 days, clinical instability (eg, hypovolemic shock), family member with an indwelling vascular access line, or being immunocompromised.22,23 The main distinctions between the 2 studies were maximal symptom duration before enrollment (72 hours in the PERC trial and 7 days in the PECARN trial) and different investigational probiotic products. In addition, the PERC trial excluded children with pancreatic dysfunction, oral or gastrointestinal surgery within the preceding 7 days, and known soy hypersensitivity. Participants lost to follow-up in the primary clinical trials were eligible for inclusion in this analysis if intravenous fluid administration status was known. The protocols for both trials recommended ORT supported by ondansetron as needed but did not specify criteria for intravenous rehydration or hospitalization.
Outcomes and Measurements
The primary outcomes, evaluated at the index visit, were (1) intravenous rehydration, defined as any crystalloid administered through a peripheral intravenous line for the purposes of rehydration; and (2) hospitalization, defined as admission to an inpatient unit outside the ED. We also collected demographic characteristics, frequency of vomiting and diarrhea in the 24 hours pre-ED visit, previous health care visit for the same illness, duration of illness, fever during the current illness (temperature ≥38.0 °C at home or in the ED, adjusted to rectal temperature by adding 1.1 °C to the axillary or 0.6 °C to the oral temperature25) or tactile temperature (PECARN), oral ondansetron, trial of ORT, intravenous fluid administration, and ED disposition. Children who received oral ondansetron within 30 minutes of ordering intravenous rehydration were not classified as having received oral ondansetron to promote ORT.
A baseline modified Vesikari Scale score was calculated based on symptoms reported at the index ED visit. The modified Vesikari Scale is a global gastroenteritis severity scale validated in our population. Scores range from 0 to 20 and are categorized as mild (0-8), moderate (9-10), or severe (≥11).22,26 The number and duration of vomiting and diarrhea episodes were categorized according to the modified Vesikari Scale score cut-points.22,26 Dehydration was assessed using the Clinical Dehydration Scale (CDS), a validated 4-item instrument with high interrater reliability (κ = 0.77).7,27 The CDS includes an assessment of general appearance, eyes (eg, sunken), mucous membranes (eg, dry), and tears. Total scores range from 0 to 8 and are grouped to correspond to dehydration severity: none (0), mild to moderate (1-4), and severe (5-8).27 The CDS scores were assigned by a trained research assistant at recruitment.
Laboratory Testing
Stool specimens were requested from all participants for enteropathogen identification. Testing was performed using the Luminex xTAG Gastrointestinal Pathogen Panel (Luminex Corp)28 that tests for viruses (adenovirus, norovirus, rotavirus), bacteria (Clostridioides difficile [formerly Clostridium] toxins A and B, enterotoxigenic Escherichia coli heat-labile toxin and heat-stable toxin, E coli O157, Salmonella, Shiga toxin-producing E coli, Shigella, Vibrio cholera, and Yersinia enterocolitica) and parasites (Cryptosporidium, Entamoeba histolytica, and Giardia). Specimens collected in the PERC study were also tested for adenovirus, astrovirus, norovirus, rotavirus, and sapovirus.29 Results were unavailable at the time of disposition.
Statistical Analysis
Demographic characteristics were combined from both trials and summarized with counts and percentages for categorical data and medians and interquartile ranges for continuous data. Bivariate analyses adjusted for site and multivariable analyses were used to explore the associations between outcome variables (ie, intravenous rehydration and hospitalization) and the following prespecified, biologically plausible covariates: sex, age in months using a priori determined groups (3 to <12 months, 12 to <24 months, 24 to <36 months, and 36 to <48 months), prior health care practitioner visit during the current illness, country, distance to hospital, infectious agent, duration of vomiting and diarrhea at the time of the index visit, frequency of vomiting and diarrheal episodes within 24 hours of the index visit, fever, ED CDS score, and oral ondansetron administration. Unadjusted bivariate and adjusted multivariable odds ratios (ORs) and 95% CIs were obtained from generalized mixed-effects logistic regression models that employed random intercepts and assumed a simple diagonal covariance structure to adjust for clinical center.
We fit additional multivariable models in order to estimate the adjusted association of infectious agents among the subset of participants from whom stool specimens were obtained. Infectious agents were categorized as negative vs isolated virus vs isolated bacteria vs virus and bacteria codetection vs parasites. In models assessing factors associated with hospitalization, the parasite category was excluded owing to an insufficient number of hospitalized participants. Children 24 months or younger in whom C difficile was detected were classified as negative given the high colonization rate and low likelihood of symptomatic causality. Finally, we fit additional multivariable models to explore the separate associations of prior health care visits with and without intravenous rehydration.
Data were analyzed using SAS/STAT software, version 9.4 (SAS Institute Inc). A type I error rate of .05 was used to reject the null hypothesis of no association. All P values were 2-tailed. Data analyses were conducted from November 2, 2018, to March 16, 2021.
Results
Participants
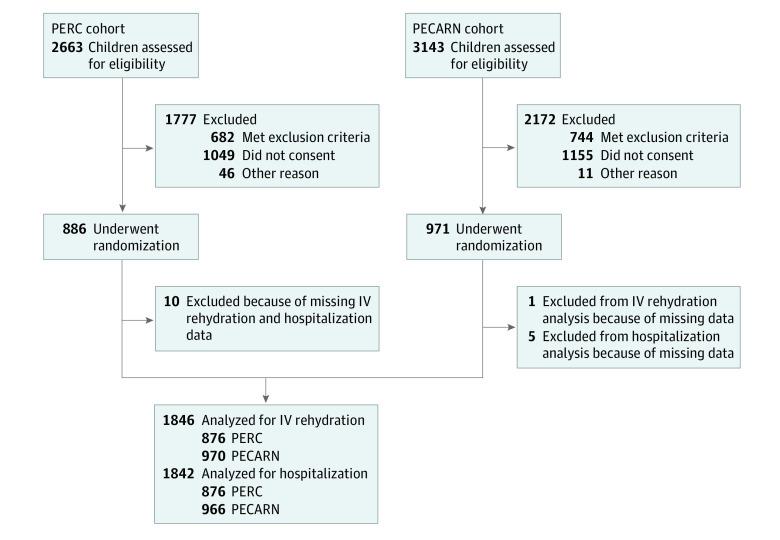
Of the 1857 participants randomized in the parent trials, we analyzed the results from 1846 children (mean [SD] age, 19.1 [11.4] months; 1007 boys [54.6%]) (Figure 1). Table 1 and Table 2 summarize demographic features. A total of 240 of 1846 participants (13.0%) received intravenous rehydration at the index ED visit, and 67 of 1846 (3.6%) were hospitalized. Oral ondansetron to promote ORT was administered to 534 of 1846 participants (28.9%): 166 of 876 (18.9%) in Canada and 368 of 970 (37.9%) in the US. When 3 or more vomiting episodes were reported, oral ondansetron was administered to 408 of 892 participants (45.7%): 141 of 428 (32.9%) in Canada and 267 of 464 (57.5%) in the US.
Figure 1. Flow Diagram of Patients Analyzed from PERC and PECARN Cohorts.
IV indicates intravenous; PECARN, Pediatric Emergency Care Applied Research Network; and PERC, Pediatric Emergency Research Canada.
Table 1. Bivariate Analysis of Variables Associated With Emergency Department Intravenous Fluid Administration.
| Variable | IV fluids during index ED visit, No./total No. (%) | Model results | ||
|---|---|---|---|---|
| No (n = 1606) | Yes (n = 240) | Unadjusted OR (95% CI)a | P valueb | |
| Sex | ||||
| Boys | 878/1007 (87.2) | 129/1007 (12.8) | 1 [Reference] | .82 |
| Girls | 727/838 (86.8) | 111/838 (13.2) | 1.03 (0.78-1.37) | |
| Age group, mo | ||||
| 3.0 to <12.0 | 548/612 (89.5) | 64/612 (10.5) | 1 [Reference] | .06 |
| 12.0 to <24.0 | 583/679 (85.9) | 96/679 (14.1) | 1.46 (1.03-2.06) | |
| 24.0 to <36.0 | 294/337 (87.2) | 43/337 (12.8) | 1.19 (0.78-1.81) | |
| 36.0 to <48.0 | 180/217 (82.9) | 37/217 (17.1) | 1.74 (1.11-2.72) | |
| ED location | ||||
| Canada | 803/876 (91.7) | 73/876 (8.3) | 1 [Reference] | .003 |
| US | 803/970 (82.8) | 167/970 (17.2) | 2.63 (1.38-5.00) | |
| Distance between home and ED, median (IQR), kmb | 8.8 (4.6-14.9) | 9.9 (4.3-21.1) | 1.11 (1.02-1.19) | .01 |
| Infectious agentc | ||||
| Negative | 607/661 (91.8) | 54/661 (8.2) | 1 [Reference] | <.001 |
| Isolated organism | ||||
| Bacteria | 91/99 (91.9) | 8/99 (8.1) | 0.92 (0.42-2.03) | |
| Virus | 666/810 (82.2) | 144/810 (17.8) | 2.81 (1.98-3.99) | |
| Virus/bacteria codetection | 26/30 (86.7) | 4/30 (13.3) | 2.42 (0.78-7.50) | |
| Parasitesd | 15/19 (78.9) | 4/19 (21.1) | 3.89 (1.18-12.81) | |
| Clinical Dehydration Scale score | ||||
| None (0) | 1062/1114 (95.3) | 52/1114 (4.7) | 1 [Reference] | <.001 |
| Mild to moderate (1-4) | 524/681 (76.9) | 157/681 (23.1) | 10.00 (6.92-14.45) | |
| Severe (5-8) | 13/40 (32.5) | 27/40 (67.5) | 69.54 (31.21-154.94) | |
| Oral ondansetron administered in the EDe | ||||
| No | 1119/1312 (85.3) | 193/1312 (14.7) | 1 [Reference] | <.001 |
| Yes | 487/534 (91.2) | 47/534 (8.8) | 0.43 (0.31-0.61) | |
| No. of diarrhea episodes in 24 h preceding index ED visit, median (IQR)b | 5.0 (4.0-8.0) | 7.0 (4.0-10.0) | 1.41 (1.24-1.61) | <.001 |
| Duration of diarrhea, h | ||||
| 1 to <96 | 1445/1648 (87.7) | 203/1648 (12.3) | 1 [Reference] | .30 |
| 96 to <120 | 76/96 (79.2) | 20/96 (20.8) | 1.49 (0.87-2.55) | |
| ≥120 | 68/83 (81.9) | 15/83 (18.1) | 1.24 (0.68-2.25) | |
| No. of vomiting episodes in 24 h preceding index ED visit, median (IQR)b | 2.0 (0.0-5.0) | 4.0 (2.0-8.0) | 1.65 (1.44-1.89) | <.001 |
| Duration of vomiting, h | ||||
| No vomiting | 417/437 (95.4) | 20/437 (4.6) | 1 [Reference] | <.001 |
| 1 to <24 | 351/405 (86.7) | 54/405 (13.3) | 2.94 (1.71-5.04) | |
| 24 to <48 | 311/367 (84.7) | 56/367 (15.3) | 3.61 (2.10-6.22) | |
| ≥48 | 403/504 (80.0) | 101/504 (20.0) | 4.79 (2.89-7.96) | |
| Preceding health care practitioner visit | ||||
| No | 1393/1556 (89.5) | 163/1556 (10.5) | 1 [Reference] | <.001 |
| Yes, but no IV rehydration | 197/261 (75.5) | 64/261 (24.5) | 2.43 (1.73-3.42) | |
| Yes, with IV rehydration | 5/16 (31.3) | 11/16 (68.8) | 15.27 (5.14-45.39) | |
| Feverf | ||||
| No | 783/861 (90.9) | 78/861 (9.1) | 1 [Reference] | <.001 |
| Yes | 820/981 (83.6) | 161/981 (16.4) | 1.86 (1.39-2.50) | |
Abbreviations: ED, emergency department; IQR, interquartile range; IV, intravenous; OR, odds ratio; PECARN, Pediatric Emergency Care Applied Research Network; PERC, Pediatric Emergency Research Canada.
ORs and CIs compare the odds of receiving IV fluids for each group vs the reference category or for an increase of 5 vomit or diarrhea episodes, or for a 10-km increase in geodetic distance between a patient’s residence and the hospital zip code. A random effect of enrolling clinical site is included in all models. Models are otherwise unadjusted.
Seven participants did not report a zip code; 6 did not report a number of vomiting or diarrhea episodes in 24 hours preceding the index ED visit.
Includes participants in whom stool testing was performed and results were available.
Parasites alone were detected in 12 participants; parasite and virus or parasite and bacterium were codetected in 7 participants.
Excluding oral ondansetron given within 30 minutes of or after ordering IV rehydration.
Fever was defined as rectal temperature ≥38.0 °C in the PERC cohort; tactile fever or temperature ≥38.0 °C adjusted to rectal temperature in the PECARN cohort during the current illness, either at home or in the ED.
Table 2. Bivariate Analysis of Index Emergency Department Variables Associated With Hospitalization.
| Variable | Admission to hospital, No./total No. (%) | Model results | ||
|---|---|---|---|---|
| No (n = 1775) | Yes (n = 67) | Unadjusted OR (95% CI)a | P valueb | |
| Sex | ||||
| Boys | 971/1005 (96.6) | 34/1005 (3.4) | 1 [Reference] | .52 |
| Girls | 804/837 (96.1) | 33/837 (3.9) | 1.17 (0.72-1.92) | |
| Age group, mo | ||||
| 3.0 to <12.0 | 583/611 (95.4) | 28/611 (4.6) | 1 [Reference] | .48 |
| 12.0 to <24.0 | 655/678 (96.6) | 23/678 (3.4) | 0.79 (0.45-1.39) | |
| 24.0 to <36.0 | 326/337 (96.7) | 11/337 (3.3) | 0.70 (0.34-1.44) | |
| 36.0 to <48.0 | 211/216 (97.7) | 5/216 (2.3) | 0.50 (0.19-1.32) | |
| ED location | ||||
| Canada | 854/876 (97.5) | 22/876 (2.5) | 1 [Reference] | .15 |
| US | 921/966 (95.3) | 45/966 (4.7) | 1.81 (0.80-4.12) | |
| Distance from home to ED, median (IQR), kmb | 8.8 (4.5-15.2) | 11.7 (5.1-31.0) | 1.23 (1.12-1.36) | <.001 |
| Infectious agentc | ||||
| Negative | 646/661 (97.7) | 15/661 (2.3) | 1 [Reference] | .004 |
| Isolated organism | ||||
| Bacteria | 97/99 (98.0) | 2/99 (2.0) | 0.88 (0.19-3.93) | |
| Virus | 765/810 (94.4) | 45/810 (5.6) | 2.89 (1.58-5.31) | |
| Virus/bacteria codetection | 29/30 (96.7) | 1/30 (3.3) | 1.89 (0.23-15.30) | |
| Parasitesd | 19/19 (100.0) | 0/19 | NA | |
| Clinical Dehydration Scale score | ||||
| None (0) | 1105/1114 (99.2) | 9/1114 (0.8) | 1 [Reference] | <.001 |
| Mild to moderate (1-4) | 633/681 (93.0) | 48/681 (7.0) | 11.84 (5.67-24.71) | |
| Severe (5-8) | 30/40 (75.0) | 10/40 (25.0) | 53.45 (19.10-149.57) | |
| Oral ondansetron administered in the EDe | ||||
| No | 1253/1308 (95.8) | 55/1308 (4.2) | 1 [Reference] | .02 |
| Yes | 522/534 (97.8) | 12/534 (2.2) | 0.47 (0.25-0.89) | |
| No. of diarrhea episodes in 24 h preceding index ED visit, median (IQR)b | 5.0 (4.0-8.0) | 8.0 (4.0-15.0) | 1.67 (1.39-2.01) | <.001 |
| Duration of diarrhea, h | ||||
| 1 to <96 | 1593/1648 (96.7) | 55/1648 (3.3) | 1 [Reference] | .15 |
| 96 to <120 | 88/96 (91.7) | 8/96 (8.3) | 2.21 (0.99-4.94) | |
| ≥120 | 79/83 (95.2) | 4/83 (4.8) | 1.28 (0.44-3.70) | |
| No. of vomiting episodes in 24 h preceding index ED visit, median (IQR)b | 2.0 (0.0-5.0) | 4.0 (1.0-8.0) | 1.50 (1.25-1.81) | <.001 |
| Duration of vomiting, h | ||||
| No vomiting | 430/437 (98.4) | 7/437 (1.6) | 1 [Reference] | .06 |
| 1 to <24 | 388/405 (95.8) | 17/405 (4.2) | 2.62 (1.07-6.43) | |
| 24 to <48 | 354/367 (96.5) | 13/367 (3.5) | 2.33 (0.91-5.99) | |
| ≥48 | 478/504 (94.8) | 26/504 (5.2) | 3.21 (1.37-7.56) | |
| Preceding health care practitioner visit | ||||
| No | 1511/1556 (97.1) | 45/1556 (2.9) | 1 [Reference] | <.001 |
| Yes, but no IV rehydration | 243/261 (93.1) | 18/261 (6.9) | 2.21 (1.24-3.94) | |
| Yes, with IV rehydration | 12/16 (75.0) | 4/16 (25.0) | 10.26 (3.11-33.86) | |
| Feverf | ||||
| No | 840/861 (97.6) | 21/861 (2.4) | 1 [Reference] | .02 |
| Yes | 935/981 (95.3) | 46/981 (4.7) | 1.93 (1.14-3.28) | |
Abbreviations: ED, emergency department; IV, intravenous; NA, not available; OR, odds ratio; PECARN, Pediatric Emergency Care Applied Research Network; PERC, Pediatric Emergency Research Canada.
ORs and CIs compare the odds of being admitted for each group vs the reference category or for an increase of 5 vomit or diarrhea episodes or for a 10-km increase in geodetic distance between a patient’s residence and the hospital zip code. A random effect of enrolling clinical site is included in all models. Models are otherwise unadjusted.
Five participants did not report a zip code; 2 did not report a number of vomiting or diarrhea episodes in 24 hours preceding the index ED visit.
Includes participants in whom stool testing was performed and results were available; patients with isolated parasites excluded from estimation of ORs due to low numbers.
Parasites alone were detected in 12 participants; parasite and virus or parasite and bacteria were codetected in 7 participants.
Excluding oral ondansetron given within 30 minutes or after ordering IV fluid.
Fever was defined as rectal temperature ≥38.0 °C in the PERC cohort; tactile fever or temperature ≥38.0 °C adjusted to rectal temperature in the PECARN cohort during the current illness, either at home or in the ED.
Variables Associated With Intravenous Rehydration
In bivariate analysis adjusted for site (Table 1), variables associated with intravenous rehydration included location of care in the US (167 of 970 [17.2%]); fever (161 of 981 [16.4%]); isolated virus (144 of 810 [17.8%]); parasites (4 of 19 [21.1%]); mild, moderate, or severe dehydration using the CDS score (mild or moderate, 157 of 681 [23.1%]; severe, 27 of 40 [67.5%]); prolonged and more frequent vomiting (≥48 hours, prolonged, 101 of 504 [20.0%]; per 5-episode increase [more frequent] OR, 1.65; 95% CI, 1.44-1.89); more frequent diarrheal episodes (unadjusted OR, 1.41; 95% CI, 1.24-1.61 per 5-episode increase); prior health care visit with intravenous rehydration (11 of 16 [68.8%]); and increased distance between home and the ED (unadjusted OR, 1.11; 95% CI, 1.02-1.19). Oral ondansetron followed by ORT was associated with lower odds of intravenous rehydration (OR, 0.43; 95% CI, 0.31-0.61).
In the multivariable model, independent variables associated with intravenous rehydration (Figure 2) were CDS scores indicative of mild to moderate (OR, 8.73; 95% CI, 5.81-13.13) and severe (OR, 34.15; 95% CI, 13.45-86.73) dehydration, care in the US (OR, 6.76; 95% CI, 3.15-14.49; relative to Canada), detection of an isolated virus (OR, 1.80; 95% CI, 1.16-2.79; relative to negative), greater number of vomiting episodes (OR, 1.66; 95% CI, 1.39-1.99 per 5-episode increase) and duration of vomiting (OR, 2.53; 95% CI, 1.39-4.61 for ≥48 hours relative to no vomiting), and prior health care visit (OR, 1.82; 95% CI, 1.20-2.77), particularly with intravenous rehydration (OR, 4.55; 95% CI, 1.32-15.72). Oral ondansetron was associated with lower odds of intravenous rehydration (OR, 0.21; 95% CI, 0.13-0.32).
Figure 2. Adjusted Odds Ratios (ORs) From Multivariable Models of Variables Associated With Intravenous (IV) Rehydration.
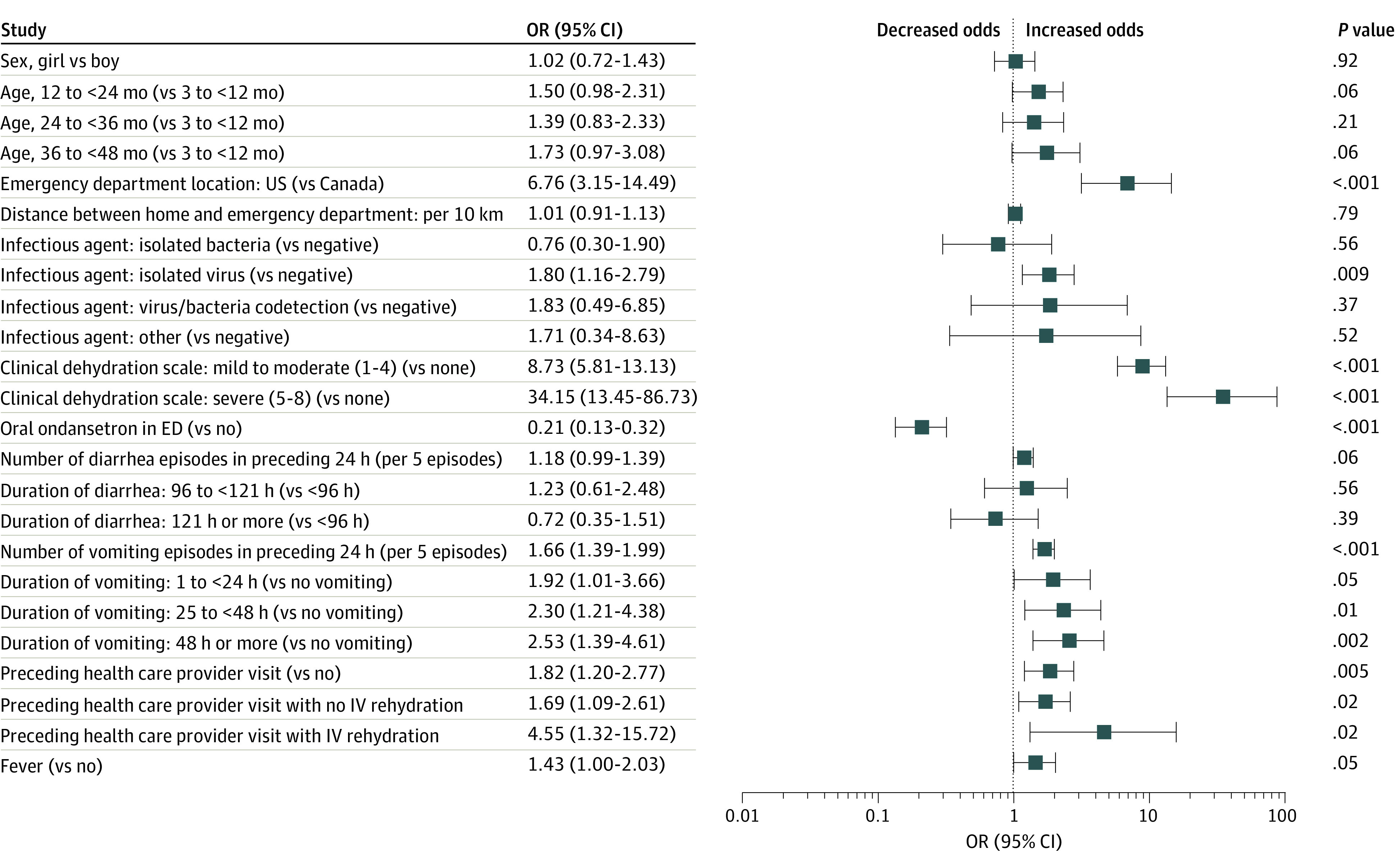
Estimates for infectious agent are from a secondary model using 1489 participants for whom stool testing results were available. The “other” category included isolated parasites in the absence of other infectious agents of interest (n = 11) and parasite or virus codetection (n = 7). Estimates for a preceding health care practitioner visit with no IV rehydration and a preceding health care practitioner visit with IV rehydration are from a secondary model using 1690 participants with information about prior health care practitioner visits. All other estimates are from a model using 1692 participants in which the largest variance inflation factor was 1.28 and intraclass correlation within sites was 0.09. ED indicates emergency department.
Variables Associated With Hospitalization
In bivariate analysis adjusted for site (Table 2), the variables associated with hospitalization included increased distance to the ED (US, OR, 1.23; 95% CI, 1.12-1.36), detection of an isolated viral enteropathogen (OR, 2.89; 95% CI, 1.58-5.31), evidence of dehydration on the CDS score (severe, OR, 53.45; 95% CI, 19.10-149.57), prolonged duration of vomiting (≥48 hours, OR, 3.21; 95% CI, 1.37-7.56), greater frequency of diarrheal (OR, 1.67 per 5-episode increase; 95% CI, 1.39-2.01) and vomiting episodes (OR, 1.50 per 5-episode increase; 95% CI, 1.25-1.81), prior health care practitioner visit (yes, with rehydration, OR, 10.26; 95% CI, 3.11-33.86), and presence of a fever (OR, 1.93; 95% CI, 1.14-3.28). Oral ondansetron followed by ORT was associated with lower odds of hospitalization (OR, 0.47; 95% CI, 0.25-0.89).
In the multivariable model, independent variables associated with hospitalization (Figure 3) included CDS scores indicative of mild to moderate (OR, 11.10; 95% CI, 5.05-24.38; P < .001) and severe (OR, 23.55; 95% CI, 7.09-78.25; P < .001) dehydration, care in the US (OR, 3.37; 95% CI, 1.36-8.40 relative to Canada; P = .009), and greater number of vomiting (OR, 1.41; 95% CI, 1.13-1.77 per 5-episode increase; P = .003) and diarrhea (OR, 1.34; 95% CI, 1.07-1.68 per 5-episode increase; P = .01) episodes. Administration of oral ondansetron was associated with a lower odds of hospitalization (OR, 0.44; 95% CI, 0.21-0.89; P = .02).
Figure 3. Adjusted Odds Ratios (ORs) From Multivariable Models of Variables Associated With Hospitalization From the Emergency Department (ED) Index Visit.
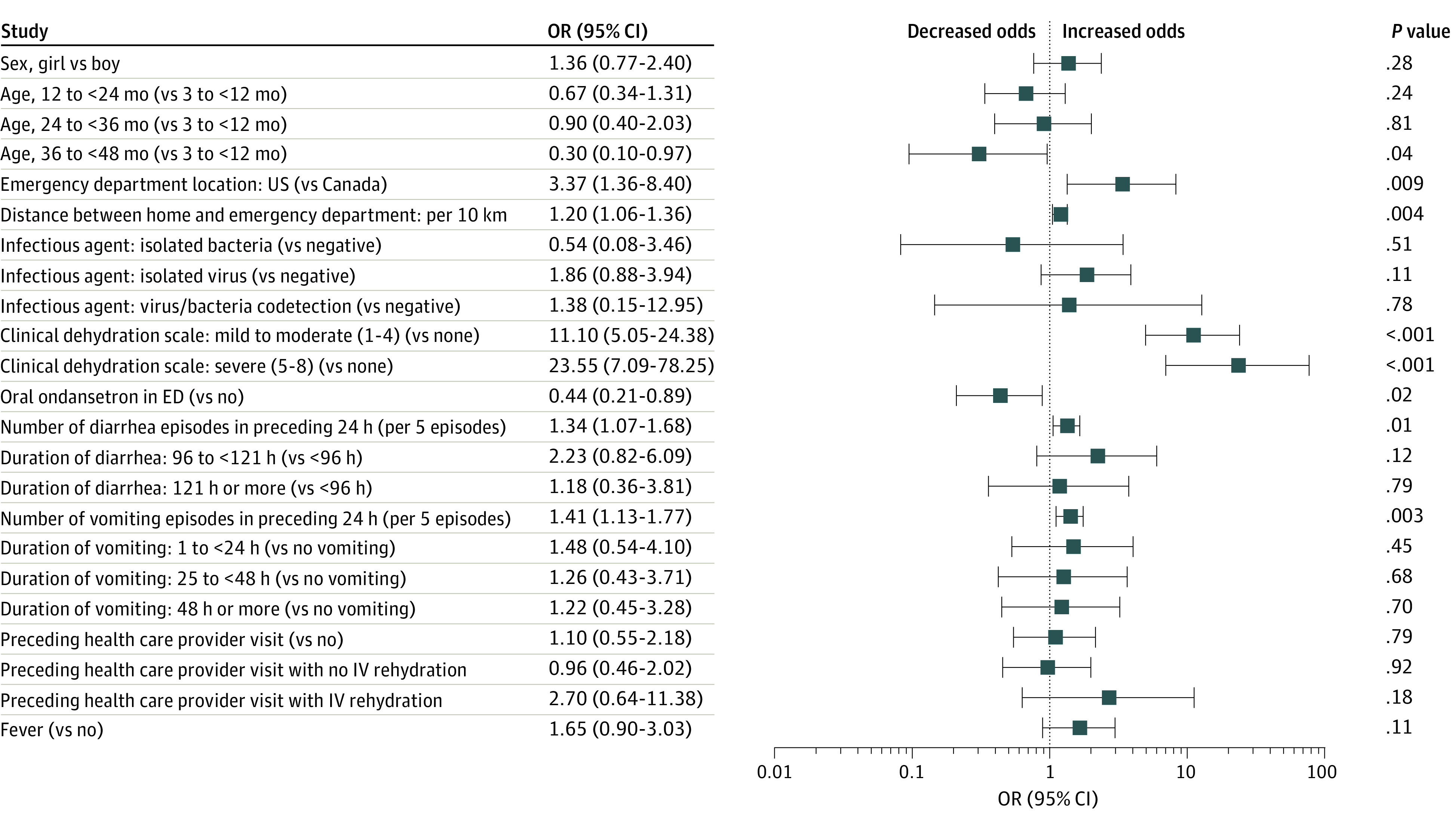
Estimates for infectious agent are from a secondary model using 1471 participants for whom stool testing results were available and excluding those with parasites. Estimates for a preceding health care practitioner visit with no intravenous (IV) rehydration and a preceding health care practitioner visit with IV rehydration are from a secondary model using 1690 participants with information about prior visits. All other estimates are from a model using 1692 participants in which the largest variance inflation factor was 1.28. Intraclass correlation within sites was 0.09.
Discussion
In this planned secondary analysis of the PERC and PECARN trials of oral probiotics in children with AGE-associated diarrhea, we identified independent variables associated with intravenous rehydration and hospitalization. Significant variables included more severe dehydration, care in the US, greater travel distance to the ED, and more vomiting episodes in the 24 hours preceding the ED visit. Oral ondansetron followed by ORT reduced the odds of receiving intravenous rehydration and hospitalization, underlying its importance in the majority of children with AGE.4,6,30 These findings can inform quality improvement initiatives to improve outcomes in at-risk children.
After adjustment for clinical characteristics, intravenous rehydration and hospitalization rates were much higher in the US. This finding is most likely explained by a previously characterized difference in willingness to initiate ORT as first-line therapy in children with moderate dehydration between emergency providers in Canada (76% willing) and the US (46% willing).31 Differences between Canada and the US, such as perception of medicolegal risks, training, hospital budgets, and parental expectations, have been highlighted as possible explanations to explain greater use of diagnostic imaging in US EDs.32 Some of these explanations may apply to intravenous rehydration and hospitalization. For example, the parents of young children in the US, when given the opportunity to make an informed decision, often opt for intravenous over ORT.9 Despite country differences, there was no association of site with outcomes. The study protocols standardized the treatment strategies and focused on the promotion of ORT, supported by ondansetron, as required. We did not explore variation in care over time, but as no major changes occurred in the recommended care of children with AGE,5 there is no reason to believe there was any variation in care over time.
The proportion of children receiving intravenous rehydration in our study was lower than reported in large retrospective studies (n = 3508 [13%] in Canada33 and n = 30 519 [26%] in the US16). Although it is encouraging to see lower overall numbers, it should be noted that a leading driver of intravenous rehydration, vomiting,34 was absent in 30% of our participants, with 51% having fewer than 3 episodes. This may have explained the low proportion of participants that received ondansetron in our study (28.9%), as ondansetron is indicated for dehydration and frequent and recent vomiting. Importantly, among participants who received intravenous rehydration, 89% were not severely dehydrated, suggesting that intravenous rehydration may be overused in pediatric EDs.
Our results are consistent with evidence that frequent vomiting is associated with intravenous rehydration.34 Although gastroenteritis severity is often characterized by the frequency and duration of diarrhea, our multivariable models showed that dehydration severity, quantified using the CDS, was more strongly associated with both intravenous rehydration and hospitalization. Although this association makes intuitive sense, clinical and laboratory assessments of dehydration in children are inaccurate.7 Cognitive bias may partly explain the strong association between higher CDS scores and intravenous rehydration. Sunken eyes and dry mucous membranes are both components of the CDS and have long been held as useful clinical signs of dehydration.35 Clinical identification of these factors may have driven intravenous rehydration, because subjective clinical measures often overestimate the degree of dehydration,36 leading to potentially unnecessary intravenous rehydration. Thus, in high-income countries, otherwise healthy children, even those with high CDS scores, in the absence of circulatory compromise should initially undergo a trial of ORT.
One approach to reduce the use of intravenous rehydration is oral ondansetron followed by a trial of ORT.37 Although clinical trials have consistently demonstrated benefit, database studies, which lack detailed clinical characteristics and timelines, have reported less positive results.38 Our study, which included timelines related to ondansetron administration, route, and timing of orders for intravenous rehydration, enabled us to ensure that oral ondansetron was administered a minimum of 30 minutes before the order for intravenous rehydration, thereby ensuring it was given and followed by ORT. This is an important concept, as one can reach incorrect conclusions when such an approach is not incorporated into analyses, because in some settings, oral ondansetron and intravenous rehydration are ordered simultaneously. Our finding associating oral ondansetron with a reduction in intravenous rehydration and hospitalization suggest that strategies promoting the appropriate use of oral ondansetron in children with AGE and nonsevere dehydration are crucial to accruing its benefits.39
Unscheduled revisit rates for children with AGE range from 7% to 18%15,38,40 and are associated with absence of a primary care provider, higher serum bicarbonate,40 greater frequency of vomiting and diarrhea,15 and administration of intravenous rehydration in the ED.15 Similarly, we found that prior ED visits, particularly those associated with intravenous rehydration, were associated with intravenous rehydration at the enrollment ED visit. These findings highlight the importance of administering intravenous rehydration based on presenting clinical features rather than previous therapies. This approach is important because caregivers of children who received intravenous rehydration are less likely to comply with ORT recommendations.41
Consistent with previous reports,38,42,43 the proportion of children with AGE admitted to the hospital was low (3.6%). Our data suggest that hospitalization was associated with more severe dehydration and care in the US. The latter association may reflect previously published differences in health care resource utilization between Canada and the US in children with AGE.31 Although we did not quantify volume of oral fluids consumed or fluid losses in the ED, a higher CDS score is independently associated with ORT failure,44 which may have influenced the decision for hospitalization. Consistent with previous reports,45,46 we found oral ondansetron to be associated with a lower odds of hospitalization, most likely through the reduction in intravenous rehydration. Thus, promoting oral ondansetron and a trial of ORT in the ED for children with nonsevere dehydration may decrease the risk of intravenous rehydration and demonstrate a strategy that caregivers can continue post–ED discharge.
Limitations
Our study has several limitations. We enrolled children presenting to tertiary care pediatric centers in high-income countries, and only a small proportion were severely dehydrated. Although baseline characteristics, such as socioeconomic status, would have helped generalize our findings, we unfortunately did not collect this information in both studies in a manner that could be integrated into a joint analysis. Therefore, our results may not be applicable to children presenting for care in rural or low-resource settings where geographic, economic, and etiologic factors may influence the need for intravenous rehydration. As this was a secondary analysis, our data may not be generalizable to patients outside the trial’s eligibility criteria. Furthermore, we were unable to ascertain the role of other potential risk factors, including insurance status, provider experience, or the ability of the child and caregiver to perform ORT. The 2 trials were conducted in countries with different populations and health care systems. An important protocol difference that could have affected results was maximal symptom duration prior to enrollment between PERC (72 hours) and PECARN (7 days). For this reason, country and duration of gastrointestinal symptoms at the index visit were included in the models to address these potential limitations. Finally, we did not describe parental expectations, a potentially influential factor in clinical decision-making. Evidence suggests that parental expectations often contradict clinical practice guidelines and reflect a preference for intravenous rehydration.9,41
Conclusions
In this study of children with AGE and minimal dehydration, independent variables associated with intravenous rehydration and hospitalization included greater dehydration, care in the US, greater travel distance to the ED, and more vomiting episodes in the 24 hours preceding the ED visit. Oral ondansetron followed by ORT was associated with a lower odds of both intravenous rehydration and hospitalization. Cost- and time-saving strategies focused on promoting successful integration of ORT and oral ondansetron into ED care for most children with AGE have the potential to reduce intravenous rehydration and hospitalizations rates.
PERC Protocol
PECARN Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405-1416. doi: 10.1016/S0140-6736(13)60222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman SB, Steiner MJ, Chan KJ. Oral ondansetron administration in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med. 2010;7(10):e1000350. doi: 10.1371/journal.pmed.1000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7-15. doi: 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases . European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-152. doi: 10.1097/MPG.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 5.King CK, Glass R, Bresee JS, Duggan C; Centers for Disease Control and Prevention . Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16. [PubMed] [Google Scholar]
- 6.van den Berg J, Berger MY. Guidelines on acute gastroenteritis in children: a critical appraisal of their quality and applicability in primary care. BMC Fam Pract. 2011;12:134. doi: 10.1186/1471-2296-12-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman SB, Vandermeer B, Milne A, Hartling L; Pediatric Emergency Research Canada Gastroenteritis Study Group . Diagnosing clinically significant dehydration in children with acute gastroenteritis using noninvasive methods: a meta-analysis. J Pediatr. 2015;166(4):908-16.e1, 6. doi: 10.1016/j.jpeds.2014.12.029 [DOI] [PubMed] [Google Scholar]
- 8.Falszewska A, Szajewska H, Dziechciarz P. Diagnostic accuracy of three clinical dehydration scales: a systematic review. Arch Dis Child. 2018;103(4):383-388. doi: 10.1136/archdischild-2017-313762 [DOI] [PubMed] [Google Scholar]
- 9.Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25(5):301-306. doi: 10.1097/PEC.0b013e3181a34144 [DOI] [PubMed] [Google Scholar]
- 10.Santosham M, Keenan EM, Tulloch J, Broun D, Glass R. Oral rehydration therapy for diarrhea: an example of reverse transfer of technology. Pediatrics. 1997;100(5):E10. doi: 10.1542/peds.100.5.e10 [DOI] [PubMed] [Google Scholar]
- 11.Cummings EA, Reid GJ, Finley AG, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain. 1996;68(1):25-31. doi: 10.1016/S0304-3959(96)03163-6 [DOI] [PubMed] [Google Scholar]
- 12.Noel M, Chambers CT, Petter M, McGrath PJ, Klein RM, Stewart SH. Pain is not over when the needle ends: a review and preliminary model of acute pain memory development in childhood. Pain Manag. 2012;2(5):487-497. doi: 10.2217/pmt.12.41 [DOI] [PubMed] [Google Scholar]
- 13.Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006;3(3):CD004390. doi: 10.1002/14651858.CD004390.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creedon JK, Eisenberg M, Monuteaux MC, Samnaliev M, Levy J. Reduction in resources and cost for gastroenteritis through implementation of dehydration pathway. Pediatrics. 2020;146(1):e20191553. doi: 10.1542/peds.2019-1553 [DOI] [PubMed] [Google Scholar]
- 15.Freedman SB, Thull-Freedman JD, Rumantir M, Atenafu EG, Stephens D. Emergency department revisits in children with gastroenteritis. J Pediatr Gastroenterol Nutr. 2013;57(5):612-618. doi: 10.1097/MPG.0b013e3182a1dd93 [DOI] [PubMed] [Google Scholar]
- 16.Rutman L, Klein EJ, Brown JC. Clinical pathway produces sustained improvement in acute gastroenteritis care. Pediatrics. 2017;140(4):e20164310. doi: 10.1542/peds.2016-4310 [DOI] [PubMed] [Google Scholar]
- 17.Torio CM, Elixhauser A, Andrews RM. Trends in potentially preventable admissions among adults and children, 2005–2010. Accessed November 3, 2020. https://www.ncbi.nlm.nih.gov/books/NBK137748/ [PubMed]
- 18.Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006-2019. J Infect Dis. 2020;222(10):1731-1739. doi: 10.1093/infdis/jiaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnochie KM, Conners GP, Lu E, Wilson C. How commonly are children hospitalized for dehydration eligible for care in alternative settings? Arch Pediatr Adolesc Med. 1999;153(12):1233-1241. doi: 10.1001/archpedi.153.12.1233 [DOI] [PubMed] [Google Scholar]
- 20.Lind CH, Hall M, Arnold DH, et al. Variation in diagnostic testing and hospitalization rates in children with acute gastroenteritis. Hosp Pediatr. 2016;6(12):714-721. doi: 10.1542/hpeds.2016-0085 [DOI] [PubMed] [Google Scholar]
- 21.Freedman SB, Williamson-Urquhart S, Farion KJ, et al. ; PERC PROGUT Trial Group . Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med. 2018;379(21):2015-2026. doi: 10.1056/NEJMoa1802597 [DOI] [PubMed] [Google Scholar]
- 22.Freedman SB, Williamson-Urquhart S, Schuh S, et al. ; Pediatric Emergency Research Canada (PERC) Gastroenteritis Study Group . Impact of emergency department probiotic treatment of pediatric gastroenteritis: study protocol for the PROGUT (Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment) randomized controlled trial. Trials. 2014;15(170):170. doi: 10.1186/1745-6215-15-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnadower D, Tarr PI, Casper TC, et al. Randomised controlled trial of Lactobacillus rhamnosus (LGG) versus placebo in children presenting to the emergency department with acute gastroenteritis: the PECARN probiotic study protocol. BMJ Open. 2017;7(9):e018115. doi: 10.1136/bmjopen-2017-018115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N Engl J Med. 2018;379(21):2002-2014. doi: 10.1056/NEJMoa1802598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alpern ER, Henretig FM. Textbook of Pediatric Emergency Medicine. 6th ed. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 26.Freedman SB, Eltorky M, Gorelick M; Pediatric Emergency Research Canada Gastroenteritis Study Group . Evaluation of a gastroenteritis severity score for use in outpatient settings. Pediatrics. 2010;125(6):e1278-e1285. doi: 10.1542/peds.2009-3270 [DOI] [PubMed] [Google Scholar]
- 27.Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201-207. doi: 10.1016/j.jpeds.2004.05.035 [DOI] [PubMed] [Google Scholar]
- 28.Wessels E, Rusman LG, van Bussel MJ, Claas EC. Added value of multiplex Luminex Gastrointestinal Pathogen Panel (xTAG® GPP) testing in the diagnosis of infectious gastroenteritis. Clin Microbiol Infect. 2014;20(3):O182-O187. doi: 10.1111/1469-0691.12364 [DOI] [PubMed] [Google Scholar]
- 29.Pang XL, Preiksaitis JK, Lee BE. Enhanced enteric virus detection in sporadic gastroenteritis using a multi-target real-time PCR panel: a one-year study. J Med Virol. 2014;86(9):1594-1601. doi: 10.1002/jmv.23851 [DOI] [PubMed] [Google Scholar]
- 30.Bhangal KK, Neen D, Dodds R. Incidence of trampoline related pediatric fractures in a large district general hospital in the United Kingdom: lessons to be learnt. Inj Prev. 2006;12(2):133-134. doi: 10.1136/ip.2005.010314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman SB, Sivabalasundaram V, Bohn V, Powell EC, Johnson DW, Boutis K. The treatment of pediatric gastroenteritis: a comparative analysis of pediatric emergency physicians’ practice patterns. Acad Emerg Med. 2011;18(1):38-45. doi: 10.1111/j.1553-2712.2010.00960.x [DOI] [PubMed] [Google Scholar]
- 32.Cohen E, Rodean J, Diong C, et al. Low-value diagnostic imaging use in the pediatric emergency department in the United States and Canada. JAMA Pediatr. 2019;e191439. doi: 10.1001/jamapediatrics.2019.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman SB, Tung C, Cho D, Rumantir M, Chan KJ. Time-series analysis of ondansetron use in pediatric gastroenteritis. J Pediatr Gastroenterol Nutr. 2012;54(3):381-386. doi: 10.1097/MPG.0b013e31822ecaac [DOI] [PubMed] [Google Scholar]
- 34.Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109(2):259-261. doi: 10.1542/peds.109.2.259 [DOI] [PubMed] [Google Scholar]
- 35.Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291(22):2746-2754. doi: 10.1001/jama.291.22.2746 [DOI] [PubMed] [Google Scholar]
- 36.Gorelick MH, Shaw KN, Baker MD. Performance of clinical signs in the diagnosis of dehydration in children. Arch Pediatr Adolesc. 1994;148(73):400. [Google Scholar]
- 37.Tomasik E, Ziółkowska E, Kołodziej M, Szajewska H. Systematic review with meta-analysis: ondansetron for vomiting in children with acute gastroenteritis. Aliment Pharmacol Ther. 2016;44(5):438-446. doi: 10.1111/apt.13728 [DOI] [PubMed] [Google Scholar]
- 38.Freedman SB, Hall M, Shah SS, et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168(4):321-329. doi: 10.1001/jamapediatrics.2013.4906 [DOI] [PubMed] [Google Scholar]
- 39.Keren R. Ondansetron for acute gastroenteritis: a failure of knowledge translation. JAMA Pediatr. 2014;168(4):308-309. doi: 10.1001/jamapediatrics.2013.5378 [DOI] [PubMed] [Google Scholar]
- 40.Freedman SB, DeGroot JM, Parkin PC. Successful discharge of children with gastroenteritis requiring intravenous rehydration. J Emerg Med. 2014;46(1):9-20. doi: 10.1016/j.jemermed.2013.04.044 [DOI] [PubMed] [Google Scholar]
- 41.Nir V, Nadir E, Schechter Y, Kline-Kremer A. Parents’ attitudes toward oral rehydration therapy in children with mild-to-moderate dehydration. ScientificWorldJournal. 2013;2013:828157. doi: 10.1155/2013/828157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendrickson MA, Zaremba J, Wey AR, Gaillard PR, Kharbanda AB. The use of a triage-based protocol for oral rehydration in a pediatric emergency department. Pediatr Emerg Care. 2018;34(4):227-232. doi: 10.1097/PEC.0000000000001070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahm A, Freedman SB, Guan J, Guttmann A. Evaluating the impact of clinical decision tools in pediatric acute gastroenteritis: a population-based cohort study. Acad Emerg Med. 2016;23(5):599-609. doi: 10.1111/acem.12915 [DOI] [PubMed] [Google Scholar]
- 44.Geurts D, Steyerberg EW, Moll H, Oostenbrink R. How to predict oral rehydration failure in children with gastroenteritis. J Pediatr Gastroenterol Nutr. 2017;65(5):503-508. doi: 10.1097/MPG.0000000000001556 [DOI] [PubMed] [Google Scholar]
- 45.Sturm JJ, Hirsh DA, Schweickert A, Massey R, Simon HK. Ondansetron use in the pediatric emergency department and effects on hospitalization and return rates: are we masking alternative diagnoses? Ann Emerg Med. 2010;55(5):415-422. doi: 10.1016/j.annemergmed.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 46.Freedman SB, Ali S, Oleszczuk M, Gouin S, Hartling L. Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries. Evid Based Child Health. 2013;8(4):1123-1137. doi: 10.1002/ebch.1932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PERC Protocol
PECARN Protocol
Statistical Analysis Plan
Data Sharing Statement