Abstract
The invasive, human-biting Asian longhorned tick, Haemaphysalis longicornis Neumann, is establishing in the United States. This tick is a threat to public health in its native range in Asia, serving as a vector of severe fever with thrombocytopenia syndrome virus and Rickettsia japonica, the agent of Japanese spotted fever. However, there is a lack of published information specifically for H. longicornis concerning the efficacy of generally recommended personal tick bite prevention measures. We, therefore, evaluated permethrin-treated clothing and formulated human skin repellent products, representing the six repellent active ingredients generally recommended for tick bite prevention by the Centers for Disease Control and Prevention (CDC), against H. longicornis nymphs from a colony established with adult ticks collected in New York state. Reluctance of H. longicornis nymphs to stay in contact with nontreated human skin precluded the use of a human skin bioassay to optimally evaluate repellency. In a Petri dish choice bioassay, all tested product formulations were highly effective with estimated repellencies ranging from 93 to 97%. In addition, we observed strong contact irritancy of a summer-weight permethrin-treated garment against H. longicornis nymphs, with 96% of introduced ticks dislodging from the vertically oriented textile within 3 min. These preliminary studies indicate that personal tick bite prevention measures currently recommended by the CDC are effective against the invasive H. longicornis. However, additional studies are needed to explore the efficacy of the evaluated products against different life stages of H. longicornis, as well as ticks collected in the field rather than reared in the laboratory.
Keywords: Haemaphysalis longicornis, repellent, permethrin-treated clothing, tick
The Asian longhorned tick, Haemaphysalis longicornis Neumann, originated in eastern Asia and later spread to Australia, New Zealand, and islands in the western Pacific (Hoogstraal et al. 1968). This invasive, human-biting tick is now establishing in the United States (Beard et al. 2018). The unexpected discovery of H. longicornis infesting a domestic sheep in Hunterdon County, NJ, in 2017 (Rainey et al. 2018) was followed by reports confirming the emergence and establishment of this species in an increasing number of states in the northeastern and south-eastern United States (Beard et al. 2018). Moreover, retrospective reexaminations of field-collected Haemaphysalis spp. specimens indicate that H. longicornis has been present, yet unrecognized in the United States since at least 2010 (Beard et al. 2018). Environmental suitability and ecological niche models based on occurrence data deduced from the native range of H. longicornis suggest that the tick has potential to continue expanding across the eastern United States and to establish along the Pacific Coast (Raghavan et al. 2019, Rochlin 2019).
As a known vector of pathogens affecting humans and domestic animals elsewhere, H. longicornis poses a yet-undetermined human and animal health threat in the United States. In Asia, H. longicornis is considered an important bridging vector of severe fever with thrombocytopenia syndrome virus (SFTSV) and the Japanese spotted fever agent, Rickettsia japonica to humans (Mahara 1997, Tabara et al. 2011, Luo et al. 2015). Moreover, a recent study from Japan indicated that saliva expelled by H. longicornis, while feeding may be involved in α−1,3-galactose (α-gal)-associated red meat allergy (Chinuki et al. 2016). Though experimental vector competence studies are still largely lacking to put the findings into context, H. longicornis has been reported to be naturally infected with various human-pathogenic Anaplasma, Borrelia, Ehrlichia, and Rickettsia bacteria in Asia (Kang et al. 2016, Liu et al. 2017, Zhuang et al. 2018). There also are concerns regarding the potential role of H. longicornis as a vector of viral pathogens in the United States, as this tick was found to harbor Powassan virus in Russia (L’vov et al. 1974); and our native tick-borne Heartland virus falls within the same family and genus (Bunyaviridae: Phlebovirus) as SFTSV (Savage et al. 2013).
In June 2018, the first recognized human bite by a H. longicornis (a nymph) in the United States was reported from Westchester County, NY, and numerous host-seeking ticks were recovered from the residential property of the tick-bite victim as well as a nearby park and trail (Wormser et al. 2020). Based on its role as a serious cattle pest and vector of disease-causing Theileria cattle parasites elsewhere (Heath 2002, 2016; Watts et al. 2016), it is not surprising that reports are emerging of heavy infestations of H. longicornis on cattle and likely involvement in transmission of Theileria orientalis Ikeda in the United States (Beard et al. 2018, Oakes et al. 2019). The parthenogenetic reproductive ability of H. longicornis, combined with its wide host range and broad habitat associations, have undoubtedly contributed to the success of its establishment and expansion in the United States (Hoogstraal et al. 1968, Beard et al. 2018, Tufts et al. 2019). These considerations, combined with the potential human health risks, highlight the need for a better understanding of personal protective and environmental tick suppression measures to successfully counter the threat H. longicornis poses in the United States. The Centers for Disease Control and Prevention (CDC) general recommendations for human tick bite prevention (CDC 2019) currently include the use of Environmental Protection Agency (EPA)-registered personal protection products, such as repellents labeled for use against ticks, permethrin sprays to treat clothing and gear, and factory-impregnated permethrin-treated clothing. Because H. longicornis was not previously a concern in the United States, this tick species was not included in the group of human-biting ticks required to be evaluated for EPA registration (EPA 2010).
There is extensive literature showing successful use of permethrin-treated uniforms or coveralls against key human-biting ticks in the United States, including Ixodes scapularis Say, Ixodes pacificus Cooley and Kohls, Amblyomma americanum (L.), Dermacentor variabilis (Say), and Dermacentor occidentalis Marx (Acari: Ixodidae) (Schreck et al. 1978, 1980, 1982, 1986; Mount and Snoddy 1983; Lane and Anderson 1984; Lane 1989). More recently, similar studies were extended to include summer-weight permethrin-treated garments available to consumers (Miller et al. 2011, Eisen et al. 2017, Prose et al. 2018, Connally et al. 2019). However, we are not aware of any published study evaluating the impact of permethrin-treated clothing on H. longicornis. Measures to prevent tick bites also include CDC-recommended, EPA-registered skin and clothing repellents containing six active ingredients: N,N-diethyl-3-methylbenzamide (DEET), picaridin, ethyl butylacetylaminopropionate (IR3535), oil of lemon eucalyptus (OLE), p-menthane-3,8-diol (PMD), and 2-undecanone (CDC 2019).
The history and efficacy of these repellents against ticks have been summarized in previous reviews (Bissinger and Roe 2010, Dolan and Panella 2011, Eisen and Dolan 2016). While the published literature includes evaluations of these skin repellents against key human-biting ticks in the United States, we are not aware of any published study on the impact of CDC-recommended active ingredients for human skin repellents against H. longicornis. Therefore, the goal of our study was to conduct a preliminary assessment of the efficiency of these personal protective measures in laboratory bioassays with H. longicornis nymphs from a laboratory colony established with ticks collected in New York state. Tick bioassays were conducted using EPA-registered over-the-counter products, formulated with active ingredients currently recommended by the CDC for personal protection against human-biting ticks (CDC 2019).
Materials and Methods
Ticks Used in Bioassays
Haemaphysalis longicornis nymphs used in bioassays were ~1 mo postmolt and from the F2 generation of a colony established with adults collected in Westchester County, NY, in August 2018, and maintained at the Medical Entomology Laboratory at the CDC in Atlanta, GA. Ixodes scapularis nymphs used in bioassays were 3–4 mo postmolt and from the F1 generation of a colony established with adults collected from multiple locations in Minnesota, and maintained at the CDC, Fort Collins, CO. The I. scapularis nymphs were included in the human skin bioassay described below to demonstrate that the volunteer was attractive to at least one tick species. All ticks used in bioassays were unfed nymphs previously unexposed to repellents or permethrin (individual ticks were used only once). Prior to use, the ticks were held in glass vials within desiccators at 22 °C, 85–95% relative humidity, with a photoperiod of 16:8 (L:D) h.
Human Skin Bioassay
The EPA-recommended human skin bioassay for evaluation of tick repellents (EPA 2010) provides a realistic method to assess the efficacy of repellent spray formulations intended for application to skin and clothing. Briefly, formulated repellent product is applied to the inner forearm of a volunteer from a marked line 3 cm above the wrist to a point near the elbow. The volunteer then places the back of the hand on a flat surface, holding the arm at an angle of 30° or more to the surface. Ticks are then placed on the wrist of the volunteer at a release point 3 cm below the marked boundary of the treatment area of the forearm. To ensure that ticks used in the trial will display the desired behavior of walking up along the forearm, they are first prescreened on a nontreated forearm. Ticks that move steadily from the release point beyond the 3-cm boundary line and upward on the nontreated forearm of the volunteer are considered ‘actively questing’ and appropriate for use in the repellency tests. Questing behavior on nontreated and treated forearms is typically recorded over a 3-min period. In treatment bioassays, ticks that cross at least 3 cm into the repellent-treated area of the forearm are considered ‘not repelled’ by the tested product.
Based on preliminary observations that the H. longicornis nymphs, despite being highly mobile and active, appeared to have limited interest in human skin; we first assessed the prescreen portion of the EPA skin bioassay on the nontreated, inner forearm of one of the authors serving as a volunteer. Alternating groups of five I. scapularis and five H. longicornis nymphs (total of 30 nymphs per species) were released and then observed over 5 min; and the distance traveled up the forearm from the release line and beyond the 3-cm boundary line was recorded for each tick. If ticks dislodged from the arm within the 5-min observation period, the total vertical distance traveled in centimeters was noted. All bioassay replicates were completed within the span of 2 h on the same day.
Horizontal Petri Dish Repellency Bioassay
This bioassay included a representative skin repellent product for each of the six active ingredients recommended by the CDC: DEET (OFF! Deep Woods Sportsmen; SC Johnson & Son, Inc., Racine, WI), picaridin (Sawyer Premium; Sawyer Products, Inc., Safety Harbor, FL), IR3535 (Coleman SkinSmart; Wisconsin Pharmacal Co, LLC, Jackson, WI), oil of lemon eucalyptus (REPEL Plant-Based Lemon Eucalyptus; WPC Brands, Inc., Bridgeton, MO), p-menthane-3,8-diol (OFF! Botanicals; SC Johnson & Son, Inc.), and 2-undecanone (Bite Blocker BioUD; Homs, LLC, Pittsboro, NC). The percentages of active ingredient varied across the products (Table 1) but were representative of the percentage most commonly used in formulated products with a given active ingredient (EPA 2019). Based on observations of the behavior of H. longicornis nymphs, we noted that they were highly active when placed on filter paper in a horizontal Petri dish and had a strong tendency to move from the center of the Petri dish toward the edges. We therefore concluded that a previously described in vitro repellency bioassay (Bissinger et al. 2009a,b; 2014) was well suited to exploit the behavior displayed by the H. longicornis nymphs in the laboratory. Briefly, in a horizontal Petri dish bioassay, ticks were introduced onto nontreated filter paper near the center of the Petri dish and given a choice to move onto nontreated filter paper versus filter paper treated (in our case) with an over-the-counter formulated tick repellent product. We conducted the bioassays in 78.5 cm2 (9.5-cm inner-diameter) glass Petri dish lids (Corning, Inc., Corning, NY) containing 2, semicircular 31.8 cm2 filter paper halves (9-cm diameter Whatman No. 1; GE Healthcare UK Ltd., Buckinghamshire, UK) joined together along the diameter. For treatment groups, one filter paper half was treated with 300 μl of formulated repellent product in a separate Petri dish and then allowed to air dry for 3 h before beginning the assay. A single container of each specific formulated repellent product was used to treat all filter papers for that product. After 3 h, treated filter paper halves were joined with nontreated filter paper halves at the center of a clean glass Petri dish. Each treated filter paper was used only for a single group of five ticks. To facilitate accurate observations of ticks in the bioassay arenas, the treated filter paper half was always on the right-hand side of the Petri dish arena. At the start of the trial, a group of five H. longicornis nymphs were introduced to the Petri dish along the margin of the treated and nontreated filter paper halves (placed on the nontreated filter paper but as near as possible to the treated filter paper). To create an arena where ticks could freely move but not escape, an O-ring (2-mm width, 81-mm inner diameter; McMaster-Carr, Cleveland, OH) was placed on top of the filter papers, and the inverted bottom of the Petri dish was used as a lid (Fig. 1). The locations of the ticks in the arenas, on nontreated versus treated filter paper, were recorded every 5 min over a 30-min period (total of six time points). For each tested repellent product, we used 10 groups of five ticks, for a total of 50 ticks and 300 time point observations per product. Side-by-side with the repellent trials, we conducted control-only trials, using two nontreated filter paper halves in the Petri dish arenas, with a total of 50 groups of five H. longicornis nymphs, for a total of 250 ticks and 1,500 total time point observations. Due to shortage of experimental ticks, the products containing the final two active ingredients (p-menthane-3,8-diol and 2-undecanone) were run together with a single side-by-side control. All bioassays were performed under fluorescent light at ~20°C and 65% relative humidity. Prior to each bioassay, ticks were removed from incubators to acclimatize to testing conditions for 30 min.
Table 1.
Over-the-counter, formulated products tested for repellency or contact irritancy against Haemaphysalis longicornis Neumann nymphs in laboratory bioassays
| Product name | Manufacturer | Active ingredient (concentration) | Formulation | EPA registration number | Lot/batch number of product |
|---|---|---|---|---|---|
| OFF! Deep Woods Sportsmen | SC Johnson & Son, Inc., Racine, WI | DEET (30%) | Aerosol | 4822-397 | P336 629374 2105 74A |
| Sawyer Premium | Sawyer Products, Inc., Safety Harbor, FL | Picaridin (20%) | Pump spray | 54287-22-58188 | Not included |
| Coleman SkinSmart | Wisconsin Pharmacal Co, LLC, Jackson, WI | IR3535 (20%) | Aerosol | 70759-4-79533 | 18103B 022490 |
| REPEL Plant-Based Lemon Eucalyptus | WPC Brands, Inc., Bridgeton, MO | Oil of Lemon Eucalyptus (30%)a | Pump spray | 305-62 | AE2188N/1046 |
| OFF! Botanicals | SC Johnson & Son, Inc., Racine, WI | p-Menthane-3,8-diolb (10%) | Pump spray | 4822-612 | W031152B |
| Bite Blocker BioUD | HOMS, LLC, Pittsboro, NC | 2-Undecanone (7.75%) | Pump spray | 82669-2 | Not included |
| Insect Shield Men’s SS Tee | Insect Shield, LLC, Greensboro, NC | Permethrin (0.52%) | Impregnated textile | 74843-2 | Not included |
Approximately 65% p-menthane-3,8-diol.
cis/trans isomer ratio: minimum 60% (+/−) cis and maximum 40% (+/−) trans.
Fig. 1.
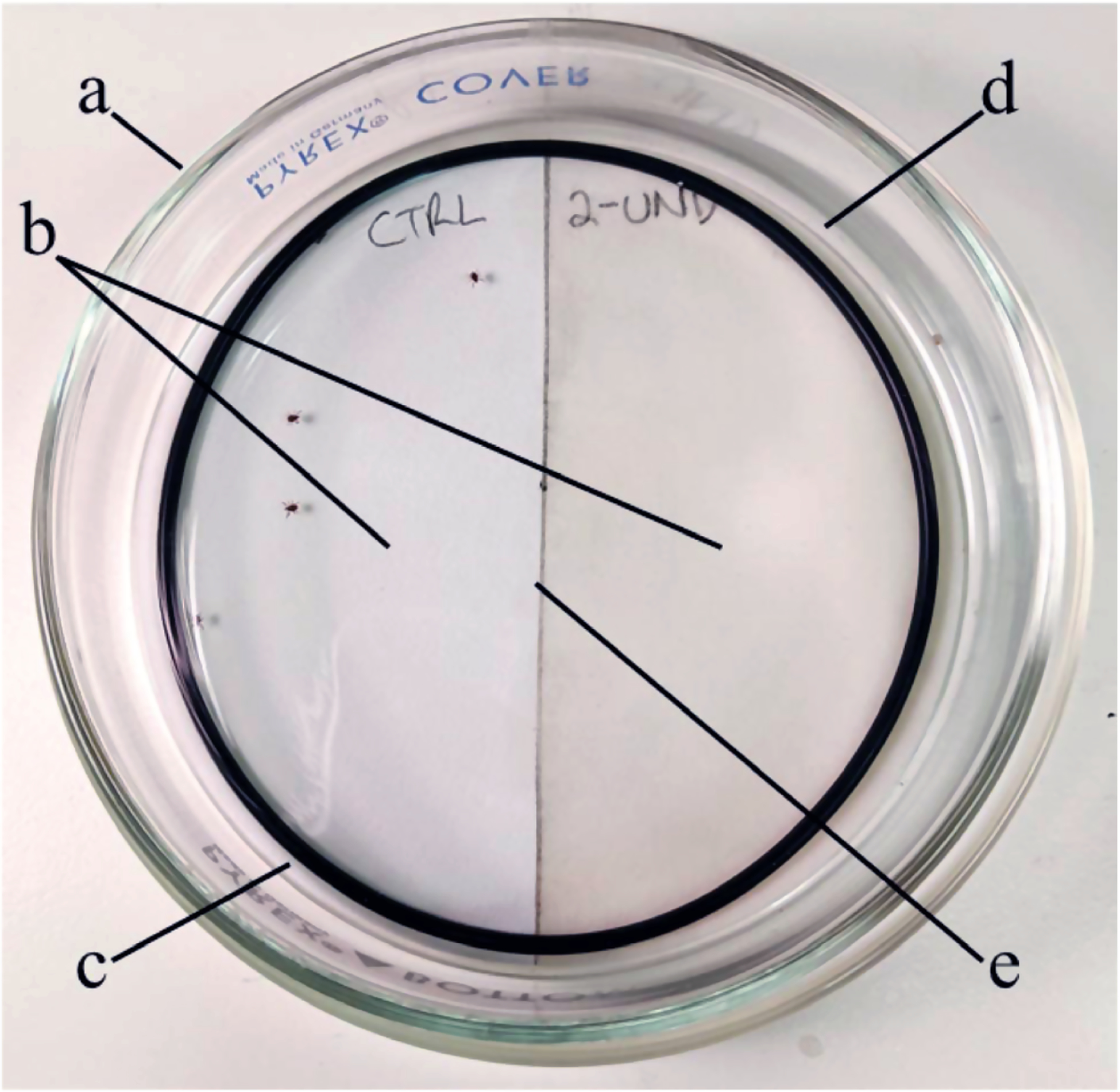
Petri dish choice bioassay arena. The arena consisted of: a) glass Petri dish base, b) two filter-paper halves (treated/control, or control/control), c) O-ring seal, d) glass Petri dish lid, and e) junction of filter-paper halves.
Vertical Contact Irritancy Bioassay
A permethrin-treated garment (0.52% permethrin [w/w]) in the form of a T-shirt (Insect Shield, LLC, Greensboro, NC) was evaluated for contact irritancy against H. longicornis nymphs according to previously described methods (Eisen et al. 2017), with modification. The purpose of the bioassay was to evaluate the behavior of ticks following their introduction to a vertically oriented permethrin-treated textile; mimicking a host-seeking tick moving from vegetation to a treated piece of clothing. Groups of five H. longicornis nymphs were placed at the center of rectangular canvas frames (20 × 25.5 cm) oriented at a 45° vertical angle and covered with textile from either the permethrin-treated T-shirt, made from 100% cotton, or a nontreated 100% cotton T-shirt control. The number of ticks remaining on the textile was recorded every minute for 5 min. We used 10 groups of five ticks for each textile type, for a total of 50 ticks per textile type. In this bioassay, contact irritancy manifested as agitated movements of ticks attempting to dislodge from the treated textile by flipping and tumbling off the bottom of the textile-covered frame. In contrast, ticks placed on a nontreated textile were expected to display normal movement and remain on the textile.
Data Analysis
To account for effects of observation time point and individual trial in the horizontal Petri dish bioassay, repellency (defined as the probability that a tick was located on the nontreated side of the Petri dish) was estimated using generalized linear mixed models assuming a binomial distribution and using a logit link function to fit the data for each product. The response was a binary variable where a success represented a tick located on the nontreated side of the Petri dish. Treatment (meaning either Petri dishes with product application in half of the dish or control-only Petri dishes) was included as a fixed effect, and a random effect was included for individual trials. Time was incorporated in two different model formulations: 1) as a fixed effect and as a random slope within individual trials and 2) as just a random slope within individual trials. Due to the large number of successes in Petri dishes with repellent product application, standard likelihood-based methods failed, and we turned to moment-based estimators as described in Perry (2017). In the vertical contact irritancy assay, tick responses to placement on permethrin-treated versus nontreated textile were compared using a one-tailed Fisher exact test for proportions and results were considered significant at α = 0.01 (adjusted for multiple observation time points using Bonferroni correction).
Results
Human Skin Bioassay
When evaluating the behavior of H. longicornis nymphs on a nontreated forearm, we found that 100% of nymphs dislodged from the arm within 1 min of being introduced just above the wrist, and 87% of the nymphs failed to move 3 cm up the forearm to where the repellency boundary line would be on a treated arm in the EPA-recommended forearm bioassay (Table 2). We, therefore, concluded that this method to assess tick repellency on human skin was not feasible for the experimental H. longicornis nymphs. In striking contrast, and as expected from their previous common use in this human skin bioassay, I. scapularis nymphs uniformly stayed in contact with the nontreated forearm over the full 5-min observation period, and more than half of the nymphs traveled >6 cm up the forearm (Table 2). Observationally, H. longicornis nymphs introduced near the wrist on nontreated skin often simply moved from the release point to the edge of the arm and then promptly dislodged from the arm. When handled, H. longicornis nymphs frequently displayed the presumably defensive behavior of contracting their legs, thereby no longer visually resembling a tick; this behavior most likely increases the likelihood of dislodging from a vertical surface.
Table 2.
Responses of Haemaphysalis longicornis Neumann and Ixodes scapularis Say nymphs when introduced onto nontreated human (forearm) skina
| Tick species | No. of ticks introduced onto the forearm at the 0 cm release mark | Maximum distance traveled up the forearm over a 5-min period | No. (%) of ticks remaining on the forearm after 1 min | No. (%) of ticks remaining on the forearm after 5 min | Location on the forearm where ticks dislodged | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) of ticks moving <3 cm up the forearmb | No. (%) of ticks moving 3–6 cm up the forearmc | No. (%) of ticks moving >6 cm up the forearmd | <3 cm up the forearm | 3–6 cm up the forearm | >6 cm up the forearm | ||||
| Haemaphysalis longicornis | 30 | 26 (86.7) | 4 (13.3) | 0 (0) | 0 (0) | N/Ae | 27 (90.0) | 3 (10.0) | 0 (0) |
| Ixodes scapularis | 30 | 7 (23.3) | 7 (23.3) | 16 (53.3) | 30 (100) | 30 (100) | N/A | N/A | N/A |
Following the protocol for the negative control (nontreated skin) in a bioassay for evaluation of tick repellents applied to human (forearm) skin as recommended by the Environmental Protection Agency (EPA 2010).
On a repellent-treated forearm, these ticks would have failed to approach the edge of the repellent-treated skin at the 3-cm mark.
On a repellent-treated forearm, these ticks would have crossed into but not passed through the 3-cm wide repellent zone.
On a repellent-treated forearm, these ticks would have crossed into and passed through the 3-cm wide repellent zone.
N/A, not applicable.
Horizontal Petri Dish Repellency Bioassay
In control-only bioassays with both filter paper halves nontreated, the overall distribution of ticks across the two halves (left/right) was slightly skewed for the early 5, 10, and 15 min time points (43–44% of ticks located on the left half) but no longer skewed for the later 20, 25, and 30 min time points (48–50% of ticks located on the left half) (Table 3). In the repellency bioassays, all six product formulations (representing CDC-recommended active ingredients for repellents to prevent tick bites) were effective against laboratory-reared H. longicornis nymphs: the percentages of ticks located on the nontreated (left) filter paper half of the arena was uniformly ≥92% across product formulations and time points, and 98–100% across time points for five of the six product formulations (Table 3). Observationally, most ticks in each bioassay completely avoided the treated filter paper portion of the arena. Often, ticks on the nontreated filter paper half probed the junction of the treated and nontreated filter paper with their front legs but ultimately remained on the nontreated filter paper.
Table 3.
Responses of Haemaphysalis longicornis Neumann nymphs to formulated repellent products in a horizontal Petri dish bioassaya
| Active ingredient of formulated product | No. of ticks usedd | Percentage of ticks located on the nontreated (left) filter paper surface | Model estimatesf | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | Percentage repellency | P-value for difference from control | ||
| None (control)b | 250 | 43 | 44 | 44 | 51 | 50 | 48 | N/Ae | N/Ae |
| DEETc | 50 | 100 | 100 | 100 | 100 | 98 | 98 | 96 | <0.0001 |
| Picaridinc | 50 | 100 | 100 | 100 | 100 | 100 | 100 | 97 | <0.0001 |
| IR3535c | 50 | 100 | 100 | 100 | 100 | 100 | 100 | 97 | <0.0001 |
| Oil of Lemon Eucalyptusc | 50 | 100 | 98 | 100 | 98 | 98 | 100 | 96 | <0.0001 |
| p-Menthane-3,8-diolc | 50 | 98 | 96 | 92 | 98 | 98 | 100 | 93 | <0.0001 |
| 2-Undecanonec | 50 | 98 | 100 | 100 | 100 | 100 | 100 | 96 | <0.0001 |
The Petri dish bioassay arena is illustrated in Fig. 1. Groups of five ticks per trial were introduced along the margin of the treated and nontreated filter paper halves (placed on the nontreated filter paper but as near as possible to the treated filter paper) and the locations of the ticks in the arena were recorded every 5 min over a 30-min period.
Both halves of the arena with nontreated filter paper.
Left half of the arena with nontreated filter paper and right half with treated filter paper.
Each product was tested in 10 trials for a total of 50 ticks per product. Cumulative control-only bioassays included 50 trials for a total of 250 ticks.
N/A, not applicable.
Based on generalized linear mixed models accounting for effects of observation time point and individual trial.
Accounting for effects of observation time point and individual trial, our model estimated repellency for each formulated product (Table 3). Since repellency did not statistically vary with observation time point (P = 0.09), we calculated marginal estimates that were averaged over observation time points and individual trials, rather than for each observation time point. Estimates of standard error incorporated variability across individual trials. Model-estimated repellency for the product formulations ranged from 93 to 97% and the statistical evaluation confirmed that H. longicornis nymphs avoided physical contact with the treated substrates for all six tested products (P < 0.0001 in all cases) when compared to the distribution of ticks in the control-only bioassays.
Vertical Contact Irritancy Bioassay
In the vertical contact irritancy bioassay, the percentage of H. longicornis nymphs remaining on the permethrin-treated textile was significantly lower than for the nontreated control textile for all five examined time points over the 5-min testing period (Fisher exact test: P < 0.001 in all cases; Table 4). After 1 min, 72% of the ticks had dislodged from the treated textile and by 3 min, 96% of the ticks had dislodged. In contrast, 84% of the ticks remained on the nontreated control textile over the full 5 min observation period. Observationally, H. longicornis nymphs encountering the permethrin-treated textile quickly displayed agitated movements by actively flipping themselves over with their legs until they dislodged from the bottom of the textile.
Table 4.
Responses of Haemaphysalis longicornis Neumann nymphs to permethrin-treated textile in a vertical contact irritancy bioassaya
| Observation time point (min) | No. (%) of ticks remaining on test textile | |
|---|---|---|
| Non-treated textile | Permethrin-treated textileb | |
| 0 (introduction) | 50 (100) | 50 (100) |
| 1 | 47 (94) | 14 (28)*** |
| 2 | 46 (92) | 9 (18)*** |
| 3 | 44 (88) | 3 (6)*** |
| 4 | 43 (86) | 2 (4)*** |
| 5 | 42 (84) | 2 (4)*** |
Groups of 5 ticks per trial were introduced onto the center of vertically (45° angle) oriented nontreated or permethrin-treated textile and the numbers still remaining in contact with the textile was recorded every 1 min over a 5-min observation period.
Statistical comparison (Fisher exact 1-tailed test) of the numbers of nymphs still in contact with permethrin-treated textile versus nontreated textile at a given observation time point; adjusted for multiple (n = 5) observation time points using Bonferroni correction: α = 0.01; P > 0.01NS; P ≤ 0.01*; P ≤ 0.005**; P ≤ 0.001***.
Discussion
The emergence of the invasive H. longicornis in the United States has raised questions regarding its potential to bite humans and transmit human pathogens, as well as whether currently recommended personal protective measures and environmental tick suppression methods will be effective against this tick. Here, we demonstrate that a suite of formulated repellent products representing the six active ingredients generally recommended against native human-biting ticks in the United States (CDC 2019) are effective against H. longicornis. Moreover, we confirm strong contact irritancy of permethrin-treated clothing for H. longicornis, with ticks actively dislodging from vertically-oriented, treated textile mimicking the use of summer-weight permethrin-treated garments. These preliminary studies were urgently needed for CDC’s evidence-based public health messaging, but additional research on personal protective measures to avoid human bites by H. longicornis is needed.
It is well understood that the protective efficacy of repellents intended for use on human skin against ticks is most appropriately evaluated in bioassays where ticks are challenged through contact with treated human skin. Initial observations of H. longicornis nymphs confined to a Petri dish revealed that we could not get them to reliably climb onto a nontreated human finger (unpublished data). The nymphs often actively moved away from the presented fingertip, and tended to curl up in a defensive, ‘seed-like’ posture when prodded to engage. This is in stark contrast to the behavior we typically see with I. scapularis nymphs, which aggressively ascend onto a nontreated fingertip whenever given the opportunity (Eisen et al. 2017). The reluctance of the H. longicornis nymphs to climb onto a nontreated fingertip precluded the use of the previously described ‘fingertip assay’ (Schreck et al. 1995) to assess repellency on a human skin substrate, as the method is based on ticks climbing onto the nontreated tip of the index finger and then moving upward along the vertically oriented finger until they encounter the repellent-treated second joint of the finger. We had hoped the forearm assay, in which ticks are introduced directly onto a nontreated human skin surface (EPA 2010), would overcome the problem. However, as shown in Table 2, not only did H. longicornis nymphs fail to display the expected behavior of moving up along the nontreated forearm of the volunteer, but all ticks actively dislodged within 1 min of being placed on the nontreated skin. In contrast, I. scapularis nymphs readily moved up the forearm of the same volunteer (Table 2). The unexpected behaviors displayed by the H. longicornis nymphs thus forced us to move to the secondary option of using an in vitro repellency bioassay.
Although our experience indicates that laboratory-reared H. longicornis nymphs are not aggressive human-biters, additional work is needed to determine whether this holds true with field-collected nymphal ticks or with other life stages, which may be more willing to stay in contact with and bite a human host. While H. longicornis is known to occasionally bite humans in its native range (Hoogstraal et al. 1968, Lee et al. 2011, Barker and Walker 2014, Yun et al. 2014, Choi et al. 2018), a global review of human-biting ticks described the species as ‘rarely reported on humans’ (Estrada-Peńa and Jongejan 1999). A few human bites by H. longicornis have been recorded in the United States (Beard et al. 2018, Wormser et al. 2020), but it is not yet clear whether this tick species will be only a sporadic human-biter or join the ranks of I. scapularis, A. americanum, and D. variabilis as a major human-biting tick across the eastern United States (Merten and Durden 2000, Goddard 2002, Rand et al. 2007, Gleim et al. 2016, Xu et al. 2016, Nieto et al. 2018, Jordan and Egizi 2019, Little et al. 2019).
Although not an optimal bioassay to evaluate the real-world performance of repellent products applied to skin, the Petri dish choice bioassay demonstrated strong repellency of all tested product formulations against H. longicornis (Table 3). These findings suggest that skin repellent products containing the six active ingredients recommended by the CDC for tick bite prevention are likely to be effective at preventing bites by H. longicornis. However, additional studies are needed to better define the impact of active ingredients and formulated repellent products on H. longicornis, including to assess the efficacy across tick life stages, concentrations of active ingredient used in different formulations, application method (aerosol, pump spray, and lotion), and time of protection postapplication. We also note that, similar to previous studies using the same Petri dish bioassay with other tick species (Bissinger et al. 2009a,b), it can take some time before the experimental ticks have moved around enough to be evenly distributed across control-only arenas: in our case the distribution of H. longicornis nymphs across the two halves of the control-only arenas remained slightly skewed over the first 15 min of the observation period but were even from 20 to 30 min. Another limitation of the Petri dish choice bioassay was that we used only a single container of each specific formulated repellent product to treat all filter papers for that product rather than using a different container of formulated repellent product for each filter paper and group of tested ticks.
Repellency for ticks exposed to a treated filter paper substrate can overestimate the repellency when compared with treated human skin for some tick species (Bissinger et al. 2014), thus, it is important to continue exploring human skin-based laboratory bioassays for repellent efficacy against H. longicornis. While human studies under field conditions would most accurately reflect repellent product performance in the real world (including realistic application and exposure to sweat, abrasion, heat, moisture, and sunlight), these studies also have the disadvantages of risk of infection with tick-borne pathogens and the spatially variable density of host-seeking ticks (Dautel et al. 2013).
In addition to the use of skin repellents, the CDC recommends permethrin treatment of clothing and outdoor gear to prevent tick bites (CDC 2019). We evaluated one representative permethrin-treated textile, in the form of a factory-impregnated T-shirt, and found the treatment to be equally effective against H. longicornis nymphs (Table 4) as was shown previously with I. scapularis and A. americanum nymphs (Eisen et al. 2017, Prose et al. 2018). Against ticks, permethrin has been shown to be a contact irritant rather than a noncontact repellent (Eisen et al. 2017). We observed H. longicornis nymphs to display a ‘hot foot’ effect very quickly following introduction onto the treated textile, and then actively dislodge by flipping and tumbling off the vertically oriented piece of textile. As with the skin repellents, additional research is needed to evaluate the impact of permethrin-treated textiles against H. longicornis. Areas of interest include the effects of: spray-on permethrin application versus factory impregnation, the use of different permethrin-treated textile types and varied washing/drying regimens, permethrin exposure to different H. longicornis life stages, and use of laboratory-reared versus field-collected ticks (Prose et al. 2018, Connally et al. 2019).
Another intriguing question is how the vigor of H. longicornis may be negatively impacted by forced contact with a permethrin-treated textile. Previous studies have shown that, following contact with permethrin-treated textile, I. scapularis loses the ability to move normally and later perishes, either before finding a host or while feeding (Miller et al. 2011, Eisen et al. 2017, Prose et al. 2018, Connally et al. 2019). Although we were severely limited in access to experimental H. longicornis larvae and nymphs, it nevertheless is worth noting that these ticks appeared to be less negatively impacted by forced contact with permethrin-treated textile for 1–5 min compared to I. scapularis (unpublished data). Further studies on the toxicity of permethrin against H. longicornis would therefore be of interest to complement our finding of strong contact irritancy.
Acknowledgments
We thank Nicole Breuner and Emma Jones of the Centers for Disease Control and Prevention for providing the I. scapularis nymphs used during our evaluations and statistical consultation, respectively. Human skin bioassays with EPA-approved tick repellent consumer products underwent ethical review by the Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, and were determined not to be subject to IRB review requirements.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of commercial products and the results of studies herein do not represent an endorsement by the authors or the Centers for Disease Control and Prevention.
References Cited
- Barker SC, and Walker AR. 2014. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 3816: 1–144. [DOI] [PubMed] [Google Scholar]
- Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, Bertone MA, et al. 2018. Multistate infestation with the exotic disease-vector tick Haemaphysalis longicornis - United States, August 2017-September 2018. MMWR. Morb. Mortal. Wkly. Rep 67: 1310–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissinger BW, and Roe RM. 2010. Tick repellents: past, present, and future. Pestic. Biochem. Physiol 96: 63–79. [Google Scholar]
- Bissinger BW, Apperson CS, Sonenshine DE, Watson DW, and Roe RM. 2009a. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp. Appl. Acarol 48: 239–250. [DOI] [PubMed] [Google Scholar]
- Bissinger BW, Zhu J, Apperson CS, Sonenshine DE, Watson DW, and Roe RM. 2009b. Comparative efficacy of BioUD to other commercially available arthropod repellents against the ticks Amblyomma americanum and Dermacentor variabilis on cotton cloth. Am. J. Trop. Med. Hyg 81: 685–690. [DOI] [PubMed] [Google Scholar]
- Bissinger BW, Schmidt JP, Owens JJ, Mitchell SM, and Kennedy MK. 2014. Activity of the plant-based repellent, TT-4302 against the ticks Amblyomma americanum, Dermacentor variabilis, Ixodes scapularis and Rhipicephalus sanguineus (Acari: Ixodidae). Exp. Appl. Acarol 62: 105–113. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2019. Preventing tick bites: https://www.cdc.gov/ticks/avoid/on_people.html. Accessed 19 November 2019.
- Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, and Morita E. 2016. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy. 71: 421–425. [DOI] [PubMed] [Google Scholar]
- Choi JY, Cho BK, Lee YB, Yu DS, Jun BC, Lee IY, and Kim JW. 2018. An uncommon presentation of human otoacariasis by Haemaphysalis longicornis. Ann. Dermatol 30: 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connally NP, Rose DA, Breuner NE, Prose R, Fleshman AC, Thompson K, Wolfe L, Broeckling CD, and Eisen L. 2019. Impact of wearing and washing/drying of permethrin-treated clothing on their contact irritancy and toxicity for nymphal Ixodes scapularis (Acari: Ixodidae) ticks. J. Med. Entomol 56: 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautel H, Dippel C, Werkhausen A, and Diller R. 2013. Efficacy testing of several Ixodes ricinus tick repellents: different results with different assays. Ticks Tick. Borne. Dis 4: 256–263. [DOI] [PubMed] [Google Scholar]
- Dolan MC, and Panella NA. 2011. A review of arthropod repellents, pp. 1–19. In Paluch GE and Coats JR (eds.), Recent developments in invertebrate repellents. American Chemical Society, Washington, DC. [Google Scholar]
- Eisen L, and Dolan MC. 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol 53: 1063–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Rose D, Prose R, Breuner NE, Dolan MC, Thompson K, and Connally N. 2017. Bioassays to evaluate non-contact spatial repellency, contact irritancy, and acute toxicity of permethrin-treated clothing against nymphal Ixodes scapularis ticks. Ticks Tick. Borne. Dis 8: 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency). 2010. Product performance test guidelines. OPPTS 810.3700: Insect repellents to be applied to human skin. United States Environmental Protection Agency, Washington, DC. [Google Scholar]
- EPA (Environmental Protection Agency). 2019. Find the repellent that is right for you (online search tool for repellent products): https://www.epa.gov/insect-repellents/find-repellent-right-you. Accessed 19 November 2019.
- Estrada-Peña A, and Jongejan F. 1999. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol 23: 685–715. [DOI] [PubMed] [Google Scholar]
- Gleim ER, Garrison LE, Vello MS, Savage MY, Lopez G, Berghaus RD, and Yabsley MJ. 2016. Factors associated with tick bites and pathogen prevalence in ticks parasitizing humans in Georgia, USA. Parasit. Vectors 9: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J 2002. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J. Agromedicine 8: 25–32. [DOI] [PubMed] [Google Scholar]
- Heath ACG 2002. Vector competence of Haemaphysalis longicornis with particular reference to blood parasites. Surveillance 29, 12–14. [Google Scholar]
- Heath A 2016. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J 64: 10–20. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H, Roberts FH, Kohls GM, and Tipton VJ. 1968. Review of Haemaphysalis (kaiseriana) Longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol 54: 1197–1213. [PubMed] [Google Scholar]
- Jordan RA, and Egizi A. 2019. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006 – 2016. PLoS One. 14: e0211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JG, Ko S, Smith WB, Kim HC, Lee IY, and Chae JS. 2016. Prevalence of Anaplasma, Bartonella and Borrelia species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci 17: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS 1989. Treatment of clothing with a permethrin spray for personal protection against the western black-legged tick, Ixodes pacificus (Acari: Ixodidae). Exp. Appl. Acarol 6: 343–352. [DOI] [PubMed] [Google Scholar]
- Lane RS, and Anderson JR. 1984. Efficacy of permethrin as a repellent and toxicant for personal protection against the Pacific Coast tick and the Pajaroello tick (Acari: Ixodidae and Argasidae). J. Med. Entomol 21: 692–702. [DOI] [PubMed] [Google Scholar]
- Lee YB, Jun JB, Kim JY, Cho BK, and Park HJ. 2011. Multiple bites from the larvae of Haemaphysalis longicornis. Arch. Dermatol 147: 1333–1334. [DOI] [PubMed] [Google Scholar]
- Little EAH, Anderson JF, Stafford KC 3rd, Eisen L, Eisen RJ, and Molaei G. 2019. Predicting spatiotemporal patterns of Lyme disease incidence from passively collected surveillance data for Borrelia burgdorferi sensu lato-infected Ixodes scapularis ticks. Ticks Tick. Borne. Dis 10: 970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Gong XY, Zheng C, Song QY, Chen T, Wang J, Zheng J, Deng HK, and Zheng KY. 2017. Molecular epidemiological survey of bacterial and parasitic pathogens in hard ticks from eastern China. Acta Trop. 167: 26–30. [DOI] [PubMed] [Google Scholar]
- Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, Xue ZF, Ma DQ, Zhang XS, Ding SJ, et al. 2015. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis 21: 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’vov DK, Leonova GN, Gromashevskii VL, Belikova NP, and Berezina LK. 1974. Isolation of the Powassan virus from Haemaphysalis neumanni Dönitz, 1905 ticks in the Maritime Territory. Vopr. Virusol 5: 538–541 [In Russian]. [PubMed] [Google Scholar]
- Mahara F 1997. Japanese spotted fever: report of 31 cases and review of the literature. Emerg. Infect. Dis 3: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten HA, and Durden LA. 2000. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol 25: 102–113. [PubMed] [Google Scholar]
- Miller NJ, Rainone EE, Dyer MC, González ML, and Mather TN. 2011. Tick bite protection with permethrin-treated summer-weight clothing. J. Med. Entomol 48: 327–333. [DOI] [PubMed] [Google Scholar]
- Mount GA, and Snoddy EL. 1983. Pressurized sprays of permethrin and deet on clothing for personal protection against the lone star tick and the American dog tick (Acari: Ixodidae). J. Econ. Entomol 76: 529–531. [DOI] [PubMed] [Google Scholar]
- Nieto NC, Porter WT, Wachara JC, Lowrey TJ, Martin L, Motyka PJ, and Salkeld DJ. 2018. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS One. 13: e0199644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes VJ, Yabsley MJ, Schwartz D, LeRoith T, Bissett C, Broaddus C, Schlater JL, Todd SM, Boes KM, Brookhart M, et al. 2019. Theileria orientalis Ikeda genotype in cattle, Virginia, USA. Emerg. Infect. Dis 25: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PO 2017. Fast moment-based estimation for hierarchical models. J. R. Statist. Soc. B 79: 267–291. [Google Scholar]
- Prose R, Breuner NE, Johnson TL, Eisen RJ, and Eisen L. 2018. Contact irritancy and toxicity of permethrin-treated clothing for Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis ticks (Acari: Ixodidae). J. Med. Entomol 55: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan RK, Barker SC, Cobos ME, Barker D, Teo EJM, Foley DH, Nakao R, Lawrence K, Heath ACG, and Peterson AT. 2019. Potential spatial distribution of the newly introduced long-horned tick, Haemaphysalis longicornis in North America. Sci. Rep 9: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey T, Occi JL, Robbins RG, and Egizi A. 2018. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol 55: 757–759. [DOI] [PubMed] [Google Scholar]
- Rand PW, Lacombe EH, Dearborn R, Cahill B, Elias S, Lubelczyk CB, Beckett GA, and Smith RP Jr. 2007. Passive surveillance in Maine, an area emergent for tick-borne diseases. J. Med. Entomol 44: 1118–1129. [DOI] [PubMed] [Google Scholar]
- Rochlin I 2019. Modeling the Asian longhorned tick (Acari: Ixodidae) suitable habitat in North America. J. Med. Entomol 56: 384–391. [DOI] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, and Nicholson WL. 2013. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg 89: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck CE, Posey K, and Smith D. 1978. Durability of permethrin as a potential clothing treatment to protect against blood-feeding arthropods. J. Econ. Entomol 71: 397–400. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Snoddy EL, and Mount GA. 1980. Permethrin and repellents as clothing impregnants for protection from the lone star tick. J. Econ. Entomol 73: 436–439. [Google Scholar]
- Schreck CE, Mount GA, and Carlson DA. 1982. Pressurized sprays of permethrin on clothing for personal protection against the lone star tick (Acari: Ixodidae). J. Econ. Entomol 75: 1059–1061. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Snoddy EL, and Spielman A. 1986. Pressurized sprays of permethrin or deet on military clothing for personal protection against Ixodes dammini (Acari: Ixodidae). J. Med. Entomol 23: 396–399. [DOI] [PubMed] [Google Scholar]
- Schreck CE, Fish D, and McGovern TP. 1995. Activity of repellents applied to skin for protection against Amblyomma americanum and Ixodes scapularis ticks (Acari: Ixodidae). J. Am. Mosq. Control Assoc 11: 136–140. [PubMed] [Google Scholar]
- Tabara K, Kawabata H, Arai S, Itagaki A, Yamauchi T, Katayama T, Fujita H, and Takada N. 2011. High incidence of rickettsiosis correlated to prevalence of Rickettsia japonica among Haemaphysalis longicornis tick. J. Vet. Med. Sci 73: 507–510. [DOI] [PubMed] [Google Scholar]
- Tufts DM, VanAcker MC, Fernandez MP, DeNicola A, Egizi A, and Diuk-Wasser MA. 2019. Distribution, host-seeking phenology, and host and habitat associations of Haemaphysalis longicornis ticks, Staten Island, New York, USA. Emerg. Infect. Dis 25: 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JG, Playford MC, and Hickey KL. 2016. Theileria orientalis: a review. N. Z. Vet. J 64: 3–9. [DOI] [PubMed] [Google Scholar]
- Wormser GP, McKenna D, Piedmonte N, Vinci V, Egizi AM, Backenson B, and Falco RC. 2020. First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis longicornis. Clin. Infect. Dis 70: 314–316. [DOI] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, and Rich SM. 2016. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis. 16: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Park C, and Han MG. 2014. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis 20: 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Du J, Cui XM, Li H, Tang F, Zhang PH, Hu JG, Tong YG, Feng ZC, and Liu W. 2018. Identification of tick-borne pathogen diversity by metagenomic analysis in Haemaphysalis longicornis from Xinyang, China. Infect. Dis. Poverty 7: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]


