Abstract
In the United States, exposure to human-biting ixodid ticks can occur while spending time on residential properties or in neighborhood green spaces as well as during recreational or occupational activities on public lands. Human-biting tick species collectively transmit >15 species of pathogenic microorganisms and the national burden of tick-borne diseases is increasing. The prospect of a new Lyme disease vaccine for use in humans provides hope for substantial reduction in the >450,000 estimated annual cases of Lyme disease but this breakthrough would not reduce cases of other tick-borne diseases, such as anaplasmosis, babesiosis, ehrlichiosis, spotted fever group rickettsiosis, and Powassan encephalitis. One intriguing question is to what extent a new Lyme disease vaccine would impact the use of personal protection measures acting broadly against tick-bites. The main tick vector for Lyme disease spirochetes in the eastern United States, Ixodes scapularis, also transmits causative agents of anaplasmosis, babesiosis, and Powassan encephalitis; and this tick species co-occurs with other human-biting vectors such as Amblyomma americanum and Dermacentor variabilis. It therefore is important that a new Lyme disease vaccine does not result in reduced use of tick-bite prevention measures, such as tick repellents, permethrin-treated clothing, and frequent tick checks. Another key issue is the continuing problem with tick exposure on residential properties, which represents a heavily used outdoor environment the residents cannot reasonably avoid and where they tend to spend large amounts of time outside. As it may not be realistic to keep up daily vigilance with personal protective measures against tick-bites on residential properties during many months of every year, homeowners may also consider the option to suppress host-seeking ticks by means of deer fencing, landscaping, vegetation management, and use of products to kill host-seeking ticks or ticks infesting rodents. When considering the full range of options for actions that can be taken to suppress host-seeking ticks on residential properties, it is clear that individual homeowners face a difficult and bewildering task in deciding what to do based on very general guidance from public health agencies (developed without the benefit of a strong evidence base) and often without ready access to local public health professionals experienced in tick control. This situation is not satisfactory but cannot be corrected without first addressing knowledge gaps regarding the impact of peridomestic tick control measures on host-seeking ticks, human tick-bites, and tick-borne diseases. In parallel with this effort, there also is a need to increase the local public health workforce with knowledge of and experience with tick control to provide better access for homeowners to sound and objective advice regarding tick control on their properties based on key characteristics of the landscaping, habitat composition, and use patterns by wild animal tick hosts as well as the residents.
Keywords: Tick, Tick-borne disease, Control, Prevention, Lyme disease vaccine
1. Background
In the United States, exposure to human-biting ixodid ticks can occur while spending time on residential properties or in neighborhood green spaces as well as during recreational or occupational activities on public lands (Falco and Fish, 1988a; Stafford, 2007; Stafford et al., 2017; Hahn et al., 2018; Mead et al., 2018; Fischhoff et al., 2019a; Jordan and Egizi, 2019). Residential properties cover small areas but represent a heavily used outdoor environment which the residents cannot reasonably avoid and where they tend to spend large amounts of time outside. In contrast, public lands cover extensive areas but represent environments where people spend less time engaging in recreational activities and can choose to avoid during parts of the year when human-biting tick species are most active. Neighborhood green spaces fall in between these two environments as they provide land with public access in the near vicinity of large numbers of residential properties, providing opportunities for daily walks or play. This decades old knowledge seemingly should have facilitated the development of effective strategies to control ticks and prevent human tick-bites and tick-borne disease. However, these efforts have not been successful as the overall burden of tick-borne diseases is still increasing in the United States (Rosenberg et al., 2018).
To make matters worse, some human-biting ixodid tick species (Amblyomma americanum, lone star tick; Amblyomma maculatum, Gulf Coast tick; and Ixodes scapularis, blacklegged tick) are expanding their ranges in the United States while other species (Dermacentor andersoni, Rocky Mountain wood tick; Dermacentor occidentalis, Pacific Coast tick; Dermacentor variabilis, American dog tick; Ixodes pacificus, western blacklegged tick; and Rhipicephalus sanguineus sensu lato (s.l.), brown dog tick) are holding steady in their historical ranges (Sonenshine, 2018; Molaei et al., 2019; Eisen and Paddock, 2020). Moreover, tick-borne diseases caused by the >15 human pathogens associated with these tick species are increasing in the United States (Rosenberg et al., 2018; Eisen and Paddock, 2020), suggesting that the frequency of human-tick encounters resulting in bites unfortunately has increased over time. Tick-borne pathogens of human concern in the United States transmitted by ixodid ticks include viral agents (Bourbon virus, Colorado tick fever virus, Heartland virus, and Powassan virus [including the deer tick virus subtype]); bacterial agents causing anaplasmosis (Anaplasma phagocytophilum), ehrlichiosis (Ehrlichia chaffeensis, Ehrlichia ewingii, Ehrlichia muris eauclairensis, and Panola Mountain Ehrlichia), Lyme disease (Borrelia burgdorferi sensu stricto [s.s.] and Borrelia mayonii), relapsing fever (Borrelia miyamotoi), spotted fever group rickettsiosis (Rickettsia parkeri and Rickettsia rickettsii), and tularemia (Francisella tularensis); and a parasite causing babesiosis (Babesia microti).
Numerous reviews and perspectives have been written about the control of human-biting tick species and prevention of tick-bites and tick-borne diseases in the United States over the years. Up to the mid-1970s, the focus was primarily on Dermacentor species, due to their roles as vectors of bacterial and viral human pathogens, and A. americanum, due to its role as a nuisance biter and potential vector of bacterial disease agents (tick control studies from this time period were later reviewed by: Mount and Haile, 1987; Haile et al., 1990; Sonenshine, 1993; Schmidtmann, 1994). From the late 1970s through the 1980s, when babesiosis and Lyme disease were emerging and Ixodes ticks were recognized as the vectors for the causative agents of these diseases, there was an increased interest in tick control and tick-bite prevention, including the use of tick repellents and permethrin-treated clothing (reviewed by: Mount and Haile, 1987; Schulze et al., 1988; Spielman, 1988; Anderson, 1989; Stafford, 1989; Haile et al., 1990; Jaenson et al., 1991; Couch and Johnson, 1992; Sonenshine, 1993). The period from the early 1990s to the early 2000s was dominated by the development and commercialization of a human Lyme disease vaccine (LYMErix), together with evaluation of personal protective measures to prevent tick-bites and development and evaluation of tick control technologies, including approaches suitable for implementation on residential properties (reviewed by: Wilson and Deblinger, 1993; Ginsberg, 1994, 2001; Piesman and Gray, 1994; Schmidtmann, 1994; Fish, 1995; Mount et al., 1997; Stafford, 1997; Golde, 1998; Schulze et al., 1998; Hayes et al., 1999; Brown and Burgess, 2001; Hu and Klempner, 2001; Poland, 2001; Spielman et al., 2001; Hayes and Schriefer, 2002; Stafford and Kitron, 2002; Wilson, 2002; Hanson and Edelman, 2003).
Following the removal of the human Lyme disease vaccine from the market in 2002, the last nearly two decades have focused on development and evaluation of: (i) natural product-based tick repellents; and (ii) technologies and products to suppress host-seeking ticks (including environmentally friendly acaricides and biological control agents), prevent ticks from feeding on key hosts, or interrupt enzootic pathogen transmission (reviewed by: Hayes and Piesman, 2003; Piesman and Gern, 2004; Ginsberg and Stafford, 2005; Ostfeld et al., 2005; Piesman, 2006a; Schulze and Jordan, 2006; Stafford, 2007; Clark and Hu, 2008; Piesman and Eisen, 2008; Fish and Childs, 2009; Bissinger and Roe, 2010; Dolan and Panella, 2011; Eisen et al., 2012; Ginsberg, 2014; Ogden et al., 2015; Eisen and Dolan, 2016; Eisen and Gray, 2016; Kugeler et al., 2016; Ginsberg et al., 2017; Kilpatrick et al., 2017; Stafford and Williams, 2017; Stafford et al., 2017; Telford, 2017; Eisen and Eisen, 2018; White and Gaff, 2018; Fischhoff et al., 2019b; Richardson et al., 2019; Rochlin et al., 2019; Eisen, 2020; R.J. Eisen and P.M. Mead, 2020; Eisen and Stafford, 2020; Rochlin and Toledo, 2020; Strnad et al., 2020). As there now is hope for a new human Lyme disease vaccine to appear in the next 5–10 yr (Comstedt et al., 2014, 2017; Gomes-Solecki et al., 2020; Nayak et al., 2020; Valneva, 2020), it is timely to reflect on how such a vaccine could be used in conjunction with other methods to prevent exposure to human-biting ticks and their associated pathogens in the United States.
Beyond a human Lyme disease vaccine, there are hard truths to face and little clarity regarding winnable battles for tick-borne diseases. Some key issues were addressed in recent perspectives: (i) the difficulty in achieving the desired goal of effective tick suppression and human tick-bite protection at low cost and with minimal personal effort, using environmentally friendly products with acceptable safety profiles for humans and pets (Eisen and Eisen, 2018; Eisen and Stafford, 2020); (ii) the lack of publicly funded and professionally staffed tick management programs, placing the burden of peridomestic tick control squarely on individual homeowners (Eisen, 2020); (iii) an inadequate industry involvement in the development and commercialization of new tick control products, outside of the pet protection market (Eisen and Stafford, 2020); and (iv) the lack of evidence for tick control approaches to not only suppress host-seeking ticks but also reduce human-tick encounters and prevent human tick-bites and tick-borne diseases (Eisen et al., 2012; Eisen and Gray, 2016; Steere et al., 2016; R.J. Eisen and P. M. Mead, 2020; Eisen and Stafford, 2020). One critical issue is the continuing challenge of designing effective and environmentally friendly interventions with acceptable cost profiles in order to achieve tick-free residential properties based on combined use of landscaping, vegetation management, and products to kill ticks infesting hosts or host-seeking ticks. Aspects of this topic have been addressed in various previous publications (Stafford, 1989; New Jersey Department of Health and Senior Services, 1999; Poland, 2001; Stafford and Kitron, 2002; Hayes and Piesman, 2003; Ward and Brown, 2004; Ginsberg and Stafford, 2005; Schulze and Jordan, 2006; Stafford, 2007; Eisen et al., 2012; Ginsberg, 2014; Ogden et al., 2015; Eisen and Eisen, 2016; Keesing and Ostfeld, 2018; Fischhoff et al., 2019b, c; Eisen and Stafford, 2020) but a comprehensive discussion of the aspirational goal to create tick-free zones on residential properties has been lacking from the recent literature. This perspective examines how a new human Lyme disease vaccine may impact the use of personal protective measures against tick-bites and how we can better control human-biting ticks in the peridomestic environment.
2. The prospect of a new Lyme disease vaccine and its potential impact on the use of personal protective measures against tick-bites
Lessons learned from the previous human Lyme disease vaccine (LYMErix), which was on the market in the United States from December 1998 to February 2002, were outlined previously (Hanson and Edelman, 2003; Shen et al., 2011; Steere and Livey, 2012). There is no doubt that a safe and effective human Lyme disease vaccine, made available to all high-risk age groups, is the only way to dramatically reduce the incidence of Lyme disease, the most common tick-borne disease in the United States, in the next 5–10 yr. The public health value of such a vaccine has been addressed in numerous publications and a human Lyme disease vaccine remains the intervention approach with the strongest evidence for potential to prevent infection with Lyme disease spirochetes (Hayes et al., 1999; Poland, 2001; Shen et al., 2011; Embers and Narasimhan, 2013; Plotkin, 2016; R.J. Eisen and P.M. Mead, 2020; Gomes-Solecki et al., 2020). However, less attention has been given to how a new human Lyme disease vaccine would fit into the overall picture of personal protective measures once the vaccine is commercially available.
The leading human Lyme disease vaccine candidate, VLA15, is a transmission-blocking OspA-based vaccine where the anti-OspA anti-bodies ingested by an infected Ixodes tick during a blood meal on a vaccinated human are intended to act on the spirochetes in the tick midgut by preventing them from completing the outer surface protein switch (from OspA to OspC) that helps the spirochetes to escape the midgut, reach the tick’s salivary glands and, following transmission via the saliva, successfully invade the human tick host (Comstedt et al., 2014, 2017; Nayak et al., 2020; Valneva, 2020). The VLA15 vaccine candidate includes OspA serotypes specific to the main causative agent of Lyme disease in the United States (Bo. burgdorferi s.s.) as well as other causative agents of Lyme borreliosis that are prevalent in Eurasia (Borrelia afzelii and Borrelia garinii). A new human Lyme disease vaccine will no doubt prevent human illness caused by Bo. burgdorferi s.s. in the United States, at a level determined by vaccine uptake in populations residing in or frequently visiting Lyme disease endemic areas together with the vaccine efficacy (should it be less than 100 % effective in preventing human infection following a bite by an infected vector tick). However, as humans are considered dead-end hosts for Lyme disease spirochetes and inconsequential blood-meal hosts for Ixodes vector tick species, it is important to remember that this vaccine will not impact the prevalence of infection with Borrelia burgdorferi sensu lato (s.l.) in vector tick populations or wild vertebrate reservoir host species. The risk for infection with Lyme disease spirochetes will therefore remain unchanged for non-vaccinated individuals. In a broader context, the human Lyme disease vaccine will have no impact on the frequency of human tick-bites or the prevalence of infection with other bacterial, viral, or parasitic disease agents in Amblyomma, Dermacentor, Haemaphysalis, Ixodes, or Rhipicephalus tick species infesting humans. Other outcomes are harder to predict with a transmission-blocking vaccine that acts primarily on the spirochetes in the tick midgut, such as the potential impact (positive, neutral, or negative) on the probability of transmission of co-infecting pathogens (Powassan virus, A. phagocytophilum, Bo. miyamotoi, or Ba. microti) by Bo. burgdorferi s. s.-infected Ixodes vector ticks feeding on vaccinated humans. Efforts to detect a broad range of human pathogens from individual ticks have revealed that a proportion (most often in the 1–10 % range) of field-collected Bo. burgdorferi s.s.-infected I. scapularis nymphs and females are infected with additional disease agents, most commonly A. phagocytophilum and Ba. microti (Nieto and Foley, 2009; Diuk-Wasser et al., 2016; Eisen and Eisen, 2018). Moreover, previous studies experimentally demonstrated simultaneous transmission of Bo. burgdorferi s.s. and A. phagocytophilum or Ba. microti by dually infected ticks (Piesman et al., 1987; Levin and Fish, 2000). A human Lyme disease vaccine blocking transmission of Bo. burgdorferi s.s. from a dually infected I. scapularis will probably not impact the transmission of a co-infecting pathogen but, due to our limited understanding of tick-pathogen interactions, this merits further study.
Perhaps the hardest outcome to predict is how access to a Lyme disease vaccine may impact the use of other personal protective measures that act more broadly against tick-bites or infection with tick-borne pathogens, such as tick repellents, permethrin-treated clothing, and regular tick checks/prompt tick removal. This unfortunately was not assessed when the LYMErix human Lyme disease vaccine was available two decades ago, or at least the results of such an assessment were not published. There is a range of different scenarios to consider. The best-case scenario is that people continue using other personal protective measures as before because they realize that bites by I. scapularis may result in infection with several other pathogens not impacted by the Lyme disease vaccine, or that they are aware of the risk of being bitten by other tick vector species carrying pathogens not impacted by the Lyme disease vaccine. In this case, a new human Lyme disease vaccine should result in a substantial reduction in Lyme disease cases without influencing case numbers for other tick-borne diseases.
The worst-case scenario is that a new Lyme disease vaccine leads to sharply decreased use of other personal protective measures, resulting in an increase in cases of other tick-borne diseases associated with bites by I. scapularis (anaplasmosis, babesiosis, Borrelia miyamotoi disease, and Powassan virus encephalitis) or other sympatric tick species, such as A. americanum (ehrlichiosis, Bourbon virus disease, and Heartland virus disease) and D. variabilis (tularemia and Rocky Mountain spotted fever) in the eastern United States; and with bites by I. pacificus (anaplasmosis and Borrelia miyamotoi disease) in the far western United States. To ensure an outcome closer to the best-case scenario, a new human Lyme disease vaccine should be accompanied by an education campaign to clarify that the risk of exposure to tick-borne disease agents other than Lyme disease spirochetes, as well as tick paralysis and allergic reactions associated with tick-bites, will remain unchanged for vaccinated individuals and that use of personal protective measures to prevent tick bites therefore are still indicated.
3. Building the evidence base for prevention of human tick-bites and tick-borne diseases
During occasional recreational activities on public lands, protection against tick-bites and tick-borne diseases could in the future be achieved through a combination of: (i) vaccination against Lyme disease; (ii) avoidance of high-risk microhabitats for tick exposure while being outside (for example, avoiding to walk along vegetated trail edges); (iii) use of personal protective measures to prevent tick-bites (applying repellents or wearing permethrin-treated clothing and checking for crawling ticks frequently when outside; removing the clothing worn outside when coming home; drying the garments at high heat to kill ticks hiding in them; and showering to increase the probability of detecting crawling ticks previously hidden under clothing); (iv) checking dogs for presence of crawling ticks when coming inside; and (v) prompt removal of ticks attached to humans (or pets) to reduce the risk of pathogen transmission. Tick-bite prevention on residential properties and in neighborhood green spaces, with daily or near daily human use, will remain more challenging because it is not feasible to avoid tick habitats in these settings and it may not be realistic to keep up daily vigilance with non-vaccine-based personal protective measures during many months of every year. In a worst-case scenario, peridomestic settings may present risk for exposure to several human-biting tick species (for example, A. americanum and I. scapularis in the Mid-Atlantic region of the United States) collectively infected with a wide array of viral, bacterial, and parasitic human pathogens (Jordan and Egizi, 2019; Eisen and Paddock, 2020).
Despite residential properties and neighborhood green spaces increasingly being recognized as important source locations for tick exposure in some parts of the United States (Falco and Fish, 1988a; Stafford, 2007; Stafford et al., 2017; Mead et al., 2018; Fischhoff et al., 2019a; Jordan and Egizi, 2019), efforts to develop and evaluate management solutions to create tick-free zones in these settings have until very recently been severely underfunded and are still too limited in the scope of tick management methods explored. Another issue is the question of who bears the responsibility for implementation of tick control extending across these landscapes. As noted previously (Eisen, 2020), a two-pronged tick management strategy is needed to account for tick management on: (i) individual properties; in the short term the sole responsibility of the homeowners; and (ii) neighborhood green spaces abutting residential areas; the responsibility of homeowner associations or local government. The most urgent issue is to find tick management solutions for the environment that is hardest to avoid and where use of personal protective measures such as repellents and permethrin-treated clothing are least likely to be used consistently when outdoors: the residential property.
Eisen and Stafford (2020) recently reviewed the options for existing tick control methods to combine in integrated tick management programs for use on single residential properties. These include: (i) fencing to prevent deer from entering the property and depositing fed immature and adult ticks; (ii) hardscaping/xeriscaping to reduce the amount of suitable tick habitat on the property; (iii) vegetation management to further reduce the portion of the property with high-quality shady and moist tick microhabitats; (iv) landscape planning, including to avoid features that provide shelter or food for deer or rodents, and to place frequently used outdoor features for human use, for example play structures and seating areas, away from high-quality tick microhabitat to reduce the risk of human-tick contact; (v) use of rodent-targeted acaricides to prevent human-biting tick species where the immatures feed on rodent pathogen reservoirs, for example I. scapularis and D. variabilis, from perpetuating enzootic pathogen transmission cycles on the property; and (vi) killing of host-seeking ticks via broadcast of conventional synthetic chemical acaricides, natural product plant-based acaricides, or entomopathogenic fungal control agents. The evidence base for these approaches to reduce the density of host-seeking ticks, and infected host-seeking ticks, on residential properties is highly variable across approaches when the methods are considered singly (strongest for broadcast of synthetic or natural acaricides and weakest for hardscaping/xeriscaping, vegetation management, and landscaping) and very limited when combining multiple approaches (reviewed by Eisen and Dolan, 2016; Stafford et al., 2017; Fischhoff et al., 2019c; Eisen and Stafford, 2020). This is explored in more detail in section 4. When instead considering the impact on human tick-bites and human tick-borne diseases, based on well-designed prospective intervention evaluations, the current evidence base is restricted to a few studies on single control methods implemented on individual residential properties to suppress host-seeking I. scapularis nymphs (barrier spraying along the lawn-woods ecotone with a synthetic chemical acaricide to kill host-seeking ticks; Hinckley et al., 2016); or use of a rodent bait-box for topical acaricide treatment to kill tick immatures on-host (Hinckley et al., 2018). None of these single approaches was found to reduce either human tick-bites or human tick-borne diseases. A few additional studies on integrated tick management approaches are underway for single or groups of residential properties, with outcome measures that include human tick-bites and human tick-borne diseases: one study combines barrier spraying of synthetic chemical acaricide along the lawn-woods ecotone with topical acaricide treatment of rodent tick hosts (N.P. Conally, 2020) and another study, the Tick Project, combines broadcast of an entomopathogenic fungal tick control agent across a larger portion of the residential property with topical acaricide treatment of rodent tick hosts (Keesing and Ostfeld, 2018). Important knowledge gaps in the evidence base for tick management to impact human health and road-blocks to progress are further addressed in sections 3.1 and 3.2.
3.1. Setting targets for tick suppression on residential properties
As noted previously (Eisen and Eisen, 2016), a target threshold for environmentally-based tick suppression that is likely to translate into reduced numbers of human tick-bites and cases of tick-borne diseases is still lacking. To be most meaningful, such a target threshold needs to be determined as a density of host-seeking ticks rather than as a percentage reduction from a highly variable baseline density of host-seeking ticks. The lack of such an a-priori tick density target for environmentally-based tick control methods to achieve also is detrimental to the process of evaluating tick management approaches in a logical and cost-effective “pipeline” manner where control methods progress, if meeting expectations for effectiveness, from less costly small-scale studies with density of host-seeking ticks as the primary outcome measure to successively larger and more costly studies with human tick-bites and human diseases as the primary outcomes. A better understanding of the linkages between the density of host-seeking ticks (and infected ticks), human tick-bites, and human disease cases will require additional extensive research including efforts that combine: (i) high-quality assessment of the density of host-seeking ticks, and infected ticks, of locally relevant human-biting species across the full range of habitats found on residential properties, and preferably also in nearby green spaces; (ii) quantification of the time people spend in these different habitats during parts of the year when ticks of different species and life stages are seeking hosts; (iii) assessment of which human behaviors present the greatest risk for contact with ticks of different species and life stages in the various habitat types; (iv) quantification of actual human-tick encounters and tick-bites with likely exposure on residential properties or nearby green spaces, including information on tick species and life stages as well as infection of recovered ticks with disease agents; and (v) documentation of human infection with tick-borne disease agents.
There is no doubt that a residential property without ticks present poses no risk for human-tick encounters and, using I. scapularis nymphs as an example, it is reasonable to assume that the likelihood of human-tick encounters is greater on a residential property with a density of 10 nymphs per 100 m2 compared to a property with a density of 0.1 nymphs per 100 m2 in the same habitat type. However, to define a target threshold value for tick suppression on residential properties, empirical data are needed to clarify the shapes of potential linear or non-linear relationships between the density of host-seeking ticks (and infected ticks) on residential properties, the frequency of recognized tick-encounters and tick-bites per resident and unit time assumed to have resulted specifically from exposure on the residential property, and the occurrence of tick-borne disease cases assumed to have been acquired via bites by infected ticks encountered on the residential property (Ginsberg, 1993; Campbell et al., 1998; Poland, 2001; Eisen et al., 2012; Eisen and Eisen, 2016). Moreover, considering that multiple human-biting tick species need to be taken into account and that some of these species have extensive geographical ranges encompassing variable ecological conditions, determining a target tick density threshold for control efforts to achieve in order to have a high probability of reducing tick-bites and tick-borne disease cases in most settings will not be an easy task.
Methods to estimate the density of host-seeking ticks were reviewed previously (Eisen and Eisen, 2016) but it is important to note here that the commonly used drag/flag sampling technique is a crude method where each individual pass of the drag/flag makes contact with only a small percentage of the host-seeking ticks present in the sampled area (<10 % for Ixodes spp. nymphs; Daniels et al., 2000; Tälleklint-Eisen and Lane, 2000). Moreover, the efficiency of drag/flag sampling is influenced by weather conditions not only on the sampling date but also for preceding days because this impacts the need for ticks to seek out protected microhabitats to rehydrate and thus also likely influences the proportion of the ticks present in the sampled area that are positioned to be able to contact the drag/flag as it moves across the substrate (Vail and Smith, 1998; Schulze et al., 2009). Two drag/flag sampling occasions per included area (e.g., residential property) during the peak activity period for each of the targeted life stages of a given tick species is considered a minimum acceptable effort to estimate the density of host-seeking ticks (CDC (Centers for Disease Control and Prevention), 2018, 2020a) but the robustness of data across sampling sites can be increased with additional sampling occasions (Dobson, 2013) or by including a large number of sampling sites. It also should be noted that the local density of host-seeking ticks is influenced by the local abundance of tick hosts as they continually encounter and thus remove ticks from the host-seeking population. Additional research is needed to clarify the requirements for tick sampling schemes capable of producing high-quality data for density of host-seeking ticks on residential properties.
3.2. Implementation challenges and study design considerations
Based on the available evidence, albeit limited, it seems reasonable to assume that a robust solution for tick suppression on residential properties will need to address all tick habitats present on the property that are used by the residents as well as require the use of a combination of approaches that each contribute to some extent to suppress host-seeking ticks or reduce the likelihood of human-tick contact. This presents two different problems: (i) the cost of an integrated tick management solution and the acceptability of all included methods and products (Eisen and Stafford, 2020); and (ii) the need for evidence of impact minimally on human tick-bites and ideally also on human tick-borne infections (Hinckley et al., 2016; Keesing and Ostfeld, 2018). The first issue refers to the well-documented problems of low willingness of homeowners to pay for tick control on residential properties (in most cases no more than $100 per year; Gould et al., 2008; Niesobecki et al., 2021), limited acceptability for broadcast of tick control products based on synthetical chemicals, and low use of some more environmentally friendly products (such as biological control agents to kill host-seeking ticks and rodent-targeted acaricides), all of which contribute to homeowners not taking action to suppress ticks on their properties (Gould et al., 2008; Connally et al., 2009; Hook et al., 2015; Niesobecki et al., 2019, 2021; Jordan and Schulze, 2020a). Of these, low willingness of homeowners to pay for tick control on residential properties is the most concerning because it, metaphorically speaking, represents a brick wall rather than a stumbling block: there is no way around the fact that only so much can be done for $100 per year to suppress ticks on an individual residential property.
The second issue refers to the optimal design of integrated tick management evaluations that include outcome measures for human-tick encounters/human tick-bites and tick-borne infections. There is no question that the best approach is a randomized study design (based on individual properties or clusters of properties, as appropriate based on the spatial reach of the intervention) including study arms representing each of the individual methods used in the integrated approach together with study arms for each of the possible combinations of these methods and at least one control arm (for example with placebo versions of all included intervention methods). Such a cluster randomized design is used in the previously mentioned Tick Project (Keesing and Ostfeld, 2018), which includes four study arms: (i) broadcast of an entomopathogenic fungal tick control agent combined with deployment of placebo rodent bait boxes without acaricide; (ii) deployment of bait boxes for topical acaricide treatment of rodent tick hosts combined with placebo broadcast of water without the fungal control agent; (iii) broadcast of the entomopathogenic fungal tick control agent combined with deployment of bait boxes for topical acaricide treatment of rodent tick hosts; and (iv) a control arm with placebo versions of both methods. As noted previously (Wilson et al., 2015; Hinckley et al., 2016; Keesing and Ostfeld, 2018), it also is desirable to double-blind a vector control intervention to both the participating homeowners and the research team members that collect ticks in the field or tick-related information via human surveys. However, this ideal scenario can be compromised when considering integrated approaches that include methods that are not possible to blind from the homeowners (for example, deer fencing, landscaping solutions, or vegetation management) or that combine more than two tick management methods, thus increasing the number of study arms needed to represent all possible combinations of individual intervention methods in treatment arms from three in the example above with two intervention methods to seven when combining three intervention methods and 15 for four intervention methods. This becomes problematic when including outcome measures, such as human-tick encounters/human tick-bites and, especially, human tick-borne infections, that require large sample sizes for participating residential properties or clusters of properties in order to achieve adequate statistical power to detect an impact of the interventions under study related specifically to the proportion of tick exposures expected to occur within rather than outside of the confines of the treatment area (the entire residential property or a portion of the property depending on the intervention method). Hinckley et al. (2016) included roughly 1350 residential properties in each of the single treatment and control arms over a 2-yr study period (total of approximately 2700 enrolled residential properties and 5400 examined “property-years”) when evaluating the potential for barrier spraying of acaricide on residential properties to reduce human tick-encounters and tick-borne diseases; and the Tick Project (Keesing and Ostfeld, 2018) included six clusters, each representing 25–40 residential properties, for each of the four study arms over a 4-yr study period (total of >750 residential properties enrolled and >3000 examined “property-years”) to assess the same outcome measures. Even with the lower volume of residential properties included in the Tick Project (roughly 200 per study arm), an ideal design to evaluate an integrated approach combining three different tick management methods would require enrolling a total of 1600 residential properties (200 per arm × 8 study arms including a single control arm) and for four different tick management methods this number rises to 3200 residential properties (200 per arm × 16 study arms including a single control arm). Moreover, depending on the included intervention methods, the studies can have minimal evaluation durations ranging from 1 to 5 yr. Studies of this magnitude are logistically very challenging as well as potentially very costly (likely in the 5–15 million U.S. dollar range depending on the cost of the intervention methods and the number of years required to assess the impact of the intervention).
One option to reduce the cost of an evaluation of an integrated tick management approach - while still retaining the ultimately most informative outcome measure, human tick-borne infections - is to relax the requirement to include treatment arms for each included individual method and all possible combinations of methods in the evaluation scheme, thus reducing the study to a single (integrated) treatment arm and a control arm (Drexler et al., 2014). Should this cost-effective evaluation strategy prove successful, then there is an evidence base for operational use of the integrated tick management approach in question, which would be a major step forward as such evidence is currently lacking for all tick-borne human diseases in the United States with the notable exception of Rocky Mountain spotted fever associated with R. sanguineus s.l. (Drexler et al., 2014). The main drawback of the approach is loss of information for which specific individual methods (or combinations of subsets of methods) contribute most strongly to reduction in human infections, should this occur. Lack of such information precludes the possibility of scaling back the number of included methods by removing those that contribute minimally to reduction in human infections, and thus reducing the overall cost of the intervention without loss of public health impact. One distinct benefit of the approach is that if an integrated tick management intervention combining multiple methods fails to reduce human infections, there is no reason to believe that any of the individual methods or combinations of fewer methods, as applied in the intervention trial, will have an impact on these outcome measures. Stated differently, should an integrated intervention fail to reduce tick-borne infections despite achieving the expected reduction in the density of host-seeking vector ticks, then both time and cost will have been saved in the overall effort to improve the evidence base for whether or not integrated tick management interventions, as well as the individual included methods or subsets of these methods, can reduce tick-borne diseases. With the notable exception of an insufficient level of deer reduction (Williams et al., 2018), there is no biological rationale for one individual tick control method to increase, rather than decrease, the density of host-seeking ticks more than temporarily.
3.3. Caveats to interpretation of negative study outcomes
A caveat to large-scale and costly intervention evaluations is that negative results may need to be interpreted cautiously unless the tick management intervention strategy under consideration has been thoroughly evaluated in a series of preceding smaller scale studies to ensure a high likelihood that it will perform as expected for the basic acarological outcome measures in the larger scale study. Otherwise, there is a risk of making broad generalizations about the value of a specific tick management method without being able to fully consider why the intervention failed and what adjustments to the implementation scheme may have changed the outcome. Unfortunately, resources have not always been available for a sufficient set of smaller scale studies to ensure a robust performance in a scaled-up study. One recent example is a tick control product in the form of a rodent-targeted topical acaricide which failed to achieve the expected reduction in density of host-seeking I. scapularis nymphs established in a previous smaller scale study (Schulze et al., 2017) when subsequently evaluated in a larger scale study (Hinckley et al., 2018). Rather than this result necessarily invalidating the intervention strategy, the problem may in this case lie with the details of the implementation scheme or the tick host communities in the respective study areas. Teasing out the specific cause(s) of a failure may require access to a broad range of quality control and ecological data, in this case the level of infestation of rodents with I. scapularis larvae (to ensure that the expected reduction in tick infestation of the targeted rodent species was achieved) and information on larval blood meal hosts for the host-seeking nymphs collected (to assess the contribution to larval feeding by host species expected to be impacted by the intervention versus host species that are not expected to be impacted).
A different type of problem is illustrated by that the intervention study by Hinckley et al. (2016) on broadcast application of a synthetic pyrethroid is sometimes referenced by others in the context of the intervention reducing density of host-seeking ticks on residential properties by more than half without having any impact on human tick-bites of the residents. This over-simplified characterization of the study misses the important point that, in part to minimize pesticide use, the broadcast application was narrowly restricted to the woods-lawn ecotone, where human-tick encounters were thought to commonly occur, and the remaining portions of the residential properties, including wooded and brushy/shrubby habitat as well as lawn and ornamental plantings, were not treated. Consequently, the density of host-seeking ticks remained unchanged on the non-treated portions of the treatment properties and a more accurate description of the study outcome is to say that reducing the density of host-seeking ticks by more than half solely along the woods-lawn ecotone on residential properties had no impact on human tick-bites of the residents. Whether or not treating all portions of the properties where ticks could be encountered would have changed the outcome for human tick-bites of the residents remains unknown. Although disappointing, negative outcomes in large-scale and costly intervention studies should lead to examination of the cause(s) of intervention failure and, when reasonable based on the findings, result in renewed smaller-scale studies to refine the implementation scheme for the tick control method(s).
4. Tick control on residential properties – practical considerations
It may seem surprising that the problem with human-biting ticks occurring commonly on residential properties, which was recognized more than three decades ago in the northeastern United States (Falco and Fish, 1988a, b; Maupin et al., 1991; Carroll et al., 1992; Stafford and Magnarelli, 1993), has not yet been successfully resolved to provide effective tick management or tick-bite prevention solutions. Delving deeper into the practical aspects of this issue, several root causes emerge that combine to create an intractable problem. Daily use of personal protective measures is unlikely to be sustained over the tick season, which may extend for many months, for outside activities on your own property. Moreover, even if a new Lyme disease vaccine, or other related methodology to induce long-lasting protection in humans against infection with Bo. burgdorferi s.l., emerges as a marketed product, exposure to I. scapularis on residential properties will remain a threat for human infection with the multiple other pathogens associated with this tick. Consequently, actions are needed that go beyond personal protective measures and rather aim to broadly suppress human-biting ticks, such as I. scapularis and A. americanum, on residential properties in order to reduce the acarological risk of exposure to tick-borne pathogens in this high-use environment. The next roadblock is that individual methods with potential to suppress ticks on residential properties often are based either on common sense approaches without strong underlying evidence for impact even on host-seeking ticks (Eisen and Dolan, 2016; Fischhoff et al., 2019c) or on approaches where reduction in the density of host-seeking ticks have failed to translate into reduction in human tick-bites and tick-borne diseases (Hinckley et al., 2016, 2018). Moreover, knowledge is lacking for which combinations of peridomestic habitats (for example, lawn, wooded ecotone, wooded areas, and ornamental plantings) and human activities (for example, gardening, other yard work, and play involving children and pets) that most commonly result in tick encounters on residential properties. These knowledge gaps lead to broad and general guidance for homeowners (Stafford, 2007; CDC (Centers for Disease Control and Prevention), 2020b), a problem compounded by the lack of local tick management programs operated by public health professionals to provide objective advice on which methods are best suited for a specific property based on its landscape and habitat characteristics (Eisen, 2020; Hornbostel et al., 2020). In conclusion, there is presently no evidence-based universal tick management solution to recommend for individual residential properties and the knowledge base is not yet robust enough to develop a set of options that account for a limited number of basic scenarios calling for different tick management approaches. The way forward will no doubt be challenging and likely needs to diverge from the current approach by homeowners and pest control firms where broadcast application of synthetic chemical acaricides often is the first action taken (Jordan and Schulze, 2020a). An alternative strategy is to initially consider other less environmentally harmful tick suppression methods, and to employ landscaping and vegetation management measures (such as brush/shrub and leaf litter removal) that can minimize the amount of broadcast acaricide needed for effective tick suppression. Not considering broader urban/suburban/exurban planning solutions to reduce human contact with tick habitat (Jackson et al., 2006; Kaup, 2018; MacDonald et al., 2019), it is instructive to consider the requirements that need to be met for ticks to be introduced onto a residential property and then survive the transition across life stages and be able to quest for hosts, and the different ways in which this can be countered.
4.1. Preventing tick hosts from entering residential properties or reducing peridomestic abundance of tick hosts
As host-seeking ticks have very limited capacity for horizontal movement, introduction of ticks onto a residential property occurs primarily via movement of tick-infested vertebrates. Ticks may be brushed or groomed off prior to attaching to the host or they may detach fully fed from the host on the residential property. The microhabitats where the ticks are introduced will depend on the spatial activity patterns of the host species they infest and whether the tick species in question has a propensity to detach during times when hosts are resting in nests or burrows, or are bedded down in vegetation. Preventing tick hosts from entering residential properties is only realistic for ungulates, such as white-tailed deer (Odocoileus virginianus), which can be kept out by fencing.
Not all major human-biting tick species feed commonly on deer and other ungulates (Eisen and Stafford, 2020), but there is evidence for moderate (47–62 %) reduction in the density of host-seeking I. scapularis nymphs on residential properties following deer fencing of areas in the range of 3–7 ha (Stafford, 1993). The impact would presumably be even stronger for tick species, such as A. americanum, that rely more heavily than I. scapularis on deer as hosts for the larval and nymphal life stages in addition to serving as key reproductive hosts for the adult life stage. Moreover, Fischhoff et al. (2019c) reported reduced levels of infestation of Peromyscus mice by larval ticks (presumably I. scapularis although this was not clearly stated) on residential properties with ≥75 % of the yard enclosed by a fence but as the type of fence was not defined in their study it is not clear to what extent this reflected fences tall enough to prevent entry by deer. There is little doubt that a deer fence has the benefit of reducing the influx of ticks onto a residential property, especially for fed female ticks which can lay in excess of one thousand eggs and thus seed the property with larval ticks which for some species, such as I. scapularis, feed frequently on small vertebrate pathogen reservoirs with home ranges falling largely within the boundaries of the property. In this way, a single fed and mated I. scapularis female detaching from a deer on a residential property can produce large numbers of larvae subsequently feeding on rodent or insectivore pathogen reservoirs and, following the molt, emerging on the residential property as host-seeking nymphs infected with the causative agents of Lyme disease, anaplasmosis, and/or babesiosis.
Deer fencing will incur a high initial cost, the full impact on host-seeking ticks will not be evident until 2–3 yr later, and there can be a temporary increase in the density of host-seeking ticks in the 1–2 yr following the installation of the fence because deer no longer contribute as hosts available to the questing ticks present on the residential property. However, once established, the protection provided by a deer fence can extend for decades with minimal annual effort and cost, and the exclusion of deer also will serve to protect ornamental plantings from deer browse. One important but still poorly understood issue is how the size of the area enclosed by the deer fence influences the level of reduction in the density of host-seeking ticks. Studies in natural areas on the impact of deer exclosures on I. scapularis (Daniels et al., 1993; Daniels and Fish, 1995; Ginsberg and Zhioua, 1999; Ginsberg et al., 2004; Perkins et al., 2006) and A. americanum (Bloemer et al., 1986, 1990; Ginsberg et al., 2002) collectively suggest that reduction in the density of host-seeking ticks is more pronounced for larger exclosures compared with smaller 1–2 ha exclosures where in some cases no effect on tick density was observed. Lack of reduction in the density of nymphal and adult ticks within small areas enclosed by deer fences may result, in part, from regular movement through or over the fences by small mammals or birds that encounter larval or nymphal ticks outside the fence and these ticks then detach, following the blood meal, within the fenced area. To clarify the value of deer fencing for residential tick control, further studies are needed to investigate the impact on the density of host-seeking I. scapularis and A. americanum of fencing (i) single residential properties in the size range from 1 to 5 ha and (ii) larger areas containing groups of residential properties. Reducing deer movement onto a property by means other than fencing, such as avoiding ornamental plants that are favored by deer or to stop feeding deer on the property, may have a similar beneficial effect as a deer fence but likely with a less pronounced impact to reduce host-seeking ticks.
Avoiding or removing features that provide harborage for rodents, such as stone walls and wood piles, may help to reduce migration of potentially tick-infested rodent pathogen reservoirs onto the property as well as keeping resident rodent populations low (Stafford, 2007; Fischhoff et al., 2019c). These actions most likely will have a negative net effect on the density of infected host-seeking ticks present on a residential property, but the impact will be restricted to tick species where the immatures commonly infest rodents, such as I. scapularis and D. variabilis, and no impact is expected for tick species that only rarely infest rodents, such as A. americanum. Minimizing food sources for small and medium-sized vertebrate tick hosts, such as food disposal in open composts and use of bird feeders (which not only provide food for birds but also for ground-dwelling mammals via bird feed falling onto the ground) should reasonably also reduce the movement of tick hosts onto and within a property. An alternative method to reduce tick hosts on a residential property, mentioned in a recent survey as practiced by subsets of homeowners in the Upper Midwest and Northeast but perhaps related more broadly to nuisance animal control (Bron et al., 2020a), is killing of rodents. Studies are needed to determine if methods aiming to reduce the use by non-deer tick hosts of residential properties result in decreased density of host-seeking ticks.
4.2. Using rodent-targeted tick control products
Another option to reduce the potential for vertebrates to introduce ticks onto a residential property, and to serve as hosts for ticks already present on the property, is to use rodent-targeted acaricides to prevent tick feeding. As noted previously (Eisen and Stafford, 2020), main benefits of this approach include containment of the pesticide to delivery devices (rather than being spread openly) and impact across the extent of the home ranges of the targeted rodent species. Two products are currently on the market for topical application of acaricide to rodents: (i) the Damminix/Thermacell Tick Tube (EcoHealth, Inc., Boston, MA, USA; Thermacell Repellents, Inc., Bedford, MA, USA), where permethrin-treated cotton balls intended for use as nesting material are offered to rodents in cardboard tubes; and (ii) the SELECT Tick Control System (Tick Box Technology Corp., Norwalk, CT, USA), where rodents are treated with fipronil as they navigate toward a food source in a bait box. Drawbacks for both products include lack of impact on host-seeking I. scapularis nymphs during the first year of treatment and, unfortunately, uneven performance across field evaluations, ranging from strong suppression of host-seeking I. scapularis nymphs in some studies to minimal impact in other studies (Mather et al., 1987, 1988; Daniels et al., 1991; Deblinger and Rimmer, 1991; Stafford, 1991, ; Ginsberg, 1992; Schulze et al., 2017; Hinckley et al., 2018; Jordan and Schulze, 2019). The underlying reasons for these performance issues remain unclear, but may include spatially variable contributions by non-target vertebrates as tick hosts, the timing of the intervention, variable ratios in the numbers of target animals present to the density of delivery devices deployed, and, for the product delivered via a bait box, fluctuations in natural rodent food sources (Eisen and Dolan, 2016; Machtinger and Li, 2019). The poor understanding of the circumstances under which the currently available products succeed or fail to suppress host-seeking I. scapularis nymphs makes it difficult to predict how the products will perform on a given individual residential property. Another notable issue is high cost for deployment of one of the products (the SELECT Tick Control System), which can only be deployed by licensed pest management professionals and has been estimated to incur an annual cost in excess of $2000 for a 1 ha residential property when applied at the high-end density recommended on the label (Jordan and Schulze, 2019). Looking to the near future, there are ongoing efforts to develop, field test, and register new products for oral treatment of rodents with acaricide to prevent tick feeding (Pelletier et al., 2020; Poché et al., 2020) or oral vaccination of rodents to protect against infection with B. burgdorferi s.s. (Stafford et al., 2020).
4.3. Reducing the extent of peridomestic habitat suitable for tick survival and modifying remaining tick habitat to make it less suitable for tick survival and host-seeking
Realizing that it is not realistic to completely block tick hosts – which may include lizards, birds, and mammals ranging in size from small insectivores and rodents to medium-sized carnivores and large ungulates – from entering residential properties or to act as hosts for ticks present on the properties, a next set of homeowner actions can focus on non-pesticide alternatives to suppress ticks on the residential property by reducing the extent of habitat suitable for tick survival and modifying remaining tick habitat to make it less suitable for tick survival and host-seeking. These actions also serve to raise awareness about where on the residential property ticks can be encountered. As noted previously (Eisen and Dolan, 2016; Fischhoff et al., 2019c), field data to support the use of common-sense landscaping and vegetation management approaches to suppress ticks are scarce and in part based on observations from studies with primary purposes other than to evaluate the impact of landscaping or vegetation management practices on the density of host-seeking ticks. A reasonable assumption, which would be easy to confirm in the field, is that host-seeking ticks are virtually absent from hardscaped portions of residential properties and occur only very infrequently in xeriscapes (Piesman, 2006b; Stafford, 2007). Other types of land cover on a residential property can be classified as: ornamental planting, vegetable garden, short-grass lawn, open area with longer grass, brushy/shrubby area, and wooded area with ground cover dominated by litter, emergent short vegetation, or brush/shrub. In addition, the ecotones among these land cover classes on residential properties merit attention as they may be preferentially used by tick hosts. Basic studies to describe the presence and density of host-seeking ticks of different species and life stages across the patchwork of land cover types (habitats) present on residential properties are still largely lacking from the United States, with the exception of early studies that focused narrowly on the transition from short-grass lawn to the lawn-woods ecotone and wooded portions of residential properties in the Northeast (Maupin et al., 1991; Carroll et al., 1992; Stafford and Magnarelli, 1993; Duffy et al., 1994; Frank et al., 1998). Such studies, together with data on human use patterns (time spent in different habitat classes engaging in different types of activities across the months of the year when host-seeking ticks are present), would be valuable to better define the risk for tick exposure within residential properties.
Field evaluations conducted specifically on residential properties in the United States to quantify the impact of vegetation management on the density of host-seeking ticks is limited to a single study showing that accumulation of leaves from leaf blowing or raking activities were associated with increased density of host-seeking I. scapularis nymphs (Jordan and Schulze, 2020b). This finding agrees with a previous study from a natural woodland setting within a forested residential community where removal of leaf litter with hand rakes and leaf blowers reduced the density of host-seeking I. scapularis nymphs by 75–77 % in the cleared areas (Schulze et al., 1995). Additional studies from natural forested areas in the northeastern United States indicate that reduction in the density of host-seeking I. scapularis adults can be achieved through mowing of understory vegetation (Wilson, 1986) or removal of invasive shrubs (Williams et al., 2009, 2017; Williams and Ward, 2010). Similarly, studies in natural forested areas in the eastern United States indicate that reduction in the density of host-seeking A. americanum can be achieved through vegetation management including removal of invasive shrubs (Allan et al., 2010) or broad removal of mid-story and understory vegetation and in some cases also leaf litter (Clymer et al., 1970; Hair and Howell, 1970; Hoch et al., 1971; Mount, 1981; Bloemer et al., 1990). However, it is not clear how vegetation management techniques such as brush/shrub removal and mowing (of lawns or other grassy areas) will perform to reduce densities of host-seeking I. scapularis and A. americanum on residential properties. Some vegetation management methods likely act primarily by reducing moist microhabitats where ticks can readily rehydrate and thus presumably will negatively impact the duration of time ticks can quest for hosts before perishing; these include leaf litter removal and mowing of lawns. Removal of brush/shrub and unmanaged long grass may have a similar but potentially less pronounced negative effect on host-seeking ticks, but also removes vegetation favored by certain tick host species for foraging and to provide cover. The latter can change both the composition of tick host species present and the abundance of individual host species on a residential property, potentially with unexpected outcomes for the density of host-seeking ticks in the short term as fewer hosts may be available to remove questing ticks from the environment. Finally, a recent study from a natural area in Canada demonstrated that use of woodchip borders along the grass-woods ecotone of a recreational trail served to reduce the density of host-seeking I. scapularis and Haemaphysalis leporispalustris at the trail margins (McKay et al., 2020).
As outlined above, the knowledge base for impact of landscaping and vegetation management on residential properties is very limited for outcomes relating to host-seeking ticks. There is an urgent need for experimental and observational field studies to evaluate common-sense recommendations aiming to reduce the density of host-seeking ticks on residential properties by: (i) negatively impacting the molting or egg-laying success of fed ticks having detached from hosts; (ii) shortening the time that unfed ticks can seek hosts before perishing; and/or (iii) removing emergent vegetation that provides opportunities for host-seeking ticks to climb and reach questing positions that may increase the likelihood of making contact with humans compared with questing at ground level. Such common-sense recommendations include adopting hardscape and xeriscape landscaping techniques with gravel pathways and mulches; using decking, tile, and gravel in areas by the house; creating wood chip, mulch, or gravel borders between lawn and woods or along stonewalls; moving children’s swing sets and sand boxes away from the woodland edge and placing them on a wood chip or mulch foundation; trimming tree branches and shrubs around the lawn edge to let in more sunlight; removing leaf litter, brush/shrub and weeds at the edge of the lawn and around stonewalls and wood piles; restricting the use of groundcover in areas frequented by family and roaming pets; broadly removing brush/shrub and leaves; and keeping lawn grass mowed short (Stafford, 2007). Generating a knowledge base for the impact of these varied recommendations on host-seeking ticks is no easy task but nevertheless should be vigorously pursued.
The impact on host-seeking ticks is readily evaluated experimentally on residential properties where homeowners are willing to participate for some of the above-mentioned actions where treatment and control plots can be assigned on each individual property, such as for mowing lawns, creating xeric borders along ecotones, removing leaf litter, or brush/shrub clearing. Other actions are less feasible to evaluate experimentally (for example, broadly adopting hardscape and xeriscape landscaping techniques or restricting the use of groundcover in areas frequented by family and roaming pets) but can be assessed by observational studies where attempts are made to determine relationships between presence or absence of a given feature and the density of host-seeking ticks. The problem then becomes deciding where on the property it is reasonable to sample ticks to assess the spatial impact of an action being considered, for example when using decking, tile, and gravel in areas by the house. Such an action may only impact the specific landscaped area or have additional impact extending into adjacent residential habitats due to changes in tick host movement patterns. Clearly, evaluating the impact of landscaping and vegetation management techniques on the density of host-seeking ticks on residential properties will require both study design ingenuity and a solid understanding of tick biology for those willing to take up the challenge. At the present time, the options for vegetation management aiming to suppress ticks on residential properties is for homeowners to do it themselves or to use a landscaping firm that provides such services, for example as part of a lawncare/landscaping package, specifically in the context of tick suppression (Jordan and Schulze, 2020a).
Related topics for which there still is inadequate knowledge on residential properties include how fed, detaching ticks are dispersed across the peridomestic landscape by their hosts, to what extent host-seeking ticks of different species and life stages move horizontally within and between distinct habitats, such as woods and lawns, and how the duration (number of days) of host-seeking activity before a tick perishes may differ among habitat types present on residential properties for different species and life stages. Laboratory experiments indicate that immatures of some tick species, such as I. scapularis and the closely related Ixodes ricinus (castor bean tick) in Europe, preferentially detach from rodent hosts during portions of the diel cycle when they presumably are resting in nests or burrows (Mather and Spielman, 1986; Matuschka et al., 1990, 1991). However, it seems likely that in a natural setting where rodents are moving around vigorously in search of food or other resources immature ticks that are adequately fed can be dislodged from moving rodent hosts and thus concentrated in microhabitats used heavily by rodents when they are outside their nests or burrows. Horizontal (lateral) movement by molted, host-seeking ticks has been evaluated experimentally for I. scapularis and I. pacificus in forested settings or fields (Falco, 1987; Daniels and Fish, 1990; Stafford, 1992; Goddard, 1993; Lord, 1993; Carroll and Schmidtmann, 1996; Lane et al., 2009), showing limited horizontal movement, typically ≤5 m, for all life stages. Similar studies are lacking from residential properties, especially in relation to the transition from woods to managed lawn. Density of host-seeking I. scapularis nymphs on the lawn has been shown to generally decrease with distance from the wooded edge over the first 5 m (Carroll et al., 1992; Stafford and Magnarelli, 1993), which can be interpreted as “spill-over” of host-seeking ticks moving from the wooded edge short distances into the lawn and thus motivating the use of a xeric border along the woods-lawn ecotone. Conversely, host-seeking nymphs on the lawn near the wooded edge may have been dislodged as fed larvae from rodents or other hosts venturing onto the lawn, followed by molting and questing in that same habitat. Assuming that the wooded ecotone provides a moister micro-environment with more varied emergent vegetation from which to select questing heights, it would seem counter-intuitive for ticks to migrate onto a short-grass lawn rather than staying at the ecotone. Carroll and Schmidtmann (1996) found that marked I. scapularis nymphs released at a woods-pasture ecotone preferentially moved into the wooded side of the ecotone rather into the pasture where cows were present, whereas no clear pattern was seen for I. scapularis females. A similar study for the woods-lawn interface on residential properties involving key human-biting tick species should prove interesting.
When considering survival of host-seeking ticks in relation to habitat type, Ginsberg and Zhioua (1996) found that I. scapularis nymphs had higher survival rates over a 4-wk period when placed in field enclosures in forested versus open habitats, whereas no such difference was seen for the more desiccation-tolerant A. americanum. A similar trend, with higher survival rates for ticks placed in field enclosures in forested versus open habitats, was reported for I. scapularis adults but surprisingly not for nymphs in another set of studies (Bertrand and Wilson, 1996, 1997). Studies on survival of host-seeking ticks would be of interest on residential properties where parts of lawns can be heavily shaded, and in some cases lawns may be occasionally watered, because differences in tick survival relative to wooded areas may be less pronounced than suggested by studies conducted in open fields. Overall, a better understanding of how host-seeking ticks of different species and life stages are distributed across the residential landscape, and why they ended up seeking a host in those specific microhabitats where they were collected – for example whether it is where they molted, if they molted in nests or burrows and then hitched a ride on a host to reach their host-seeking location, or if they migrated on their own from the spot where they molted to the host-seeking location – would be useful to predict the outcomes of specific landscaping or vegetation management techniques on the density of host-seeking ticks.
4.4. Killing host-seeking ticks through broadcast of acaricides or biological control agents
Although there is little doubt that the individual actions described in sections 4.1–4.3 each would contribute to some extent to reduce the cumulative number of tick questing-days on a residential property, the combinations of actions individual homeowners are willing to invest in may not suffice to create tick-free peridomestic environments without resorting to broadcast of products designed to kill host-seeking ticks. Currently available broadcast products fall into three categories: synthetic chemical acaricides, natural product plant-based acaricides, and biological control agents in the form of entomopathogenic fungus. As noted in previous reviews (Eisen and Dolan, 2016; Eisen and Stafford, 2020), each of these product groups have distinct benefits and drawbacks.
Synthetic chemical acaricide products have long-lasting residual efficacy against host-seeking ticks in the field (≥ 6 wk for I. scapularis nymphs), which reduces both the number of applications needed to achieve protection over the tick season and the cost of the intervention. These products also provide high levels of tick suppression (typically >80 % across tick species) irrespective of habitat and weather conditions (see reviews by: Stafford, 2007; Eisen and Dolan, 2016; White and Gaff, 2018; and recent publications by Jordan et al., 2017; Schulze and Jordan, 2020a; Bron et al., 2020b). However, synthetic chemical acaricides act broadly against arthropods, including beneficial insects (Ginsberg et al., 2017), which is cause for concern if they are used year after year in area-wide applications that cover all peridomestic tick habitats where their use is allowable based on label instructions. This raises the question of which habitats on a residential property to treat, and how frequently to treat them, in order to minimize acaricide use and environmental impact while still achieving a level of tick suppression that reduces the risk of tick-bites for the residents. Another knowledge gap is how well homeowners and pest control firms perform in broadcast application of acaricides, relative to the high expectations for killing efficacy set for the same products and application methods in research studies as outlined above (Hornbostel et al., 2020). Related to this is the question of how the complexity of the ground vegetation and the spray equipment used impact the penetration of an acaricide to contact host-seeking ticks, regardless of who the spray operator is. Lawn grass and leaf litter without abundant emergent vegetation or downed branches present habitats on residential properties where residual acaricide sprays are likely to effectively contact host-seeking ticks (even with a low-pressure sprayer). Habitats on residential properties with higher complexity of the ground vegetation, such as areas with high grasses, herbaceous plants, or shrubs, present a greater challenge to penetrate in order for the acaricide to adequately reach the microhabitats where the ticks are moving around, especially as the growing season progresses and foliage becomes more dense. At present, there is a knowledge gap for how low- or high-pressure spraying of synthetic chemical acaricide products, or use of granular formulations, perform in different habitat types encountered on residential properties, including for the seasonal timing of acaricide applications.
Natural acaricide products containing plant oils or active ingredients derived from plants may in some cases have more favorable environmental profiles than synthetic chemical acaricides (Ginsberg et al., 2017) and thus are better suited to be used widely across tick habitats on residential properties from year to year. However, these products tend to be less stable in the environment and therefore require more frequent applications (every 2–3 wk) to sustain high killing efficacy for host-seeking ticks (see review by Eisen and Dolan, 2016; and a recent publication by Schulze and Jordan, 2020b). The intervention cost consequently will be higher compared to synthetic chemical acaricide products. Another caveat is that some “minimum risk natural products” marketed for tick control but exempt from registration by the United States Environmental Protection Agency - the “25b exempt products” - appear to have poor tick killing efficacy (Dyer et al., 2020).
Entomopathogenic fungal control products (such as Met52, containing Metarhizium anisopliae; Novozymes Biologicals, Inc., Salem, VA, USA) are considered environmentally friendly, with limited impact on non-target organisms (Fischhoff et al., 2017), and thus can be used widely across tick habitats on residential properties from year to year. However, there is a 3–7 d time lag from field application until the fungus starts impacting the treated ticks, and the duration of effective tick killing after field application is no longer than 2–3 wk (Benjamin et al., 2002; Hornbostel et al., 2005; Bharadwaj and Stafford, 2010; Stafford and Allan, 2010; Schulze and Jordan, 2020b). Similar to natural acaricide products, this creates a need for frequent applications with higher cost compared to synthetic chemical acaricide products. Moreover, the impact of weather conditions on the effectiveness of entomopathogenic fungal control products remains poorly understood and field evaluations in the United States have to date focused almost exclusively on I. scapularis.
Homeowners face several questions as part of a decision to treat their properties with a broadcast product to kill host-seeking ticks: whether to apply an over-the-counter product themselves or use a pest control firm for the implementation; which type of tick control product and application equipment to use; which areas of the residential property to treat; and when during the year to start treatments and how often to re-apply the tick control product. The answers to some of these questions will depend on which tick species are present on the residential property, for example only A. americanum, only I. scapularis, or both species. If homeowners choose the do-it-yourself route the information sources sought likely will be a combination of general guidance found on websites provided by federal, state, or local health agencies, university extension services, or other entities. It may not be an easy task to translate the general guidance into actions appropriate for a specific residential property. If homeowners instead choose to use a pest control firm to suppress ticks by broadcast application of synthetic chemical acaricides, natural product acaricides, or entomopathogenic fungus, then the details of the implementation, including where and how often to spray, will likely be guided in part by the service packages offered by the firm (Jordan and Schulze, 2020a).
4.5. Bringing it all together
After considering, in sections 4.1–4.4, the full range of options available to incorporate into a strategy for suppression of host-seeking ticks on residential properties, it is clear that individual homeowners face a difficult and bewildering task in deciding what to do (Fig. 1; Hornbostel et al., 2020). As noted previously (Eisen and Stafford, 2020), this task is further complicated by that some actions will impact all human-biting tick species (for example, broadcast of acaricides), whereas other actions may impact some tick species more strongly than other species (for example, removal of leaf litter and lawn mowing should have a stronger impact on desiccation-sensitive species such as I. scapularis compared with hardier species such as A. americanum and D. variabilis), and some actions will have no or minimal impact on certain tick species due to their host preferences (for example, deer fencing will not impact D. variabilis and rodent-targeted acaricides will not impact A. americanum).
Fig. 1.
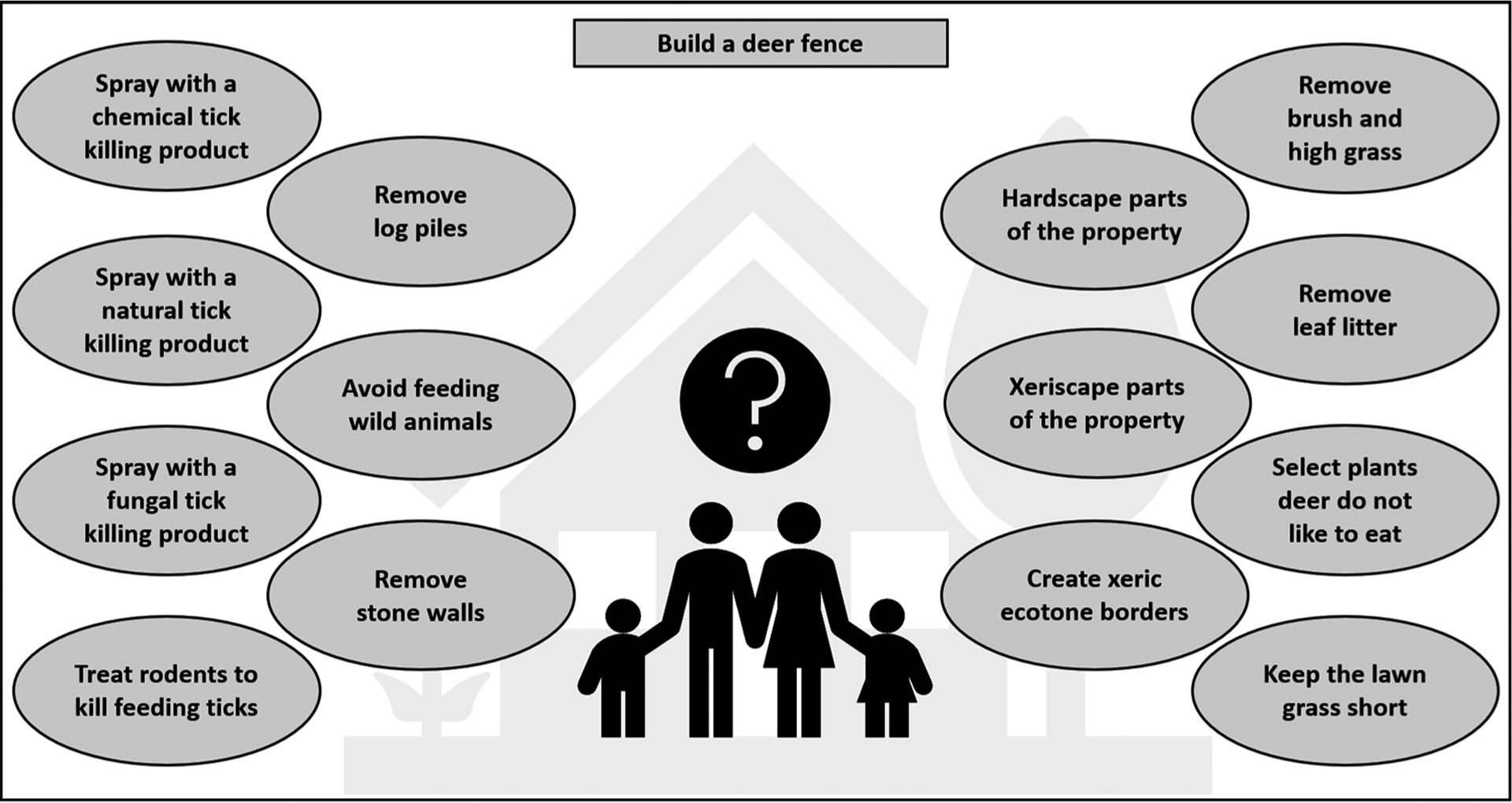
The bewildering choices facing homeowners trying to create tick-free residential properties.
No single action that is both acceptable to the homeowner and sustainable over time is likely to solve the problem with ticks in the peridomestic environment. The evidence base for outcomes relating to host-seeking ticks on residential properties is non-existent to inadequate across single actions other than broadcast of synthetic chemical acaricides, and even in that case questions remain regarding what portions of the property need to be treated and how to minimize the amount of acaricide applied over the tick season while still sustaining an acceptable level of tick suppression throughout the risk period. A few studies on integrated tick management approaches have examined outcomes for host-seeking ticks specifically on residential properties (Stafford and Allan, 2010; Williams et al., 2018) and a few other studies are underway (Keesing and Ostfeld, 2018; N.P Connally, 2020), but with a single exception (Stafford and Allan, 2010) these studies have focused narrowly on combinations of broadcast of acaricides or entomopathogenic fungus to kill host-seeking ticks and use of rodent-targeted acaricides. Additional studies have examined outcomes for host-seeking ticks on residential properties for integrated tick management approaches that, in addition to broadcast of acaricides or entomopathogenic fungus to kill host-seeking ticks and use of rodent-targeted acaricides, also included area-wide deer-targeted control measures (Schulze et al., 2007, 2008; Williams et al., 2018).
To sum up the current situation in the United States, evidence for impact on human tick-bites or tick-borne diseases is still lacking for environmentally-based approaches to suppress ticks on residential properties and homeowners are essentially forced to make decisions regarding how to reduce the risk for tick bites on their residential properties based on very general guidance and without ready access to local public health professionals experienced in tick control. This situation obviously is not satisfactory but cannot reasonably be corrected without a major push from federal agencies to: (i) develop a strong research program aiming to fill the knowledge gaps outlined above for existing and emerging tick suppression approaches on residential properties, including evaluations progressing from outcomes dealing with host-seeking ticks to human tick-bites and tick-borne diseases; and (ii) increase the local public health workforce with knowledge of and experience with tick control to provide better access for homeowners to sound and objective advice regarding tick control on their properties based on key characteristics of the landscaping, habitat composition, and use patterns by wild animal tick hosts as well as the residents.
Acknowledgments
I am grateful to Rebecca Eisen, Alison Hinckley, and Paul Mead of the Centers for Disease Control and Prevention for the discussions that helped to shape the thinking leading up to this Perspective paper.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions of this study are by the author and do not necessarily represent the views of the Centers for Disease Control and Prevention. The mention of commercial products does not represent an endorsement by the author or the Centers for Disease Control and Prevention.
References
- Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, Orrock JL, 2010. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proc. Natl. Acad. Sci. U. S. A 107, 18523–18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF, 1989. Preventing Lyme disease. Rheum. Dis. Clin. N. Am 15, 757–766. [PubMed] [Google Scholar]
- Benjamin MA, Zhioua E, Ostfeld RS, 2002. Laboratory and field evaluation of the entomopathogenic fungus Metarhizium anisopliae (Deuteromycetes) for controlling questing adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 39, 723–728. [DOI] [PubMed] [Google Scholar]
- Bertrand MR, Wilson ML, 1996. Microclimate-dependent survival of unfed adult Ixodes scapularis (Acari: Ixodidae) in nature: life cycle and study design implications. J. Med. Entomol 33, 619–627. [DOI] [PubMed] [Google Scholar]
- Bertrand MR, Wilson ML, 1997. Microhabitat-independent regional differences in survival of unfed Ixodes scapularis nymphs (Acari: Ixodidae) in Connecticut. J. Med. Entomol 34, 167–172. [DOI] [PubMed] [Google Scholar]
- Bharadwaj A, Stafford KC III, 2010. Evaluation of Metarhizium anisopliae Strain F52 (Hypocreales: Clavicipitaceae) for control of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 47, 862–867. [DOI] [PubMed] [Google Scholar]
- Bissinger BW, Roe RM, 2010. Tick repellents: past, present, and future. Pestic. Biochem. Physiol 96, 63–79. [Google Scholar]
- Bloemer SR, Snoddy EL, Cooney JC, Fairbanks K, 1986. Influence of deer exclusion on populations of lone star ticks and American dog ticks (Acari: Ixodidae). J. Econ. Entomol 79, 679–683. [DOI] [PubMed] [Google Scholar]
- Bloemer SR, Mount GA, Morris TA, Zimmerman RH, Barnard DR, Snoddy EL, 1990. Management of lone star ticks (Acari: Ixodidae) in recreational areas with acaricide applications, vegetative management, and exclusion of white-tailed deer. J. Med. Entomol 27, 543–550. [DOI] [PubMed] [Google Scholar]
- Bron GM, Fernandez MDP, Larson SR, Maus A, Gustafson D, Tsao JI, Diuk-Wasser MA, Bartholomay LC, Paskewitz SM, 2020a. Context matters: contrasting behavioral and residential risk factors for Lyme disease between high-incidence states in the northeastern and midwestern United States. Ticks Tick. Dis 11, 101515. [DOI] [PubMed] [Google Scholar]
- Bron GM, Lee X, Paskewitz SM, 2020b. Do-it-yourself tick control: granular gamma-cyhalothrin reduces Ixodes scapularis (Acari: Ixodidae) nymphs in residential backyards. J. Med. Entomol 57 10.1093/jme/tjaa212. [DOI] [PubMed] [Google Scholar]
- Brown RN, Burgess EC, 2001. Lyme borreliosis. In: Williams ES, Barker IK (Eds.), Infectious Diseases of Wild Mammals Iowa State University Press, Ames, IA, USA, pp. 435–454. [Google Scholar]
- Campbell GL, Fritz CL, Fish D, Nowakowski J, Nadelman RB, Wormser GP, 1998. Estimation of the incidence of Lyme disease. Am. J. Epidemiol 148, 1018–1026. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Schmidtmann ET, 1996. Dispersal of blacklegged tick (Acari: Ixodidae) nymphs and adults at the woods-pasture interface. J. Med. Entomol 33, 554–558. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Ginsberg HS, Hyland KE, Hu R, 1992. Distribution of Ixodes dammini (Acari: Ixodidae) in residential lawns on Prudence Island, Rhode Island. J. Med. Entomol 29, 1052–1055. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2018. Surveillance for Ixodes scapularis and Pathogens Found in This Tick Species in the United States. Accessed 23 October 2020. https://www.cdc.gov/ticks/resources/TickSurveillance_Iscapularis-P.pdf.
- CDC (Centers for Disease Control and Prevention), 2020a. Guide to the Surveillance of Metastriate Ticks (Acari: Ixodidae) and Their Pathogens in the United States. Accessed 23 October 2020. https://www.cdc.gov/ticks/pdfs/Tick_surveillance-P.pdf.
- CDC (Centers for Disease Control and Prevention), 2020b. Preventing Ticks in the Yard. Accessed 23 October 2020. https://www.cdc.gov/ticks/avoid/in_the_yard.html.
- Clark RP, Hu LT, 2008. Prevention of Lyme disease and other tick-borne infections. Infect. Dis. Clin. N. Am 22, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clymer BC, Howell DE, Hair JA, 1970. Environmental alteration in recreational areas by mechanical and chemical treatment as a means of lone star tick control. J. Econ. Entomol 63, 504–509. [DOI] [PubMed] [Google Scholar]
- Comstedt P, Hanner M, Schüler W, Meinke A, Lundberg U, 2014. Design and development of a novel vaccine for protection against Lyme borreliosis. PLoS One 9, e113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstedt P, Schüler W, Meinke A, Lundberg U, 2017. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PLoS One 12, e0184357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connally NP, 2020. The Backyard Integrated Tick Managment (BITM) Study. Accessed 23 October, 2020. https://tickipmwg.files.wordpress.com/2018/08/connally_ipm_mar2018-publish.pdf.
- Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, Heimer R, 2009. Peridomestic Lyme disease prevention - results of a population-based case-control study. Am. J. Prev. Med 37, 201–206. [DOI] [PubMed] [Google Scholar]
- Couch P, Johnson CE, 1992. Prevention of Lyme disease. Am. J. Hosp. Pharm 49, 1164–1173. [PubMed] [Google Scholar]
- Daniels TJ, Fish D, 1990. Spatial distribution and dispersal of unfed larval Ixodes dammini (Acari: Ixodidae) in southern New York. Environ. Entomol 19, 1029–1033. [Google Scholar]
- Daniels TJ, Fish D, 1995. Effect of deer exclusion on the abundance of immature Ixodes scapularis (Acari: Ixodidae) parasitizing small and medium-sized mammals. J. Med. Entomol 32, 5–11. [DOI] [PubMed] [Google Scholar]
- Daniels TJ, Fish D, Falco RC, 1991. Evaluation of host-targeted acaricide for reducing risk of Lyme disease in southern New York State. J. Med. Entomol 28, 537–543. [DOI] [PubMed] [Google Scholar]
- Daniels TJ, Fish D, Schwartz I, 1993. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease risk by deer exclusion. J. Med. Entomol 30, 1043–1049. [DOI] [PubMed] [Google Scholar]
- Daniels TJ, Falco RC, Fish D, 2000. Estimating population size and drag sampling efficiency for the blacklegged tick (Acari: Ixodidae). J. Med. Entomol 37, 357–363. [DOI] [PubMed] [Google Scholar]
- Deblinger RD, Rimmer DW, 1991. Efficacy of a permethrin-based acaricide to reduce the abundance of Ixodes dammini (Acari: Ixodidae). J. Med. Entomol 28, 708–711. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vannier E, Krause PJ, 2016. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 32, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ADM, 2013. Ticks in the wrong boxes: assessing error in blanket-drag studies due to occasional sampling. Parasit. Vectors 6, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Panella NA, 2011. A review of arthropod repellents. In: Paluch GE, Coats JR (Eds.), Recent Developments in Invertebrate Repellents. American Chemical Society, Washington, D.C., USA, pp. 1–19. [Google Scholar]
- Drexler N, Miller M, Gerding J, Todd S, Adams L, Dahlgren FS, Bryant N, Weis E, Herrick K, Francies J, Komatsu K, Piontkowski S, Velascosoltero J, Shelhamer T, Hamilton B, Eribes C, Brock A, Sneezy P, Goseyun C, Bendle H, Hovet R, Williams V, Massung R, McQuiston JH, 2014. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One 9, e112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DC, Clark DD, Campbell SR, Gurney S, Perello R, Simon NM, 1994. Landscape patterns of abundance of Ixodes scapularis (Acari: Ixodidae) on Shelter Island, New York. J. Med. Entomol 31, 875–879. [DOI] [PubMed] [Google Scholar]
- Dyer MC, Requintina MD, Berger KA, Puggioni G, Mather TN, 2020. Evaluating the effects of minimal risk natural products for control of the tick, Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 57 10.1093/jme/tjaa188. [DOI] [PubMed] [Google Scholar]
- Eisen L, 2020. Stemming the rising tide of human-biting ticks and tickborne diseases, United States. Emerg. Infect. Dis 26, 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Dolan MC, 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol 53, 1063–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, 2016. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J. Med. Entomol 53, 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Gray JS, 2016. Lyme borreliosis prevention strategies: United States versus Europe. In: Braks MAH, Van Wierer SE, Takken W, Sprong H (Eds.), Ecology and Prevention of Lyme Borreliosis. Wageningen Academic Publishers, Wageningen, The Netherlands, pp. 429–450. [Google Scholar]
- Eisen RJ, Eisen L, 2018. The blacklegged tick, Ixodes scapularis: an increasing health concern. Trends Parasitol. 34, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Mead PM, 2020. Prevention of Lyme Disease. UpTo Date article, Accessed 23 October, 2020. https://www.uptodate.com/contents/prevention-of-lyme-disease.
- Eisen RJ, Paddock CD, 2020. Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol 57 10.1093/jme/tjaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Stafford KC III, 2020. Barriers to effective tick management and tick-bite prevention in the United States (Acari: Ixodidae). J. Med. Entomol 57 10.1093/jme/tjaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Piesman J, Zielinski-Gutierrez E, Eisen L, 2012. What do we need to know about Lyme disease ecology to prevent Lyme disease in the northeastern United States? J. Med. Entomol 49, 11–22. [DOI] [PubMed] [Google Scholar]
- Embers ME, Narasimhan S, 2013. Vaccination against Lyme disease: past, present, and future. Front. Cell. Infect. Microbiol 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, 1987. Abundance of the Deer Tick, Ixodes dammini (Acari: Ixodidae), in a Lyme Disease Endemic Area of Southern New York State. Ph.D. dissertation. Fordham University, Bronx, N.Y., USA. [Google Scholar]
- Falco RC, Fish D, 1988a. Ticks parasitizing humans in a Lyme disease endemic area of southern New York State. Am. J. Epidemiol 128, 1146–1152. [DOI] [PubMed] [Google Scholar]
- Falco RC, Fish D, 1988b. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am. J. Epidemiol 127, 826–830. [DOI] [PubMed] [Google Scholar]
- Fischhoff IR, Keesing F, Ostfeld RS, 2017. The tick biocontrol agent Metarhizium brunneum (= M. anisopliae) (strain F52) does not reduce non-target arthropods. PLoS One 12, e0187675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Bowden SE, Keesing F, Ostfeld RS, 2019a. Systematic review and meta-analysis of tick-borne disease risk factors in residential yards, neighborhoods, and beyond. BMC Infect. Dis 19, 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Keesing F, Ostfeld RS, 2019b. Risk factors for bites and diseases associated with black-legged ticks: a meta-analysis. Am. J. Epidemiol 188, 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Keesing F, Pendleton J, DePietro D, Teator M, Duerr STK, Mowry S, Pfister A, LaDeau SL, Ostfeld RS, 2019c. Assessing effectiveness of recommended residential yard management measures against ticks. J. Med. Entomol 56, 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D, 1995. Environmental risk and prevention of Lyme disease. Am. J. Med 98 (Suppl 4A), S2–S9. [DOI] [PubMed] [Google Scholar]
- Fish D, Childs E, 2009. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricde to white-tailed deer: background and rationale. Vector-borne Zoonotic Dis. 9, 357–364. [DOI] [PubMed] [Google Scholar]
- Frank DH, Fish D, Moy FH, 1998. Landscape features associated with Lyme disease risk in a suburban residential environment. Landscape Ecol. 13, 27–36. [Google Scholar]
- Ginsberg HS, 1992. Ecology and Management of Ticks and Lyme disease at Fire Island National Seashore and Selected Eastern National Parks. Scientific monograph NPS/NRSUNJ/NRSM-92/20. United States Department of the Interior, National Park Service, Denver, CO, USA. [Google Scholar]
- Ginsberg HS, 1993. Transmission risk of Lyme disease and implications for tick management. Am. J. Epidemiol 138, 65–73. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, 1994. Lyme disease and conservation. Conserv. Biol 8, 343–353. [Google Scholar]
- Ginsberg HS, 2001. Integrated pest management and allocation of control efforts for vector-borne diseases. J. Vector Ecol 26, 32–38. [PubMed] [Google Scholar]
- Ginsberg HS, 2014. Tick control. Trapping, biocontrol, host management, and other alternative strategies. In: Sonenshine DE, Roe RM (Eds.), Biology of Ticks, second edition, Vol. 2. Oxford University Press, New York, NY, USA, pp. 409–444. [Google Scholar]
- Ginsberg HS, Stafford KC III, 2005. Management of ticks and tick-borne diseases. In: Goodman JL, Dennis DT, Sonenshine DE (Eds.), Tickborne Diseases of Humans. ASM Press, Washington, D.C., USA, pp. 65–86. [Google Scholar]
- Ginsberg HS, Butler M, Zhioua E, 2002. Effect of deer exclusion by fencing on abundance of Amblyomma americanum (Acari: Ixodidae) on Fire Island, New York, USA. J. Vector Ecol 27, 215–221. [PubMed] [Google Scholar]
- Ginsberg HS, Zhioua E, Mitra S, Fischer J, Buckley PA, Verret F, Underwood HB, Buckley FG, 2004. Woodland type and spatial distribution of nymphal Ixodes scapularis (Acari: Ixodidae). Environ. Entomol 33, 1266–1273. [Google Scholar]
- Ginsberg HS, Bargar TA, Hladik ML, Lubelczyk C, 2017. Management of arthropod pathogen vectors in North America: minimizing adverse effects on pollinators. J. Med. Entomol 54, 1463–1475. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Zhioua E, 1996. Nymphal survival and habitat distribution of Ixodes scapularis and Amblyomma americanum ticks (Acari: Ixodidae) on Fire Island, New York, USA. Exp. Appl. Acarol 20, 533–544. [Google Scholar]
- Ginsberg HS, Zhioua E, 1999. Influence of deer abundance on the abundance of questing adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 36, 376–381. [DOI] [PubMed] [Google Scholar]
- Goddard J, 1993. Ecological studies of adult Ixodes scapularis (Acari: Ixodidae) in Central Mississippi: lateral movement of adult ticks. J. Med. Entomol 30, 824–826. [DOI] [PubMed] [Google Scholar]
- Golde WT, 1998. A vaccine for Lyme disease: current progress. Infect. Med 15, 40–42. [Google Scholar]
- Gomes-Solecki M, Arnaboldi PM, Backenson PB, Benach JL, Cooper CL, Dattwyler RJ, Diuk-Wasser M, Fikrig E, Hovius JW, Laegreid W, Lundberg U, Marconi RT, Marques AR, Molloy P, Narasimhan S, Pal U, Pedra JHF, Plotkin S, Rock DL, Rosa P, Telford SR III, Tsao J, Yang XF, Schutzer SE, 2020. Protective immunity and new vaccines for Lyme disease. Clin. Infect. Dis 70, 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould LH, Nelson RS, Griffith KS, Hayes EB, Piesman J, Mead PS, Cartter ML, 2008. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector-Borne Zoonotic Dis 8, 769–776. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Bjork JKH, Neitzel DF, Dorr FM, Whitemarsh T, Boegler KA, Graham CB, Johnson TL, Maes SE, Eisen RJ, 2018. Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks Tick. Dis 9, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile DG, Mount GA, Cooksey LM, 1990. Computer simulation of management strategies for American dog ticks (Acari: Ixodidae) and Rocky Mountain spotted fever. J. Med. Entomol 27, 686–696. [DOI] [PubMed] [Google Scholar]
- Hair JA, Howell DE, 1970. Lone Star Ticks: Their Biology and Control in Ozark Recreational Areas. Oklahoma Agricultural Experiment Station, Stillwater, OK, USA: ) Bulletin. B-679. [Google Scholar]
- Hanson MS, Edelman R, 2003. Progress and controversy surrounding vaccines against Lyme disease. Exp. Rev. Vaccines 2, 683–703. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Piesman J, 2003. How can we prevent Lyme disease? N. Engl. J. Med 348, 2424–2430. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Schriefer ME, 2002. Vaccination against Lyme borreliosis. In: Gray JS, Kahl O, Lane RS, Stanek S (Eds.), Lyme Borreliosis: Biology, Epidemiology and Control. CABI Publishing, New York, NY, USA, pp. 281–300. [Google Scholar]
- Hayes EB, Maupin GO, Mount GA, Piesman J, 1999. Assessing the prevention effectiveness of local Lyme disease control. J. Public Health Manage. Pract 5, 84–92. [DOI] [PubMed] [Google Scholar]
- Hinckley AF, Meek JI, Ray JAE, Niesobecki SA, Connally NP, Feldman KA, Jones EH, Backenson PB, White JL, Lukacik G, Kay AB, Miranda WP, Mead PS, 2016. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J. Clin. Dis 214, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley A, Niesobecki S, Hook S, Connally N, Heimer R, Meek J, 2018. Effectiveness of a rodent-targeted approach to prevent Lyme and other tick-borne diseases in humans. In: Abstract, 15th International Conference on Lyme Borreliosis and Other Tick-Borne Diseases. Atlanta, GA, USA, 11–14 September, 2018. [Google Scholar]
- Hoch AL, Barker LW, Hair JA, 1971. Further observations on the control of lone star ticks (Acarina: Ixodidae) through integrated control procedures. J. Med. Entomol 8, 731–734. [DOI] [PubMed] [Google Scholar]
- Hook SA, Nelson CA, Mead PS, 2015. U.S. public’s experience with ticks and tick-borne diseases: results from national HealthSyles surveys. Ticks Tick. Dis 6, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbostel VL, Zhioua E, Benjamin MA, Ginsberg HS, Ostfeld RS, 2005. Pathogenicity of Metarhizium anisopliae (Deuteromycetes) and permethrin to Ixodes scapularis (Acari: Ixodidae) nymphs. Exp. Appl. Acarol 35, 301–316. [DOI] [PubMed] [Google Scholar]
- Hornbostel VL, Krall RK, Reid JJ, Schappach JL, Volpe S, Connally NP, 2020. Spray safe, play safe: story-based films increase homeowner confidence about backyard tick management. J. Med. Entomol 57 10.1093/jme/tjaa230. [DOI] [PubMed] [Google Scholar]
- Hu LT, Klempner MS, 2001. Update on the prevention, diagnosis, and treatment of Lyme disease. Adv. Intern. Med 46, 247–275. [PubMed] [Google Scholar]
- Jackson LE, Hilborn ED, Thomas JC, 2006. Towards landscape design guidelines for reducing Lyme disease risk. Int. J. Epidemiol 35, 315–322. [DOI] [PubMed] [Google Scholar]
- Jaenson TGT, Fish D, Ginsberg HS, Gray JS, Mather TN, Piesman J, 1991. Methods for control of tick vectors of Lyme borreliosis. Scand. J. Infect. Dis 77 (Suppl), S151–S157. [PubMed] [Google Scholar]
- Jordan RA, Egizi A, 2019. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One 14, e0211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RA, Schulze TL, 2019. Ability of two commercially available host-targeted technologies to reduce abundance of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol 56, 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RA, Schulze TL, 2020a. Availability and nature of commercial tick control services in three Lyme disease endemic states. J. Med. Entomol 57, 807–814. [DOI] [PubMed] [Google Scholar]
- Jordan RA, Schulze TL, 2020b. Artificial accumulation of leaf litter in forest edges on residential properties via leaf blowing is associated with increased numbers of host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs. J. Med. Entomol 57, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Jordan RA, Schulze TL, Eisen L, Dolan MC, 2017. Ability of three general use, over-the-counter pesticides to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Am. Mosq. Contr. Assoc 33, 50–55. [DOI] [PubMed] [Google Scholar]
- Kaup B, 2018. The making of Lyme disease: a political ecology of ticks and tick-borne illness in Virginia. Environ. Sociol 4, 381–391. [Google Scholar]
- Keesing F, Ostfeld RS, 2018. The tick project: testing environmental methods of preventing tick-borne diseases. Trends Parasitol. 34, 447–450. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Dobson ADM, Levi T, Salkeld DJ, Swei A, Ginsberg HS, Kjemtrup A, Padgett KA, Jensen PM, Fish D, Ogden NH, Diuk-Wasser MA, 2017. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Phil. Trans. R. Soc. B 372, 20160117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Jordan RA, Schulze TL, Griffith KS, Mead PS, 2016. Will culling white-tailed deer prevent Lyme disease? Zoonoses Publ. Health 63, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Mun J, Stubbs HA, 2009. Horizontal and vertical movements of host-seeking Ixodes pacificus (Acari: Ixodidae) nymphs in a hardwood forest. J. Vector Ecol 34, 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Fish D, 2000. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect. Immun 68, 2183–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, 1993. Mortality of unfed nymphal Ixodes dammini (Acari: Ixodidae) in field exclosures. Environ. Entomol 22, 82–87. [Google Scholar]
- MacDonald AJ, Larsen AE, Plantinga AJ, 2019. Missing the people for the trees: identifying coupled natural–human system feedbacks driving the ecology of Lyme disease. J. Appl. Ecol 56, 354–364. [Google Scholar]
- Machtinger ET, Li AY, 2019. Tick control bait box use by Peromyscus spp. influenced by habitat placement but raises questions on disease ecology. Ecosphere 10, e02972. [Google Scholar]
- Mather TN, Spielman A, 1986. Diurnal detachment of immature deer ticks (Ixodes dammini) from nocturnal hosts. Am. J. Trop. Med. Hyg 35, 182–186. [DOI] [PubMed] [Google Scholar]
- Mather TN, Ribeiro JMC, Spielman A, 1987. Lyme disease and babesiosis acaricide focused on potentially infected ticks. Am. J. Trop. Med. Hyg 36, 609–614. [DOI] [PubMed] [Google Scholar]
- Mather TN, Ribeiro JMC, Moore SI, Spielman A, 1988. Reducing transmission of Lyme disease spirochetes in a suburban setting. Ann. N.Y. Acad. Sci 539, 402–403. [Google Scholar]
- Matuschka FR, Richter D, Fischer P, Spielman A, 1990. Time of repletion of subadult Ixodes ricinus ticks feeding on diverse hosts. Parasitol. Res 76, 540–544. [DOI] [PubMed] [Google Scholar]
- Matuschka FR, Richter D, Spielman A, 1991. Differential detachment from resting hosts of replete larval and nymphal Ixodes ticks. J. Parasitol 77, 341–345. [PubMed] [Google Scholar]
- Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J, 1991. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am. J. Epidemiol 133, 1105–1113. [DOI] [PubMed] [Google Scholar]
- McKay R, Talbot B, Slatculescu A, Stone A, Kulkarni MA, 2020. Woodchip borders at the forest ecotone as an environmental control measure to reduce questing tick density along recreational trails in Ottawa, Canada. Ticks Tick Borne Dis. 11, 101361. [DOI] [PubMed] [Google Scholar]
- Mead P, Hook S, Niesobecki S, Ray J, Meek J, Delorey M, Prue C, Hinckley A, 2018. Risk factors for tick exposure in suburban settings in the northeastern United States. Ticks Tick Borne Dis. 9, 319–324. [DOI] [PubMed] [Google Scholar]
- Molaei G, Little EA, Williams SC, Stafford KC III, 2019. Bracing for the worst – range expansion of the lone star tick in the northeastern United States. N. Engl. J. Med 381, 2189–2192. [DOI] [PubMed] [Google Scholar]
- Mount GA, 1981. Control of lone star ticks through vegetative management. J. Econ. Entomol 74, 173–175. [DOI] [PubMed] [Google Scholar]
- Mount GA, Haile DG, 1987. Computer simulation of area-wide management strategies for the lone star tick, Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol 24, 523–531. [DOI] [PubMed] [Google Scholar]
- Mount GA, Haile DG, Daniels E, 1997. Simulation of management strategies for the blacklegged tick, Ixodes scapularis (Acari: Ixodidae) and the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol 34, 672–683. [DOI] [PubMed] [Google Scholar]
- Nayak A, Schüler W, Seidel S, Gomez I, Meinke A, Comstedt P, Lundberg U, 2020. Broadly protective multivalent OspA vaccine against Lyme borreliosis, developed based on surface shaping of the C-terminal fragment. Infect. Immun 88, e00917–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Jersey Department of Health and Senior Services, 1999. Assessment and Management of Vector Tick Populations in New Jersey. New Jersey Department of Health and Senior Services, Trenton, NJ, USA. [Google Scholar]
- Niesobecki S, Hansen A, Rutz H, Mehta S, Feldman K, Meek J, Niccolai L, Hook S, Hinckley A, 2019. Knowledge, attitudes, and behaviors regarding tick-borne disease prevention measures in endemic areas. Ticks Tick Borne Dis. 10, 101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesobecki S, Rutz H, Niccolai L, Hook S, Meek J, Hansen A, Feldman K, Hinckley A, 2021. Willingness to pay for select tickborne disease prevention measures in endemic areas. J. Public Health Manage. Pract In Press. [DOI] [PubMed] [Google Scholar]
- Nieto NC, Foley JE, 2009. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife, and Ixodes ricinus-complex ticks. Vector Borne Zoonotic Dis. 9, 93–102. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Schofield SW, 2015. Methods to prevent tick bites and Lyme disease. Clin. Lab. Med 35, 883–889. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Price A, Hornbostel VL, Benjamin MA, Keesing F, 2005. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience 56, 383–394. [Google Scholar]
- Pelletier J, Rocheleau J‑P, Aenishaenslin C, Beaudry F, Masson GD, Lindsay LR, Ogden NH, Bouchard C, Leighton PA, 2020. Evaluation of fluralaner as an oral acaricide to reduce tick infestation in a wild rodent reservoir of Lyme disease. Parasit. Vectors 13, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SE, Cattadori IM, Tagliapietra V, Rizzoli AP, Hudson PJ, 2006. Localized deer absence leads to tick amplification. Ecology 87, 1981–1986. [DOI] [PubMed] [Google Scholar]
- Piesman J, 2006a. Strategies for reducing the risk of Lyme borreliosis in North America. Int. J. Med. Microbiol 296 (Suppl. 1), S17–S22. [DOI] [PubMed] [Google Scholar]
- Piesman J, 2006b. Response of nymphal Ixodes scapularis, the primary tick vector of Lyme disease spirochetes in North America, to barriers derived from wood products or related home and garden items. J. Vector Ecol 31, 412–417. [DOI] [PubMed] [Google Scholar]
- Piesman J, Eisen L, 2008. Prevention of tick-borne diseases. Annu. Rev. Entomol 53, 323–343. [DOI] [PubMed] [Google Scholar]
- Piesman J, Gern L, 2004. Lyme borreliosis in Europe and North America. Parasitology 129, S191–S220. [DOI] [PubMed] [Google Scholar]
- Piesman J, Gray JS, 1994. Lyme disease/Lyme borreliosis. In: Sonenshine DE, Mather TN (Eds.), Ecological Dynamics of Tick-Borne Zoonoses. Oxford University Press, New York, NY, USA, pp. 327–350. [Google Scholar]
- Piesman J, Hicks TC, Sinsky RJ, Obiri G, 1987. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. J. Clin. Microbiol 25, 2012–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, 2016. Need for a new Lyme disease vaccine. N. Engl. J. Med 375, 911–913. [DOI] [PubMed] [Google Scholar]
- Poché DM, Franckowiak G, Clarke T, Tseveenjav B, Polyakova L, Poché RM, 2020. Efficacy of a low dose fipronil bait against blacklegged tick (Ixodes scapularis) larvae feeding on white‑footed mice (Peromyscus leucopus) under laboratory conditions. Parasit. Vectors 13, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland GA, 2001. Prevention of Lyme disease: a review of the evidence. Mayo Clin. Proc 76, 713–724. [DOI] [PubMed] [Google Scholar]
- Richardson M, Khouja C, Sutcliffe K, 2019. Interventions to prevent Lyme disease in humans: a systematic review. Prev. Med. Rep 13, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin I, Toledo A, 2020. Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol 69, 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin I, Ninivaggi DV, Benach JL, 2019. Malaria and Lyme disease - the largest vector-borne US epidemics in the last 100 years: success and failure of public health. BMC Publ. Health 19, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, Hooks H, Partridge SK, Visser SN, Beard CB, Petersen LR, 2018. Vital signs: trends in reported vectorborne disease cases — United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep 67, 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmann ET, 1994. Ecologically based strategies for controlling ticks. In: Sonenshine DE, Mather TN (Eds.), Ecological Dynamics of Tick-Borne Zoonoses. Oxford University Press, New York, NY, USA, pp. 240–280. [Google Scholar]
- Schulze TL, Jordan RA, 2006. Assessment and Management of Vector Tick Populations in New Jersey. A Guide for Pest Management Professionals, Land Managers, and Public Health Officials. Freehold Township Health Department, Freehold, NJ, USA. [Google Scholar]
- Schulze TL, Jordan RA, 2020a. Early season applications of bifenthrin suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Med. Entomol 57, 797–800. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, 2020b. Synthetic pyrethroid, natural product, and entomopathogenic fungal acaricide product formulations for sustained early season suppression of host-seeking Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Med. Entomol 57 10.1093/jme/tjaa248. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Parkin WE, Bosler EM, 1988. Vector tick populations and Lyme disease. A summary of control strategies. Ann. N. Y. Acad. Sci 539, 204–211. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW, 1995. Suppression of Ixodes scapularis (Acari: Ixodidae) following removal of leaf litter. J. Med. Entomol 32, 730–733. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW, 1998. Availability and nature of commercial tick control services in established and emerging Lyme disease areas of New Jersey. J. Spirochetal Tick-borne Dis 4, 44–48. [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, Healy SP, Jahn MB, Piesman J, 2007. Integrated use of 4-poster passive topical treatment devices for deer, targeted acaricide applications, and Maxforce TMS bait boxes to rapidly suppress populations of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol 44, 830–839. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Dolan MC, Dietrich G, Healy SP, Piesman J, 2008. Ability of 4-poster passive topical treatment devices for deer to sustain low population levels of Ixodes scapularis (Acari: Ixodidae) after integrated tick management in a residential landscape. J. Med. Entomol 45, 899–904. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, Hung RW, 2009. Precipitation and temperature as predictors of the local abundance of Ixodes scapularis (Acari: Ixodidae) nymphs. J. Med. Entomol 46, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Williams M, Dolan MC, 2017. Evaluation of the SELECT Tick Control System (TCS), a host-targeted bait box, to reduce exposure to Ixodes scapularis (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J. Med. Entomol 54, 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen AK, Mead PS, Beard CB, 2011. The Lyme disease vaccine - A public health perspective. Clin. Infect. Dis 52, S247–S252. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, 1993. Biology of Ticks, Vol. 2. Oxford University Press, New York, NY, USA. [Google Scholar]
- Sonenshine DE, 2018. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Publ. Health 15, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A, 1988. Prospects for suppressing transmission of Lyme disease. Ann. N.Y. Acad. Sci 539, 212–220. [DOI] [PubMed] [Google Scholar]
- Spielman A, Pollack RJ, Kiszewski AE, Telford SR III, 2001. Issues in public health entomology. Vector-borne Zoonotic Dis. 1, 3–19. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, 1989. Lyme disease prevention: personal protection and prospects for tick control. Conn. Med 53, 347–351. [PubMed] [Google Scholar]
- Stafford KC III, 1991. Effectiveness of host-targeted permethrin in the control of Ixodes dammini (Acari: Ixodidae). J. Med. Entomol 28, 611–617. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, 1992. Oviposition and larval dispersal of Ixodes dammini (Acari: Ixodidae). J. Med. Entomol 29, 129–132. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, 1993. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) with exclusion of deer by electric fencing. J. Med. Entomol 30, 986–996. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, 1997. Pesticide use by licensed applicators for the control of Ixodes scapularis (Acari: Ixodidae) in Connecticut. J. Med. Entomol 34, 552–558. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, 2007. Tick Management Handbook: An Integrated Guide for Homeowners, Pest Control Operators, and Public Health Officials for the Prevention of Tick-Associated Disease, 2nd edition. The Connecticut Agricultural Experiment Station, Bull. No. 1010, New Haven, CT, USA. [Google Scholar]
- Stafford KC III, Allan SA, 2010. Field applications of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae F52 (Hypocreales: Clavicipitaceae) for the control of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol 47, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, Kitron U, 2002. Environmental management for Lyme borreliosis control. In: Gray JS, Kahl O, Lane RS, Stanek S (Eds.), Lyme Borreliosis: Biology, Epidemiology and Control. CABI Publishing, New York, NY, USA, pp. 301–334. [Google Scholar]
- Stafford KC III, Magnarelli LA, 1993. Spatial and temporal patterns of Ixodes scapularis in southeastern Connecticut. J. Med. Entomol 30, 762–771. [DOI] [PubMed] [Google Scholar]
- Stafford KC III, Williams SC, 2017. Deer-targeted methods: a review of the use of topical acaricides for the control of ticks on white-tailed deer. J. Integr. Pest Manage 8, 19. [Google Scholar]
- Stafford KC III, Williams SC, Molaei G, 2017. Integrated pest management in controlling ticks and tick-associated diseases. J. Integr. Pest Manage 8, 28. [Google Scholar]
- Stafford KC III, Williams SC, van Oosterwijk JG, Linske MA, Zatechka S, Richer LM, Molaei G, Przybyszewski C, Wikel SK, 2020. Field evaluation of a novel oral reservoir-targeted vaccine against Borrelia burgdorferi utilizing an inactivated whole-cell bacterial antigen expression vehicle. Exp. Appl. Acarol 80, 257–268. [DOI] [PubMed] [Google Scholar]
- Steere AC, Livey I, 2012. Lyme disease vaccines. In: Plotkin SA, Orenstein WA, Offit PA (Eds.), Vaccines, sixth edition. Elsevier, Philadelphia, PA, USA, pp. 1122–1132. [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JWR, Li X, Mead PS, 2016. Lyme borreliosis. Nat. Rev. Dis. Primers 2, 16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad M, Grubhoffer L, Rego ROM, 2020. Novel targets and strategies to combat borreliosis. Appl. Microbiol. Biotechnol 104, 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tälleklint-Eisen L, Lane RS, 2000. Efficiency of drag sampling for estimating population sizes of Ixodes pacificus (Acari: Ixodidae) nymphs in leaf litter. J. Med. Entomol 37, 484–487. [DOI] [PubMed] [Google Scholar]
- Telford SR III, 2017. Deer reduction is a cornerstone of integrated deer tick management. J. Integr. Pest Manage 8, 25. [Google Scholar]
- Vail SG, Smith G, 1998. Air temperature and relative humidity effects on behavioral activity of blacklegged tick (Acari: Ixodidae) nymphs in New Jersey. J. Med. Entomol 35, 1025–1028. [DOI] [PubMed] [Google Scholar]
- Valneva, 2020. Valneva and Pfizer Announce Collaboration to Co-develop and Commercialize Lyme disease Vaccine, VLA15. Accessed 23 October, 2020. https://valneva.com/press-release/valneva-and-pfizer-announce-collaboration-to-co-develop-and-commercialize-lyme-disease-vaccine-vla15/.
- Ward SE, Brown RD, 2004. A framework for incorporating the prevention of Lyme disease transmission into the landscape planning and design process. Landsc. Urban Plan 66, 91–106. [Google Scholar]
- White A, Gaff H, 2018. Review: application of tick control technologies for blacklegged, lone star, and American dog ticks. J. Integr. Pest Manage 9, 12. [Google Scholar]
- Williams SC, Ward JS, 2010. Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environ. Entomol 39, 1911–1921. [DOI] [PubMed] [Google Scholar]
- Williams SC, Ward JS, Worthley TE, Stafford KC III, 2009. Managing Japanese barberry (Ranunculales: Berberidaceae) infestations reduces blacklegged tick (Acari: Ixodidae) abundance and infection prevalence with Borrelia burgdorferi (Spirochaetales: Spirochaetaceae). Environ. Entomol 38, 977–984. [DOI] [PubMed] [Google Scholar]
- Williams SC, Linske MA, Ward JS, 2017. Long-term effects of Berberis thunbergii (Ranunculales: Berberidaceae) management on Ixodes scapularis (Acari: Ixodidae) abundance and Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) prevalence in Connecticut, USA. Environ. Entomol 46, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Williams SC, Stafford KC III, Molaei G, Linske MA, 2018. Integrated control of nymphal Ixodes scapularis: effectiveness of white-tailed deer reduction, the entomopathogenic fungus Metarhizium anisopliae, and fipronil-based rodent bait boxes. Vector Borne Zoonotic Dis. 18, 55–64. [DOI] [PubMed] [Google Scholar]
- Wilson ML, 1986. Reduced abundance of adult Ixodes dammini (Acari: Ixodidae) following destruction of vegetation. J. Econ. Entomol 79, 693–696. [DOI] [PubMed] [Google Scholar]
- Wilson ME, 2002. Prevention of tick-borne diseases. Med. Clin. N. Am 86, 219–238. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Deblinger RD, 1993. Vector management to reduce the risk of Lyme disease. In: Ginsberg HS (Ed.), Ecology and Environmental Management of Lyme Disease. Rutgers University Press, New Brunswick, NJ, USA, pp. 126–156. [Google Scholar]
- Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, Lindsay SW, 2015. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 31, 380–390. [DOI] [PubMed] [Google Scholar]


