Key Points
Question
Among patients taking a direct oral anticoagulant (DOAC) for atrial fibrillation and/or venous thromboembolism, what are the outcomes following concurrent acetylsalicylic acid (ASA, or aspirin) use?
Findings
In this registry-based cohort study, one-third of patients treated with DOACs were also taking ASA. Comparing 2 propensity score–matched groups of 1047 patients without a history of heart valve replacement or recent acute coronary syndrome, patients treated with combination therapy had significantly higher rates of bleeding and hospitalizations for bleeding; there was no observed difference in thrombosis rates.
Meaning
Many patients using DOAC therapy may be using concomitant ASA, which may increase bleeding risk with uncertain therapeutic benefit.
This cohort study evaluates whether dual therapy with acetylsalicylic acid and a therapeutically dosed direct oral anticoagulant increases rates of bleeding.
Abstract
Importance
It is unclear how many patients treated with a direct oral anticoagulant (DOAC) are using concomitant acetylsalicylic acid (ASA, or aspirin) and how this affects clinical outcomes.
Objective
To evaluate the frequency and outcomes of prescription of concomitant ASA and DOAC therapy for patients with atrial fibrillation (AF) or venous thromboembolic disease (VTE).
Design, Setting, and Participants
This registry-based cohort study took place at 4 anticoagulation clinics in Michigan from January 2015 to December 2019. Eligible participants were adults undergoing treatment with a DOAC for AF or VTE, without a recent myocardial infarction (MI) or history of heart valve replacement, with at least 3 months of follow-up.
Exposures
Use of ASA concomitant with DOAC therapy.
Main Outcomes and Measures
Rates of bleeding (any, nonmajor, major), rates of thrombosis (stroke, VTE, MI), emergency department visits, hospitalizations, and death.
Results
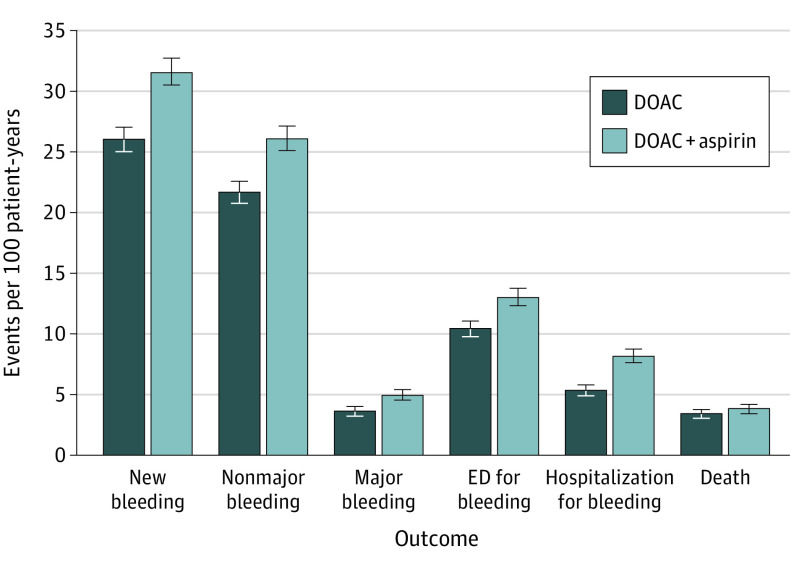
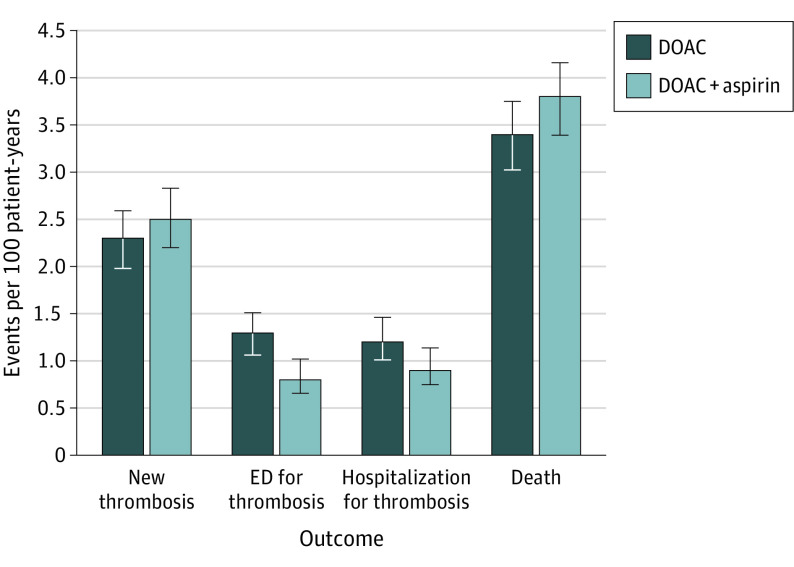
Of the study cohort of 3280 patients (1673 [51.0%] men; mean [SD] age 68.2 [13.3] years), 1107 (33.8%) patients without a clear indication for ASA were being treated with DOACs and ASA. Two propensity score–matched cohorts, each with 1047 patients, were analyzed (DOAC plus ASA and DOAC only). Patients were followed up for a mean (SD) of 20.9 (19.0) months. Patients taking DOAC and ASA experienced more bleeding events compared with DOAC monotherapy (26.0 bleeds vs 31.6 bleeds per 100 patient years, P = .01). Specifically, patients undergoing combination therapy had significantly higher rates of nonmajor bleeding (26.1 bleeds vs 21.7 bleeds per 100 patient years, P = .02) compared with DOAC monotherapy. Major bleeding rates were similar between the 2 cohorts. Thrombotic event rates were also similar between the cohorts (2.5 events vs 2.3 events per 100 patient years for patients treated with DOAC and ASA compared with DOAC monotherapy, P = .80). Patients were more often hospitalized while undergoing combination therapy (9.1 vs 6.5 admissions per 100 patient years, P = .02).
Conclusion and Relevance
Nearly one-third of patients with AF and/or VTE who were treated with a DOAC received ASA without a clear indication. Compared with DOAC monotherapy, concurrent DOAC and ASA use was associated with increased bleeding and hospitalizations but similar observed thrombosis rate. Future research should identify and deprescribe ASA for patients when the risk exceeds the anticipated benefit.
Introduction
Acetylsalicylic acid (ASA), or aspirin, is commonly used for a variety of clinical indications including the primary prevention of coronary artery disease (CAD),1,2 for management of stable ischemic heart disease,3 in peripheral arterial disease (PAD),4,5 and/or for the secondary prevention of stroke after noncardioembolic stroke or transient ischemic attack.6,7 The combination of ASA with oral anticoagulation can be indicated for patients with certain devices (eg, left ventricular assist devices) as well as for patients with nonvalvular atrial fibrillation and/or venous thromboembolism (VTE) who experience an acute coronary syndrome (ACS) and/or undergo percutaneous coronary intervention (PCI).8,9 Aside from these situations and select other clinical scenarios, there is evidence that the combination of ASA and an oral anticoagulant may increase bleeding, without a significant reduction in thrombotic outcomes.10,11,12,13 Recent guidelines14 recommend against prophylactic ASA use for patients at an increased risk of bleeding with concurrent use of anticoagulants. With hundreds of thousands of patients15 on combination therapy with an anticoagulant and antiplatelet medication, it is critical to develop a better understanding of which patients treated with oral anticoagulation should also receive ASA.
Consistent with other studies, we have previously shown10 excess bleeding with combination ASA and warfarin therapy in patients who do not have a clear indication for ASA. However, it is unknown if dual therapy with ASA and a therapeutically dosed direct oral anticoagulant (DOAC) similarly increases bleeding. Based on the results of the COMPASS trial,16 increased bleeding could be hypothesized but the magnitude of effect for therapeutic DOACs is uncertain. Compared with dual therapy with ASA and warfarin, one could hypothesize a lower rate of bleeding in patients taking ASA plus DOAC given the overall safer profile of DOAC medications as compared with warfarin.17 However, some studies have reported higher rates of gastrointestinal bleeding with DOACs as compared with warfarin.18,19,20,21 When combining treatment doses of DOACs with ASA, it is plausible that overall bleeding rates could be elevated beyond those seen with combination ASA and warfarin.
We determined the frequency of concomitant ASA use for patients treated with DOACs for nonvalvular atrial fibrillation or VTE in an observational practice-based setting. Furthermore, we compared the patient characteristics and outcomes of concurrent DOAC-ASA use with DOAC monotherapy.
Methods
Study Design and Participants
A registry was created of adult patients newly starting a DOAC (apixaban, dabigatran, edoxaban, or rivaroxaban) and of patients transitioning to a DOAC from warfarin at 4 medical centers in the state of Michigan. These data were collected as part of the Blue Cross Blue Shield of Michigan–sponsored Michigan Anticoagulation Quality Improvement Initiative (MAQI2). This study is a collaborative of outpatient anticoagulation clinics in Michigan that includes both academic and community practices; all forms of health insurance are included.22 This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.23
For this study, data were assessed between January of 2015 and December of 2019. Included patients started DOAC therapy either without recent anticoagulant use or in transition from warfarin therapy. For our primary analysis, patients were excluded from our study if they had less than 3 months of follow-up data available, a myocardial infarction (MI) within 6 months prior to DOAC initiation, and/or history of heart valve replacement. Use of ASA was defined based on medications assessed at the time of DOAC initiation. Patients meeting the study inclusion criteria and not on ASA were included in the DOAC monotherapy group while those on any dose of ASA were included in the DOAC combination therapy group. Only 1 of the 4 centers participating in this study routinely enrolls patients treated with DOACs in anticoagulation clinic monitoring. More specifically, 1 of the 4 centers uses anticoagulant specialty pharmacists to educate patients and review DOAC prescriptions for indication, drug interactions, and dosing based on weight or renal function. Therefore, medication prescriptions largely reflect the clinical practice from a wide group of clinicians (eg, primary care, cardiology, hematology).
Data Collection and Outcome Measures
Data were abstracted from the time of DOAC initiation through the earliest time of either DOAC discontinuation, transition to an alternative anticoagulant, patient death, the patient being lost to follow-up, or the end of the study period. The data collection was performed by trained abstractors using standardized data collection forms. Exposures and most outcomes were verified through random chart audits that were performed by the coordinating center (University of Michigan) to ensure that the abstracted data matched the information contained in the primary electronic medical record. This was done as part of routine practice of the MAQI2 registry. This study was approved by the institutional review board at each of the participating centers. Because of the nature of this study, a waiver of informed consent was granted by the institutional review boards at each participating center and the coordinating center.
The data registries were designed to require entry to key data elements, including demographic data, DOAC dosing, and details about any adverse events (eg, location of a bleeding event). Through combined use of wide-ranging validation rules during data entry and an automated program that identifies missing information and prompts for completion and correction, there were no missing data in the variables used in the analysis.
For each patient, comprehensive data were collected at study enrollment. This included patient demographics, including race as defined by the participant, as recorded in the medical record. We also collected information on patient comorbidities, bleeding and thrombosis risk factors, histories of bleeding or thrombosis, and concomitant medications (including antiplatelet therapies other than ASA). Concomitant medications assessed included nonsteroidal anti-inflammatory drugs and other non-ASA antiplatelet therapies. After enrollment, charts were abstracted at approximately 6-month intervals.
The primary study outcome was the rate of bleeding events (which included any patient-reported bleeding event, regardless of severity). Secondary bleeding outcomes included major bleeding as defined by the International Society on Thrombosis and Hemostasis,24 nonmajor bleeding (defined as any bleeding event that did not meet criteria for major bleeding), emergency department (ED) visits for bleeding, and hospitalizations related to bleeding. Major bleeding included outcomes of fatal bleeding, life-threatening bleeding, and intracranial or intraspinal bleeding. Life-threatening bleeding included intracranial bleeding, a 5 g per dL drop in hemoglobin, more than 4 units of blood transfused, or bleeding that required surgical intervention. Secondary thrombotic outcomes included ischemic strokes, transient ischemic attack, VTE, ACS/MI, ED visits for thrombosis, and hospitalizations for thrombosis. Mortality data were also collected. A hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, and drugs/alcohol concomitantly (HAS-BLED) score,25 modified to exclude ASA use and labile international normalized ratio, and a Charlson Comorbidity Index score26 was calculated for each patient at the time of enrollment.
Statistical Analysis
Based on clinical and demographic factors (eFigure 1 in the Supplement), propensity scores were generated as a probability of receiving DOAC plus ASA through a logistic regression model. The propensity score was used to produce matched DOAC plus ASA and DOAC groups using an optimal matching method (1:1).27 The optimal matching selects all matches simultaneously and without replacement to minimize the total absolute difference in propensity score across all matches. A standardized difference of less than 0.1 was used to indicate a negligible difference in the covariates between the groups. Covariates that maintained residual differences between the groups were included in subsequent Poisson models.
After the matched cohorts were created (Table 1), we compared event rates between the 2 outcome groups (DOAC plus ASA and DOAC monotherapy) using Poisson regression. These analyses were performed for each of our primary outcomes listed in Table 2.
Table 1. Characteristics of Patients Receiving Combination DOAC and Aspirin vs DOAC Monotherapy Before and After Propensity Score Matchinga.
| Characteristic | Matching, No. (%) | |||||
|---|---|---|---|---|---|---|
| Before | After | |||||
| DOAC (n = 2173) | DOAC+ASA (n = 1107) | Standard difference | DOAC (n = 1047) | DOAC+ASA (n = 1047) | Standard difference | |
| DOAC | ||||||
| Apixaban | 1189 (54.7) | 681 (61.5) | −0.132 | 646 (61.7) | 653 (62.4) | −0.014 |
| Dabigatran | 165 (7.6) | 82 (7.4) | 0.007 | 93 (8.9) | 69 (6.6) | 0.092 |
| Edoxaban | 2 (0.1) | 2 (0.2) | 0.001 | 1 (0.1) | 1 (0.1) | 0.000 |
| Rivaroxaban | 817 (37.6) | 342 (30.9) | 0.133 | 307 (29.3) | 324 (31) | −0.034 |
| DOAC doseb | ||||||
| Low | 337 (16.0) | 225 (21.0) | 0.140 | 206 (19.7) | 221 (21.1) | 0.037 |
| High | 1769 (84.0) | 845 (79.0) | 841 (80.3) | 826 (78.9) | ||
| Demographics | ||||||
| Age, mean (SD), y | 66.3 (15.4) | 72.0 (11.1) | 0.421 | 71.9 (12.0) | 71.9 (11.1) | 0.002 |
| Gender (% male) | 1032 (47.6) | 641 (57.9) | −0.203 | 603 (57.6) | 603 (57.6) | 0.000 |
| BMIc >30 kg/m2 | 1045 (49.3) | 564 (52.1) | −0.071 | 564 (53.9) | 553 (52.8) | 0.021 |
| Alcohol or drug use | 136 (6.3) | 86 (7.8) | −0.060 | 68 (6.5) | 83 (7.9) | −0.056 |
| Tobacco use | ||||||
| Former | 721 (33.2) | 487 (44.0) | −0.215 | 446 (42.6) | 462 (44.1) | −0.032 |
| Current | 189 (8.7) | 81 (7.3) | 0.050 | 70 (6.7) | 77 (7.4) | −0.025 |
| Indication | ||||||
| AF/Aflutter | 1316 (60.6) | 868 (78.4) | −0.383 | 822 (78.5) | 815 (77.8) | 0.015 |
| DVT/PE | 876 (40.3) | 264 (23.9) | 0.348 | 235 (22.5) | 253 (24.2) | −0.037 |
| Both | 19 (0.9) | 25 (2.3) | −0.121 | 10 (1.0) | 21 (2.0) | −0.092 |
| Comorbidities | ||||||
| CAD | 320 (14.7) | 497 (44.9) | −0.709 | 294 (28.1) | 469 (44.8) | −0.389 |
| Cancer | 483 (22.2) | 280 (25.3) | −0.074 | 258 (24.6) | 267 (25.5) | −0.020 |
| CHF | 318 (14.6) | 291 (26.3) | −0.290 | 230 (22.0) | 273 (26.1) | −0.103 |
| Chronic liver disease | 74 (3.4) | 49 (4.4) | −0.057 | 37 (3.5) | 48 (4.6) | −0.054 |
| CKD | 343 (15.8) | 244 (22.0) | −0.161 | 221(21.1) | 234 (22.4) | −0.032 |
| Diabetes mellitus | 471 (21.7) | 358 (32.3) | −0.265 | 318 (30.4) | 350 (33.4) | −0.069 |
| History of falls | 120 (5.5) | 90 (8.1) | −0.115 | 64 (6.1) | 87 (8.3) | −0.087 |
| Hypercoagulable state | 62 (2.9) | 14 (1.3) | 0.121 | 6 (0.6) | 11 (1.1) | −0.035 |
| HTN | 1143 (52.6) | 696 (62.9) | −0.229 | 689 (65.8) | 681 (65.0) | 0.016 |
| PAD | 97 (4.5) | 115 (10.4) | −0.242 | 72 (6.9) | 110 (10.5) | −0.140 |
| Prior PCI/CABG | 128 (5.9) | 260 (23.5) | −0.502 | 120 (11.5) | 242 (23.1) | −0.341 |
| History of bleeding or thrombosisd | ||||||
| Bleeding, d | ||||||
| ≤30 | 92 (4.2) | 42 (3.8) | 0.021 | 42 (4.0) | 40 (3.8) | 0.010 |
| >30 | 115 (5.3) | 61 (5.5) | −0.018 | 61 (5.8) | 60 (5.7) | 0.004 |
| History of embolism (not DTE/PE) | 28 (1.3) | 19 (1.7) | −0.033 | 14 (1.3) | 18 (1.7) | −0.031 |
| Prior | ||||||
| CVA/TIA | 217 (10.0) | 171 (15.5) | −0.158 | 154 (14.7) | 161 (15.4) | −0.020 |
| DVT/PE | 232 (10.7) | 106 (9.6) | 0.000 | 98 (9.4) | 103 (9.8) | −0.016 |
| GIB | 126 (5.8) | 72 (6.5) | −0.029 | 71 (6.8) | 69 (6.6) | 0.008 |
| Remote MI (>6 mo) | 86 (4.0) | 167 (15.1) | −0.397 | 75 (7.2) | 161 (15.4) | −0.284 |
| Medications | ||||||
| Aspirin, mg | ||||||
| ≤100 | N/A | 997 (90.1) | N/A | N/A | 948 (90.5) | N/A |
| >100 | N/A | 110 (9.9) | N/A | N/A | 99 (9.5) | N/A |
| Estrogen/progesterone | 46 (2.1) | 6 (0.5) | 0.139 | 15 (1.4) | 6 (0.6) | 0.074 |
| Non-ASA antiplatelet | 53 (2.4) | 40 (3.6) | −0.056 | 45 (4.3) | 36 (3.4) | 0.051 |
| NSAID | 94 (4.3) | 39 (3.5) | 0.038 | 32 (3.1) | 38 (3.6) | −0.029 |
| Months of follow-up, median (IQR) | 12 (6, 24) | 12 (6, 30) | 0.020 | 12 (6, 30) | 12 (6, 30) | 0.870 |
| Other, mean (SD) | ||||||
| Modified HAS-BLEDe | 2.0 (1.2) | 2.6 (1.1) | 0.451 | 2.4 (1.2) | 2.6 (1.2) | 0.123 |
| CCI | 4.0 (2.1) | 5.1 (1.9) | 0.522 | 4.8 (1.9) | 5.1 (1.9) | 0.129 |
Abbreviations: AF, atrial fibrillation; Aflutter, atrial flutter; ASA, acetylsalicylic acid or aspirin; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; CKD, chronic kidney disease; CVA, cerebrovascular accident; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; GIB, gastrointestinal bleed; HAS-BLED, hypertension abnormal renal/liver function stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; HTN, hypertension; IQR, interquartile range; MI, myocardial infarction; N/A, not applicable; NSAID, nonsteroidal anti-inflammatory drug; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PE, pulmonary embolism; TIA, transient ischemic attack.
Denominator is equal to No. at the top of the column.
Low dose defined as a total daily dose of dabigatran <300 mg, apixaban <10 mg, rivaroxaban <20 mg, edoxaban <60 mg. High dose is considered a total daily dose of dabigatrain ≥300 mg, apixaban ≥10 mg, rivaroxaban ≥20 mg, and edoxaban ≥60 mg.
Body mass index calculated as weight in kilograms divided by height in meters squared.
Bleeding history as assessed at the time of warfarin initiation.
HAS-BLED score modified to exclude aspirin use.
Table 2. Outcomes of DOAC vs DOAC Plus ASA.
| Outcome | No. per 100 patient-years (95% CI) | P value | |
|---|---|---|---|
| DOAC (n = 1047) | DOAC+ASA (n = 1047) | ||
| New thrombosis | 2.30 (1.98-2.59) | 2.50 (2.20-2.83) | .80 |
| Ischemic/embolic stroke | 0.90 (0.71-1.08) | 0.70 (0.55-0.90) | .31 |
| TIA | 0.20 (0.10-0.27) | 0.10 (0.05-0.20) | .58 |
| PE | 0.20 (0.14-0.33) | 0.20 (0.09-0.26) | .67 |
| DVT | 0.40 (0.28-0.53) | 0.90 (0.70-1.07) | .07 |
| MI | 0.10 (0.05-0.20) | 0.10 (0.02-0.12) | .51 |
| New bleed | 26.00 (25.05-27.06) | 31.60 (30.54-32.75) | .009 |
| Major | 3.59 (3.23-3.98) | 4.95 (4.52-5.41) | .09 |
| Fatal | 0.06 (0.02-0.13) | 0.05 (0.02-0.12) | .82 |
| Intracranial or intraspinal | 0.39 (0.28-0.53) | 0.33 (0.23-0.46) | .75 |
| Life threatening | 1.05 (0.86-1.27) | 1.42 (1.20-1.67) | .38 |
| Nonmajor | 21.70 (20.77-22.60) | 26.10 (25.14-27.15) | .02 |
| ED visit | 11.50 (10.84-12.18) | 13.80 (13.05-14.52) | .14 |
| For bleeding | 10.40 (9.77-11.04) | 13.00 (12.31-13.74) | .08 |
| For clotting | 1.30 (1.06-1.51) | 0.80 (0.65-1.02) | .12 |
| Hospitalization | 6.50 (6.03-7.04) | 9.10 (8.51-9.70) | .02 |
| For bleeding | 5.30 (4.87-5.78) | 8.20 (7.62-8.75) | .006 |
| For clotting | 1.20 (1.01-1.46) | 0.90 (0.75-1.14) | .31 |
| Death | 3.40 (3.02-3.75) | 3.80 (3.39-4.16) | .76 |
Abbreviations: ASA, acetylsalicylic acid or aspirin; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; ED, emergency department; MI, myocardial infarction; PE, pulmonary embolism; TIA, transient ischemic attack.
Patients with CAD, PAD, prior PCI/CABG, or remote MI were included for the primary analysis. However, a predefined subgroup analysis was performed to isolate those patients without any CAD, PAD, prior MI, prior PCI/CABG (eFigure 2 in the Supplement, Sensitivity Analysis 1). Given the potential for ASA use to be dynamic, an additional sensitivity analysis was performed where patients were assigned to groups based on their enrollment medications and patients who later stopped or started ASA were excluded (eFigure 2 in the Supplement, Sensitivity Analysis 2). While our initial analysis was matched based on the dose of the DOACs, we also conducted a sensitivity analysis (eFigure 2 in the Supplement, Sensitivity Analysis 3) where we did not match on DOAC dose, given that DOAC dosing may change longitudinally, with indication, and for multiple other clinical factors. It was unclear how DOAC dose could affect results. We did not match on specific DOAC drugs.
For each of the 3 sensitivity analyses outlined above, we repeated the propensity match. We then assessed event rates of matched cohorts generated from each unique set of inclusion and exclusion criteria. A 2-sided P < .05 was considered significant for all comparisons. All statistical analyses were carried out using SAS version 9.4 and R 3.4.1.
Results
The study cohort consisted of 3280 patients without a clear indication for ASA (1673 [51.0%] men, mean [SD] age of 68.2 [13.3] years). Over the study period, 1107 of 3280 (33.8%) patients were treated with DOACs plus ASA compared with 2173 of 3280 (66.3%) patients treated with DOAC monotherapy. The percentage of patients on combination therapy did not change significantly over time (eFigure 3 in the Supplement). Low-dose ASA (dose ≤100 mg) was used by 997 (90.1%) of all patients on ASA.
Two propensity score–matched cohorts of 1047 patients were analyzed (DOAC plus ASA and DOAC only). After propensity score matching there were limited outstanding differences between the outcome groups (Table 1; eFigure 2 in the Supplement), which were then included in the Poisson models.
Patients were followed up for a median (interquartile range) of 12 (6, 30) months. As shown in Table 2, matched patients taking DOAC plus ASA experienced more total bleeding events compared with DOAC monotherapy (26.0 bleeds vs 31.6 bleeds per 100 patient years, P = .01). Specifically, patients on combination therapy had significantly higher rates of nonmajor bleeding compared with those on DOAC monotherapy. Major bleeding rates were not statistically different between the two groups (Figure 1). Major bleeding subtypes (fatal, intracranial/intraspinal, and life threatening) were not statistically different. Major rates of gastrointestinal bleeding showed no statistically significant difference but minor gastrointestinal bleeds were increased with combination therapy (4.6 bleeds vs 3.0 bleeds per 100 patient years, P = .03; eTable 1 in the Supplement). Thrombotic event rates were overall low but similar between the groups (Figure 2). Patients were more often hospitalized on combination therapy vs DOAC monotherapy (9.0 vs 6.5 admissions per 100 patient years, P = .02). More specifically, hospitalizations for bleeding were higher with DOAC plus ASA compared with DOAC monotherapy while hospitalizations for thrombotic outcomes were similar. Mortality and ER visits for bleeding rates were similar between the 2 groups.
Figure 1. Bleeding Outcomes for DOACs vs DOACs plus ASA.
Figure reflects outcomes from propensity matched cohorts. Bleed is an aggregate outcome including major and nonmajor bleeding. Abbreviations: ASA, acetylsalicylic acid or aspirin; DOAC, direct oral anticoagulant; ED, emergency department.
Figure 2. Thrombosis Outcomes for DOACs vs DOACs plus ASA.
Figure reflects outcomes from propensity matched cohorts. Thrombosis is an aggregate outcome including ischemic/embolic stroke, transient ischemic attack, pulmonary embolism, deep vein thrombosis, and/or myocardial infarction. Abbreviations: ASA, acetylsalicylic acid or aspirin; DOAC, direct oral anticoagulant; ED, emergency department.
Sensitivity Analyses
In the first sensitivity analysis, excluding patients with any history of MI, CAD, PAD, or PCI/CABG, we compared 516 matched pairs instead of 1047 matched pairs included in the primary analysis. Bleeding rates were similarly elevated, but the increase was no longer statistically significant (eTable 2 in the Supplement).
In the second sensitivity analysis, excluding patients who started or stopped ASA after study enrollment, we compared 621 matched pairs. Emergency department visits and hospitalizations for bleeding were significantly increased for patients treated with DOAC plus ASA (14.1 ED visits vs 10.3 ED visits per 100 patient years, P = .04; 9.6 admissions vs 5.8 admissions per 100 patient years, P = .02, respectively). Otherwise there were no significant differences between the 2 groups (eTable 3 in the Supplement) for other outcomes.
For the third sensitivity analysis, where DOAC dose was not included in the propensity score match, we again compared 1047 matched pairs. The findings were largely unchanged from those of the primary analysis. However, the outcome of all hospitalizations no longer showed a statistically significant increase for patients receiving combination therapy. A statistically higher rate of ED visits for clotting was observed for DOAC monotherapy compared with DOAC plus ASA (1.47 vs 0.82 ER visits per 100 patient years, P = .04, eTable 4 in the Supplement) but overall rates of thrombosis were similar to those from the primary analysis.
Discussion
In this large, registry-based cohort study of patients treated with DOACs for AF and/or VTE, one-third of patients were taking concomitant ASA without a well-defined therapeutic indication. Treatment with combination DOAC and ASA therapy was associated with increased bleeding rates. In particular, nonmajor bleeding was increased, as were admissions related to bleeding. Rates of thrombotic outcomes appeared similar in the 2 treatment groups.
In our sensitivity analyses limiting the study to patients without any apparent vascular indication for ASA (eTable 1 in the Supplement, n = 516 matched pairs) while taking a DOAC and to patients who maintained their treatment group assignment (eTable 2 in the Supplement, n = 621 matched pairs), outcomes were similar to those of the primary analysis. Despite lower power to detect statistically significant differences, the point estimates in all 3 sensitivity analyses supported the findings of the primary analysis. When a specific DOAC dose was not included in the propensity score match (eTable 3 in the Supplement), we did observe a statistically higher rate of ED visits for thrombosis but no difference in overall thrombosis rates, thrombosis rates by clotting subtype, and admissions for thrombosis. This finding is of unclear clinical significance and merits ongoing study; overall we did not observe a clear thrombotic benefit with combination therapy.
There remains limited evidence on how to optimally combine DOACs with ASA outside the setting of an ACS and/or PCI procedure. The randomized OAC-ALONE trial28 evaluated oral anticoagulation with or without concomitant antiplatelet therapy for patients with stable CAD and atrial fibrillation. This study was terminated early due to low patient enrollment and accordingly was underpowered to answer this question. Additionally, only one-quarter of the patients were prescribed DOAC therapy. The subsequent AFIRE trial29 similarly studied patients with stable coronary artery disease (history of PCI with stenting, CAD, or history of CABG) and atrial fibrillation, evaluating rivaroxaban monotherapy compared with rivaroxaban and antiplatelet therapy. This randomized study was conducted in Japan and was terminated early due to increased mortality with combination therapy. Rivaroxaban was noninferior for the efficacy end point and superior for the safety end point.29 The AFIRE trial was similar in size and results to our practice-based population. Notable differences in our study as compared with the AFIRE trial include no mortality difference in our population, limiting the analysis to patients on ASA vs other antiplatelet agents, broad DOAC medication and dose use, inclusion of patients with VTE, and a largely White population. Therefore, it is probable that the baseline thrombotic risk differed between the 2 populations. Ultimately, while emerging data seem to suggest that anticoagulant monotherapy alone may be sufficient for some patients, further confirmation of these findings is necessary.
It is important to acknowledge that there are numerous patient subgroups and clinical scenarios where the role of combination therapy compared with that of anticoagulant monotherapy has not been sufficiently studied. Examples include patients with vascular stents, myeloproliferative neoplasms, poorly controlled vascular risk factors, and thrombophilias. In such scenarios, shared decision-making and individualized care should remain standard.
We did not see a substantial decline in the number of patients on combination therapy over time (eFigure 3 in the Supplement). This may reflect the overall lack of data on how to optimally manage such patients, especially those with multiple vascular risk factors. The proportion of patients taking combination DOAC plus ASA therapy, about one-third, is similar to that observed in our prior analysis of patients treated with warfarin plus ASA.10 It is notable that about half of the patients receiving combination therapy had no apparent vascular indication to suggest a possible need to add ASA (eTable 1 in the Supplement). It is possible that such patients would benefit from efforts to deprescribe ASA to patients on systemic anticoagulation.
Strengths and Limitations
Strengths of the present study include use of real-world patients with robust statistical methods, including use of a propensity score match. We were not able to fully match on all included variables but those with residual differences were included in the Poisson regression models (eFigure 1 in the Supplement). While ASA use was not randomized, the propensity score match based on numerous covariates allowed us to reduce the impact of selection biases and compare 2 matched groups. The study included patients with atrial fibrillation but offers unique insight on this topic by including patients anticoagulated for VTE. Additionally, several sensitivity analyses supported our study findings. The present study largely included patients newly prescribed DOACs (745 of 1047, 71.2%) but also included patients who switched to DOACs from warfarin, making it generalizable to routine clinical practice. Patients were followed for relevant clinical outcomes for a median of 1 year but up to 5 years using predefined forms for data abstraction, and random medical record audits were conducted to verify data accuracy. Patients received routine anticoagulation care and were managed by a spectrum of medical specialties.
Limitations of the study include those inherent to the registry design including the potential for unmeasured or uncaptured confounding variables. While abstractors have extensive training, come largely from clinical backgrounds, and have well defined variables to assess for, we did not specifically assess interrater reliability for this study. Routine audits have not found significant discrepancies between our registry and the primary electronic medical record. Patients were only in the state of Michigan. While we still believe the results are generalizable, it was a geographically limited population. Use of ASA was specifically assessed at the time of study enrollment and some patients had been on ASA for some time prior to study entry. While attention was given to ASA use in follow-up, it is possible that changes in this nonprescription medication were not well captured in the registry. The sensitivity analysis limiting the study to patients who maintained their ASA use throughout the study period supported the findings of the primary analysis (Sensitivity Analysis 2). It is possible that not all clinical outcomes were captured, especially if patients received their care outside our research collaborative and this outside medical care was not subsequently documented in follow-up. Therefore, the observed event rates may underestimate the true event rates.
To have sufficient power to answer the study question, we evaluated patients with both AF and VTE. While we matched on indication for anticoagulation, it is not certain if patients with VTE and AF have different net risk with combination therapy. While we believe this study reflects actual clinical practice, all of our centers are led by anticoagulation experts who regularly engage in quality improvement activities pertaining to anticoagulation. It is possible that this would bias the results compared with other centers without these resources. Finally, the overall rates of thrombosis and many bleeding subtypes were low. Accordingly, this study is likely underpowered to make conclusions about thrombotic outcomes and outcomes of some bleeding subtypes. It is also not able to distinguish outcomes based on the specific type of DOAC.
Conclusions
Concurrent use of a DOAC and ASA in patients without a recent ACS or heart valve replacement is common and associated with increased bleeding and related hospitalization. Further research is needed to determine if select high-risk patient subgroups derive a net benefit from combination therapy. Efforts should be made to help clinicians identify and deprescribe ASA for patients taking a DOAC without an indication for ASA.
eTable 1. Rates of Gastrointestinal Bleeding (per 100 patient years) for DOAC versus DOAC+ASA
eTable 2. Outcomes of DOAC versus DOAC+ASA (rates per 100 patient years) for Patients Without Any History of MI, CAD, PAD, PCI/CABG
eTable 3. Outcomes of DOAC versus DOAC+ASA (rates per 100 patient years) for Patients Continuously on ASA or Not on ASA Throughout Follow-up
eTable 4. Outcomes of DOAC versus DOAC+ASA (rates per 100 patient years) Not Matching on DOAC Dose
eFigure 1. Standardized Difference Plot
eFigure 2. Study Schema
eFigure 3. Percent of Patients Without Recent Myocardial Infarction or Valve Replacement on Aspirin and DOACs by Year
References
- 1.Bibbins-Domingo K, US Preventive Services Task Force . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 2.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e637S-e668S. doi: 10.1378/chest.11-2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):3097-3137. doi: 10.1161/CIR.0b013e3182776f83 [DOI] [PubMed] [Google Scholar]
- 4.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686-e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso-Coello P, Bellmunt S, McGorrian C, et al. Antithrombotic therapy in peripheral artery disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e669S-e690S. doi: 10.1378/chest.11-2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 7.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e601S-e636S. doi: 10.1378/chest.11-2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-e425. doi: 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 9.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e531S-e575S. doi: 10.1378/chest.11-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer JK, Li Y, Gu X, et al. Association of adding aspirin to warfarin therapy without an apparent indication with bleeding and other adverse events. JAMA Intern Med. 2019;179(4):533-541. doi: 10.1001/jamainternmed.2018.7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg BA, Kim S, Piccini JP, et al. ; ORBIT-AF Investigators and Patients . Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation. 2013;128(7):721-728. doi: 10.1161/CIRCULATIONAHA.113.002927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson SG, Rogers K, Delate T, Witt DM. Outcomes associated with combined antiplatelet and anticoagulant therapy. Chest. 2008;133(4):948-954. doi: 10.1378/chest.07-2627 [DOI] [PubMed] [Google Scholar]
- 13.So CH, Eckman MH. Combined aspirin and anticoagulant therapy in patients with atrial fibrillation. J Thromb Thrombolysis. 2017;43(1):7-17. doi: 10.1007/s11239-016-1425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douketis JD. Combination warfarin-ASA therapy: which patients should receive it, which patients should not, and why? Thromb Res. 2011;127(6):513-517. doi: 10.1016/j.thromres.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 16.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 17.Chai-Adisaksopha C, Hillis C, Isayama T, Lim W, Iorio A, Crowther M. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;13(11):2012-2020. doi: 10.1111/jth.13139 [DOI] [PubMed] [Google Scholar]
- 18.Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320(21):2221-2230. doi: 10.1001/jama.2018.17242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. doi: 10.1136/bmj.h1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ETTL. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145(1):105-112.e15. doi: 10.1053/j.gastro.2013.02.041 [DOI] [PubMed] [Google Scholar]
- 21.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 22.Barnes GD, Kline-Rogers E. Engaging with quality improvement in anticoagulation management. J Thromb Thrombolysis. 2015;39(3):403-409. doi: 10.1007/s11239-015-1184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 25.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173-180. doi: 10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. doi: 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumura-Nakano Y, Shizuta S, Komasa A, et al. ; OAC-ALONE Study Investigators . Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139(5):604-616. doi: 10.1161/CIRCULATIONAHA.118.036768 [DOI] [PubMed] [Google Scholar]
- 29.Yasuda S, Kaikita K, Akao M, et al. ; AFIRE Investigators . Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381(12):1103-1113. doi: 10.1056/NEJMoa1904143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Rates of Gastrointestinal Bleeding (per 100 patient years) for DOAC versus DOAC+ASA
eTable 2. Outcomes of DOAC versus DOAC+ASA (rates per 100 patient years) for Patients Without Any History of MI, CAD, PAD, PCI/CABG
eTable 3. Outcomes of DOAC versus DOAC+ASA (rates per 100 patient years) for Patients Continuously on ASA or Not on ASA Throughout Follow-up
eTable 4. Outcomes of DOAC versus DOAC+ASA (rates per 100 patient years) Not Matching on DOAC Dose
eFigure 1. Standardized Difference Plot
eFigure 2. Study Schema
eFigure 3. Percent of Patients Without Recent Myocardial Infarction or Valve Replacement on Aspirin and DOACs by Year