Key Points
Question
What is the prevalence of incidentally detected signs of chronic intracranial hypertension (IH) on magnetic resonance imaging (MRI) among outpatients undergoing brain MRI for any clinical indication, and are these radiographic signs associated with papilledema?
Findings
In this cross-sectional study of 296 consecutive patients undergoing brain MRI, 49% of patients had at least 1 MRI finding suggestive of chronic IH. However, the prevalence of papilledema detected by ocular fundus photography performed at the time of MRI was only 2%.
Meaning
This study’s findings indicate that MRI signs of chronic IH are common among patients undergoing brain MRI but are rarely associated with papilledema.
Abstract
Importance
Magnetic resonance imaging (MRI) signs of intracranial hypertension (IH) are traditionally associated with idiopathic intracranial hypertension (IIH), but these signs are also detected among individuals with primary headaches and among asymptomatic individuals without papilledema.
Objective
To examine the prevalence of MRI signs of IH among consecutive outpatients undergoing brain MRI for any clinical indication and to explore their association with papilledema.
Design, Setting, and Participants
This prospective cross-sectional study of outpatients undergoing brain MRI at 1 outpatient imaging facility was conducted between August 1, 2019, and March 31, 2020, with ocular fundus photographs taken concurrently. Radiographic images from consecutive adult patients who were undergoing brain MRI and able to participate in fundus photography were analyzed for MRI signs of IH. A univariate analysis using either Fisher exact tests or t tests was performed.
Main Outcomes and Measures
Prevalence of MRI signs of IH and prevalence of papilledema detected on ocular fundus photographs. Radiographic signs of IH included empty sella, optic nerve head protrusion, posterior scleral flattening, increased perioptic cerebrospinal fluid, optic nerve tortuosity, enlarged Meckel caves, cephaloceles, cerebellar tonsillar descent, and bilateral transverse venous sinus stenosis.
Results
A total of 388 patients were screened for eligibility; of those, 92 patients were excluded (58 declined participation, 16 were unable to consent, 14 were unable to complete fundus photography, and 4 completed MRI and fundus photography twice, so their second set of findings was removed). Among the 296 patients included in the study, the median age was 49.5 years (interquartile range, 37.8-62.0 years), and 188 patients (63.5%) were female. The most common indication for MRI was surveillance of a brain neoplasm (82 patients [27.7%]). Investigations of headaches (26 patients [8.8%]) and disorders of intracranial pressure (4 patients [1.4%]) were uncommon. At least 1 radiographic sign of IH was present in 145 patients (49.0%). Among 296 total study patients, 98 patients (33.1%) had empty sella, 47 patients (15.9%) had enlarged Meckel caves, 32 patients (10.8%) had increased perioptic cerebrospinal fluid, 23 patients (7.8%) had optic nerve tortuosity, 2 patients (0.7%) had scleral flattening, and 4 patients (1.4%) had cephaloceles. Bilateral transverse venous sinus stenosis was present in 6 of 198 patients (3.0%). Five patients (1.7%) had papilledema. Compared with patients without papilledema, those with papilledema had a significantly higher body mass index and history of IIH, in addition to an increased prevalence of empty sella, optic nerve tortuosity, and transverse venous sinus stenosis detected on MRI. The prevalence of papilledema increased from 2.8% among patients with at least 1 MRI sign of IH to 40.0% among patients with 4 or more MRI signs of IH.
Conclusions and Relevance
Magnetic resonance imaging signs of IH were common among patients undergoing brain MRI in this study but rarely associated with papilledema. The management of patients with incidentally detected signs of IH likely does not require systematic lumbar puncture unless concerning symptoms or papilledema are present.
This cross-sectional study examines the prevalence of incidentally detected signs of intracranial hypertension on magnetic resonance imaging and assesses whether these radiographic signs are associated with papilledema among adult patients.
Introduction
Signs of intracranial hypertension (IH) detected on magnetic resonance imaging (MRI), such as empty sella, increased perioptic cerebrospinal fluid (CSF), optic nerve tortuosity, and transverse venous sinus stenosis (TVSS) are typically associated with idiopathic intracranial hypertension (IIH) but are also present among patients with primary headache syndromes and asymptomatic individuals.1,2,3,4 The prevalence of empty sella in a general patient population is estimated to be between 8% and 35% and is more common in women and obese individuals.5,6 The presence of empty sella is often automatically associated with IIH, a disorder of chronic IH of unknown origin that manifests as headache, papilledema (ie, disc edema from increased intracranial pressure), and vision loss from papilledema if untreated.4,7
Patients with incidentally detected MRI signs of IH are frequently referred for neurologic or neuro-ophthalmologic consultations and often undergo invasive testing procedures, such as lumbar puncture, because of concern about IIH. Excess diagnosis of IIH frequently occurs among obese women with chronic headaches because of diagnostic errors, such as inaccurate ophthalmoscopic examination and cognitive biases.8,9 This common inaccurate diagnosis is concerning given the association between IIH and obesity, particularly because estimates indicate that approximately 50% of US adults are predicted to be obese by 2030.10 The rate of IIH doubled in England between 2002 and 2016, corresponding with a 442% increase in hospital admissions and a 6-fold increase in direct health care costs.11
The prevalence of radiographic signs of IH among patients undergoing brain MRI is currently unknown. Furthermore, the prevalence of papilledema among patients with MRI signs of IH has not been examined to date. We aimed to prospectively identify the prevalence and importance of MRI signs of IH and their association with the presence or absence of papilledema among outpatients undergoing brain imaging for any clinical indication at 1 imaging facility.
Methods
This study was approved by the institutional review board of Emory University and adhered to the tenets of the Declaration of Helsinki.12 Written informed consent was obtained from all participants.
Patients
Consecutive patients aged 18 years or older who were undergoing a clinically indicated brain MRI assessment at 1 outpatient imaging facility (Emory Brain Health Center) were invited to participate. Patients unable to sit upright or fixate their gaze for fundus photography were excluded. Immediately after their MRI, patients underwent nonmydriatic fundus photography of both eyes and were asked about the presence of headaches, visual symptoms, and ocular history. Patient medical records were reviewed for ocular, neurologic, and medical history, including previous neurosurgery. Clinical indications for MRI and demographic characteristics, including age, sex, self-reported race/ethnicity, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) were recorded.
Magnetic Resonance Imaging Analysis
Magnetic resonance imaging was performed in the supine position on the same 3-T unit (MAGNETOM Skyra; Siemens Healthineers) using a standard head coil. Although protocols and specific acquisition parameters varied by clinical indication for MRI, all imaging contained unenhanced sagittal T1-weighted and axial T2-weighted sequences. Patients undergoing contrast-enhanced imaging received a standard dose (0.2 mL/kg body weight [0.1 mmol/kg]) of intravenous gadolinium-based contrast agent followed by acquisition of contrast-enhanced T1-weighted sequences.
One masked subspecialty-certified neuroradiologist (A.M.S.) systematically reviewed all imaging for 10 radiographic signs of IH that are often associated with IIH using established criteria previously validated among patients with IIH.13,14,15 Radiographic signs of IH included empty sella (defined as a pituitary grade of ≥3),15 protrusion of the optic nerve head, flattening of the posterior sclera, increased perioptic CSF, vertical tortuosity of the intraorbital optic nerve, enlarged Meckel caves, cephaloceles, and cerebellar tonsillar ectopia more than 5 mm below the foramen magnum (Figure). For patients undergoing contrast-enhanced imaging, the presence of bilateral TVSS with at least 50% narrowing14 and optic nerve head enhancement was also determined.
Figure. Examples of Magnetic Resonance Imaging Signs of Intracranial Hypertension.
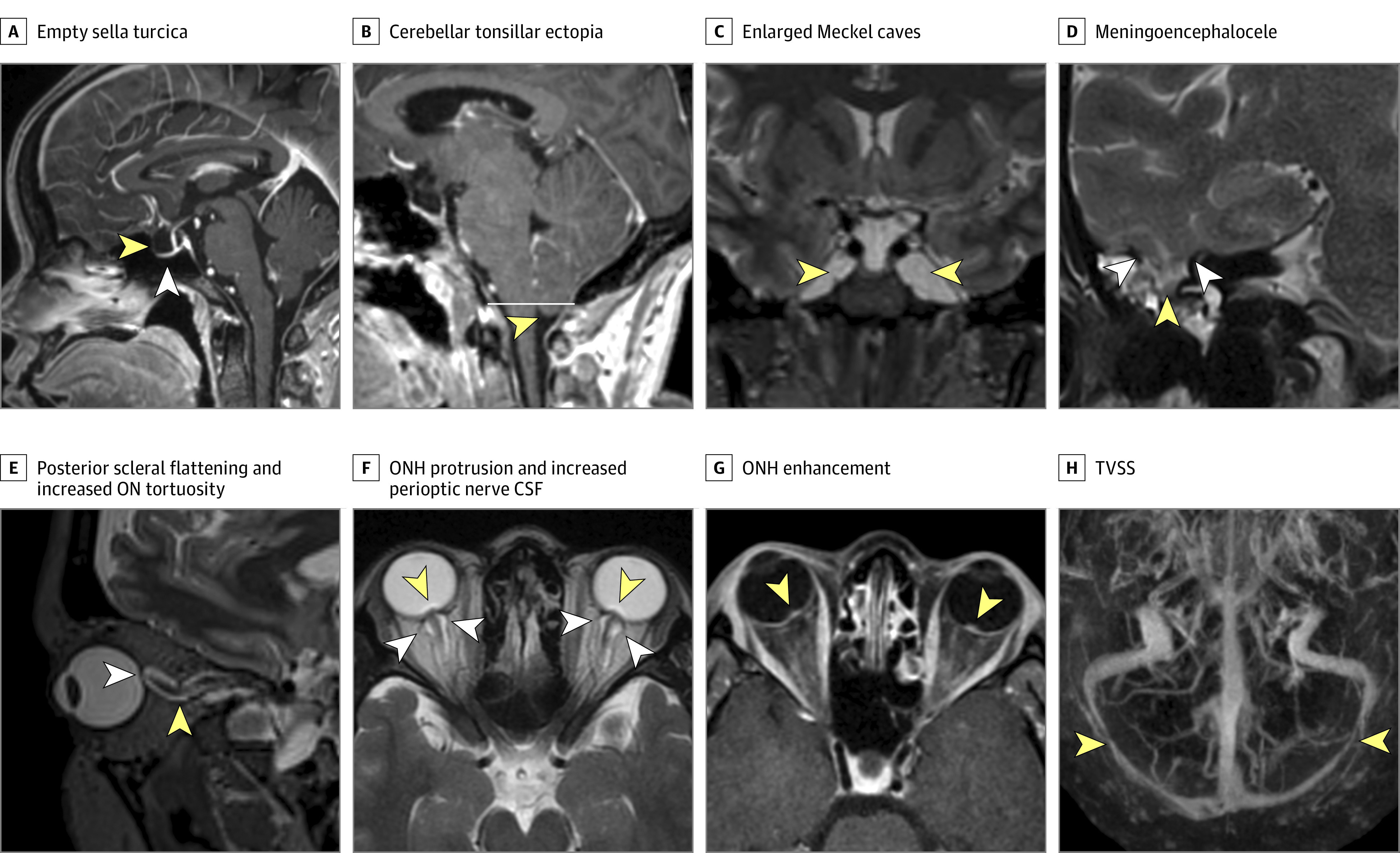
A, Sagittal postcontrast T1-weighted image. The yellow arrowhead illustrates an expanded cerebrospinal fluid (CSF)–filled empty-appearing sella turcica. The white arrowhead illustrates a flattened appearance of the pituitary gland against the floor of the sella turcica. B, Sagittal postcontrast T1-weighted image. The arrowhead illustrates the low-lying position but normal rounded morphology of the cerebellar tonsils 5 mm below the foramen magnum (white line). C, Coronal T2-weighted image. Arrowheads show abnormally enlarged CSF-filled Meckel caves bilaterally. D, Coronal T2-weighted image through the right temporal lobe. Yellow arrowheads illustrate margins of defects in the tegmen mastoideum transmitting CSF and a portion of the right inferior temporal lobe (white arrowhead). Fluid within adjacent mastoid air cells indicates an associated CSF leak. E, Oblique sagittal reconstruction of volumetric T2-weighted image in the plane of the optic nerve (ON) illustrates posterior scleral flattening (white arrowhead) and increased vertical tortuosity of the intraorbital ON sheath complex (yellow arrowhead). F, Axial T2-weighted image illustrates protrusion of the prelaminar ONs (yellow arrowheads), indicating severe papilledema and distension of the ON sheaths by CSF (white arrowheads) bilaterally. G, Axial postcontrast T1-weighted image illustrating enhancement of the prelaminar ONs bilaterally (arrowheads), indicating optic disc edema. H, Cranio-caudal maximum intensity projection of a postcontrast magnetic resonance venogram. Arrowheads illustrate smooth tapered severe stenosis of the distal transverse sinuses bilaterally. ONH indicates ON head; TVSS, transverse venous sinus stenosis.
Fundus Photography and Analysis
Fundus photography was performed using a commercially available US Food and Drug Administration–approved tabletop nonmydriatic ocular fundus camera (Kowa nonmyd α-DIII; Kowa Optimed) via a previously described method.16 Photographs centered at the optic disc, macula, and major retinal vasculature of each eye were obtained without pharmacologic pupillary dilation by a trained medical student (B.I.M.). This procedure typically took less than 5 minutes, creating minimal disruption to patients’ schedules.
Two neuro-ophthalmologists (N.J.N. and V.B.) independently reviewed all fundus photographs. The quality of photographs was rated using a previously described 5-point grading scale.16,17 The frequencies of high-quality photographs (grades 4 and 5) and photographs with no diagnostic value (grade 1) were determined. Photographs were classified as normal, optic nerve edema, or other optic nerve abnormalities. Patients with abnormal fundus photographs were evaluated by neuro-ophthalmologists from the Emory Eye Center, who confirmed the diagnosis of papilledema.
Statistical Analysis
Statistical analysis was performed using R software, version 3.6.3 (R Foundation for Statistical Computing). Univariate analysis was conducted using Fisher exact tests or 2-tailed unpaired t tests, as appropriate. The significance threshold was set at P = .05.
Results
Between August 1, 2019, and March 31, 2020, 388 consecutive outpatients underwent a clinically indicated brain MRI assessment and were screened for eligibility by a member of the study team (B.S.C. or B.I.M.). Fourteen patients were excluded because they were unable to sit unsupported in an upright position or fixate their gaze to allow fundus photography; informed consent was unable to be obtained from 16 patients, and 58 patients declined to participate. A total of 300 patients agreed to participate. Four patients completed MRI and fundus photography twice, and their second set of MRI results and fundus photos was excluded.
In total, 296 consecutive patients were included in analysis (Table 1). Most patients were female (188 women [63.5%] and 108 men [36.5%]), with a median age of 49.5 years (interquartile range, 37.8-62.0 years). A total of 196 patients (66.2%) were overweight or obese (BMI ≥25). At the time of the MRI, 124 patients (41.9%) reported a history of headaches. Four patients (1.4%) had a previous diagnosis of IIH.
Table 1. Demographic Characteristics and Magnetic Resonance Imaging Findings of Study Patients.
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| All patients (N = 296) | Patients without papilledema (n = 291) | Patients with papilledema (n = 5) | ||
| Age, median (IQR), y | 49.5 (37.8-62.0) | 50 (38.0-62.0) | 40 (33.0-47.0) | .41 |
| Female sex | 188 (63.5) | 186 (63.9) | 2 (40.0) | .36 |
| Race/ethnicity | ||||
| White | 167 (56.4) | 164 (56.4) | 3 (60.0) | >.99 |
| Black or African American | 88 (29.7) | 86 (29.6) | 2 (40.0) | .66 |
| Hispanic or Latinx | 5 (1.7) | 5 (1.7) | 0 | >.99 |
| Asian | 9 (3.0) | 9 (3.1) | 0 | >.99 |
| Other or unknownb | 27 (9.1) | 27 (9.3) | 0 | >.99 |
| BMI | ||||
| Median (IQR) | 27.6 (23.9-32.0) | 27.5 (23.8-32.0) | 37.6 (30.1-38.8) | .04 |
| Score range | ||||
| 25-29 (Overweight) | 91 (30.7) | 90 (30.9) | 1 (20.0) | >.99 |
| 30-34 (Moderate obesity) | 58 (19.6) | 57 (19.6) | 1 (20.0) | >.99 |
| ≥35 (Severe obesity) | 47 (15.9) | 44 (15.1) | 3 (60.0) | .03 |
| Self-reported history of headache | 124 (41.9) | 121 (41.6) | 3 (60.0) | .65 |
| History of IIH | 4 (1.4) | 2 (0.7) | 2 (40.0) | .001 |
| No. of MRI signs of IH | ||||
| 0 | 151 (51.0) | 150 (51.5) | 1 (20.0) | .21 |
| 1 | 97 (32.8) | 97 (33.3) | 0 | .18 |
| 2 | 32 (10.8) | 31 (10.7) | 1 (20.0) | .44 |
| 3 | 11 (3.7) | 10 (3.4) | 1 (20.0) | .17 |
| ≥4 | 5 (1.7) | 3 (1.0) | 2 (40.0) | .002 |
| MRI signs of IH | ||||
| Empty sella | 98 (33.1) | 94 (32.3) | 4 (80.0) | .006 |
| Increased perioptic CSF | 32 (10.8) | 30 (10.3) | 2 (40.0) | .09 |
| Optic nerve tortuosity | 23 (7.8) | 20 (6.9) | 3 (60.0) | .004 |
| Scleral flattening | 2 (0.7) | 2 (0.7) | 0 | >.99 |
| ONH protrusion | 0 | 0 | 0 | NA |
| ONH enhancement | 0 | 0 | 0 | NA |
| Enlarged Meckel caves | 47 (15.9) | 45 (15.5) | 2 (40.0) | .18 |
| Cephaloceles | 4 (1.4) | 3 (1.0) | 1 (20.0) | .07 |
| Cerebellar tonsillar ectopia | 1 (0.3) | 1 (0.3) | 0 | >.99 |
| Bilateral TVSSc | 6/198 (3.0) | 4/194 (2.1) | 2/4 (50.0) | .005 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CSF, cerebrospinal fluid; IH, intracranial hypertension; IIH, idiopathic intracranial hypertension; IQR, interquartile range; MRI, magnetic resonance imaging; NA, not applicable; ONH, optic nerve head; TVSS, transverse venous sinus stenosis.
Comparison of patients with and without papilledema.
Specific races and ethnicities included in the other category were not available.
A total of 198 patients completed contrast-enhanced imaging (194 patients without papilledema and 4 patients with papilledema).
The most common indication for MRI was surveillance of a brain neoplasm (82 patients [27.7%]) followed by investigation of a nonheadache neurologic symptom (58 patients [19.6%]) and diagnosis or monitoring of multiple sclerosis, neuromyelitis optica spectrum disorders, or myelin oligodendrocyte glycoprotein–associated diseases (55 patients [18.6%]) (Table 2). Few patients received an MRI for investigation of a new or worsening headache (26 individuals [8.8%]) or for a known disorder of raised intracranial pressure (4 individuals [1.4%]).
Table 2. Indications for Magnetic Resonance Imaging Among Study Patients.
| Indication | Patients, No. (%) (N = 296) |
|---|---|
| Surveillance of brain neoplasma | 82 (27.7) |
| Investigation of nonheadache neurologic symptomb | 58 (19.6) |
| Diagnosis or monitoring of MS, NMOSD, or MOGAD | 55 (18.6) |
| Investigation of seizure or preoperative imaging for epilepsy surgery | 53 (17.9) |
| Investigation of new or worsening headache | 26 (8.8) |
| Surveillance of cerebral vascular malformation | 9 (3.0) |
| Investigation of disorder of increased intracranial pressurec | 4 (1.4) |
| Follow up of non-MS, non-NMOSD, or non-MOGAD white matter abnormality or lesion | 4 (1.4) |
| Follow-up of neurosarcoidosis | 3 (1.0) |
| Follow-up of stroke | 2 (0.7) |
Abbreviations: MOGAD, myelin oligodendrocyte glycoprotein–associated disease; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder.
Magnetic resonance imaging was requested for surveillance, monitoring, or follow-up of a known brain neoplasm or as part of the patient’s treatment plan.
Includes aphasia, ataxia, cognitive impairment or dementia, dizziness, dysarthria, dystonia, numbness, parkinsonism, spasms, syncope, and weakness.
Includes aqueductal stenosis, hydrocephalus, and ventriculomegaly.
A total of 145 patients (49.0%) had at least 1 MRI sign of IH, and 5 patients (1.7%) had at least 4 MRI signs of IH (Table 1). The most common MRI sign of IH was empty sella (98 patients [33.1%]) followed by enlarged Meckel cave (47 patients [15.9%]). Orbital signs of IH were less frequent, including increased perioptic CSF (32 patients [10.8%]), optic nerve tortuosity (23 patients [7.8%]), and posterior scleral flattening (2 patients [0.7%]). Cephalocele and cerebellar tonsillar ectopia were both rare. A total of 198 patients (66.9%) received contrast as part of their MRI assessment and were examined for the presence of bilateral TVSS, which was present in only 6 patients (3.0%). None of the 198 patients had optic nerve head enhancement.
Of 592 eyes belonging to 296 patients, nonmydriatic fundus photographs were unable to be obtained for 6 eyes. Most photographs were of good quality (grade ≥4) (Table 3).
Table 3. Quality of Ocular Fundus Photographs Obtained From Study Patients.
| Quality gradea | Patients, No. (%) (N = 296) | |
|---|---|---|
| Right eye | Left eye | |
| Unable to obtain | 1 (0.3) | 5 (1.7) |
| 1 | 6 (2.0) | 7 (2.4) |
| 2 | 10 (3.4) | 16 (5.4) |
| 3 | 33 (11.1) | 50 (16.9) |
| 4 | 95 (32.1) | 104 (35.1) |
| 5 | 151 (51.0) | 114 (38.5) |
Quality grading scale was previously published.16,17 Quality grades ranged from 1 to 5, with 1 indicating inadequate for any diagnostic purpose, 2 indicating unable to exclude all emergent findings, 3 indicating able to exclude emergent findings only, 4 indicating not ideal but able to exclude subtle findings, and 5 indicating ideal quality.
Five patients (1.7%) had papilledema detected on fundus photography, including 2 patients with a history of IIH and worsening headaches, 2 patients with glioblastoma, and 1 patient with a history of temporal lobe–onset seizures secondary to a meningoencephalocele (Table 1). Among those 5 patients, the most common MRI signs of IH were empty sella (4 patients [80.0%]), optic nerve tortuosity (3 patients [60.0%]), increased perioptic CSF (2 patients [40.0%]), and enlarged Meckel caves (2 patients [40.0%]). None of the 5 patients with papilledema exhibited cerebellar tonsillar ectopia or scleral flattening. Only 4 of 5 patients with papilledema completed contrast-enhanced MRI; 2 of those patients had bilateral TVSS (1 patient with IIH, and 1 patient with glioblastoma). As the number of MRI signs of IH increased, the prevalence of papilledema also increased. The prevalence of papilledema was 2.8% among patients with at least 1 MRI sign of IH, increasing to 40.0% among patients with at least 4 MRI signs of IH (Table 4).
Table 4. Association Between Prevalence of Papilledema and Number of Magnetic Resonance Imaging Signs of Intracranial Hypertension.
| No. of MRI signs of IH | Patients without papilledema, No. (%) (n = 291) | Patients with papilledema, No. (%) (n = 5) | Prevalence of papilledema, No./total No. (%) | P value |
|---|---|---|---|---|
| ≥0 | 291 (100.0) | 5 (100.0) | 5/296 (1.7) | >.99 |
| ≥1 | 141 (48.5) | 4 (80.0) | 4/145 (2.8) | .21 |
| ≥2 | 44 (15.1) | 4 (80.0) | 4/48 (8.3) | .003 |
| ≥3 | 13 (4.5) | 3 (60.0) | 3/16 (18.8) | .001 |
| ≥4 | 3 (1.0) | 2 (40.0) | 2/5 (40.0) | .002 |
Abbreviations: IH, intracranial hypertension; MRI, magnetic resonance imaging.
Patients with papilledema had a significantly higher BMI compared with patients without papilledema (median [interquartile range], 37.6 [30.1-38.8] vs 27.5 [23.8-32.0], respectively) and history of IIH (2 of 5 patients [40.0%] vs 2 of 291 patients [0.7%]) (Table 1). Radiographic signs of IH that were significantly more prevalent among patients with papilledema included empty sella, optic nerve tortuosity, and TVSS. Other radiographic signs and demographic characteristics did not significantly differ between those with and without papilledema, in particular age, sex, self-reported race, or the presence of headaches. Similar results were observed when excluding the 82 patients with a history of brain tumors.
Discussion
To our knowledge, this cross-sectional prospective study is the first to determine the prevalence of MRI signs of IH among a cohort of outpatients undergoing brain MRI for any clinical indication. Radiographic signs of IH were common, with almost one-half of patients having at least 1 sign. Interpretation of nonmydriatic fundus photographs obtained at the time of MRI indicated that papilledema was rare, occurring in only 1.7% of all patients. Significantly more patients with papilledema had 2 or more MRI signs of IH compared with patients without papilledema. The prevalence of papilledema increased as the number of MRI signs of IH increased, from 2.8% among patients with at least 1 sign of IH to 40.0% among patients with at least 4 signs of IH.
While papilledema was rare among patients in the present study, the prevalence of papilledema that was unexplained by other imaging findings, such as space-occupying lesions, was rarer. Only 3 patients with papilledema did not have a space-occupying lesion to account for their papilledema, representing 1.0% of the total study patients. One patient with papilledema underwent MRI for investigation of temporal lobe seizures and was found to have a dural osseous defect associated with herniation of the left temporal lobe. Seizures owing to encephaloceles of the temporal lobe are increasingly recognized as potential rare presentations of IIH.18,19 Patients with temporal lobe–onset seizures owing to temporal encephaloceles have MRI findings that are similar to those of patients with IIH but do not develop typical manifestations of IH over time.18,20,21
Tortuous optic nerves and TVSS were found in significantly more patients with papilledema vs without papilledema. A previous systematic review reported that TVSS was the most useful sign of IIH because of its high pooled sensitivity (97%) and specificity (93%).1 Orbital findings, such as optic nerve head protrusion, posterior scleral flattening, optic nerve tortuosity, and distension of the optic nerve sheath, were previously found to be more specific for IIH (with pooled specificities of 99%, 98%, 90%, and 89%, respectively) but to be less sensitive (with pooled sensitivities of 36%, 66%, 43%, and 58%, respectively) compared with TVSS.1
Recent studies have focused on using a combination of MRI findings to identify patients who may have the rare occurrence of IIH without papilledema.3,22 According to 2013 criteria,7 in the absence of papilledema or sixth nerve palsy, the diagnosis of IIH without papilledema can be suggested by a combination of opening pressure of 25 cm CSF or higher and the presence of at least 3 imaging findings, including empty sella, flattening of the posterior aspect of the globes, distension of the perioptic subarachnoid space with or without optic nerve tortuosity, and TVSS. One study found that the combination of any 3 of these 4 imaging findings was almost 100% specific in diagnosing IIH without papilledema among adult patients with chronic headaches and elevated CSF opening pressure, but this combination had a sensitivity of only 64%.3 In the present study, the same imaging criteria were 99% specific in identifying patients with papilledema of any origin but had a sensitivity of only 20%. However, patients with IIH without papilledema are not at risk of vision loss, and the management of their condition is most often nonurgent, is conservative, and is aimed entirely at headache control.19
The findings of the present study challenge the widespread practice of performing systematic investigations among all patients with incidentally detected MRI signs of IH. Although the prevalence of empty sella was significantly higher among patients with papilledema compared with those without papilledema, the findings of this study suggest that the presence of empty sella does not automatically indicate a diagnosis of IIH. Patients are often referred for urgent neurologic or neuro-ophthalmologic assessment of other symptoms of IH and for examination of the ocular fundus to detect papilledema. Such assessments are important, as patients with undiagnosed papilledema are at risk of permanent vision loss. Therefore, ocular fundoscopic examination to identify possible papilledema is of greater priority than proceeding to lumbar puncture.16,23 However, many neurologists are not comfortable with examination of the ocular fundus,24 and the correct diagnosis of papilledema is not always easy, even among trained practitioners.8,9 It is likely that the incorporation of nonmydriatic ocular fundus cameras in neurology clinics would enable routine examination of the ocular fundus in nonophthalmology settings,23,24 which could perhaps be facilitated by automatic screening of photographs for the presence of papilledema using artificial intelligence.25,26
The findings of the present study suggest that the identification of patients who require urgent assessment could be determined by a combination of clinical (ocular fundus examination) and imaging criteria, including the number of MRI signs of IH detected. Novel radiographic signs, such as the angle of deflection that occurs in the optic nerve under conditions of IH, may also prove useful.27 For many patients with incidentally detected signs of IH, the MRI findings may indicate a previous state of IH, and aggressive workup for increased IH may not be required. Many MRI signs of IH persist even after the patient’s intracranial pressure normalizes. Signs of IH owing to bony erosion, such as empty sella and enlarged Meckel caves, are likely to persist after intracranial pressure normalizes, whereas signs of IH owing to mechanical deformations, such as posterior scleral flattening, increased perioptic CSF, and optic nerve tortuosity, may improve.1 These differences in evolution over time support the view that relying on MRI signs of increased intracranial pressure is unlikely to be helpful in determining current intracranial pressure status. In this situation, papilledema is the most useful surrogate marker of increased intracranial pressure and can help to determine which patients need more invasive procedures to assess their intracranial pressure status.
Strengths and Limitations
This study has several strengths. Primary strengths include the study’s prospective design and its use of nonmydriatic fundus photography to detect papilledema at the time of MRI. Previous studies estimating the prevalence of MRI signs of IH were retrospective or focused on only 1 sign, such as empty sella.28,29,30 Other studies used a case-control design to assess the importance of different MRI signs among patients with IIH.31,32,33 The drawback of these approaches is that intracranial pressure could not be determined at the time of MRI and was only estimated by a lumbar puncture performed around the time of MRI. Nonmydriatic fundus photography addresses this problem by documenting papilledema, the definitive clinical sign of ongoing IH, and has been found to be safe, reliable, and comfortable for both the patient and the operator.17,23,24 We did not include lumbar puncture in our study protocol because we did not seek to determine the predictive value of papilledema for increased intracranial pressure. However, 3 patients with papilledema (2 patients with IIH and 1 patient with temporal lobe–onset seizures) received lumbar punctures as part of their clinical care after detection of papilledema. All 3 patients were confirmed to have high opening pressure, further supporting the utility of ocular fundus photography.
This study also has several limitations. Emory Healthcare is a tertiary and quaternary health care service with several highly specialized neurologic and neurologic surgical programs. Although the study’s findings may not be representative of a general population of patients with neurologic conditions undergoing clinically indicated brain MRI, all MRI assessments were performed at 1 outpatient imaging facility (independent of the hospital and emergency department) in which MRI is routinely conducted. We did not perform subanalyses comparing the frequency of MRI signs of IH based on the clinical indication for MRI because of low patient numbers in most groups. However, because 27.7% of the patients included in this study underwent MRI for surveillance of a brain neoplasm, we repeated the analysis after excluding the 82 patients with a history of brain neoplasia and compared findings among this subgroup with those of the group of patients without a history of brain neoplasia. The results were unchanged, confirming that the inclusion of patients with a current or previous brain neoplasia is unlikely to have substantial implications for our findings. Overall, MRI signs of chronic IH were similarly prevalent in patients with or without brain tumors.
Another limitation is that 33.1% of the patients in the study did not complete a contrast-enhanced assessment because the imaging protocol was tailored to the clinical indication for MRI; therefore, the imaging results could not be evaluated for the presence of TVSS. It is therefore possible that the prevalence of TVSS in the full cohort may have been higher. However, restricting study participation to only patients who completed a contrast-enhanced MRI or magnetic resonance venography may have biased the results to patients being evaluated for possible cerebral venous sinus stenosis, IIH, or high intracranial pressure. In addition, we had to terminate the study after enrolling only 300 patients because of the SARS-CoV-2 pandemic. We could not perform a multivariate analysis and control for confounding variables, such as BMI or history of IIH, because of the small number of patients with papilledema in the study. We were also unable to determine the predictive value of individual radiographic signs of IIH. However, given the low rate of papilledema (1.7%) in this patient population, increasing the number of included patients would not have been likely to change the study’s main results. Further research with a larger cohort will be beneficial in answering the questions that this study has been unable to address.
Conclusions
In this study, MRI signs of IH were common among patients undergoing brain MRI for any clinical indication and may have represented either ongoing or previous IH. The presence of papilledema indicating current IH was rare but increased with the number of MRI signs of IH. In particular, the presence of empty sella, tortuous optic nerves, and TVSS occurred more frequently among patients with papilledema. In patients with incidentally detected MRI signs of IH, prompt clinical assessment for signs and symptoms of IH, including ocular fundus examination, is preferable to systematically proceeding to lumbar puncture. Further research is needed to determine which combination of MRI signs and clinical factors has the highest predictive value for papilledema, and such research would be useful to guide decision-making regarding patient selection for urgent assessments.
References
- 1.Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 2015;35(4):400-411. doi: 10.1097/WNO.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 2.Kelly LP, Saindane AM, Bruce BB, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg. 2013;115(8):1215-1219. doi: 10.1016/j.clineuro.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallery RM, Rehmani OF, Woo JH, et al. Utility of magnetic resonance imaging features for improving the diagnosis of idiopathic intracranial hypertension without papilledema. J Neuroophthalmol. 2019;39(3):299-307. doi: 10.1097/WNO.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 4.Saindane AM, Lim PP, Aiken A, Chen Z, Hudgins PA. Factors determining the clinical significance of an “empty” sella turcica. AJR Am J Roentgenol. 2013;200(5):1125-1131. doi: 10.2214/AJR.12.9013 [DOI] [PubMed] [Google Scholar]
- 5.Chiloiro S, Giampietro A, Bianchi A, et al. Diagnosis of endocrine disease: primary empty sella: a comprehensive review. Eur J Endocrinol. 2017;177(6):R275-R285. doi: 10.1530/EJE-17-0505 [DOI] [PubMed] [Google Scholar]
- 6.Guitelman M, Garcia Basavilbaso N, Vitale M, et al. Primary empty sella (PES): a review of 175 cases. Pituitary. 2013;16(2):270-274. doi: 10.1007/s11102-012-0416-6 [DOI] [PubMed] [Google Scholar]
- 7.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159-1165. doi: 10.1212/WNL.0b013e3182a55f17 [DOI] [PubMed] [Google Scholar]
- 8.Fisayo A, Bruce BB, Newman NJ, Biousse V. Overdiagnosis of idiopathic intracranial hypertension. Neurology. 2016;86(4):341-350. doi: 10.1212/WNL.0000000000002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stunkel L, Newman-Toker DE, Newman NJ, Biousse V. Diagnostic error of neuro-ophthalmologic conditions: state of the science. J Neuroophthalmol. 2021;41(1):98-113. doi: 10.1097/WNO.0000000000001031 [DOI] [PubMed] [Google Scholar]
- 10.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 11.Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye (Lond). 2019;33(3):478-485. doi: 10.1038/s41433-018-0238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48(8):521-527. doi: 10.1007/s00234-006-0095-y [DOI] [PubMed] [Google Scholar]
- 14.Riggeal BD, Bruce BB, Saindane AM, et al. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology. 2013;80(3):289-295. doi: 10.1212/WNL.0b013e31827debd6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging. 2000;12(6):808-813. doi: [DOI] [PubMed] [Google Scholar]
- 16.Bruce BB, Lamirel C, Biousse V, et al. Feasibility of nonmydriatic ocular fundus photography in the emergency department: phase I of the FOTO-ED study. Acad Emerg Med. 2011;18(9):928-933. doi: 10.1111/j.1553-2712.2011.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamirel C, Bruce BB, Wright DW, Delaney KP, Newman NJ, Biousse V. Quality of nonmydriatic digital fundus photography obtained by nurse practitioners in the emergency department: the FOTO-ED study. Ophthalmology. 2012;119(3):617-624. doi: 10.1016/j.ophtha.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Poles J, Toledano R, Jimenez-Huete A, et al. Epilepsy associated with temporal pole encephaloceles: an unrecognized manifestation of idiopathic intracranial hypertension? Clin Neuroradiol. Published online October 15, 2020. doi: 10.1007/s00062-020-00969-0 [DOI] [PubMed] [Google Scholar]
- 19.Chen BS, Newman NJ, Biousse V. Atypical presentations of idiopathic intracranial hypertension. Taiwan J Ophthalmol. 2021;11(1):25-38. doi: 10.4103/tjo.tjo_69_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell ZM, Hyer JM, Lauzon S, Bonilha L, Spampinato MV, Yazdani M. Detection and characteristics of temporal encephaloceles in patients with refractory epilepsy. AJNR Am J Neuroradiol. 2018;39(8):1468-1472. doi: 10.3174/ajnr.A5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbach H, Jamneala G, Mader I, Egger K, Yang S, Altenmuller D. Temporal lobe epilepsy due to meningoencephaloceles into the greater sphenoid wing: a consequence of idiopathic intracranial hypertension? Neuroradiology. 2018;60(1):51-60. doi: 10.1007/s00234-017-1929-5 [DOI] [PubMed] [Google Scholar]
- 22.Kohli AA, Vossough A, Mallery RM, et al. Magnetic resonance imaging findings in pediatric pseudotumor cerebri syndrome. Pediatr Neurol. 2019;99:31-39. doi: 10.1016/j.pediatrneurol.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irani NK, Bidot S, Peragallo JH, Esper GJ, Newman NJ, Biousse V. Feasibility of a nonmydriatic ocular fundus camera in an outpatient neurology clinic. Neurologist. 2020;25(2):19-23. doi: 10.1097/NRL.0000000000000259 [DOI] [PubMed] [Google Scholar]
- 24.Biousse V, Bruce BB, Newman NJ. Ophthalmoscopy in the 21st century: the 2017 H. Houston Merritt lecture. Neurology. 2018;90(4):167-175. doi: 10.1212/WNL.0000000000004868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biousse V, Newman NJ, Najjar RP, et al. ; BONSAI (Brain and Optic Nerve Study with Artificial Intelligence) Study Group . Optic disc classification by deep learning versus expert neuro-ophthalmologists. Ann Neurol. 2020;88(4):785-795. doi: 10.1002/ana.25839 [DOI] [PubMed] [Google Scholar]
- 26.Milea D, Najjar RP, Zhubo J, et al. ; BONSAI Group . Artificial intelligence to detect papilledema from ocular fundus photographs. N Engl J Med. 2020;382(18):1687-1695. doi: 10.1056/NEJMoa1917130 [DOI] [PubMed] [Google Scholar]
- 27.Chen BS, Asnafi S, Lin MY, et al. Optic nerve angle in idiopathic intracranial hypertension. J Neuroophthalmol. Published online June 19, 2020. doi: 10.1097/WNO.0000000000000986 [DOI] [PubMed] [Google Scholar]
- 28.Debnath J, Ravikumar R, Sharma V, et al. “Empty sella” on routine MRI studies: an incidental finding or otherwise? Med J Armed Forces India. 2016;72(1):33-37. doi: 10.1016/j.mjafi.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foresti M, Guidali A, Susanna P. Primary empty sella: incidence in 500 asymptomatic subjects examined with magnetic resonance. Article in Italian. Radiol Med. 1991;81(6):803-807. [PubMed] [Google Scholar]
- 30.Hardjasudarma M, White KE, Nandy I, Burns PL. Sellar emptiness on routine magnetic resonance imaging. South Med J. 1994;87(3):340-343. doi: 10.1097/00007611-199403000-00008 [DOI] [PubMed] [Google Scholar]
- 31.Delen F, Peker E, Onay M, Altay CM, Tekeli O, Işıkay CT. The significance and reliability of imaging findings in pseudotumor cerebri. Neuroophthalmology. 2018;43(2):81-90. doi: 10.1080/01658107.2018.1493514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann J, Huppertz HJ, Schmidt C, et al. Morphometric and volumetric MRI changes in idiopathic intracranial hypertension. Cephalalgia. 2013;33(13):1075-1084. doi: 10.1177/0333102413484095 [DOI] [PubMed] [Google Scholar]
- 33.Maralani PJ, Hassanlou M, Torres C, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012;67(7):656-663. doi: 10.1016/j.crad.2011.12.002 [DOI] [PubMed] [Google Scholar]


