Key Points
Question
Did the 2016 Food and Drug Administration’s warnings and removal of indications for oral quinolones in acute, uncomplicated urinary tract infection (uUTI), acute sinusitis, and acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) result in decreases in their use?
Findings
In a nationally representative sample of privately insured patients, we noted immediate decreases in quinolone use for uUTI and significant but less robust decreases for sinusitis and AE-COPD. However, quinolones continued to comprise 19% of treated uUTI and 15% of acute exacerbations of AE-COPD.
Meaning
Although label changes and their announcements resulted in reduction on oral quinolone use, opportunities for improvement remain.
This interupted time series study analyzes the association of the implementation of oral quinolone safety warnings and indication restrictions with trends in prescriptoin practices and usage among patients with uncomplicated urinary tract infection, acute sinusitis, and acute exacerbation of chronic obstructive pulmonary disease.
Abstract
Importance
In May 2016, due to concerns of the risks outweighing the benefits, the US Food and Drug Administration (FDA) removed systemic quinolones’ indications for acute, uncomplicated urinary tract infection (uUTI), acute sinusitis (AS), and acute exacerbation of chronic obstructive pulmonary disease (AE-COPD). How the change influenced oral quinolone use is unknown.
Objective
To assess the association of oral quinolone safety warnings and indication restrictions with use.
Design, Setting, and Participants
This interrupted time series (January 2015-November 2018) analysis of the monthly prevalence of oral quinolone-treated infection episodes used a national sample of privately insured patients in outpatient care from the IBM MarketScan Database and included adults with antibiotic treatment of new uUTI, AS, or AE-COPD episodes, excluding patients with conditions that complicate infections, previous hospitalization, or other infections.
Exposures
Time before and after May 2016 when the FDA mandated label changes.
Main Outcomes and Measures
Monthly oral quinolone use prevalence by each condition before and after the label changes, overall and stratified by prescriber specialty.
Results
In January 2015, quinolone prevalence among antibiotic-treated uUTI episodes (n = 652 235) was 41.6% (95% CI, 40.6%-42.5%); AS (n = 1 742 248) was 8.3% (95% CI, 7.9%-8.6%), and AE-COPD (n = 22 817) was 31.9% (95% CI, 30.3%-33.4%). Before the label changes, trends in monthly quinolone prevalence were nearly flat. The month of the label changes we noted an immediate reduction for uUTI (−7.2%; 95% CI, −8.6% to −5.8%); and to a lesser extent for AS (−1.2%; 95% CI, −1.5% to −0.9%) and AE-COPD (−2.6%; 95% CI, −4.1% to −1.1%), and continued monthly declines thereafter. Falsification tests confirmed an immediate decrease after the label change of quinolone use for uUTI but more obscured effects for AS and AE-COPD. Treatment shifted mostly to first-line (eg, nitrofurantoin in uUTI, amoxicillin in AS, macrolides in AE-COPD) and other second-line agents but use of not recommended antibiotics also increased (eg, tetracyclines in AE-COPD). Prescribing preferences varied, but significant reductions were seen across all prescriber specialties. At the end of the study period, quinolone was used for 19.2% of treated uUTIs, 2.9% of treated AS, and 14.6% of treated AE-COPD episodes.
Conclusions and Relevance
Label changes and their announcements was associated with an immediate reduction in oral quinolone use for uUTI and to a lesser extent for AS and AE-COPD. Quinolones continued to contribute a considerable proportion of treatments for uUTI and AE-COPD episodes at the end of the study period, pointing to opportunities for further improvement.
Introduction
Since their introduction in the 1980s, fluoroquinolones have evolved to one of the most popular antibiotics, used for a wide range of infections.1,2,3 With increased use, concerns about adverse events have emerged.1,2,4,5 In July 2008, the US Food and Drug Administration (FDA) issued a black box warning related to tendinitis and tendon rupture associated with quinolone use.6,7 Several professional societies subsequently advocated against quinolone use in acute, uncomplicated urinary tract infection (uUTI)8 and acute bacterial sinusitis (AS).9,10,11 In 2014, the US Department of Veteran Affairs released guidelines that discouraged the use of quinolones for acute bacterial exacerbation of chronic bronchitis among patients with chronic obstructive pulmonary disease (AE-COPD).12
Concerns about the continued use of quinolones for uncomplicated infections and additional emerging safety information led the FDA to convene an advisory committee meeting in November 2015 to discuss regulatory action. The committee reviewed evidence on disabling and potentially permanent adverse effects involving the tendons, muscles, joints, and central nervous system and concluded that risks do not support quinolone use for AS, uUTI, and AE-COPD.13,14,15,16,17,18,19 In May 2016, the FDA announced its decision to constrain quinolones’ indication for these uncomplicated infections to those for whom no alternative treatment is available.6,20 In July 2016, respective quinolone label changes for oral and injectable forms were approved.6
Several regulatory mechanisms are available to ensure the safe use of medications, including label changes, communication plans, and risk mitigation and evaluation, which in their most restrictive form can limit access to a drug. In the case of quinolones, label changes were expanded beyond warnings to advise restricted use in the 3 most prevalent indications for quinolones. Efforts to enforce on-label use were limited, however, to communication strategies (black box warning, medication guide, and press releases), with unclear effectiveness in changing prescribing behavior.
One recent study21 has reported decreasing trends in overall quinolone use in inpatient settings following the labeling change, whereas another study22 found an insignificant effect for the target indications at a single outpatient center. No information from a national representative sample is available. Evidence on the effectiveness of FDA action as well as the epidemiology of safe use of quinolones is valuable for both regulatory and clinical decision-making. The goal of this study was to evaluate the association of the FDA’s 2016 label changes limiting indications for uUTI, AS, and AE-COPD with oral quinolone use.
Methods
Data Source and Study Design
This study used an interrupted time-series (ITS) design to compare monthly prescribing of quinolones for uUTI, AS, and AE-COPD before and after FDA action. Data were ascertained from the IBM MarketScan Commercial Claims and Medicare Supplemental Database between 2005 and 2018. The data set provides longitudinal information on health care utilization of a national sample of privately insured employees, retirees, and their dependents in the US. It includes detail on diagnoses and procedures associated with inpatient and outpatient encounters as well as outpatient pharmacy medication dispensing of more than 100 million patients.
The University of Florida institutional review board exempted this study from review because all data used were deidentified.
Study Population
The study included 3 distinct cohorts of adults with uUTI, AS, or AE-COPD. Patients entered 1 of the 3 cohorts when they had a new outpatient encounter for 1 of the 3 conditions (eTable 1 in the Supplement) following at least 90 days without such diagnosis (index day). We required participants to have continuous insurance enrollment and drug coverage for at least 1 year (for uUTI, AE-COPD) or 2 years (for AS) before and 30 days after the index day. This period was used to exclude episodes that were associated with conditions that might indicate more complicated infections.
Eligible patients also had to fill an oral antibiotic prescription within 5 days of the index uUTI, AS, or AE-COPD diagnosis. This time window allowed for common scenarios, including antibiotics prescribed by phone before patients were seen by clinicians or delays in filling prescriptions. We excluded episodes with antibiotic dispensing claims with invalid days of supply (≤0) or days of supply greater than 30.
To avoid situations where antibiotics might have been prescribed for other concomitant infections or potential resistant bacteria, we excluded patient episodes with any acute infection (eTable 2 in the Supplement) or with any hospitalization within 90 days before and 30 days after the index day.23,24 Patients were allowed to reenter the study with a new episode if all inclusion and exclusion criteria were met.
Based on FDA definitions on the label revision and previously published work, we developed claims-based coding algorithms for each of the 3 target indications. The uUTI cohort included women aged 19 years or older with an outpatient encounter indicating acute cystitis, unspecified cystitis, or urinary tract infection with unspecified site (eTable 1 in the Supplement).23 To be selected, the uUTI episode had to have no diagnoses of urologic abnormalities or other comorbidities that can complicate uUTIs such as diabetes within 1 year prior (eTable 3 in the Supplement).23 Eligible patients for the AS cohort were aged 19 years or older with an outpatient encounter for AS and no diagnosis of chronic sinusitis during the 2-year look-back period (eTable 1 in the Supplement).25
Patients with AE-COPD were aged 40 years or older and had at least 1 outpatient encounter with a diagnoses of COPD, at least 1 dispensing of an inhaled COPD medication or oral theophylline, and no diagnosis of asthma (eTable 1 in the Supplement) within 1 year.26,27 A moderate exacerbation of COPD was defined as an outpatient or emergency department visit using previously specified algorithms (eTable 1 in the Supplement) and at least 1 new prescription of an oral corticosteroid within 7 days of this encounter defined based on absence of a refill flag on the pharmacy claim and no other oral corticosteroid prescription fill within 90 days before.26,27 We did not examine mild or severe exacerbations of COPD because a mild condition does not require antibiotics and severe exacerbations often require hospitalization which obscures capture of antibiotic use.
Antibiotic Use
If more than 1 antibiotic was prescribed in an episode, the earliest filled prescription was assigned to the episode as the primary treatment because subsequent prescriptions might reflect nonresponse to the initial therapy. Antibiotics were categorized according to drug class and current recommendation as first line, second line, or not recommended.8,11,28,29,30
Statistical Analysis
For each indication, we summarized episodes into calendar months based on index date and calculated the monthly prevalence of episodes treated with oral quinolones per all antibiotic-treated episodes. For the formal evaluation of the FDA label change, we truncated our historical look-back period to a start in January 2015, when initial decreases in quinolone use had plateaued and the population was relatively stable. We then used segmented ordinary least-square regression models with Newey-West autocorrelation adjustment to evaluate changes in levels (ie, immediate effect from April 2016 to May 2016) and slopes (ie, trend effect) before (from January 2015 to April 2016, 16 data points) and after (May 2016 to November 2018, 31 data points) the label change (eMethods 1 in the Supplement).31,32,33
We also conducted stratified analyses by prescriber specialty obtained from the index outpatient encounter.34 We assumed the clinician who diagnosed the index indications was the same person who prescribed antibiotics.
To account for earlier effects of publicity of the FDA advisory committee meeting, announcement of the FDA decision to implementation of the labeling change, we conducted 2 sensitivity analyses that excluded from the ITS model a phase-in period from November 2015 (date of FDA advisory committee meeting) to July 2016 (FDA approval of new label) and from May 2016 (FDA decision to require label change) to July 2016.
To evaluate spill-over effects, we replicated the analyses in adults with community-acquired pneumonia (CAP), which was not targeted by the label change. We also examined secular changes in the treatment of skin and subcutaneous tissue infections as a negative control, because quinolones are rarely used for this indication (eMethods 2 in the Supplement).
Finally, we used joinpoint regression (eMethods 3 in the Supplement) and ITS models with a series of alternative hypothetical interruption points as falsification methods to test our ITS results.35,36,37,38 The joinpoint method uses a data-driven approach to identify change points (agnostic of any prespecified hypothesis) and thus, on overlap between change points identified by the software with our hypothesized change point of the time of label change can corroborate ITS model specification.
All analyses were conducted with SAS statistical software (version 9.4; SAS Institute, Inc) and the actest function in STATA SE software (version 16.1, STATA Corp) was used to determined lag for the models.
Results
The descriptive analyses included 3 048 534 episodes of UTI, 6 935 820 episodes of AS, and 95 306 episodes of AE-COPD respectively (eFigure 1 in the Supplement). Visual examination of quinolone use prevalence showed reductions for AE-COPD and AS during early study years and fairly stable use for uUTI (eFigure 2 in the Supplement). Introduction of clinical guidelines, which recommended against oral quinolone use as first-line agents, had minimal, if any proximate association with oral quinolone prescribing, whereas use appeared to drop after the FDA label change.
During our study period from 2015 to 2018, monthly rates for each indication and the proportion treated with antibiotics remained relatively stable across study years (eFigure 3 in the Supplement). The demographic composition of the study cohorts remained similar as well, with slight decreases in age for the uUTI and AE-COPD cohorts (eTable 4 in the Supplement).
Uncomplicated UTI
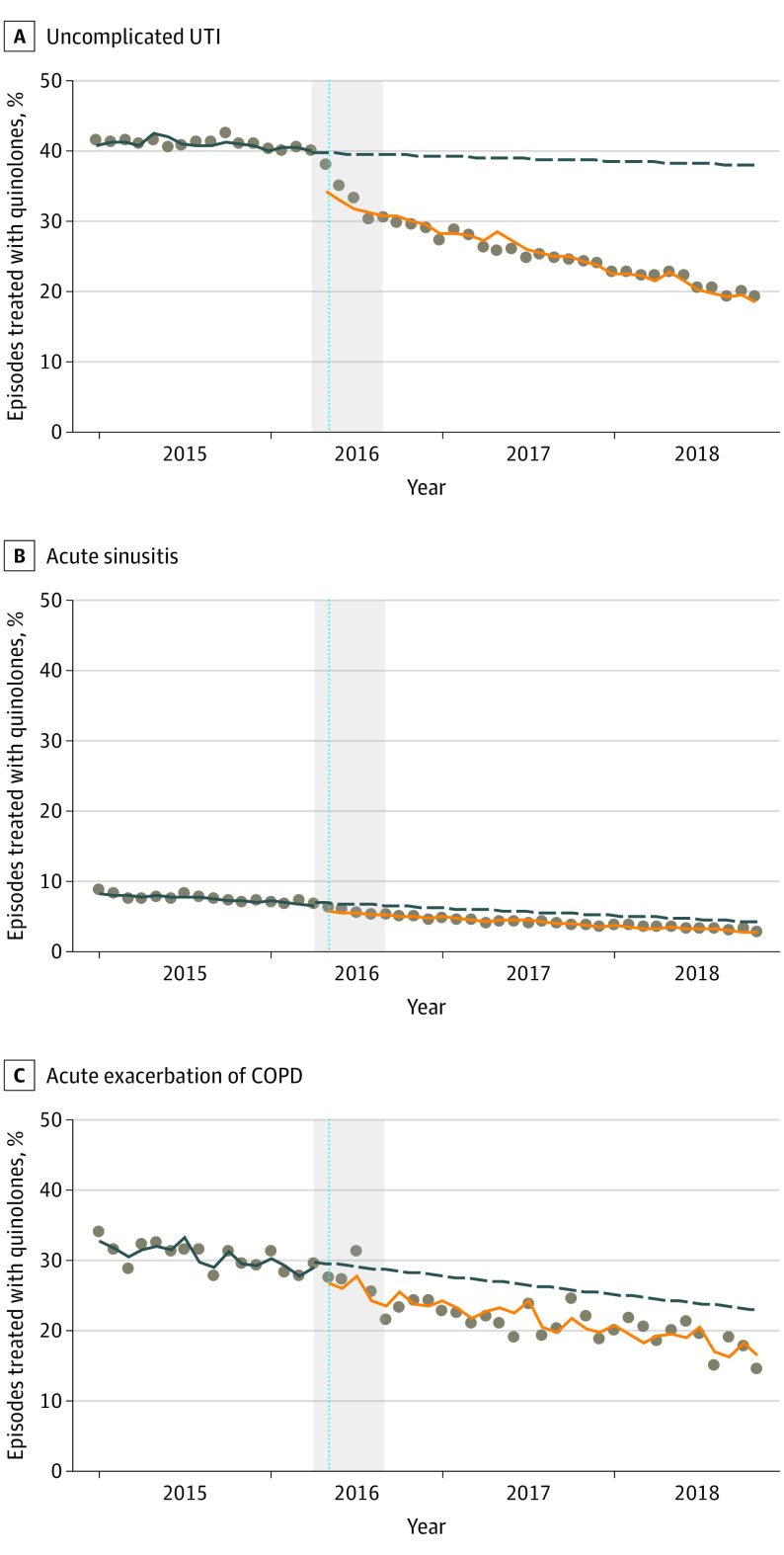
From 2015 to 2016, oral quinolones were the most commonly prescribed antibiotic for uUTI, with monthly prevalence only decreasing marginally by 0.06% per month before the FDA’s call for restricted indications. The label change resulted in an immediate drop (from April 2016 to May 2016) in quinolone use prevalence (−7.21%; 95% CI, −8.62% to −5.80%), followed by a continuous downward trend thereafter (−0.41% per month; 95% CI, −0.51% to −0.31%). At the end of the study period, oral quinolones continued to account for 20% of treated uUTI episodes (Figure 1) (Table). Oral quinolone prescribing for uUTI by internists was roughly double that of obstetrician-gynecologists both before and after the label change. Significant reductions were seen across all prescriber types.
Figure 1. Association of Label Changes With Oral Quinolone Use by Medical Condition (Interrupted Time Series Analysis).
UTI Indicates urinary tract infection; COPD, chronic obstructive pulmonary disease. Data points represent monthly prevalence of oral quinolone use between January 2015 and November 2018. The vertical dashed lines indicate May 2016 when US Food and Drug Administration (FDA) required quinolone label changes. Grey areas show the period from November 2015 (FDA advisory committee meeting) to July 2016 (FDA-approved label changes submitted by pharmaceutical companies). Regression lines show the trends of oral quinolone use before (blue lines) and after (orange lines) label changes. Dashed regression lines (blue) are the predicted trends of oral quinolone use after May 2016 assuming no changes in prelabel change trends.
Table. Association of Label Changes on Oral Quinolone Use Overall and by Prescriber Specialty—Interrupted Time-series Analyses.
| Variable | Estimated quinolone prevalence and changes in trend, % (95% CI) | Mean monthly prevalence | |||||
|---|---|---|---|---|---|---|---|
| Prevalence in January 2015 | Change | ||||||
| Average monthly before May 2016 | Immediate after May 2016 | Trend after May 2016 | Before | After | Change, % | ||
| Uncomplicated UTI | 41.6 (40.6 to 42.5) | −0.06 (−0.11 to −0.01) | −7.21 (−8.62 to −5.80) | −0.41 (−0.51 to −0.31) | 40.9 | 25.7 | −37.2 |
| Emergency physician | 35.6 (33.1 to 38.1) | 0.09 (−0.08 to 0.26) | −8.12 (−10.32 to −5.92) | −0.57 (−0.77 to −0.37) | 34.8 | 20.2 | −42.0 |
| Family physician | 45.7 (44.1 to 47.2) | −0.06 (−0.15 to 0.03) | −7.32 (−8.75 to −5.89) | −0.42 (−0.54 to −0.3) | 45.4 | 29.9 | −34.1 |
| Internist | 53.6 (52.1 to 55.1) | −0.16 (−0.20 to −0.12) | −5.92 (−7.09 to −4.75) | −0.32 (−0.41 to −0.23) | 52.0 | 37.2 | −28.5 |
| Nurse practitioner | 35.2 (31.5 to 39.0) | 0.12 (0.05 to 0.19) | −9.37 (−11.24 to −7.50) | −0.54 (−0.64 to −0.44) | 35.6 | 21.0 | −41.0 |
| Obstetrician/gynecologist | 25.2 (23.2 to 27.3) | 0.04 (−0.11 to 0.19) | −5.15 (−7.03 to −3.27) | −0.23 (−0.39 to −0.07) | 26.6 | 18.6 | −30.1 |
| Physician assistant | 39.9 (37.1 to 42.7) | −0.17 (−0.35 to 0.01) | −6.16 (−8.21 to −4.11) | −0.35 (−0.55 to −0.15) | 40.1 | 24.5 | −38.9 |
| Urologista | 31.0 (25.5 to 36.4) | −0.16 (−0.53 to 0.21) | −5.91 (−10.08 to −1.74) | −0.14 (−0.53 to 0.25) | 32.4 | 21.0 | −35.2 |
| Others | 38.0 (35.8 to 40.2) | −0.14 (−0.20 to −0.08) | −6.12 (−7.45 to −4.79) | −0.29 (−0.40 to −0.18) | 36.6 | 22.6 | −38.3 |
| Unknown | 40.0 (39.1 to 40.9) | −0.11 (−0.14 to −0.08) | −8.14 (−9.63 to −6.65) | −0.35(−0.44 to −0.26) | 38.1 | 22.1 | −42.0 |
| Acute sinusitis | 8.3 (7.9 to 8.6) | −0.09 (−0.12 to −0.06) | −1.23 (−1.52 to −0.94) | 0 (−0.04 to 0.04) | 7.5 | 4.2 | −44.0 |
| Emergency physician | 5.7 (4.8 to 6.6) | −0.02 (−0.09 to 0.05) | −2.24 (−2.87 to −1.61) | −0.04 (−0.11 to 0.03) | 5.9 | 2.6 | −55.9 |
| Family physician | 8.9 (8.6 to 9.9) | −0.08 (−0.11 to −0.05) | −1.24 (−1.54 to −0.94) | −0.02 (−0.05 to 0.01) | 8.1 | 4.7 | −42.0 |
| Internist | 11.5 (11.1 to 11.9) | −0.13 (−0.16 to −0.1) | −1.19 (−1.51 to −0.87) | 0.02 (−0.02 to 0.06) | 10.7 | 6.9 | −35.5 |
| Nurse practitioner | 6.2 (5.5 to 6.9) | −0.08 (−0.13 to −0.03) | −1.30 (−1.75 to −0.85) | 0.02 (−0.03 to 0.07) | 5.5 | 2.7 | −50.9 |
| Otolaryngologist | 10.9 (9.9 to 11.9) | −0.05 (−0.11 to 0.01) | −1.88 (−2.73 to −1.03) | −0.08 (−0.15 to −0.01) | 10.2 | 6.0 | −41.2 |
| Physician assistant | 5.6(4.8 to 6.3) | 0 (−0.07 to 0.07) | −0.84 (−1.68 to 0) | −0.09 (−0.17 to −0.01) | 5.4 | 3.0 | −44.4 |
| Others | 6.4 (6.0 to 6.9) | −0.06 (−0.1 to −0.02) | −1.10 (−1.45 to −0.75) | −0.01 (−0.06 to 0.04) | 6.3 | 3.7 | −41.3 |
| Unknown | 5.5 (5.1 to 5.9) | −0.08 (−0.1 to −0.06) | −0.98(−1.18 to −0.78) | 0.02(−0.01 to 0.05) | 4.8 | 2.4 | −50.0 |
| Acute exacerbation COPD | 31.9 (30.3 to 33.4) | −0.21 (−0.31 to −0.11) | −2.58 (−4.05 to −1.11) | −0.09 (−0.21 to 0.03) | 30.5 | 21.6 | −29.2 |
| Family physiciana | 30.6 (28.5 to 32.8) | −0.33 (−0.44 to −0.22) | −1.91 (−3.42 to −0.4) | 0.14 (0 to 0.28) | 28.6 | 21.6 | −24.5 |
| Internista | 34.1 (27.9 to 40.3) | −0.49 (−0.82 to −0.16) | −1.90 (−5.43 to 1.63) | 0.23 (−0.11 to 0.57) | 32.7 | 22.5 | −31.2 |
| Pulmonologista | 21.7 (15.0 to 28.4) | 0.53 (0.35 to 0.71) | −11.1 (−13.17 to −9.03) | −0.69 (−0.96 to −0.42) | 27.8 | 17.8 | −36.0 |
| Othersa | 33.5 (31.0 to 36.0) | −0.35 (−0.57 to −0.13) | 0.07 (−3.39 to 3.53) | −0.11 (−0.38 to 0.16) | 31.6 | 21.9 | −30.7 |
| Unknown | 34.9 (28.8 to 40.9) | −0.12 (−0.34 to 0.10) | −2.89 (−4.63 to −1.15) | −0.25 (−0.5 to 0) | 31.9 | 22.3 | −29.8 |
Abbreviations: COPD, chronic obstructive pulmonary disease; UTI, urinary tract infection.
Some data points had less than recommended observations (<100 observations). Others includes prescribers in multispecialty physician groups, physicians not classified elsewhere, allergists/immunologists (in the acute sinusitis cohort) and emergency physicians (in the COPD population).
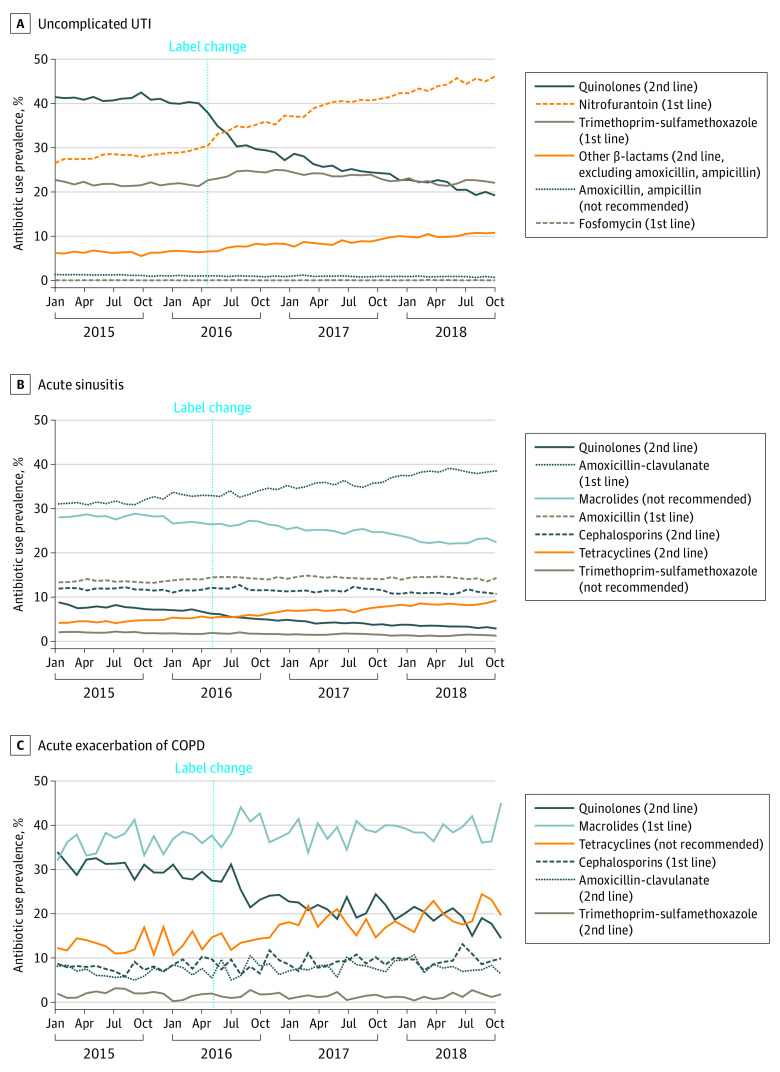
The drop in oral quinolone use was met with a shift to first-line antibiotics (Figure 2; eTable 5 in the Supplement), eg, nitrofurantoin showed an immediate increase (2.99%; 95% CI, 2.40%-3.58%) and a continued upward trend (0.29% per month; 95% CI, 0.24%-0.34%). Antibiotics in the not-recommended group remained low throughout the study period.
Figure 2. Trends in Oral Antibiotic Use Prevalence by Medical Condition.
UTI Indicates urinary tract infection; COPD, chronic obstructive pulmonary disease.
Acute Sinusitis
Monthly quinolone prescribing prevalences for AS were smaller than those for uUTI. This notwithstanding, the label change was associated with an immediate reduction (−1.23%, 95% CI, −1.52% to −0.94%). At the end of the study period, oral quinolones were used in less than 5% of AS episodes (Figure 1) (Table). Although quinolone use prevalence dropped by about half across all prescribers, otolaryngologists and internists retained roughly double the prevalence compared with nurse practitioners, physician assistants, and emergency physicians (Table).
Therapeutic preferences began to shift prior to the FDA label changes in favor of amoxicillin-clavulanate. This change appeared to be more closely linked to the drop in use of other agents that are not currently recommended, such as macrolides and trimethoprim-sulfamethoxazole (Figure 2; eTable 5 in the Supplement). Second-line therapy, such as cephalosporins showed a modest immediate increase following the label change (0.44%; 95% CI, 0.19%-0.69).
Acute Exacerbation of COPD
At the beginning of the study period, oral quinolones were used to treat more than 40% of AE-COPD episodes, but by January 2015, prevalence had dropped to 32%. The label change was followed by an immediate decline (−2.58%; 95% CI −4.05% to −1.11%) (Figure 1) (Table). At the end of the study period, oral quinolones continued to constitute nearly 15% of antibiotics prescribed for AE-COPD (Table).
Removal of oral quinolones’ AE-COPD indication was associated with an immediate change in amoxicillin/clavulanate use prevalence (1.05%; 95% CI, 0.31%-1.79%) (Figure 2; eTable 5 in the Supplement). Tetracyclines, which are not currently recommended for AE-COPD, showed an upward trend (0.20% per month; 95% CI, 0.07%-0.33%) but no immediate change.
After accounting for the phase-in and lag periods in the sensitivity analyses, the estimates for immediate drops in quinolone prescribing after the label change increased (eTable 6 in the Supplement). We noted a significant association of the label change with quinolone prescribing for CAP in the outpatient setting, suggesting a spill-over effect (eFigure 4, eTable 6 in the Supplement). The analysis of our negative control population with skin infections showed no appreciable change in antibiotic prescribing (eTable 6 in the Supplement).
Finally, the results from the joinpoint analyses searching for natural break points without prespecified hypotheses yielded break points with confidence intervals that overlapped with our tested change point for each of the 3 indications, but confidence intervals for AS and AE-COPD were wide and suggested decreases preceding the FDA label change announcement (eTable 7, eFigure 5 in the Supplement). Interrupted time-series falsification tests confirmed that reductions in quinolone use following the announcement of the label change were consistently extreme for all 3 conditions relative to the variability in quinolone over time before the policy change (eFigure 6 and eTable 8 in the Supplement).
Discussion
Drug therapy selection is fundamentally driven by risk-benefit considerations. Clinicians are informed on drug risk-benefit through a myriad of sources, including primary evidence in the peer-reviewed literature, industry marketing, expert opinion, clinical practice guidelines, peer feedback, and personal experience. Considerations for 1 drug must be weighed against alternatives and patient-specific factors must be taken into account. This is highly dynamic, with new information emerging constantly and done in the context of evolving federal and state regulations, starting with FDA approval and labeling, which changes far less often than the scientific literature.
We sought to assess whether the actions taken by the FDA in 2016 was significantly associated with the prescribing of oral quinolones. Our nationally representative data indicate that oral quinolone prescribing for uUTI, AS, and AE-COPD cohorts dropped significantly, both immediately and with sustained downward trends following the FDA’s interventions. These reductions varied by indication, with uUTI showing the most marked declines. These changes were commensurate with the opportunity for improvement, as quinolones were much more commonly used for uUTI than they were for AS prior to 2016. They contrast (qualitatively) with a lack of change in quinolone prescribing trends before and after the introduction of clinical practice guidelines. We surmise that the greater changes following the FDA’s actions may well have been the result of more intense publicity that better penetrated the public and the medical community, which was already aware of mounting risks and growing concern over litigation. Additional FDA safety communications after May 2016 that also reiterated the label change may have further contributed to decreases in quinolone prescribing, but our follow-up time was too short to investigate these associations individually.6,39,40 Interestingly, the label changes appeared to be associated with changes in use beyond the targeted indications to treatment selection for CAP, suggesting that risk-benefit assessments by prescribers were altered on a broader level.
These data also speak to the continued opportunities for improvement. Oral quinolones continue to comprise a fifth of antibiotic prescriptions for uUTI and 15% of antibiotic prescriptions for AE-COPD at the end of the study period. Because claims data do not provide insights into disease severity, drug sensitivities, or other reasons for prescribing oral quinolones over the recommended alternatives, it is not possible to ascertain the appropriateness of quinolone prescribing. This might be particularly the case for AS with its low prescribing prevalence at the end of the study period. Variations in practice patterns across specialties suggest that these may not be the only driving forces for much of the continued quinolone prescribing in uncomplicated infections. For example, urologists would be more likely to treat more complicated UTIs than primary care prescribers and it is unlikely that antibiotic sensitivities are substantially higher in primary care physician practices. Yet, prescribing of oral quinolones by urologists was markedly lower than that of internists and family physicians. Antibiotic prescribing has also been shown to have significant regional variations.41 This suggests that factors other than underlying disease are important drivers of quinolone prescribing. These factors need to be better understood to foster broad adoption of best practices. Though we saw a shift mostly to first-line antibiotics, reasons for increased use of not recommended antibiotics, also warrant further examination.
Limitations
This study had limitations that should be acknowledged. First, we had no control groups that were not influenced by the label changes because FDA regulation affects the entire US. Using another antibiotic that was not regulated by the label change was not feasible, because use of other antibiotics was likely indirectly affected by declining quinolone use. However, we did not find any changes in antibiotic prescribing for skin infections—a condition where quinolone is rarely prescribed.
The ITS design’s ability to support causal inferences depends on accurate detection of the time when outcomes trends change, which in turn depends on the regression model’s ability to accurately capture preintervention and postintervention trends. The design is also stronger if the effect of the intervention is abrupt rather than gradual and if changes in the population or other external factors as causes for the observed change can be excluded. Three sensitivity analyses supported the robustness of our ITS models. We accounted for a more gradual effect of the label change by excluding the time period between the advisory committee recommendation and final FDA approval of the revised labels, which resulted in a more pronounced estimate of the abrupt change in prescribing prevalence. Empirical detection of change points with joinpoint regression and ITS falsification tests also corroborated our findings.
Oral quinolone prescribing trends may well have been affected by other interventions, including publication of clinical practice guidelines, antibiotic stewardship programs, increased awareness of quinolone-associated adverse events, changes in the study population and the emergence of quinolone-resistant bacteria. To our knowledge, no other such causal pathways coincided with FDA action in May 2016, and visual inspection of effects of guideline releases does not suggest appreciable influence, which is consistent with previous findings.23,42 This notwithstanding, the label change occurred in the context of several years of emerging evidence and related guidances and our findings may not generalize to regulatory action that does not build on similar history. Due to the strong restrictions imposed for study entry to focus on isolated uncomplicated infection episodes, we also expect that the composition of the study population was homogenous throughout the study period.
Second, this analysis only captured treatment in the outpatient setting resulting in pharmacy dispensing of antibiotics, which however, should represent the most AS, uUTI, and AE-COPD episodes. Our results may further not be generalizable to patients in public health insurance or the uninsured. Finally, the assessment of these 3 conditions is susceptible to the accuracy of diagnosis codes. We used previously validated algorithms to identify infection episodes and evaluated up to 2 years of historical data to capture conditions that could complicate the evaluated infections in an effort to ensure that only uncomplicated acute infection episodes were evaluated.
Conclusions
The 2016 label changes by the FDA and associated announcements had a significant association with oral quinolone use for uUTI, but the results were less robust for AS and AE-COPD. This association was seen across all studied prescriber specialties. Quinolone treatment continued to contribute considerable proportion of treated uUTI and AE-COPD episodes, pointing to sustained opportunities for improvement.
eMethods 1. Model specification for interrupted time series analysis
eMethods 2. Specifications for the sensitivity analysis testing spill-over effects on treatment of community-acquired pneumonia and examination of a negative control cohort of with skin infections.
eMethods 3. JoinPoint regression specifications
eFigure 1. Flow diagram for inclusion and exclusion of enrollees to assemble study cohorts
eFigure 2. Trends of disease rate for the four study conditions over time
eFigure 3. Time trends of oral quinolone prevalence across all treated episodes by three main medical conditions
eFigure 4. Trends in oral antibiotic use prevalence among adults with community-acquired pneumonia
eFigure 5. Breakpoints and trend lines fitted by Joinpoint regression
eFigure 6. Distribution of hypothetical change points surrounding the tested label change
eTable 1. ICD-9-CM and ICD-10-CM* codes used to define study cohorts and excluded conditions
eTable 2. Infection categories based on Clinical Classification Software (CCS) for ICD-9-CM and Clinical Classification Software Refined (DXCCSR) for ICD-10-CM
eTable 3. ICD-9-CM and ICD-10-CM* codes used for exclusion of patients with potentially complicated urinary tract infections
eTable 4. Demographic characteristics of the three study cohorts
eTable 5. Impact of label changes on antibiotic use by medical condition– interrupted time-series analyses
eTable 6. Sensitivity analyses for interrupted time series models when excluding the phase-in period surrounding the labeling change (Nov 2015 to Jul 2016), shortening the pre-period (May 2015 to April 2016) and testing for spillovers effects in quinolone use for community acquired pneumonia
eTable 7. Joinpoint regression results
eTable 8. Monthly aggregated input data for the interrupted time series analysis of the three study cohorts
References
- 1.Pham TDM, Ziora ZM, Blaskovich MAT. Quinolone antibiotics. Medchemcomm. 2019;10(10):1719-1739. doi: 10.1039/C9MD00120D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correia S, Poeta P, Hébraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: where do we stand? J Med Microbiol. 2017;66(5):551-559. doi: 10.1099/jmm.0.000475 [DOI] [PubMed] [Google Scholar]
- 3.Hao D, Kiss G, Grubb W, Cohen S, Levin D, Sakr A. Spinal cord neuromodulation therapy for levofloxacin-reinduced complex regional pain syndrome and neurotoxicity: a case report. A A Pract. 2018;11(6):158-159. doi: 10.1213/XAA.0000000000000769 [DOI] [PubMed] [Google Scholar]
- 4.Pitiriga V, Vrioni G, Saroglou G, Tsakris A. The impact of antibiotic stewardship programs in combating quinolone resistance: a systematic review and recommendations for more efficient interventions. Advances in Therapy. 2017;34(4):854-865. [DOI] [PubMed] [Google Scholar]
- 5.Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med. 2005;118(3):259-268. doi: 10.1016/j.amjmed.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Accessed 10 Mar, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics
- 7.US Food and Drug Administration . Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs [ciprofloxacin (marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended-release (marketed as Cipro XR and Proquin XR), gemifloxacin (marketed as Factive), levofloxacin (marketed as Levaquin), moxifloxacin (marketed as Avelox), norfloxacin (marketed as Noroxin), and ofloxacin (marketed as Floxin)]. Accessed June 23, 2020. http://wayback.archive-it.org/7993/20170112032310/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm
- 8.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 9.Aring A, Chan M. Acute rhinosinusitis in Adults. American Family Physician. 2011;83(9):1057-1063. [PubMed] [Google Scholar]
- 10.Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America . IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72-e112. doi: 10.1093/cid/cis370 [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg. 2015;152(4):598-609. doi: 10.1177/0194599815574247 [DOI] [PubMed] [Google Scholar]
- 12.US Department of Veterans Affairs . VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. Accessed March 26, 2020. https://www.healthquality.va.gov/guidelines/CD/copd/VADoDCOPDCPG2014.pdf
- 13.US Food and Drug Administration . Summary Minutes of the Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Risk and Safety Management Advisory Committee November 5, 2015. Accessed June 23, 2020. http://wayback.archive-it.org/7993/20170113044503/http:/www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM475246.pdf
- 14.Szarfman A, Chen M, Blum MD. More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med. 1995;332(3):193. doi: 10.1056/NEJM199501193320319 [DOI] [PubMed] [Google Scholar]
- 15.Alves C, Mendes D, Marques FB. Fluoroquinolones and the risk of tendon injury: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2019;75(10):1431-1443. doi: 10.1007/s00228-019-02713-1 [DOI] [PubMed] [Google Scholar]
- 16.Cheng JZ, Sodhi M, Etminan M, Carleton BC. Fluoroquinolone use and risk of carpal tunnel syndrome: a pharmacoepidemiologic study. Clin Infect Dis. 08 2017;65(4):684-686. [DOI] [PubMed] [Google Scholar]
- 17.Etminan M, Brophy JM, Samii A. Oral fluoroquinolone use and risk of peripheral neuropathy: a pharmacoepidemiologic study. Neurology. 2014;83(14):1261-1263. doi: 10.1212/WNL.0000000000000846 [DOI] [PubMed] [Google Scholar]
- 18.Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ. 2018;360:k678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CC, Lee MT, Chen YS, et al. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med. 2015;175(11):1839-1847. doi: 10.1001/jamainternmed.2015.5389 [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration . FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. Accessed June 23, 2020. https://www.fda.gov/media/97602/download
- 21.Yarrington ME, Anderson DJ, Dodds Ashley E, et al. Impact of FDA black box warning on fluoroquinolone and alternative antibiotic use in southeastern US hospitals. Infect Control Hosp Epidemiol. 2019;40(11):1297-1300. [DOI] [PubMed] [Google Scholar]
- 22.Bratsman A, Mathias K, Laubscher R, Grigoryan L, Rose S. Outpatient fluoroquinolone prescribing patterns before and after US FDA boxed warning. Pharmacoepidemiol Drug Saf. 2020;29(6):701-707. doi: 10.1002/pds.5018 [DOI] [PubMed] [Google Scholar]
- 23.Durkin MJ, Keller M, Butler AM, et al. An Assessment of Inappropriate Antibiotic Use and Guideline Adherence for Uncomplicated Urinary Tract Infections. Open Forum Infect Dis. 2018;5(9):ofy198. doi: 10.1093/ofid/ofy198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubberke ER, Olsen MA, Stwalley D, et al. Identification of Medicare recipients at highest risk for clostridium difficile infection in the US by population attributable risk analysis. PLoS One. 2016;11(2):e0146822. doi: 10.1371/journal.pone.0146822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudmik L, Xu Y, Kukec E, Liu M, Dean S, Quan H. A validated case definition for chronic rhinosinusitis in administrative data: a Canadian perspective. Int Forum Allergy Rhinol. 2016;6(11):1167-1172. [DOI] [PubMed] [Google Scholar]
- 26.Stanford RH, Lau MS, Li Y, Stemkowski S. External validation of a COPD risk measure in a commercial and Medicare population: the COPD treatment ratio. J Manag Care Spec Pharm. 2019;25(1):58-69. doi: 10.18553/jmcp.2019.25.1.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanford RH, Nag A, Mapel DW, et al. Validation of a new risk measure for chronic obstructive pulmonary disease exacerbation using health insurance claims data. Ann Am Thorac Soc. 2016;13(7):1067-1075. [DOI] [PubMed] [Google Scholar]
- 28.Hooton T, Gupta K. Acute simple cystitis in women. Accessed Mar 17, 2020. https://www.uptodate.com/contents/acute-simple-cystitis-in-women#H207461713
- 29.Patel Z, Hwang P. Uncomplicated acute sinusitis and rhinosinusitis in adults: Treatment. Accessed Mar 17, 2020. https://www.uptodate.com/contents/uncomplicated-acute-sinusitis-and-rhinosinusitis-in-adults-treatment#H17857080
- 30.Sethi S, Murphy T. Management of infection in exacerbations of chronic obstructive pulmonary disease. 2020; https://www.uptodate.com/contents/management-of-infection-in-exacerbations-of-chronic-obstructive-pulmonary-disease. Accessed Mar 17, 2020.
- 31.Delamou A, Ayadi AME, Sidibe S, et al. Effect of Ebola virus disease on maternal and child health services in Guinea: a retrospective observational cohort study. Lancet Glob Health. 2017;5(4):e448-e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jandoc R, Burden AM, Mamdani M, Lévesque LE, Cadarette SM. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol. 2015;68(8):950-956. doi: 10.1016/j.jclinepi.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 33.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. doi: 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 34.Tran P, Winterstein A, Wang X, Rhew K, Antonelli P.. Appropriateness of otic quinolone use among privately insured US patients. Otolaryngol Head Neck Surg. 2020;162(1):102-107. [DOI] [PubMed] [Google Scholar]
- 35.Jones CM, Einstein EB, Compton WM. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010-2016. JAMA. 2018;319(17):1819-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170(15):1337-1345. doi: 10.1001/archinternmed.2010.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner DR, Heer E, Sutherland RL, et al. National trends in colorectal cancer incidence among older and younger adults in Canada. JAMA Netw Open. 2019;2(7):e198090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CY, Bussing R, Hartzema AG, Shuster JJ, Segal R, Winterstein AG. Stimulant use following the publicity of cardiovascular safety and the introduction of patient medication guides. Pharmacoepidemiol Drug Saf. 2016;25(6):678-686. [DOI] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration . FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients. Accessed Jan 3, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics
- 40.US Food and Drug Administration . FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics requires label changes. Accessed Jan 3, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-reinforces-safety-information-about-serious-low-blood-sugar-levels-and-mental-health-side
- 41.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308-1316. doi: 10.1093/cid/civ076 [DOI] [PubMed] [Google Scholar]
- 42.Crocker A, Alweis R, Scheirer J, Schamel S, Wasser T, Levingood K. Factors affecting adherence to evidence-based guidelines in the treatment of URI, sinusitis, and pharyngitis. J Community Hosp Intern Med Perspect. 2013;3(2). doi: 10.3402/jchimp.v3i2.20744 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Model specification for interrupted time series analysis
eMethods 2. Specifications for the sensitivity analysis testing spill-over effects on treatment of community-acquired pneumonia and examination of a negative control cohort of with skin infections.
eMethods 3. JoinPoint regression specifications
eFigure 1. Flow diagram for inclusion and exclusion of enrollees to assemble study cohorts
eFigure 2. Trends of disease rate for the four study conditions over time
eFigure 3. Time trends of oral quinolone prevalence across all treated episodes by three main medical conditions
eFigure 4. Trends in oral antibiotic use prevalence among adults with community-acquired pneumonia
eFigure 5. Breakpoints and trend lines fitted by Joinpoint regression
eFigure 6. Distribution of hypothetical change points surrounding the tested label change
eTable 1. ICD-9-CM and ICD-10-CM* codes used to define study cohorts and excluded conditions
eTable 2. Infection categories based on Clinical Classification Software (CCS) for ICD-9-CM and Clinical Classification Software Refined (DXCCSR) for ICD-10-CM
eTable 3. ICD-9-CM and ICD-10-CM* codes used for exclusion of patients with potentially complicated urinary tract infections
eTable 4. Demographic characteristics of the three study cohorts
eTable 5. Impact of label changes on antibiotic use by medical condition– interrupted time-series analyses
eTable 6. Sensitivity analyses for interrupted time series models when excluding the phase-in period surrounding the labeling change (Nov 2015 to Jul 2016), shortening the pre-period (May 2015 to April 2016) and testing for spillovers effects in quinolone use for community acquired pneumonia
eTable 7. Joinpoint regression results
eTable 8. Monthly aggregated input data for the interrupted time series analysis of the three study cohorts