Abstract
Background and aims
Patients with COVID-19 infection presents with a broad clinical spectrum of symptoms and complications. As a consequence nutritional requirements are not met, resulting in weight- and muscle loss, and malnutrition. The aim of the present study is to delineate nutritional complaints, the (course of the) nutritional status and risk of sarcopenia of COVID-19 patients, during hospitalisation and after discharge.
Methods
In this prospective observational study in 407 hospital admitted COVID-19 patients in four university and peripheral hospitals, data were collected during dietetic consultations. Presence of nutrition related complaints (decreased appetite, loss of smell, changed taste, loss of taste, chewing and swallowing problems, nausea, vomiting, feeling of being full, stool frequency and consistency, gastric retention, need for help with food intake due to weakness and shortness of breath and nutritional status (weight loss, BMI, risk of sarcopenia with SARC-F ≥4 points) before, during hospital stay and after discharge were, where possible, collected.
Results
Included patients were most men (69%), median age of 64.8 ± 12.4 years, 60% were admitted to ICU at any time point during hospitalisation with a median LOS of 15 days and an in-hospital mortality rate of 21%. The most commonly reported complaints were: decreased appetite (58%), feeling of being full (49%) and shortness of breath (43%). One in three patients experienced changed taste, loss of taste and/or loss of smell. Prior to hospital admission, 67% of the patients was overweight (BMI >25 kg/m2), 35% of the patients was characterised as malnourished, mainly caused by considerable weight loss. Serious acute weight loss (>5 kg) was showed in 22% of the patents during the hospital stay; most of these patients (85%) were admitted to the ICU at any point in time. A high risk of sarcopenia (SARC-F ≥ 4 points) was scored in 73% of the patients during hospital admission.
Conclusion
In conclusion, one in five hospital admitted COVID-19 patients suffered from serious acute weight loss and 73% had a high risk of sarcopenia. Moreover, almost all patients had one or more nutritional complaints. Of these complaints, decreased appetite, feeling of being full, shortness of breath and changed taste and loss of taste were the most predominant nutrition related complaints. These symptoms have serious repercussions on nutritional status. Although nutritional complaints persisted a long time after discharge, only a small group of patients received dietetic treatment after hospital discharge in recovery phase.
Clinicians should consider the risks of acute malnutrition and sarcopenia in COVID-19 patients and investigate multidisciplinary treatment including dietetics during hospital stay and after discharge.
Keywords: COVID-19, Nutritional status, Sarcopenia, Anorexia, ICU, Dietitian
1. Introduction
The World Health Organization (WHO) stated on 11th March 2020 that COVID-19 had become a worldwide pandemic, caused by the Coronavirus SARS-CoV-2, also called ‘severe acute respiratory syndrome coronavirus, SARS-CoV-2’ [1]. Acute respiratory complications due to severe COVID-19 infection, regularly require hospital admission and even treatment at an Intensive Care Unit (ICU) is regularly necessary. Patients with COVID-19 infection presents with a broad clinical spectrum of symptoms and complications such as fever, coughing, nasal cold, shortness of breath, pain during breathing, sore throat, general malaise, fatigue, general pain symptoms, headache, muscle ache, stomach-ache, decreased appetite (anorexia), decreased taste (ageusia), changed taste, decreased smell (anosmia), nausea, vomiting and diarrhea [2,3]. Several of these symptoms are associated with reduced nutrient intake, increased energy expenditure or decreased nutrient absorption. As consequence nutritional requirements are often not being met, resulting in weight- and muscle loss, and malnutrition. Malnutrition negatively affects the recovery of patients and increases the risk of morbidity and mortality [4,5]. Acute muscle loss contributes to further development of sarcopenia, an ongoing age-related loss of muscle mass, strength and physical function [6]. Sarcopenia is an important cause of morbidity and mortality in elderly and ICU patients where it is associated with higher comorbidities and increased length of ICU stay [7,8]. Additionally, sarcopenia is also often found in diabetic and obese patients whom are prone for COVID-19 infection [5].
In a recent study comprising 114 COVID-19 hospital admitted patients, 42% of the nursing ward patients and 67% of the ICU patients were malnourished [9]. Critically ill patients who are admitted to ICU for at least 48 h are known to be at risk for malnutrition and patients that survive long term ICU admission often suffer from long-term consequences due to ICU-acquired weakness [10,11].
Currently, there is limited knowledge about the nutritional complaints that coincide with, and influences the nutritional status and risk at sarcopenia of COVID-19 patients. The aim of the present study is to delineate nutrition related complaints, the (course of the) nutritional status and risk of sarcopenia of COVID-19 patients, during hospitalization and after discharge. Additionally, because nutrition related complaints and disease course may affect patients’ nutrient intake, we describe the current dietetic consultation and nutritional treatment of COVID-19 patients during and after hospitalization in a combined cross-sectional and longitudinal approach.
2. Material & methods
2.1. Design
This prospective observational study (COVOED-study) was conducted between April 27th and December 31st 2020 in three Dutch hospitals: Amsterdam University Medical Centers (Amsterdam UMC; locations AMC and VUmc), Franciscus Gasthuis & Vlietland (Rotterdam) and Zuyderland Medical Center (Heerlen, Sittard-Geleen). The study protocol was reviewed by the medical ethics review board of VU University Medical Center (IRB00002991), who decided that the Medical Research Involving Human Subjects Act (WMO) did not apply for this study. This study was included in the Amsterdam UMC Corona units Clinical Trial Center. A general informed consent was provided by the patients to use medical data for research purposes.
2.2. Patients
We included adult patients with an active COVID-19 infection necessitating hospital admission either at a (COVID) nursing ward or Intensive Care Unit (ICU) and whom received dietetic consultation, between 27th April and 31st December 2020. Patients were monitored during hospitalisation and after discharge in primary care setting, rehabilitation and elderly care centers, when dietetic care was indicated to be continued. Data collection ceased when the dietetic treatment was completed, patients died or were transferred to another hospital that was not participating in this study.
2.3. Data collection
Dietitians collected the data prospectively during the dietetic consultations with the patients. If direct consultation was not possible (due to isolation, limited consciousness or other factors hampering an interview such as fatigue and shortness of breath or sedation at ICU), the data was collected by telephone consultation, by questioning the treating nurse or derived from the electronic patient files. Castor Electronic Data Capture (version: Castor EDC 2020.2, Amsterdam, The Netherlands), a web-based electronic platform, was used for data management.
2.4. General data
The following items were collected: age, sex, length of hospital stay (LOS) (nursing ward stay and ICU stay), medication use (dexamethasone, propofol, morphine), support therapy (oxygen administration, ventilatory support, renal function replacement therapy), comorbidities (diabetes mellitus, kidney insufficiency, delirium), nutritional information (consistency of oral nutrition, use and type of enteral or parenteral nutrition, type of tube) and hospital discharge data (subdivided in in-hospital mortality, transfer to home or another hospital, rehabilitation- or elderly care center). Patients were characterized as ‘ICU patients’ if they were admitted to the ICU at any time point during hospital admission. Patients were marked as ‘nursing ward patients’ if they remained at the nursing ward during their total hospital stay.
2.5. Nutritional status
Nutritional status of the patients was assessed by Body Mass Index (BMI), weight loss before, during and after hospitalisation. Body weight was measured and body height was asked for. BMI was calculated (weight/height2) and categorised as described by WHO [12]: underweight BMI <18.5 kg/m2, normal weight BMI 18.5–25 kg/m2, overweight BMI 25–30 kg/m2, obesity BMI 30–40 kg/m2 and morbid obesity BMI >40 kg/m2. Patients were categorised as malnourished when the BMI was <18.5 kg/m2 or when recent weight loss was present: >5% in 1 week and/or >10% in 1 month and/or >10% lower than their regular body weight.
Risk of sarcopenia was assessed with the SARC-F questionnaire once during hospital admission during first dietetic consultation, and after discharge by the dietitian in primary care setting, rehabilitation and elderly care centers. A total score of 4 or more indicated an increased risk of sarcopenia [13,14].
2.6. Nutrition related complaints
The following data on nutrition related complaints was collected during the dietetic consultations: presence of loss of taste (ageusia), changed taste, loss of smell (anosmia), difficult chewing or swallowing, nausea, vomiting, feeling of being full, decreased appetite (anorexia), stool frequency and consistency, gastric retention (according to intern protocols defined as >250 ml), extreme fatigue or weakness necessitating need for help with food intake, and shortness of breath. For appetite, a visual analogue scale (1–100) was used [15]. For stool consistency, the Bristol Stool Chart [16].
2.7. Data analysis
Descriptive analyses were conducted to describe both the patient population using proportion, medians with interquartile ranges (IQR) and means with standard deviations (SD) for the general patient characteristics (i.e. age, sex, total LOS, LOS at nursing ward or ICU), as well as for the anthropometric data and nutrition related complaints. Statistical analysis was conducted in SPSS (IBM SPSS Statistics 26).
While individual patients were monitored longitudinally, data were accumulated per time point in a cross-sectional manner to adequately describe the actual situation at different time points. For instance, a dietitian that was first consulted in week 4 of hospital stay contributes to the prevalence of a nutritional complaint in week 4.
3. Results
3.1. Patient characteristics
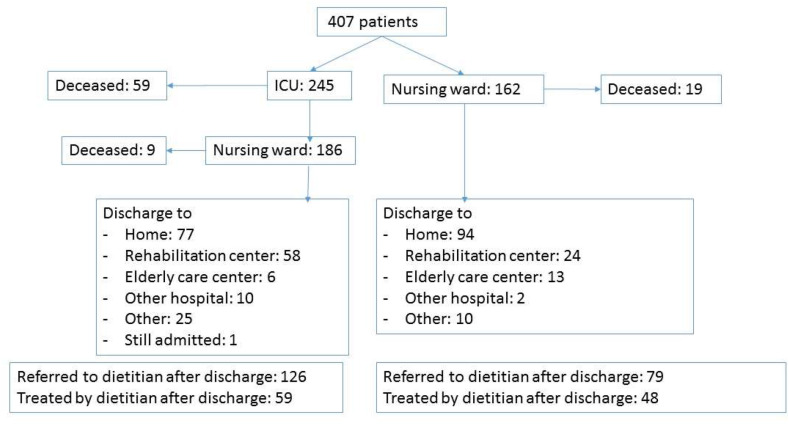
As displayed in Table 1 and flowchart in Fig. 1 , 407 patients were included in this study; 245 (60%) at the ICU and 162 (40%) at the nursing ward. In the university medical centers 77% of the patients were included and in the peripheral hospitals 23% of the patients. Roughly two-thirds of the patients were male and the mean age was 64.8 ± 12.4 years. Most patients (61%) were in the 41–70 years of age group. This was also the case for the patients whom were admitted to the ICU at any point during the hospital stay.
Table 1.
General patient characteristics.
| Characteristics | Total group (N = 407) | ICU patients (N = 245) | Nursing ward patients (N = 162) |
|---|---|---|---|
| Sex | |||
| Men | 280 (69%) | 185 (76%) | 95 (59%) |
| Women | 127 (31%) | 60 (24%) | 67 (41%) |
| Age (mean ± SD) | 64.8 ± 12.4 | 62.9 ± 11.6 | 67.7 ± 13.1 |
| ≤40 years | 18 (4%) | 13 (5%) | 5 (3%) |
| 41–70 years | 250 (61%) | 161 (66%) | 89 (55%) |
| ≥70 years | 139 (34%) | 71 (29%) | 68 (42%) |
| Hospital | |||
| Amsterdam UMC, location AMC | 157 (39%) | 110 (45%) | 47 (29%) |
| Amsterdam UMC, location VUmc | 156 (38%) | 100 (41%) | 56 (35%) |
| Franciscus & Vlietland Hospital | 43 (11%) | 22 (9%) | 21 (13%) |
| Zuyderland Medical Center | 51 (13%) | 13 (5%) | 38 (24%) |
| In-hospital mortality | 87 (21%) | 68 (28%) | 19 (12%) |
| Length of total hospital stay in days (LOS) (median, IQR) | 15 (7; 26) | 19 (12; 32) | 8 (5; 15) |
Fig. 1.
Flowchart of hospital admission, in-hospital mortality, discharge information and referral to the dietitian after discharge.
In-hospital mortality was 21% and the median length of hospital stay was 15 days. The ICU group had a higher in-hospital mortality rate compared with the nursing ward group (28% versus 12%) and even so for LOS (median 19 (12; 32) versus 8 [5,15] days).
In the ICU approximately 70% of the patients were sedated, usually with (caloric) propofol, during the first week of ICU admission. Morphine administration was needed in 73% of the ICU patients at some point, 72% of the ICU patients were treated with dexamethasone, 33% of the ICU patients had diabetes mellitus, 28% had signs of renal failure, 10% in the ICU patients renal function replacement therapy was initiated and 17% experienced gastric retention complaints. The number of delirious ICU patients increased from 5% in first week of admission to 36% in the 5th and 6th week of ICU-stay (in comparison to 12% of delirium in nursing ward patients).
For 205 out of the 320 survived patients (63%), dietetic treatment was indicated to be continued after hospital discharge. In 28 patients data could not be collected due to several reasons (readmission to the hospital (n = 3), passing away (n = 1), transfer to a different hospital which is not included in the study (n = 32) or other reason (n = 2)). In 107 out of the remaining 167 patients, the dietetic treatment in primary-, revalidation-of elderly care were registered. Data of 23 patients could not be included because the first dietetic appointment was planned at moment of data-analysis and 37 patients did not start the dietary treatment to the following reasons; appointment cancellation by patient itself (n = 4), lack of dietetic application or transfer (n = 14) or unknown reasons (n = 19).
3.2. Nutritional status
Data on nutritional status of included COVID-19 patients is described in Table 2 . At hospital admission 67% of the patients was overweight (BMI >25 kg/m2). The percentage of patients with (morbid) obesity was not different in de ICU and hospital ward patients. Considerable weight loss prior to hospital admission was seen in 25% of the patients, who were therefore characterised as malnourished. During hospital stay, 21% of the patients showed serious acute weight loss (>5 kg); most of these patients (85%) were admitted to the ICU at any point in time.
Table 2.
Nutritional status of hospital admitted COVID-19-patients.
| Total group (N = 407) | ICU patients (N = 245) | Nursing ward patients (N = 162) | |
|---|---|---|---|
| BMI at admission |
aN = 399 28.3 ± 5.8 |
aN = 243 29.0 ± 5.8 |
aN = 156 27.2 ± 5.8 |
| <18.5 kg/m2 | 4 (1%) | 0 | 4 (3%) |
| 18.5–25 kg/m2 | 126 (31%) | 63 (26%) | 63 (40%) |
| 25–30 kg/m2 | 146 (36%) | 102 (42%) | 44 (28%) |
| 30–40 kg/m2 | 108 (27%) | 66 (27%) | 42 (27%) |
| >40 kg/m2 | 15 (4%) | 12 (5%) | 3 (2%) |
| Malnourished at admission |
aN = 247 87 (35%) |
aN = 113 39 (34%) |
aN = 134 48 (36%) |
| Weight loss in the past month or past week or compared to regular weight (at admission) | aN = 246 | aN = 113 | aN = 133 |
| <5% | 162 (66%) | 74 (66%) | 88 (66%) |
| 5–10% | 57 (23%) | 26 (23%) | 31 (23%) |
| >10% | 27 (11%) | 13 (11%) | 14 (11%) |
| In hospital weight loss | aN = 290 | aN = 158 | aN = 132 |
| No weight loss to -1kg | 166 (57%) | 66 (42%) | 100 (76%) |
| −1 tot −5 kg | 60 (21%) | 34 (22%) | 26 (19%) |
| −5 to −10 kg | 37 (13%) | 32 (20%) | 5 (4%) |
| More than 10 kg | 27 (9%) | 26 (16%) | 1 (1%) |
| Weight change after hospital discharge compared to discharge weight |
0–3 weeks (first month) after discharge aN = 91 |
4–7 weeks (second month) after discharge aN = 40 |
3–5 months after discharge aN = 17 |
| Weight gain >1 kg | 28 (31%) | 18 (45%) | 9 (53%) |
| No weight loss to −1 kg | 38 (42%) | 10 (25%) | 4 (24%) |
| −1 tot −5 kg | 22 (24%) | 7 (18%) | 4 (24%) |
| −5 to −10 kg | 3 (3%) | 4 (10%) | 0 |
| More than 10 kg | 0 | 1 | 0 |
Data were not fully available in all patients: the N within the table depicts the number of patients with available data.
In Table 3 the risk of sarcopenia assessed by SARC-F questionnaire during admission and after discharge is shown. 73% of the patients were at high risk of sarcopenia during hospital admission. In the first dietetic consultation after discharge dietitians measured a high sarcopenia risk in 56% of the patients and 1 month after discharge this percentage was 21% in a small subgroup of 28 patients.
Table 3.
SARC-F in COVID-19 patients during hospital admission and after hospital discharge.
| Question | Total group (during admission) (aN = 219) | At first consultation after discharge (aN = 68) | 1 month later (aN = 28) |
|---|---|---|---|
| Strength; How much difficulty do you have in lifting and carrying 10 lb/5 kg? | |||
| None | 50 (23%) | 20 (29%) | 12 (43%) |
| Some | 68 (31%) | 24 (35%) | 11 (39%) |
| A lot of/unable | 101 (46%) | 24 (35%) | 5 (18%) |
| Assistance in walking; How much difficulty do you have walking across a room? | |||
| None | 64 (29%) | 26 (38%) | 20 (71%) |
| Some | 75 (34%) | 26 (38%) | 6 (21%) |
| A lot, use of aids or unable | 80 (37%) | 18 (27%) | 3 (11%) |
| Rise from a chair; How much difficulty do you have transferring from a chair or bed? | |||
| None | 78 (36%) | 29 (43%) | 22 (79%) |
| Some | 87 (40%) | 26 (38%) | 4 (14%) |
| A lot or unable without help | 54 (24%) | 13 (19%) | 2 (7%) |
| Climb stairs; How much difficulty do you have climbing a flight of 10 stairs? | |||
| None | 19 (10%) | 11 (16%) | 9 (32%) |
| Some | 44 (20%) | 30 (44%) | 17 (61%) |
| A lot or unable | 153 (70%) | 27 (40%) | 2 (7%) |
| Falls; How many times have you fallen in the past year? | |||
| None | 169 (77%) | 50 (74%) | 22 (79%) |
| 1–3 falls | 43 (19%) | 15 (22%) | 6 (21%) |
| ≥4 falls | 7 (3%) | 3 (4%) | 0 |
| Total score (mean ± SD) | 5.3 ± 2.6 | 4.2 ± 2.6 | 2.4 ± 2.2 |
| ≥4 points | 159 (73%) | 38 (56%) | 6 (21%) |
Data were not fully available in all patients: the N within the table depicts the number of patients with available data.
3.3. Nutrition related complaints in nursing ward patients
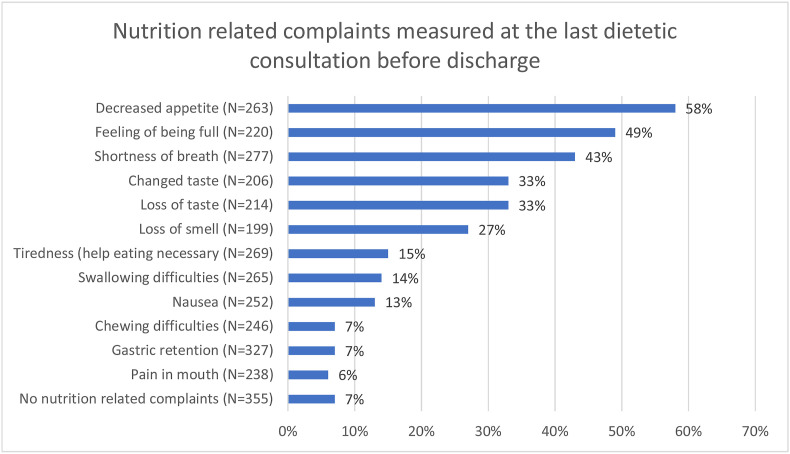
The nutrition related complaints (per week of hospital admission) during hospitalisation and after discharge are shown in the Supplemental Table S1. Figure 2 shows the frequency of the nutrition related complaints at the last dietetic consultation before discharge. Only a small minority of patients (7%) did not report any nutrition related complaints at any time point during hospitalisation. Most reported complaints were: a decreased appetite (58%), feeling of being full (49%) and shortness of breath (43%). One in three patients experienced changed taste, loss of taste and/or loss of smell. The change and loss of taste were usually reported by the same patients (25%), whereas 6% only had a changed taste and 6% only had loss of taste.
Fig. 2.
Nutrition related complaints, measured at the last dietetic consultation before discharge. ∗Data were not fully available in all patients: the N within the figure depicts the number of patients with available data.
The patients had infrequent bowel movements; the median daily stool frequency was 1 (0; 1) and 35% of the patients had no daily stools. The consistency of the stools was often soft; the Bristol Stool Chart showed that 7% of patients had type 1–3 (hard droppings), 17% had type 4 or 5 (normal consistency) and 41% had type 6 or 7 (soft/watery).
3.4. Development of nutrition related complaints in time
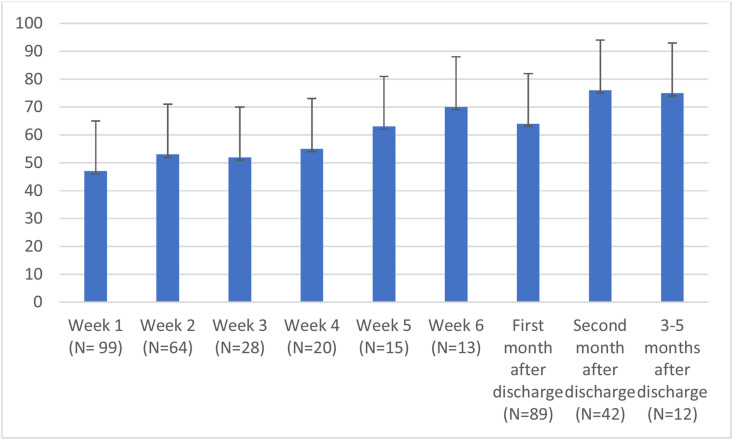
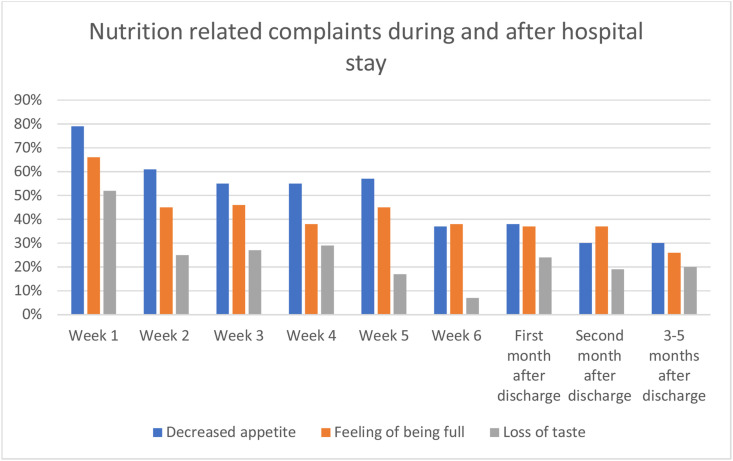
In Fig. 3 it is shown that the mean visual analog score of appetite was and remained low (<75%), especially in the first weeks of hospital stay. The development of the most common nutrition related complaints in time during hospital admission and after discharge is shown in Fig. 4 . The information on number of cases per time point is reported in the Supplemental Table S1. Loss of taste was experienced by half the patients in the first week of hospital admission and 24% in the weeks after discharge. Even 3–5 months after discharge 20% of the patients experienced loss of taste.
Fig. 3.
Mean visual analog scale appetite score (0–100) during hospital stay and after discharge.
Fig. 4.
Most common nutrition related complaints during hospital admission at nursing ward and after hospital discharge: decreased appetite, feeling of being full and loss of taste.
3.5. Dietetic treatment
During hospital stay, in 68% of the patients the consistency of the meals had to be adjusted to a soft, pureed or fluid consistency. After discharge, normal diet could, as a rule, be reintroduced at short notice. Oral nutritional supplements (ONS) was required in 53% of the patients at any moment during hospital stay and in 70% of the ICU patients information on the tube feeding was reported. In 81% this was applied by nasogastric tubes, in the remaining patients a naso-jejunal tube was necessary. In 71% of the tube-fed patients, polymeric tube feeding was prescribed, whereas in 29% semi-elementary tube feeding was provided. In five patients Parenteral Nutrition (PN) was applied. Only a small number of patients (16%), of those seen by the dietitian, did not receive any kind of medical nutrition (ONS or tube feeding) during hospital stay.
The mean number of dietetic consultations during hospital stay was 2.6 ± 2.6 and ranged from 1 to 17. After discharge this was 2.3 ± 1.4 consultations with a range from 1 to 6. In 72% of the patients the dietetic treatment started in the first week of admission. After discharge the dietetic treatment was in 52% of the patients resumed in the first week after discharge but in 10% of the patients after more than a month after discharge. The median duration of the dietetic treatment after discharge was 2 weeks with a range of 1–35 weeks. In 53% of the patients the treatment was completed within a month after discharge and in 32% of the patients within three months after discharge.
4. Discussion
COVID-19 has become a pandemic in a very short time, affecting millions of patients on a global scale. Our study demonstrates clearly that COVID-19 is associated with a large number of nutrition related complaints, acute malnutrition and a high risk of sarcopenia in hospitalized patients. Here, almost all COVID-19 patients (93%) seen by the dietitian experienced symptoms like decreased appetite, feeling of being full, shortness of breath and change or loss of taste. A large proportion of these patients (35%) showed acute malnutrition on admission to the hospital, based on substantial weight loss, lost further weight during hospital stay and were at high risk of developing sarcopenia (74%).
COVID-19 affects patients of all ages, but has more severe clinical consequences for adult and elderly patients. The patients that we included in our study had high mortality rates and fell typically in the higher age groups with more than 95% being older than 40 and 30% being older than 70 years of age. Additionally, our data fit with the common notion that hospitalized patients are most often overweight, obese or diabetic [17]. We collected information on medication that is commonly used in COVID-19. Propofol is used in severely affected patients to allow mechanical ventilatory support. Notably, propofol has a high nutritional content where an average daily dose may add up to one-third of the total daily calories [18]. Morphine was administered to relieve pain associated with breathing and facilitate respiration itself. However, morphine slows gastro-intestinal transit inducing nutritional related complaints like nausea and constipation. Thirdly, dexamethasone is used in COVID-patients whom require oxygen therapy to improve outcome [19]. Corticosteroid often increase plasma glucose levels necessitating tailored nutrition and insulin therapy which can be a challenge in patients who are already diabetic.
Despite the fact that the majority of our patients were overweight or obese, more than a third of the patients suffered from substantial weight loss before hospital admission. This indicates that COVID-19 has a profound negative impact on nutritional status already before admission. This is reinforced by the finding that in 22% of the patients, mainly ICU patients, loosed more than 5 kg extra body weight during hospital stay. This percentage is probably an underestimation because weight at discharge was not recorded in the patients who died or were transferred to another hospital from the ICU. It has been demonstrated that in-hospital malnutrition is associated to hospital length of stay (LOS), in-hospital mortality, and re-admission rate [[20], [21], [22]]. Although studies specifically assessing the role of a deranged nutritional status in this type of patient are still sparse, our findings confirm earlier data. Li et al. evaluated the nutritional status of 182 elderly inpatients with COVID-19 using the Mini Nutritional Assessment (MNA) and found that 28% were at risk for malnutrition and 53% were malnourished [23]. Bedock et al. showed in a small study (n = 114) that overall prevalence of malnutrition was 42% (moderate: 24%, severe: 18%). The prevalence of malnutrition reached 67% in patients admitted from ICU [9]. It must be emphasized that also patients with a BMI in the overweight or obese range have a risk on sarcopenia. This is aggravated by a disease-related weight loss that contributes to the risks and complications described above [24]. This is further supported by our SARC-F questionnaire data showing tremendous risk on sarcopenia in the different domains although oxygen dependency may have worsened these results since the risk decreased rapidly after discharge.
In general, disease results in multiple nutrition-related problems such as changes in appetite, taste and energy expenditure and changes in taste. The ensuing malnutrition negatively affects the recovery of patients and increases the risk of morbidity and mortality [4,5]. It is to be expected that nutrition-related complaints are more prevalent in more severe ill and thus hospitalized patients. However our data, showing that more than 90% of patients experience some form of nutritional complaints during hospitalization, emphasize the severe impact of COVID-19 on nutritional intake. We found loss of appetite to be most prevalent and not loss of smell and taste. These were at the lower boundaries as reported earlier: 33–68% with female dominance [25]. Besides we found a relative large proportion of patients to have soft or watery stools, but reported stool frequency was low, suggesting that there was not often a real diarrhea problem in our study population. This supports data from other studies showing a wide incidence range of diarrhea from 2 to 50% [26]. Also here, it should be taken into account that oxygen dependency may have contributed to some of the nutritional-related complaints that are described in Fig. 2. Notably, nutrition-related complaints persisted after hospital discharge, which indicates that dietary follow-up may be indicated. In this study 63% of the patients were referred for dietetic care after hospital discharge. We managed to record the dietetic treatment of half of these patients, the prevalence of de nutrition related complaints remained present for a long period of time. Based on the results of this study and the fact that this patient group is also at high risk of the Post Intensive Care Syndrome (PICS), dietetic and physiotherapeutic treatment should be considered in all patients at discharge [27].
Our study has some limitations. First, it was a challenge to organise appropriate dietetic care in the hospital and after discharge due to isolation, hygiene and lockdown measures. Secondly and as a consequence of the former, it was not possible to measure the body composition and muscle strength. Therefore, future projects need to determine the impact of the inadequate nutritional intake, disease severity and immobilisation on the amount and loss of muscle mass and strength in COVID-19 patients. Although, the results of this study may suggest a high prevalence of sarcopenia and sarcopenic obesity, imaging techniques such as repetitive computed tomography scans need to be used to quantify muscle mass. Thirdly, we were unable to include information about ethnicity in our cohort. However, the two hospitals that contributed mostly to the patient inclusion are located in the urban area of Amsterdam and are known to have a diverse multi-ethnic patient population.
Finally, patients in this study were included based on a dietetic consultation and may not represent all COVID-19 patients admitted to hospital. However, the included patient group is comparable to hospital COVID-19 populations described by others for sex, age, prevalence of overweight and obesity (BMI), and comorbidities [28]. The in-hospital mortality at the ICU was 28%, which corresponds to reported COVID-19 ICU mortality [29].
5. Conclusion
In conclusion, one in five admitted COVID-19 patients suffered from serious acute weight loss and 73% had a high risk of sarcopenia. Moreover, almost all patients had one or more nutritional complaints. Of these complaints, decreased appetite, feeling of being full and changed taste and loss of taste were the most predominant nutrition related complaints. These symptoms have serious repercussions on nutritional status. Although nutritional complaints persisted a long time after discharge, only a small group of patients received dietetic treatment after hospital discharge in recovery phase.
Clinicians should consider the risks of acute malnutrition and sarcopenia in COVID-19 patients and investigate multidisciplinary treatment including dietetics during hospital stay and after discharge.
Contribution to authorship
NW, HK, PW, MS and AvB designed the research; NW, DK, MJ, JJ, FT and LvA conducted the research; HK, LK and NW analyzed data; NW, HK, LK, PW, MS and AvB wrote the article; NW and HK had primary responsibility for the final content and have shared first authorship of this article. All authors critically reviewed the manuscript and approved the final manuscript.
Funding statement
This study is performed largely without funding. NW and HK received a grant from Nutricia to perform data-analyses and to write the manuscript.
Declaration of competing interest
Both first authors NW and HK received a grant from Nutricia to be able to perform this study. All authors report no conflict of interests. ICMJE disclosure of interest forms are submitted as supporting information.
Acknowledgement
Authors like to thank all the ICU and COVID-nursing ward dietitians of the four participating hospitals for collecting the data and import data into Castor.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2021.03.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Janiaud P., Axfors C., Van’t Hooft J., Saccilotto R., Agarwal A., Appenzeller-Herzog C. The worldwide clinical trial research response to the COVID-19 pandemic - the first 100 days. F1000Research. 2020;9:1193. doi: 10.12688/f1000research.26707.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges do Nascimento I.J., Cacic N., Abdulazeem H.M., von Groote T.C., Jayarajah U., Weerasekara I. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020; Mar;9(4) doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; Apr;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguila E.J.T., Lontok M.A.D., Francisco C.P.D. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. Vol. 18. 2020. Follow your gut: challenges in nutritional therapy during the COVID-19 pandemic; pp. 2638–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D. Vol. 39. Clinical Nutrition; Edinburgh, Scotland): 2020. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection; pp. 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019, Jan;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kizilarslanoglu M.C., Kuyumcu M.E., Yesil Y., Halil M. Sarcopenia in critically ill patients. J Anesth. 2016, Oct;30(5):884–890. doi: 10.1007/s00540-016-2211-4. [DOI] [PubMed] [Google Scholar]
- 8.Joyce P.R., O'Dempsey R., Kirby G., Anstey C. A retrospective observational study of sarcopenia and outcomes in critically ill patients. Anaesth Intensive Care. 2020, May;48(3):229–235. doi: 10.1177/0310057X20922234. [DOI] [PubMed] [Google Scholar]
- 9.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020, Dec;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pryor L., Ward E., Cornwell P., O'Connor S., Chapman M. Patterns of return to oral intake and decannulation post-tracheostomy across clinical populations in an acute inpatient setting. Int J Lang Commun Disord. 2016, Sep;51(5):556–567. doi: 10.1111/1460-6984.12231. [DOI] [PubMed] [Google Scholar]
- 11.Singer P., Berger M.M., Van den Berghe G., Biolo G., Calder P., Forbes A. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009, Aug;28(4):387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 12.WHO Website World Health organisation (WHO) http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [internet]. [cited 2021 Feb 24]. Available from:
- 13.Bahat G., Yilmaz O., Merve Oren M., Beaudart C. Cross-cultural adaptation and validation of the SARC-F to assess sarcopenia: methodological report from European union geriatric medicine society sarcopenia special interest group. Eur Geriatr Med. 2018;9:23–28. doi: 10.1007/s41999-017-0003-5. [DOI] [PubMed] [Google Scholar]
- 14.Visser M., Schaap L., Hobbelen H., Perkisas S., Sipers M. Sarcopenie: screening en diagnose. Ned Tijdschr Geneeskd. 2020:D3824. [Google Scholar]
- 15.Stubbs R.J., Hughes D.A., Johnstone A.M., Rowley E., Reid C., Elia M. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000, Oct;84(4):405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 16.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997, Jan 8;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 17.Kwok S., Adam S., Ho J.H., Iqbal Z., Turkington P., Razvi S. Obesity: a critical risk factor in the COVID-19 pandemic. Clin Obes. 2020, Dec;10(6) doi: 10.1111/cob.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousie E., van Blokland D., Lammers H.J.W., van Zanten A.R.H. Relevance of non-nutritional calories in mechanically ventilated critically ill patients. Eur J Clin Nutr. 2016, Dec;70(12):1443–1450. doi: 10.1038/ejcn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021, Feb;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson L., Chittams J., Griffith C., Compher C. Malnutrition identified by academy of nutrition and dietetics/American society for parenteral and enteral nutrition is associated with more 30-day readmissions, greater hospital mortality, and longer hospital stays: a retrospective analysis of nutrition assessment data in a major medical center. JPEN - J Parenter Enter Nutr. 2018, Jul;42(5):892–897. doi: 10.1002/jpen.1021. [DOI] [PubMed] [Google Scholar]
- 21.Norman K., Pichard C., Lochs H., Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal E., Ferguson M., Banks M., Batterham M., Bauer J., Capra S. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: results from the Nutrition Care Day Survey 2010. Clin Nutr. 2013; Oct;32(5):737–745. doi: 10.1016/j.clnu.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020, Jun;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gualtieri P., Falcone C., Romano L., Macheda S., Correale P., Arciello P. Body composition findings by computed tomography in SARS-CoV-2 patients: increased risk of muscle wasting in obesity. Int J Mol Sci. 2020; Jun;21(13) doi: 10.3390/ijms21134670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2020, Jul;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Tol B., Dettling D., Kruizenga H., Pellegrom S., Major M., Siebel M. Customised care: post intensive care syndrome. Kompass Nutr Diet [Internet] 2021;1(1):27–30. https://www.karger.com/DOI/10.1159/000513300 [Google Scholar]
- 28.Fedele D., De Francesco A., Riso S., Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutrition. 2021, Jan;81:111016. doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020, Mar;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.