Abstract
Introduction
Infection and inflammation of the reproductive tract by Chlamydia trachomatis (CT) are recognized as significant risk factors for male infertility. This study aimed to evaluate CT infection and its effects on seminal parameters and cytokines in asymptomatic patients with teratozoospermia.
Material and methods
Semen samples from one hundred four male patients were collected, and CT detection was performed by polymerase chain reaction (PCR). The quality (volume, sperm concentration, pH, motility, morphology, and leucocytes) of the semen was measured by standard procedures recommended by the World Health Organization (WHO). Pro-inflammatory cytokines [interleukin (IL)-1 β, IL-6, IL-8, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ)], as well as anti-inflammatory cytokines (IL-4, IL-10), were determined by using enzyme-linked immunosorbent assay (ELISA). The frequency of CT infection was expressed as a percentage. Descriptive statistics were used for comparison of cytokines from infertile men, and then the Mann-Whitney U test was applied for the contrast of seminal parameters and cytokines from CT-infected versus non-CT infected men.
Results
A ratio of 33/104 (31.7%) patients were positive for CT infection. The ejaculate of positive CT infection was found to have increased pH (pH = 7.65 in non-CT infected vs. 7.94 CT-infected men; p = 0.026). High levels of pro-inflammatory cytokines were found in the population studied; however, infected males were noted to have high levels of IL-1 β [184.66 (0-3985.33 pg/ml), p = 0.001] and IL-6 [87.8 (0-1042.8 pg/ml), p = 0.001].
Conclusions
CT infection increased seminal pH, as well as IL-1 β and IL-6 cytokines, suggesting a potential role of infection and inflammation in asymptomatic patients with teratozoospermia.
Keywords: Chlamydia trachomatis, inflammation, male infertility, teratozoospermia, cytokines, semen
Introduction
Infertility is a major global problem of reproductive health. It is defined as the inability of sexually active couples to achieve pregnancy after 12 consecutive months of unprotected sex [1, 2]. Unfortunately, in many countries, infertility is under-diagnosed. It is estimated that 15% of all couples are infertile, and it is identified that a male factor plays a role in about half of the cases [3].
Furthermore, about 70% of cases of male infertility are idiopathic [2], and multiple factors are associated with the rest, such as genetic, hormonal, inadequate lifestyle, psychological stress, pro-inflammatory cytokines, as well as sexually transmitted infections (STIs), including Chlamydia trachomatis (CT) [4, 5]. CT is an intracellular Gram-negative bacterium that causes infertility, although it may also cause urethritis and prostatitis in men [6, 7]. In male infertility, there is considerable variability in the rate of CT infection, ranging from 0 to 90.3%, and, in general, its prevalence is high in developing countries, such as Mexico [7].
Infections impair male fertility by different mechanisms including damaging spermatogenesis and loss of sperm function [5], and induce immunological responses where pro-inflammatory cytokines and other immunoregulatory molecules are involved by interacting with cell types [8, 9], causing chronic inflammation and damage to spermatozoa [6, 8]. There are few reports concerning the relationship between CT infection, alteration in seminal parameters [10], and elevated levels of pro-inflammatory cytokines as causes of male infertility [11].
For this reason, this study aims to investigate the CT infection and its effects on seminal parameters and cytokines in asymptomatic patients with teratozoospermia.
Material and methods
Study design and population
The present study was conducted at the “Hospital Militar de Especialidades de la Mujer y Neonatología” of the National Defense Ministry (SEDENA), Mexico City.
The Institutional Human Research Ethical Committee approved the protocol and the informed consent forms. All experiments were examined and approved by the appropriate ethics committee and therefore were performed in accordance with the ethical guidelines of the Declaration of Helsinki, and the official Mexican Standard NOM-012-SSA3-2012.
One hundred four semen samples were obtained between January and November 2016 from military men aged 22 to 49 years old. Men were eligible for participation if they 1) were male partners from couples attending the clinic for inability to conceive within at least one year of unprotected regular sexual intercourse, 2) underwent sperm parameters analysis, and 3) provided semen by masturbation. Additionally, a clinical examination was done.
Semen analysis and criteria
Specimens were allowed to liquefy at room temperature for 30 minutes; then, their macroscopic and microscopic examinations were performed according to the World Health Organization (WHO) [1]. The men were considered to have teratozoospermia if the percentage of spermatozoa with normal morphology was ≤ 4%.
Detection of Chlamydia trachomatis by PCR
Semen samples were collected, and DNA extraction was performed using the DNeasy Blood and Tissue Kit (QIAGEN Ltd., U.K.), according to the manufacturer’s instructions. The amplification was carried out using specific primers KL1 and KL2 of CT [9]. The β-globin gene was used as a positive control for DNA extraction and PCR methods.
Cytokines in seminal plasma
Cytokine determinations were performed on seminal plasma (dilution 1 : 1) per duplicate, using sandwich enzyme-linked immunosorbent assay (ELISA) for the pro-inflammatory cytokines interleukin (IL)-1β (range: 8-1000 pg/ml), IL-6 (range: 24-1500 pg/ml), IL-8 (range: 16-1000 pg/ml), tumor necrosis factor α (TNF-α) (range: 31-2000 pg/ml) and interferon γ (IFN-γ) (range: 8-3000 pg/ml), as well as for the anti-inflammatory cytokines IL-4 (range: 16-1000 pg/ml), and IL-10 (range: 23-3000 pg/ml) (PeproTech, USA). Standard curves were developed for each cytokine according to the manufacturer’s instructions.
Statistical analysis
The frequency of CT infection was expressed as a percentage. Descriptive statistics were used for comparison of cytokines from infertile men. For continuous data, the nonparametric Mann-Whitney U test was applied for the contrast of seminal parameters and cytokines from CT-infected versus non-CT infected men. Data was analyzed by SPSS statistical software, version 24 (IBM Corp., USA) and Microsoft Excel (Windows 10). In all cases, a statistical significance was assumed when p < 0.05.
Results
In this study, a ratio of 33/104 (31.7%) asymptomatic teratozoospermic patients were positive for CT infection.
Table 1 summarizes seminal parameters in non-CT-infected and CT-infected asymptomatic patients with teratozoospermia. Interestingly, CT-infected men showed an increased pH in semen. The concentrations of seminal cytokines were high in the population studied (Table 2). The levels of pro-inflammatory cytokines IL-1β and IL-6 in the CT-infected group were higher than in the non CT-infected group (Table 3).
Table 1.
Seminal parameters in the population studied
| Parameter | Non CT-infected (n = 71) | CT-infected (n = 33) | Significance p | Normal parameters |
|---|---|---|---|---|
| Volume (ml) | 3.56 ±1.44 | 3.1 ±0.84 | 0.761 | 1.5-5 ml |
| Total sperm number (106/ejaculate) | 67.58 ±55.03 | 75.99 ±51.48 | 0.426 | > 40 million |
| Sperm concentration (millions/ml) | 21.36 ±17.92 | 29.21 ±25.16 | 0.371 | > 20 million/ml |
| pH | 7.65 ±0.61 | 7.94 ±0.60 | 0.026 | 7.2-7.8 |
| Progressive motility (% A+B) | 39.88 ±20.93 | 48.11 ±16.92 | 0.123 | ≥ 32% |
| Normal morphology (%) | 1.85 ±1.17 | 2.58 ±1.5 | 0.055 | ≤ 4 % |
| Leucocytes (106) | 1.32 ±1.71 | 0.78 ±0.72 | 0.189 | ≤ 1 million |
Values are media ± SD. Significance refers to Mann-Whitney test for difference between groups; the significance level is p ≤ 0.05. The normal parameters are those considered in the WHO laboratory manual for the examination and processing of human semen, Handbook, 5th ed., WHO 2010. CT – Chlamydia trachomatis
Table 2.
Descriptive statistics of seminal pro-inflammatory cytokines in the population studied
| Cytokine (pg/ml) | Mean (SD) | Median (range) | Percentile (25-75) | Fertile men | Infertile men |
|---|---|---|---|---|---|
| IFN-γ | 731.92 ±1279.12 | 180.25 (0-7406) | 0-951.5 | 0 (0-130)a | 593 (192-1665)d |
| IL-1β | 327.42 ±736.88 | 28 (0-3985.33) | 0-198.41 | 0.70 (0.01-9)b | 5.66 (0.1-334.8)b |
| IL-6 | 224.23 ±500.77 | 31.8 (0-2994.8) | 0-205.8 | 11.98 (2.5-102)b | 48.91 (6.9-2634)b |
| TNF-α | 0.9457 ±6.63 | 0 (0-43.5) | 0-0 | 2.76 (0.55-10.3)b | 18.42 (0.8-360)b |
| IL-8 | 1477.61 ±5087.01 | 336.64 (0-31950) | 199.12-336.64 | 441.84 (215.4-767)b | 1975.34 (186.9-6754)b |
| IL-4 | 43.88 ±52.63 | 27.5 (0-212.5) | 7.18-71.87 | 12.6 ±3.8c | 8.4 ±3.9c |
| IL-10 | 16.06 ±92.34 | 0 (0-617) | 0-0 | 2.81e | 5.2 (2.7-7.7)f |
Concentration of cytokines reported by aPolitch et al., 2007, bMoretti et al., 2008, cOmu et al., 1999, dSeshadri et al., 2009, eJiang et al. 2016, fMartínez-Prado and Camejo, 2009 for comparison. IFN-γ – interferon-γ, TNF-α – tumor necrosis factor-α, IL-4 – interleukin-4, IL-1β – interleukin-1β, IL-6 – interleukin-6, IL-8 – interleukin-8. Values are median (range) in pg/ml in all results, except the reference values of Omu et al., 1999, which are mean ±SD.
Table 3.
Levels of seminal pro-inflammatory cytokines in non-Chlamydia trachomatis (CT)-infected and CT-infected patients
| Cytokine (pg/ml) | Non CT-infected (n = 71) | CT-infected (n = 33) | Significance p |
|---|---|---|---|
| IFN-γ | 494 (0-3394) | 154 (0-7406) | 0.320 |
| IL-4 | 33.75 (0-212.5) | 26.25 (0-91.25) | 0.052 |
| IL-1β | 0 (0-1304) | 184.66 (0-3985.33) | 0.001 |
| IL-6 | 0 (0-2994) | 87.8 (0-1042.8) | 0.001 |
| TNF-α | 0 (0-108.5) | 0 (0-43.5) | 0.687 |
| IL-8 | 342.35 (0-31950) | 364.85 (0-14817.1) | 0.724 |
| IL-10 | 0 (0-1177) | 0 | 0.202 |
IFN-γ – interferon-γ, IL-4 – interleukin-4, IL-1β – interleukin-1β, IL-6 – interleukin-6, TNF-α – tumor necrosis factor-α, IL-8 – interleukin-8. Values are the median (range). Significance refers to Mann-Whitney U test for difference between both groups, the significance level is p ≤ 0.05.
Discussion
Recently, infection and inflammation of the male reproductive tract have been recognized as severe risk factors of male infertility, where CT infection is among the most prevalent sexually transmitted disease in the world [12-14]. This study examined the relationship between CT infection and its effects on seminal parameters and inflammatory markers.
Firstly, a CT infection was found in 31.7% of the semen samples from asymptomatic teratozoospermic men. This prevalence rate is very similar to other reports from Mexico and Latin America, which demonstrated a prevalence of 31.9% [7], and 38.6% [15] in infertile patients, respectively; while in a specific population with teratozoospermia the prevalence was reported to be 35% [7].
In terms of sperm quality, according to the WHO criteria [1], the population herein studied was similar to other reports [10, 16, 17] because they also presented a higher pH value related to the CT infection (pH = 7.65 vs. 7.94). Recent findings suggest that CT infection may influence a change in pH to alkaline values in the female reproductive tract [18]. It has been observed that an alkaline pH favors a shift in the seminal microbiota, such as Lactobacillus, in healthy men [19]. Microbial imbalance harms semen parameters, including sperm motility and morphology, and the host becomes susceptible to other infections and diseases [19].
Concerning the immunological response, the results are consistent with other studies that have reported the presence of high levels of diverse cytokines in seminal plasma from infertile men, such as the pro-inflammatory cytokines IL-6 [20-23], TNF-α [18, 19, 21, 22, 25], IL-8 [19, 25, 26], IL-1β [20, 25], and IFN-γ [29], as well as the anti-inflammatory cytokines IL-4 [24] and IL-10 [30], among others [29, 30]. As in the case of most infections, the immune system is a crucial element in the body’s attempts to eradicate pathogens such as Chlamydia trachomatis, which infect the epithelial cells and produce tissue damage. CT infection is characterized by clinical manifestations from subclinical disease to a robust inflammatory response; unfortunately, 50% of men are asymptomatic which may lead to repeated transmission [6, 14]. Chronic inflammation by infections can lead to urethritis, epididymitis, epididymo-orchitis, and potentially prostatitis and infertility in men [6, 8, 31]; nevertheless, this process has been poorly studied.
Interestingly, the activation of immunological responses due to infection was evidenced in this study, as well as others. Other authors have suggested that the CT infection often causes tissue lesions that stimulate IL-1, which in turn activates polymorphonuclear cells (PMN) and macrophages, and subsequently, the production of IL-6, IL-8, IL-10, TNF-α, and IFN-γ is induced [8].
The IL-6 level in the seminal plasma has also been observed to be higher in infertile men [17, 33] compared to fertile men [34, 35]; however, the results found herein are the highest so far reported. Other authors have suggested that IL-6 is involved in lipid peroxidation (LP). This conclusion was confirmed due to the detection of a byproduct (malondialdehyde, MDA) added to IL-6, simultaneously [36]. These reactions activate oxidative stress, which contributes to male infertility [8].
It is essential to consider that the population studied here consisted of infertile military men, whose condition contributes to presence of several neurochemical, endocrinological, and immunological alterations due to stress, as reported by other authors in military communities [36, 37]. This may also have contributed to modulated inflammatory cytokine levels. For example, studies on hypercortisolemia in military trainees suggest that this hormone also contributes to infertility due to decreases of testosterone by the repetition of psychological stress [37], and more in socially dominant subjects [38]. In addition, cortisol has been found to increase IgE synthesis in response to IL-4, which suppresses the TNF-α production by monocytes [37]. The CT infection probably is chronic and accompanied by other pathogens in semen which are responsible for altered pro-inflammatory cytokine levels and an inadequate response of Th2 cytokines such as IL-10 and IL-4.
Furthermore, both low and non-significant levels of IFN-γ and TNF-α were found. This suggests that most of these patients were suffering from chronic infection with CT, as reported by Feodorova et al. [10]. It is proposed that IFN-γ participates in the activation of the innate immune response at the beginning of the infection [6, 38]. CT infection exerted its effect on motility at high concentrations, as reported by other authors [39]; hence, it may be assumed that this cytokine is no longer protecting against the chronic CT infection.
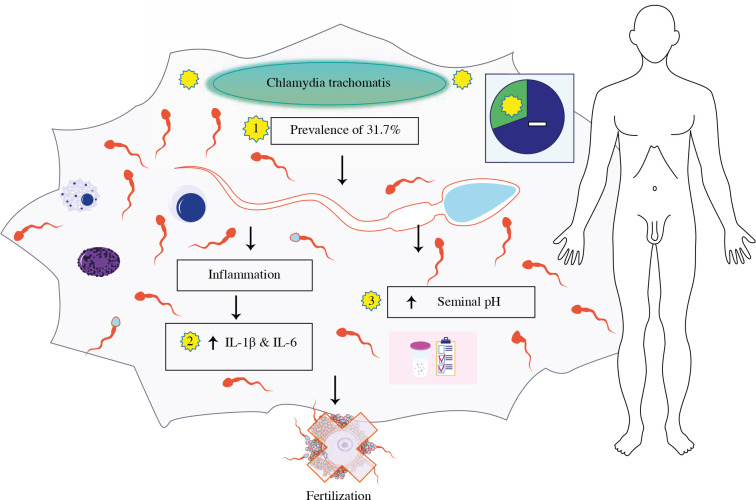
Taken together, the results suggest a strong pro-inflammatory response and a possible chronic CT infection, which can cause effects on the health of men and lead to pathologies such as chronic prostatitis with subfertility [40]. In conclusion, CT infection causes immunological responses because it increases IL-1β and IL-6, also increasing seminal pH (see Fig. 1), suggesting a potential role of infection and inflammation in asymptomatic patients with teratozoospermia.
Fig. 1.
Chlamydia trachomatis infection increases seminal pH, also interleukin (IL)-1β and IL-6 pro-inflammatory cytokines in asymptomatic patients with teratozoospermia. Image modified from Motifolio Toolkit licence (http://www.motifolio.com)
Acknowledgments
The authors are grateful for the availability of patients attending at the Hospital Militar de Especialidades de la Mujer y Neonatología, SEDENA, Mexico City.
Footnotes
The authors declare no conflict of interest.
Funding
This work has been funded by the transdisciplinary SIP research project of the Instituto Politécnico Nacional, SIP number 20190296 and the CONACYT scholarship number 2018-000068-02NACF-24430 for doctoral studies of Elvia Pérez-Soto.
References
- 1.World Health Organization, Department of Reproductive Health and Research (2010): WHO laboratory manual for the examination and processing of human semen, 5th ed. WHO Press, Switzerland. [Google Scholar]
- 2.Vander BM, Wyns C (2018): Fertility and infertility: definition and epidemiology. Clin Biochem 62: 2-10. [DOI] [PubMed] [Google Scholar]
- 3.Schuppe H, Pilatz A, Hossain H, et al. (2017): Urogenital infection as a risk factor for male infertility. Dtsch Aerzteblatt 114: 339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou D, Zhou X, Zhong X, et al. (2013): Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril 100: 1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Ezzy AIA (2016): Effect of torch agents and Chlamydia trachomatis on reproductive parameters and fertility hormones of Iraqi infertile males. Asian J Pharm Clin Res 9: 47-56. [Google Scholar]
- 6.Redgrove KA, McLaughlin EA (2014): The role of the immune response in Chlamydia trachomatis infection of the male genital tract: A double-edged sword. Front Immunol 5: 1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-Hurtado M, Velazco-Fernández M, Pedraza-Sánchez MJE, et al. (2017): Molecular detection of Chlamydia trachomatis and semen quality of sexual partners of infertile women. Andrologia e12812: 1-6. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Rana M, Qiu E, et al. (2018): Role of oxidative stress, infection and inflammation in male infertility. Andrologia 50: 1-13. [DOI] [PubMed] [Google Scholar]
- 9.Mahony JB, Luinstra KE, Sellors JW, et al. (1992): Confirmatory polymerase chain reaction testing for Chlamydia trachomatis in first-void urine from asymptomatic and symptomatic men. J Clin Microbiol 30: 2241-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feodorova V, Sultanakhmedov E, Saltykov Y, et al. (2018): First detection of Chlamydia trachomatis “Swedish” variant (nvCT) in a Russian couple with infertility. Open Microbiol J 12: 343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Marzoqi AH, Al-taee ZM, Ahmed KN (2014): Profiles among asthenospermic men with Chlamydia trachomatis infections: concentrations and significance of multiplex seminal fluid cytokine and other immunologic factors. Int J Sci Nat 5: 103-108. [Google Scholar]
- 12.Loveland KL, Klein B, Pueschl D, et al. (2017): Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front Endocrinol (Lausanne) 8: 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gdoura R, Kchaou W, Ammar-Keskes L, et al. (2008): Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl 29: 198-206. [DOI] [PubMed] [Google Scholar]
- 14.Samplaski MK, Domes T, Jarvi KA (2014): Chlamydial infection and its role in male infertility. Adv Androl 2014: 1-11. [Google Scholar]
- 15.Vigil P, Morales P, Tapia A, et al. (2002): Chlamydia trachomatis infection in male partners of infertile couples: incidence and sperm function. Andrologia 34: 155-161. [DOI] [PubMed] [Google Scholar]
- 16.Kokab A, Akhondi MM, Sadeghi RM, et al. (2010): Raised inflammatory markers in semen from men with asymptomatic Chlamydial infection. J Androl 31: 114-120. [DOI] [PubMed] [Google Scholar]
- 17.Marvast Dehghan L, Aflatoonian A, Talebi A, et al. (2016): Semen inflammatory markers and Chlamydia trachomatis infection in male partners of infertile couples. Andrologia 48: 729-736. [DOI] [PubMed] [Google Scholar]
- 18.Ziklo N, Huston WM, Hocking JS, et al. (2016): Chlamydia trachomatis genital tract infections: when host immune response and the microbiome collide. Trends Microbiol 24: 750-765. [DOI] [PubMed] [Google Scholar]
- 19.Baud D, Pattaroni C, Vulliemoz N, et al. (2019): Sperm microbiota and its impact on semen parameters. Front Microbiol 10: 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moretti E, Cosci I, Spreafico A, et al. (2009): Semen characteristics and inflammatory mediators in infertile men with different clinical diagnoses. Int J Andrology 6: 637-646. [DOI] [PubMed] [Google Scholar]
- 21.Shukla KK, Agnihotri S, Gupta A, et al. (2013): Significant association of TNFα and IL-6 gene with male infertility–an explorative study in Indian populations of Uttar Pradesh. Immunol Lett 156: 30-37. [DOI] [PubMed] [Google Scholar]
- 22.Aghazarian A, Stancik I, Pflüger H, Lackner J (2013): Influence of pathogens and moderate leukocytes on seminal interleukin (IL)-6, IL-8, and sperm parameters. Int Urol Nephrol 45: 359-365. [DOI] [PubMed] [Google Scholar]
- 23.Hćrvig KK, Kierkegaard L, Lund R, et al. (2018): Is male factor infertility associated with midlife low-grade inflammation? A population based study. Hum Fertil 21: 146-154. [DOI] [PubMed] [Google Scholar]
- 24.Omu AE, Al-Qattan F, Al-Abdul-Hadi FM, et al. (1999): Seminal immune response in infertile men with leukocytospermia: Effect on antioxidant activity. Eur J Obstet Gynecol Reprod Biol 86: 195-202. [DOI] [PubMed] [Google Scholar]
- 25.Paradisi R, Capelli M, Mandini M, et al. (1996): Increased levels of interferon-gamma in seminal plasma of infertile men. Andrologia 28: 157-161. [DOI] [PubMed] [Google Scholar]
- 26.Eldamnhoury EM, Elatrash GA, Rashwan HM, et al. (2018): Association between leukocytospermia and semen interleukin-6 and tumor necrosis factor-alpha in infertile men. Andrology 6: 775-780. [DOI] [PubMed] [Google Scholar]
- 27.Eggert-Kruse W, Kiefer I, Beck C, et al. (2007): Role for tumor necrosis factor alpha (TNF-α) and interleukin 1-beta (IL-1β) determination in seminal plasma during infertility investigation. Fertil Steril 4: 810-823. [DOI] [PubMed] [Google Scholar]
- 28.Seshadri S, Bates M, Vince G, et al. (2009): The Role of Cytokine Expression in Different Subgroups of Subfertile Men. Am J Reprod Immunol 62: 275–282. [DOI] [PubMed] [Google Scholar]
- 29.Havrylyuk A, Chopyak V, Boyko Y, et al. (2015): Cytokines in the blood and semen of infertile patients. Cent Eur J Immunol 40: 337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez-Prado E, Camejo Bermúdez MI (2010): Expression of IL-6, IL-8, TNF alpha, IL-10, HSP60, anti-HSP-60 antibodies, and anti-sperm antibodies, in semen of men with leukocytes and/or bacteria. Am J Reprod Immunol 63: 233-243. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, Zheng T, Huang J, et al. (2016): Association of semen cytokines with reactive oxygen species and histone transition abnormalities. J Assist Reprod Genet 9: 1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadi MH, Mirsalehian A, Bahador A (2016): Association of Chlamydia trachomatis with infertility and clinical manifestations: a systematic review and meta-analysis of case-control studies. Infect Dis (Auckl) 48: 517-523. [DOI] [PubMed] [Google Scholar]
- 33.Politch JA, Tucker L, Bowman FP, et al. (2007): Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod 22: 2928-2935. [DOI] [PubMed] [Google Scholar]
- 34.Koçak I, Yenisey Ç, Dündar M, et al. (2002): Relationship between seminal plasma interleukin-6 and tumor necrosis factor α levels with semen parameters in fertile and infertile men. Urol Res 30: 263-267. [DOI] [PubMed] [Google Scholar]
- 35.Moretti E, Cosci I, Spreafico A, et al. (2009): Semen characteristics and inflammatory mediators in infertile men with different clinical diagnoses. Int J Andrology 6: 637-646. [DOI] [PubMed] [Google Scholar]
- 36.Camejo MI, Segnini A, Proverbio F (2001): Interleukin-6 (IL-6) in seminal plasma of infertile men, and lipid peroxidation of their sperm. Arch Androl 47: 97-101. [DOI] [PubMed] [Google Scholar]
- 37.Bernton E, Hoover D, Galloway R (2006): Adaptation to chronic stress in military trainees. Ann N Y Acad Sci 774: 217-231. [PubMed] [Google Scholar]
- 38.Helmunt HD, Buchtal J, Gutberlet I, et al. (1997): Social hierarchy and adrenocortical stress reactivity in men. Psychoneuroendocrinology 22: 643-650. [DOI] [PubMed] [Google Scholar]
- 39.Carrasquel G, Camejo MI, Michelangeli F, et al. (2014): IFN-gamma alters the human sperm membrane permeability to Ca2+. Syst Biol Reprod Med 60: 21-27. [DOI] [PubMed] [Google Scholar]
- 40.Cai T, Wagenlehner FME, Mondaini N, et al. (2014): Effect of human papillomavirus and Chlamydia trachomatis co-infection on sperm quality in young heterosexual men with chronic prostatitis-related symptoms. BJU 14: 281-287. [DOI] [PubMed] [Google Scholar]