Summary
Most patients with Alzheimer’s disease (AD) present with amnestic problems, but a significant proportion, over-represented in young-onset cases, have atypical phenotypes including predominant visual, language, executive, behavioural, or motor dysfunction. In the past, these individuals were often diagnosed late; however, availability of CSF and PET biomarkers of AD pathologies and incorporation of atypical forms of AD into new diagnostic criteria increasingly allows them to be more confidently diagnosed early in their illness. This in turn allows patients to be offered tailored information, appropriate care and support, and individualized treatment plans. These advances will provide improved access to clinical trials, which often exclude atypical phenotypes. Research into atypical AD has revealed previously unrecognised neuropathologic heterogeneity across the AD spectrum. Neuroimaging, genetic, biomarker, and basic science studies are providing important insights into the factors that may drive selective vulnerability of differing brain networks, with potential mechanistic implications for understanding typical late-onset AD.
Introduction
Alzheimer’s disease (AD) is defined by amyloid-β plaques and neurofibrillary tangles, which can be detected post-mortem or in vivo with biomarkers.1 The most common clinical presentation of sporadic AD (i.e., typical AD dementia) is a slowly progressive amnestic disorder reflecting predominant early distribution of neurofibrillary tangle pathology in medial temporal-lobe structures that eventually evolves into an amnestic-predominant, multi-domain dementia. However, non-amnestic phenotypes are characterised based upon initial, dominant difficulties in visual, language, executive, behavioural and motor domains. These presentations (‘atypical AD’) disproportionately affect individuals with young onset dementia whose symptoms begin before age 65 years.2
AD dementia may not be recognized in younger patients with non-amnestic symptoms or lacking ‘typical’ hippocampal volume loss. In a neuropathologically confirmed cohort of young-onset AD, 53% with atypical presentations were misdiagnosed compared with 4% of patients with typical symptoms.3, 4 Given their younger age and unusual symptoms, patients with non-amnestic AD may have their symptoms attributed to life stresses, or new-onset psychiatric illness Neuropsychological assessment should be individualized for atypical AD and interpreted in context of the overall profile. For example, memory or executive-function tests with visual or numerical demands present particular challenges to patients with visual/spatial phenotypes.
Beyond diagnostic delays and deployment of unnecessary tests, non-memory symptoms of AD correlate with significant morbidity and consequential autonomy and quality-of-life issues, often in working-age people with dependents. AD services are typically tailored to the needs of older patients, often not addressing the specific needs of atypical AD patients requiring treatment plans tailored to their symptoms and stage of life.
Biomarkers allow for improved detection of non-amnestic phenotypes in vivo. Recent biomarker studies, in addition to emerging findings from neuropathologically defined AD subtypes, provide insights into the pathogenesis of both typical and atypical AD including regional vulnerability and opportunities for earlier diagnosis.
Here, we review (1) clinical features of atypical AD and common scenarios regarding delayed diagnosis; (2) advances in biomarkers and quantitative neuropathology; 3) key aspects of individualized treatment approaches; and (4) unique opportunities provided by atypical phenotypes to better understand AD.
Epidemiology
Age-standardised prevalence of dementia over age 60 is ~5–7% worldwide.5 To date, no population-based studies of atypical AD exist. Limited studies from dementia clinics estimate a prevalence of AD of 15–65/100,000 in the 45-to-64 age range6. Approximately 8–13% present with visual or motor difficulties, 7–9% with language difficulties, and 2% with executive dysfunction.7, 8 Atypical variants represent one third of young-onset cases compared to 6% of late-onset AD8; however, atypical late-onset cases may be less likely to be referred to academic centres. While the proportion of atypical cases may be lower in older populations, larger numbers of people with late-onset AD suggest the absolute number of atypical cases may be higher in the older population. There are few studies comparing younger versus older atypical AD.
Sex distributions may vary by phenotype with evidence of modest overrepresentations of women in visual/spatial and motor presentations, possibly reflecting increased AD prevalence in women.9, 10 Behavioural presentations may be more common in men, while there is limited evidence of either sex being overrepresented for language and executive presentations.7, 11 There is scarce evidence on survival in atypical AD.
Atypical clinical phenotypes of Alzheimer’s disease
Visual-spatial:
Posterior cortical atrophy (PCA) refers to a clinic-radiologic syndrome previously termed ‘Benson syndrome’, most commonly attributable to AD pathology (75–100% of cases).12, 13 PCA patients typically present in their sixth or seventh decade; of 302 patients, 82% had young onset dementia.10 Core features of PCA include difficulty with space and object perception; simultanagnosia, optic ataxia, and oculomotor apraxia (Balint syndrome); dyscalculia, dysgraphia, left-right confusion, and finger agnosia (Gerstmann syndrome); constructional, dressing, and/or limb apraxia; environmental agnosia; and alexia, with relative preservation of other cognitive domains (table 1).13, 14 A dorsal, visuospatial-led variant of PCA with elements of simultanagnosia predominates, with ventral (visuoperceptual) variants exhibiting letter-by-letter reading, and/or apperceptive prosopagnosia and caudal (primary visual) variants less commonly documented.14 Predominant right lateralised atrophy in PCA is associated with dressing apraxia,15 whereas left lateralised PCA is associated with elements of Gerstmann syndrome. Early symptoms include problems with driving including minor damage to one side of the car, dressing, judging distances, and negotiating familiar environments and stairs, escalators, and patterned flooring.16 Visual impairments include difficulties perceiving objects in the periphery or amidst clutter, and becoming lost on a page while reading.17, 18 Incongruent findings on visual acuity and field testing may prompt suspicion of functional illness.
Table 1:
Clinical features of atypical phenotypes and common scenarios regarding delay or misdiagnosis (red flags)
| Clinical features | Diagnostic red flags | |
|---|---|---|
| PCA-AD | • Space and/or object perception difficulties • Simultanagnosia, optic ataxia, and oculomotor apraxia • Dyscalculia, dysgraphia, left-right confusion, finger agnosia • Constructional, dressing, and/or limb apraxia • Environmental agnosia • Reading difficulties • Face perception difficulties • Relatively spared anterograde memory, speech, nonvisual language, executive function and behaviour |
• Repeated appointments with eye specialists • Repeatedly changing prescription of glasses • Diagnosed with ocular condition • May undergo unnecessary surgeries (e.g., cataract removal) • May be diagnosed as functional |
| lvPPA | • Impaired single-word retrieval • Impaired sentence repetition • Phonologic errors • Spared single-word comprehension • Spared motor speech • Absence of frank agrammatism |
• Due to aphasia, may be misdiagnosed as having a stroke even in the absence of neuroimaging changes • May be misdiagnosed as another form of PPA |
| bvAD | • Progressive deterioration of behaviour and cognition • Features of bvFTD (apathy, disinhibition, loss of empathy, less commonly perserverative or compulsive behaviour, hyperorality and dietary changes) • Executive deficits with relative sparing of memory and visuospatial functions |
• May be misdiagnosed as bvFTD • May receive a psychiatric diagnosis |
| dexAD | • Predominant decline in core executive cognitive function: working memory, cognitive flexibility, inhibition in the absence of predominant behavioural features | • May receive a psychiatric diagnosis • Mimic dysexecutive problems seen in vascular dementia with co-existing AD |
| CBS-AD | • Parkinsonism • Myoclonus • Apraxia • Cortical sensory deficit • Alien limb • Executive, visuospatial, and language dysfunction |
• May be misdiagnosed as Parkinson’s disease or other parkinsonian disorder |
Abbreviations: AD Alzheimer’s disease; PCA Posterior cortical atrophy; lvPPA logopenic variant primary progressive aphasia; dexAD dysexecutive Alzheimer’s; bvAD behavioural Alzheimer’s; CBS corticobasal syndrome; bvFTD: behavioural variant Frontotemporal Dementia
Recent consensus criteria introduced syndromic- and disease-level descriptions. Syndromic-level descriptions specify key neuropsychological-inclusion criteria and supportive neuroimaging features comprising occipital-parietal or occipito-temporal atrophy/hypometabolism on MRI/FDG-PET. Disease-level descriptions incorporate molecular biomarker or neuropathologic evidence to classify individuals by underlying pathology; e.g. distinguishing PCA-AD from PCA due to non-AD pathology. Motor features, including limb rigidity, myoclonus, and tremor, may reflect underlying non-AD pathology but are also seen in PCA-AD,13, 15 while early hallucinations and rapid eye movement-sleep behaviour disorder may be suggestive of PCA-Lewy body disease (LBD). Rapid clinical progression and cortical restricted diffusion on MRI suggest underlying prion disease. The FDG pattern in PCA overlaps with LBD which can lead to diagnostic confusion.19 The pattern of amyloid PET deposition in PCA resembles typical AD, in contrast to regional, particularly occipital involvement on FDG and tau PET. As PCA progresses, deficits in episodic and working memory and language emerge, though early word-finding difficulties may be apparent.16, 20 Depression, anxiety and other neuropsychiatric symptoms in PCA overlap with typical AD.21
Language:
Patients with progressive aphasia, which can remain isolated for years prior to the development of impairments in other domains, are defined as having Primary Progressive Aphasia (PPA). Frontotemporal lobar degeneration and atypical AD were the most common underlying pathologies in early PPA studies. Current clinical PPA diagnostic criteria emphasize progressive language impairment with relatively spared memory, visual abilities, and behaviour.22 There are three major PPA subtypes: non-fluent/agrammatic (nfvPPA), semantic (svPPA), and logopenic (lvPPA) variants. AD pathology is most commonly associated with lvPPA; a large amyloid PET study in PPA provided data consistent with neuropathologic studies, with amyloid PET positivity in 86% of 443 lvPPA cases, 20% of 333 nfvPPA cases, and 16% of 401 svPPA cases. Of these, the majority were under 70 years and 49% female consistent with typically young onset presentation in these syndromes.23
Patients with lvPPA often have word-finding difficulty, sentence-repetition deficits and phonological impairments without impairments of motor speech and single word comprehension (table 1).24 In lvPPA, anomia is common, but unlike in svPPA object knowledge and single word comprehension are typically preserved. Speech may appear hesitant, but in contrast to nfvPPA, lvPPA patients do not have prominent agrammatic or telegraphic speech or motor speech deficits. MRI and FDG-PET scans typically show evidence of left hemisphere-lateralized, posterior-temporal, and inferior-parietal atrophy/hypometabolism. The presence of posterior temporal and parietal atrophy distinguishes lvPPA from FTLD which can also have asymmetric temporal atrophy. Tau PET studies show asymmetric, left-hemisphere predominant temporoparietal signal in most lvPPA cases.25
While some cases of PPA have clear, isolated language problems, others have varying degrees of additional memory and executive dysfunction, particularly later in the disease course. The initial lvPPA neuropsychological profile may ultimately evolve into multi-domain ‘AD dementia’26 featuring memory, executive, and visuospatial dysfunction, often with limb apraxia, acalculia, and other elements of Gerstmann syndrome. Behavioural symptoms including anxiety may be accompanied by depression, irritability, or agitation.
Executive and Behavioural:
‘Frontal AD’ originally described cases with primary executive dysfunction and frontal-lobe neurofibrillary tangle pathology compared to typical AD, noting that none of these cases had major behavioural change.27 ‘Frontal/frontal-variant AD’ has since described patients presenting with either dysexecutive or behaviour-predominant syndromes.7, 28, 29 Two distinct clinical phenotypes, dysexecutive (dexAD) and behavioural AD (bvAD), were subsequently informed by group studies.11, 30
Dysexecutive AD
DexAD primarily presents with a dysexecutive syndrome involving working memory, cognitive flexibility/set shifting, inhibitory control deficits, and rarely behavioural symptoms (table 1).11, 30 Early features include impaired multi-tasking, planning, and project completion, e.g., problems playing board games, following directions and recipes, and organizing calendars. DexAD is now recognized as a distinct, predominantly young-onset atypical AD phenotype in patients with positive AD biomarkers.30
DexAD is associated with parieto-frontal atrophy and relatively preserved medial temporal regions compared to amnestic phenotypes.11, 30 These parieto-frontal brain regions overlap with the working-memory network corresponding to spatial patterns of tau PET signal.31 Atrophy occurs in the parietal lobe but may be subtle. In patients with dexAD, FDG-PET scans show frontal and parietal hypometabolism. The frontal hypometabolism may lead to diagnostic confusion with frontotemporal degeneration. Unique executive profiles are observed in AD and bvFTD, involving disproportionate working-memory and inhibition deficits, respectively.32
Impaired core executive functions lead to a multidomain dysfunctional pattern on neuropsychological testing. While depressive and anxiety disorders comprise a substantial proportion of misdiagnosed dexAD patients and neuropsychiatric symptoms may be more evident relative to typical AD,11 behavioural and personality changes are typically not reported, the exception being apathy.
Behavioural AD
A primary behavioural syndrome mimicking behavioural-variant frontotemporal dementia (bvFTD)33 is a relatively rare clinical manifestation of AD.7, 12, 34–37 Of 532 consecutive AD patients presenting to an academic memory clinic, 2% reported predominant frontal behavioural features. 75% of the predominantly behavioural cases were male with a mean age of onset of 49.7 Clinicopathologic series determined AD as the causative neuropathology in 7–20% of clinically diagnosed bvFTD cases.12, 34–37 Subsequent studies describe demographic, clinical, and neuroimaging features of patients with a behaviour-predominant clinical presentation and autopsy or biomarker confirmation of underlying AD (bvAD).11, 12, 34, 35, 37, 38 Symptoms typically start in the sixth or seventh decade. In contrast to bvFTD, cognitive symptoms often pre-date behavioural change,33 apathy is more common than disinhibition or loss of empathy, perseverative/compulsive and eating changes are relatively uncommon, and behavioural changes are generally less marked (table 1). Conversely, delusions and hallucinations are more common in bvAD than in bvFTD, though occur in a minority of patients.36
Paradoxically, atrophy/hypometabolism on MRI/FDG-PET primarily focuses on ‘classical’ AD regions including posterior cingulate/precuneus and hippocampus/medial temporal lobe.11, 37 Variable frontal involvement, intermediate between bvFTD and ‘typical’ amnestic AD, shows more predilection for dorsal than ventral frontal regions,37 consistent with clinical changes (apathy>disinhibition, executive dysfunction).
An intermediate behavioural profile, including prominent apathy, early cognitive deficits and temporo-parietal predominant involvement on MRI or FDG-PET, characterises bvAD (compared to bvFTD).
Amyloid and tau biomarkers (biofluid or PET) allow distinction of dexAD and bvAD from dysexecutive and behavioural presentations due to frontotemporal degeneration.
Motor dysfunction
Corticobasal syndrome (CBS), characterised by motor and sensory symptoms, typically correlates with corticobasal degeneration (CBD) pathology. However, 15–50% of cases are attributable to AD12, 39, 40,41, 42
Proposed core clinical features for CBS include limb rigidity, bradykinesia, dystonia, myoclonus, apraxia, cortical sensory deficit and alien limb phenomenon (table 1).40, 42, 43 Executive, visuospatial, and language dysfunction are proposed core or supportive features of CBS. 40, 42, 43 CBS may be due to several pathologies; prominent episodic memory and visuospatial/visuoperceptual deficits, frequent myoclonus and logopenic type aphasia are suggestive of AD while prominent executive dysfunction, nfvPPA and/or supranuclear gaze palsies suggest non-AD pathology. 9, 42, 44 Autopsy and neuroimaging studies have found relative preservation of superior frontal regions contrasted by greater occipital and temporo-parietal volume loss in CBS-AD compared to CBD.9, 41 Asymmetric clinical syndromes or atrophy patterns do not distinguish AD from CBD or other causes of CBS. Biofluid and PET biomarkers of amyloid and tau support identification of underlying AD pathology in vivo, with tau PET showing asymmetric involvement of peri-rolandic cortex, often spared in other AD variants.
Over the course of CBS-AD, variable initial signs may progress to apraxia, myoclonus, gait disorder, visuospatial, language and memory symptoms. While prominent apathy and disinhibition may be suggestive of non-AD pathology,42, 44 the CBS-AD neuropsychiatric profile has yet to be characterised comprehensively.
Overlapping presentations
Phenotype-overlap is recognized in criteria (e.g. PCA-plus14). Both PCA and CBS involve limb apraxia and visuospatial dysfunction13, 14, 38, 40, 43 and may encompass biparietal, apraxic and dyscalculic AD variants.14 The language profile of CBS40, 42 overlaps with lvPPA. Verbal working-memory difficulty features prominently in lvPPA and dexAD.
Biomarkers to diagnose atypical AD
The extent and regional deposition of the neuropathologic hallmarks of both typical and atypical AD differ. Contemporary criteria for both typical and atypical AD dementia45, 46 require molecular evidence for these neuropathologies, which, in vivo, depends on imaging or fluid biomarkers. These biomarkers are particularly relevant in atypical AD, where the underlying pathology is challenging to recognize clinically. With predominantly younger patients, false positives (i.e., asymptomatic age-related AD pathology) are less likely to occur.
Structural MRI:
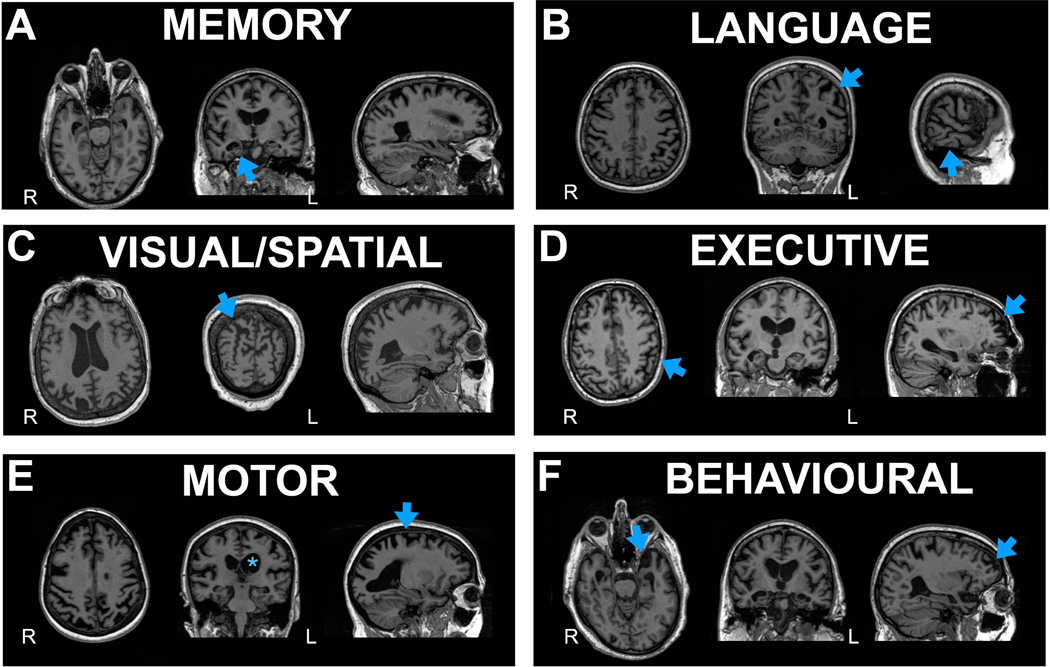
Typical AD atrophy begins in the medial-temporal lobe and spreads to the lateral-temporal and parietal cortices. In atypical AD atrophy is usually most prominent in regions corresponding to clinical symptoms, often sparing the hippocampus early in the disease. See Figure 1 for patterns.
Figure 1: MRI across AD phenotypes.
A. Memory (Typical amnestic); blue arrows highlighting hippocampal atrophy
B. Language (logopenic variant primary progressive aphasia); blue arrow highlighting left temporal-parietal atrophy
C. Visual/Spatial (posterior cortical atrophy); blue arrow indicating parieto-occipital atrophy
D. Executive (Dysexecutive); blue arrows indicating frontoparietal atrophy
E. Motor (corticobasal syndrome); asterisk highlighting left greater than right hemisphere atrophy and arrow indicating atrophy around the motor cortex
F. Behavioural with arrows demonstrating temporal>frontal atrophy
FDG-PET:
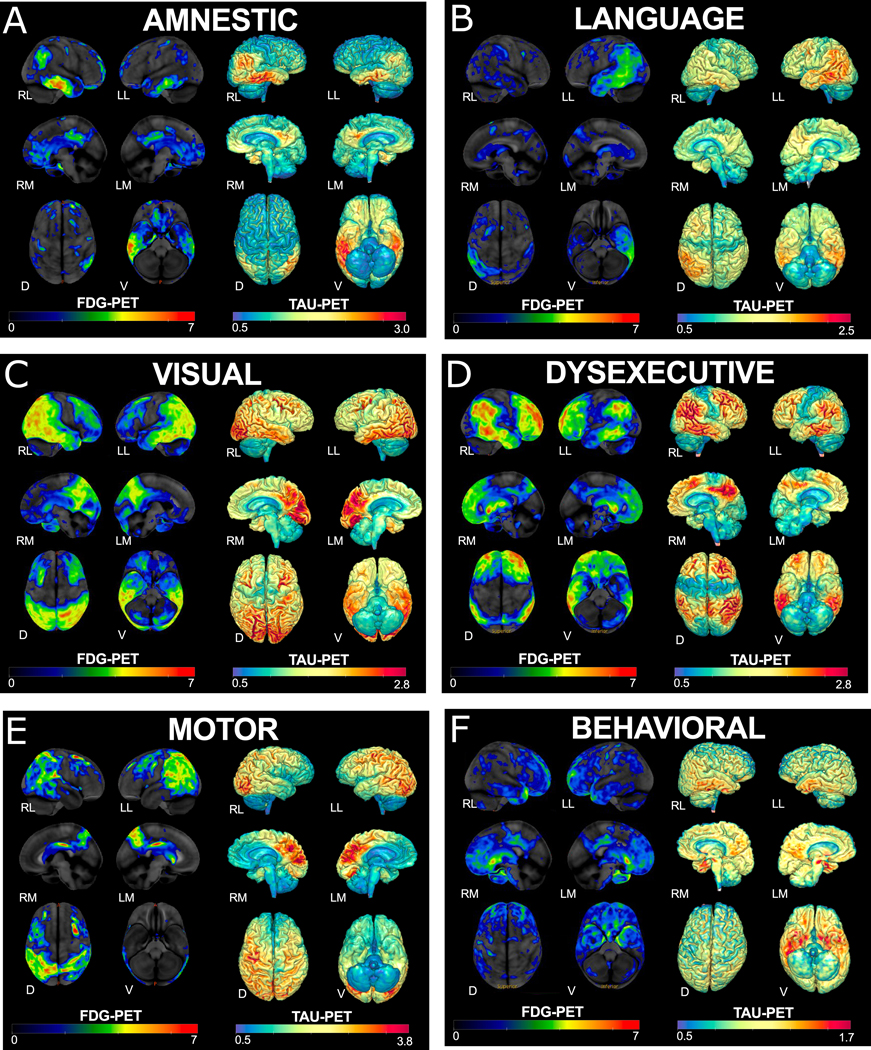
FDG-PET can aid in diagnosing AD dementia, particularly in differentiating AD from FTD. Hypometabolism patterns on FDG-PET reflect clinical deficits across atypical AD variants and distinguish between typical and atypical AD (figure 2).
Figure 2: FDG and tau PET across AD phenotypes.
FDG-PET on left and tau PET on right of representative cases of each AD phenotype: A) Memory (Typical amnestic); B) Language (logopenic variant primary progressive aphasia); C) Visual/Spatial (posterior cortical atrophy); D) Executive (Dysexecutive); E) Motor (corticobasal syndrome); F) Behavioural.The z-scores, relative to a normative database, of pons intensity normalized FDG-PET scans for each individual are displayed on stereotactic surface projections using Cortex ID (GE Healthcare). Red colour indicates greater hypometabolism. The cerebellar crus intensity normalized Tau-PET scan (Tauvid; AV1451; flortaucipir F18; Avid Radiopharmaceuticals, Eli Lilly and Co.) is overlaid on the grey matter segmentations of each subject’s own T1 weighted structural MRI scan. Red colour indicates higher intensity of tracer. RL- Right Lateral, LL – Left Lateral, RM–RightMedial, LM – Left Medial, D – Dorsal, V-Ventral
Amyloid PET:
While amyloid PET is clinically approved as a diagnostic test, it is predominantly used in research settings. In typical AD, amyloid deposition occurs diffusely throughout neocortex, with early involvement of posteromedial cortices and relative sparing of medial temporal, primary sensorimotor and visual cortices. Importantly, unlike other imaging modalities, amyloid distribution is similar between atypical and typical AD.
Tau PET:
The U.S. Food & Drug Administration recently approved F18-flortaucipir to image tau pathology in AD. While flortaucipir and several other tau-specific tracers are available, imaging is rarely accessible outside the research setting. As opposed to amyloid PET, tau PET deposition patterns reflect the anatomical areas producing the clinical phenotype and overlap with regional FDG-PET hypometabolism and atrophy. Figure 2 reflects example tau PET patterns across phenotypes. In atypical AD, tau PET does not conform to a typical pattern and the pattern may have utility in distinguishing typical and atypical phenotypes. Tau negative cognitive disorders, even in the context of a positive amyloid scan, may suggest different underlying non-AD pathologies.31
Longitudinal Imaging:
Longitudinal atrophy patterns diverge by phenotype with greatest medial temporal atrophy in typical AD, occipito-parietal/occipito-temporal atrophy in PCA,20 and left temporal atrophy in lvPPA. Across PCA, lvPPA and bvAD/dexAD, regions of greatest baseline atrophy are particularly affected over time, though converge across temporoparietal and dorsolateral prefrontal regions.47 While baseline tau PET corresponds closely to clinical phenotype and atrophy pattern, longitudinal tau accumulation occurs in frontal regions in atypical variants and typical AD.48
Fluid Biomarkers
Tau and amyloid PET give information on regional distribution and burden of tau and amyloid-β. In contrast CSF or plasma biomarkers allow for indirect detection of these pathologies: CSF Aβ42 concentration and Aβ42/Aβ40 ratios correlate inversely with cerebral amyloid-β plaque burden, and concentrations of total and phosphorylated tau (p-tau) correlate with intensity of neurodegeneration and neurofibrillary-tangle pathology respectively, both in typical and atypical AD.49 Combining CSF Aβ42 and p-tau181 gives a sensitivity and specificity of ~90% for distinguishing AD from non-AD pathologies.50 While fluid and imaging molecular diagnostics correlate fairly well, CSF and plasma biomarkers may show changes earlier in the disease course than amyloid or tau PET, and conversely show earlier plateau with disease progression.51
Few studies have directly compared the profiles of typical and atypical AD. CSF phenotypic differences may include increased tau in atypical phenotypes,52 with mixed evidence of whether p-tau differs between variants.53, 54 CSF concentrations of synaptic proteins (neurogranin, SNAP-25, synaptotagmin-1) and neurofilament light (NFL) increase in atypical AD,54, 55 noting that normal age-related rise in NFL needs to be considered.56 CSF proteomics approaches reveal various biological pathways involved in AD varying from hemostasis, lipoprotein and extracellular matrix,57 possibly underpinning phenotypic AD variation. Recent advances in blood-based biomarkers of amyloid-β, tau, p-tau and NFL, and proteomic-approach biomarkers in plasma are preliminary in atypical AD.
AD and NIA-AA research criteria, IWG-2 criteria
While traditional AD criteria focused on amnestic deficits, the NIA-AA dementia 2011 and IWG-2 AD 2014 criteria acknowledged non-amnestic (i.e., atypical) phenotypes.45, 46 The IWG-2 criteria describes posterior, logopenic, and frontal variants of AD and requires biomarker confirmation of AD pathology (CSF, PET or mutation status), while the NIA-AA criteria describe executive, visual, and language presentations with different levels of certainty based on biomarker abnormalities. Applying these criteria requires adoption of diagnostic algorithms extending beyond detection of amnestic deficits and use of biomarkers where possible.
Neuropathological underpinnings
Despite differences in their extent and regional deposition, accumulation of amyloid-β plaques and neurofibrillary tangles are neuropathological hallmarks of both typical and atypical AD (figure 3).58 While clinical criteria subdivide atypical AD into several canonical syndromes, neuropathological studies have also investigated AD spectrum predicated on regional neuropathologic involvement.
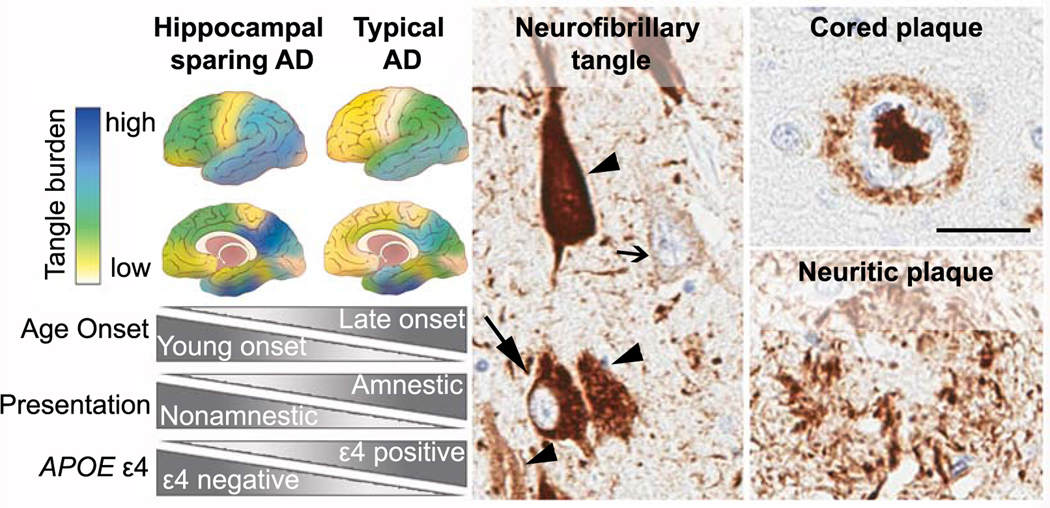
Figure 3:
(Left panel) Neuropathologic subtypes of AD are characterized by distribution of neurofibrillary tangle pathology. Illustrations depict the hippocampal sparing subtype with greater cortical pathology relative to sparing of the hippocampus. The typical AD subtype demonstrates expected patterns of both limbic and cortical involvement. Disproportionate differences in age onset, clinical presentation, and APOE ε4 positivity are observed between hippocampal sparing AD and typical AD. (Middle panel) Abnormal accumulation of intracellular tau pathology is observed with increasing severity from pre-tangles (open arrow) to mature tangles (closed arrow). As the neuron dies, a remnant of the tau pathology remains in the extracellular space as ghost tangles (arrowhead). (Right panel) Top: Classic cored plaques are typically observed in AD brains. Bottom: Neuritic plaques can be readily observed using tau antibodies, but may be more easily distinguished by silver stain or thioflavin-S microscopy (not shown).(Scale bar 50μm; the PHF-1 tau antibody was a kind gift from Peter Davies; the 6F/3D amyloid-β antibody was purchased from Dako)
Neuropathological AD criteria utilize scoring systems for severity of cortical neuritic plaques (CERAD)59 and topography of amyloid-β plaque pathology using Thal phase,60 with tangle distribution scored using Braak stage.61 These scoring systems designed for typical AD rely on a predictable sequence of neuropathologic spread not always observed in atypical AD. Quantitative assessment of tangle density in a larger AD cohort identified several subtypes including limbic predominant (not shown) and hippocampal sparing (figure 3).4, 62 Non-amnestic presentations are relatively uncommon at 11% in typical AD, compared to 38% of hippocampal sparing AD cases.62 The tangle density in the cortex and nucleus basalis of Meynert (i.e., cholinergic hub) in hippocampal sparing AD exceeds that in the relatively spared hippocampal-amygdala region.62 Further, an inverse relationship exists between younger onset and greater neuronal loss in the nucleus basalis of Meynert. Given widespread cholinergic projections throughout corticolimbic structures,63 pathologies in specific nuclei within the cholinergic hub may confer vulnerability to neocortical tangle pathology in non-amnestic AD phenotypes.62, 64
Atypical, non-amnestic AD phenotypes are most commonly observed in the hippocampal sparing AD subtype, although typical AD patterns at autopsy may reflect late-stage concurrent hippocampal involvement.4 Contribution of oligomeric amyloid-β species cannot be ruled out; however, overwhelming evidence points to tau pathology as the major contributor to domain-specific functional consequences in AD.53, 62, 65 Tangle density in PCA is greatest in primary visual cortex and visual association areas, with lesser hippocampal involvement relative to typical AD.13 In PPA due to AD, neuronal loss and tau pathology are seen in temporoparietal structures.66 Asymmetry of AD pathology was inconsistently observed at the individual level in PPA cases, but when observed, it appears to be more specific to tangle than neuritic plaque pathology.67 CBS cases with underlying AD pathology have greater perirolandic tau and nigral neuronal loss with less temporal pathology than typical AD.9 While asymmetric clinical presentation of motor symptoms was observed, the relationship to asymmetry of pathology was precluded by routine unavailability of both hemispheres.9 This highlights the mutually beneficial relationship between the macroscopic information provided by neuroimaging and the microscopic provided by neuropathology studies.68
Some patients with atypical dementia syndromes and AD pathology exhibit co-existing cerebrovascular disease and LBD pathology, but are not thought to play a major role in atypical AD.53, 62, 69 The frequency of LBD is lower in hippocampal sparing AD (14%) compared to typical AD (26%),62 but these estimates do not account for amygdala predominant Lewy bodies often seen in end-stage AD. TDP-43 pathology in limbic regions is frequently found in typical AD (60%),70 more so than non-amnestic phenotypes (42%) or hippocampal-sparing AD cases (21%).4, 67, 69 The lower frequency of co-existing pathologies in non-amnestic phenotypes or hippocampal sparing AD cases may be age-related noting that atypical AD cases are often younger.62 Microscopic inspection often reveals an overall greater burden of tangle pathology of vulnerable cortical regions compared to typical AD.13, 30, 62 This likely reflects the fact that atypical forms of AD are more common in younger patients, who generally have a greater tau burden.71 The reasons for this are not well-understood, but younger individuals may exhibit greater inflammatory reactions to amyloid or a different genetic profile, resulting in more tangles, or increasing age may correlate with higher risk of multiple co-pathologies, that may result in dementia accompanied by a lesser burden of specific pathologies. Morphological differences of amyloid- β plaque pathology may play a role in atypical AD, as recently identified ‘coarse-grained’ plaques in young-onset AD do not contain the classic amyloid-β core and have a poorly organized microglial response.72
Etiology of atypical AD
Genetics
Of autosomal dominant AD cases, a small proportion have atypical phenotypes.73 Beyond case reports, canonical atypical AD phenotypes apparently do not associate with autosomal dominant mutations, and it is not common practice to offer clinical genetic testing without a compelling family history. Despite the apolilpoprotein E (APOE) ε4 allele being the strongest genetic risk factor for sporadic AD and lowering the age of symptom onset, patients with atypical phenotypes10, 74 are less likely to carry APOE ε4 than those with a typical presentation.75 The relative rarity of these presentations render it challenging to conduct large-scale genetic studies with adequate power; however, a GWAS in PCA identified candidate genes implicated in developmental and intercellular communication processes in visual and central nervous systems, findings requiring replication and validation.10
Functional brain networks
AD-phenotypic extremes highlight our limited understanding of the disease-mechanism underlying such heterogeneity. Amyloid-β deposition is thought to precede accumulation of tau, regional atrophy, and clinical symptoms, but clinical phenotype broadly corresponds to regional atrophy and tau deposition, not amyloid-β deposition.76 These spatiotemporal discrepancies suggest that mechanisms leading to amyloid deposition are distinct from those leading to tau deposition, neurodegeneration, and symptom development. Well-documented cognitive variability is reflected in differential network disruptions between clinically defined phenotypes.70 Variability in tau patterns coincide with functional networks31 suggesting heterogeneity in symptoms, atrophy, and tau may be explained by differential effects on functional brain networks.77 A widely accepted model explaining the relationship between tau and networks is the seed-based templating or prion-like spread of tau across functionally connected brain regions.78 For such a mechanism to account for phenotypic heterogeneity, there must be variable initiation, selective spread, or a common site with diverse connections with variable, complex spreading patterns (e.g., locus coeruleus).79
While amyloid is also associated with functional network properties of the brain,80 network properties are more general (e.g., hubness or overall connectivity) and do not directly relate to variably impaired cognitive abilities in AD. The association between hubness and amyloid-β may relate to variations in metabolic or other local tissue factors, imparting selective vulnerability.81 In line with seed-based templating for amyloid, others report observing sequential spread in cortical amyloid-β,82 but this contrasts with pathologic observations.60 If both amyloid-β and tau accumulate via the same seed-based mechanism within functional brain networks, it is uncertain why they have variable relationships to clinical phenotypic heterogeneity. One possible explanation is that oligomeric species of amyloid align with neurodegeneration83 but extracellular amyloid plaque deposition measured with PET62 does not.
The cascading network-failure theory of AD is an alternative model that explains both the uniform amyloid-β and variable tau distributions via functional networks but allows different network properties to account for the observed spatiotemporal differences.31 The large-scale neural networks associated with clinical phenotype are marked by tau, but general compensatory network functions performed by brain hubs are marked by amyloid. Such modular failure and global compensation are features of complex networks like power grids and may be a general disease mechanism in the brain.84 Such models do not preclude the co-existence of seed-based templating, but they are also not dependent upon them.
Amyloid-β may also potentiate tau pathology and neurodegeneration potentially explaining temporal differences, but the mechanistic link between amyloid-β and tau accounting for regional and phenotypic discrepancies between them is currently unknown.
Other associations or molecular mechanisms
The global amyloid-β distribution seen in both atypical and typical AD prompts consideration of additional factors influencing the clinicoradiological profile. Recently, altered inflammatory response has received increased attention. Relative to typical AD, a small study of PCA-AD documented C11-PBR28 PET binding (a marker of activated microglia and astrocytes) increased in parieto-occipital and reduced in entorhinal regions.85 Genes implicated in immune processes and phagocytosis may carry comparable or reduced risk for PCA.10 Disproportionate glial activation in superior parietal-versus-temporal regions was noted in atypical relative to typical AD.86 Yet, evidence is limited for differential glial burden or abnormality between individual language-predominant or CBS compared to typical phenotypes.9, 87
Treatment of Atypical Alzheimer’s disease
Pharmacological management strategies for atypical and typical AD overlap. Acetylcholinesterase-inhibitor medications are indicated. Limited studies of young-onset AD, in which these phenotypic variants are overrepresented, suggest a similar treatment response relative to late-onset AD.88 Less data for memantine exist, but a trial of memantine is reasonable when indicated at the moderate-to-severe dementia stage. As with other dementias, antidepressant drugs may alleviate patients’ depression, behavioural symptoms, or anxiety, but evidence is limited. Treatment for parkinsonism, seizures, dystonia, or myoclonus may be appropriate for individual patients.
Resources for typical AD often do not cover the unique challenges faced by atypical AD patients. A multidisciplinary approach targeted to individual patients’ symptoms and specific phenotype can improve functional status and quality of life.89 Approaches to maximize function in atypical AD are largely derived from small studies. Compensatory strategies may mitigate reading loss and environmental disorientation in PCA,17, 90 and word-retrieval interventions can benefit lvPPA patients (table 2).91 Many atypical AD patients may find research participation empowering, particularly given delays to diagnosis and lack of public and professional understanding, although appropriate study outcomes are required.
Table 2:
Syndrome-specific education and non-pharmacological treatment approaches in atypical AD
| Phenotype specific education/recommendations | Non-pharmacological treatment | |
|---|---|---|
| PCA-AD | • Early discussion of driving safety is a priority. Most PCA patients will not be safe to drive • Patients may have a high risk of becoming lost • Occupational and daily routines may be very susceptible to progressive visual loss, in many cases despite preserved insight • Can be appropriate grounds for registration as severely sight impaired, or legally blind in order to obtain appropriate services • Most patients become functionally blind leading to a high falls risk |
• Occupational therapist experienced in dealing with low vision can assist in identifying compensation strategies for vision issues • Aids and adaptations to support diminished reading and navigation based on minimizing visual clutter and strategic use of contrast may help • Adaptive equipment designed for those with low vision may be appropriate (talking watch, cane, typoscope, audiobooks) with careful appreciation of concurrent nonvisual symptoms |
| lvPPA | • May have difficulty communicating their diagnosis and needs, prompting use of aphasia awareness/medical cards. • Communication difficulties may lead to social isolation due to increased anxiety |
• Speech-language therapy can help maximize independence in communication and lessen frustration • Evidence of lexical retrieval based on self-cueing, reading, repetition and recall in lvPPA • May use repetition for words that present the biggest challenge • Practice talking around words |
| bvAD | • Increase risk of financial losses and susceptibility to scams • Determining driving safety considering relevant skills (judgement/inhibition, praxis, visuospatial) |
• Counselling the patient and family to focus on simple instructions (i.e. one step rather multistep commands) • Avoid multi-tasking, environmental and emotional distractions • Emphasize approaches to facilitate sequential processing and reliance on highly learned strategies to improve daily task performance • Redirection techniques to mitigate and prevent behavioural symptoms |
| dexAD | • Majority will develop symptoms during working years. Referral to occupational medicine or counseling regarding job loss/disability may be needed | |
| CBS-AD | • Mobility and balance difficulties may lead to a high falls risk • Motor and visual symptoms have particular implications for daily functioning. • Communication and swallowing difficulties may pose challenges to maintaining social function and nutrition. |
• Interdisciplinary teams may include physical therapy, occupational therapy, and speech and language therapy-based approaches, with an emphasis on risk management and maximising functional status. |
Individuals living with young-onset dementia and their families/households often experience particular challenges compared to late-onset dementia. Given the substantial overlap with young-onset AD, such challenges affect many individuals with atypical AD. Patients are still likely to be working, more likely to have children living at home, and more likely to also be providing care for their own parents. These needs are frequently unaddressed by government services targeting older individuals, which are often only available to those over ages 60 to 65. Providing access to important services regardless of age is a necessary step which will benefit patients with atypical AD. Syndrome-specific support and education for patients and their families can be found at https://www.raredementiasupport.org/, a resource used by atypical AD patients worldwide.92
Conclusions and future directions
While recognized for many years, AD biomarkers and novel neuropathologic approaches have refined our understanding of the phenotypic breadth of atypical AD. Increasing use of AD biomarkers in clinical practice and greater recognition of diverse phenotypes can ensure early diagnosis, timely treatment, and appropriate support. Atypical AD overlaps with young-onset AD, and there is increasing focus on ensuring appropriate resources and support for these individuals. Studying phenotypic heterogeneity in AD is key to disentangling mechanisms underlying clinico-radiologic as well as neuropathologic variability, particularly regarding relative sparing of memory function and medial temporal regions. While patients with atypical AD are in many ways ideal for clinical trials, e.g., having fewer co-pathologies, current trials in AD emphasize memory and patients with atypical AD may not fulfil entry criteria. Similar to typical AD research, nearly all atypical AD studies disproportionately feature Caucasian populations. Forthcoming research should describe these syndromes in more diverse, representative populations.93, 94 Multi-centre studies, such as the Longitudinal Early-onset AD Study (LEADS, www.leads-study.org),95 are now underway, but more research into atypical AD is needed in this important patient group to determine the mechanisms behind the focal onset, whether there is a link to early brain development, and the appropriate outcome measures to facilitate clinical trials.
Panel – Current Gaps in Knowledge.
Why focal onset?
Evidence from prospective studies on location initiation is limited, largely owing to challenges of investigating atypical AD during the preclinical phase. Neuropathological studies of atypical AD provide preliminary evidence of selective vulnerability. Selective spread has received support from recent longitudinal multi-centre investigation estimating regional atrophy differences between atypical versus typical AD persisting across disease stages. Further research is required on the role of common sites such as the locus coeruleus mediating disease spread. There are age related-changes in large-scale network configurations, which are associated with Alzheimer’s pathophysiology, therefore, there may be windows of vulnerability for networks associated with atypical Alzheimer’s disease at younger age (for example, see brain development section below), in contrast to typical Alzheimer’s disease where the network problem is focused on the hippocampus which occurs at a later date
Is there a link to brain development and/or other factors?
Greater frequency of self-reported learning disabilities have been documented in atypical AD, including language-learning disabilities in lvPPA and mathematical/visual in PCA, implying that networks subserving these abilities may be developmentally vulnerable to age-related pathology.94, 96 The link between learning disabilities and later life neurodegenerative disease in a corresponding neural network suggest a vulnerability or compensation may predispose to later life neurodegeneration. Work in this area is still preliminary and further research is necessary. Regarding other associations, further investigations might relate exogenous factors and neuroinflammation to coexisting pathology and regional vulnerability.
Response to pharmacological therapy and appropriate outcomes for trials
Information on differential response to acetylcholinesterase inhibitors in atypical relative to typical AD is limited, though may be of key interest given differential involvement of the nucleus basalis of Meynert in neuropathologically defined subtypes. While patients with atypical AD may be good candidates for clinical trials, these largely emphasize memory outcomes and atypical AD patients may not fulfil inclusion criteria. Questions on appropriate outcomes include whether these should reflect deficits that are relatively common across atypical phenotypes (for example, working memory), and/or be adapted to mitigate confounds presented by atypical symptoms (e.g. joint visual/verbal presentation of episodic memory stimuli). The LEADS (Longitudinal Early-onset AD study (LEADS, www.leads-study.org) study and other international studies plan to answer these questionns in the years to come.
Acknowledgments
Funding
This work was supported by U01 AG057195 (to LGA, MC, BCD, GDR, MEM) and R01 AG054449(JGR, MEM). The funder had no role in the preparation of this document.
Disclosures
JGR receives research funding from the NIH and serves as an assistant editor for Neurology.
KXXY is funded by the Alzheimer’s Society, grant number 453 (AS-JF-18–003).
LA served as a paid consultant for Biogen and receives research support from the NIH, and the Alzheimer’s Association.
JMS acknowledges the support of the National Institute for Health Research University College London Hospitals Biomedical Research Centre, ARUK (ARUK-PG2017–1946), Weston Brain Institute (UB170045), Medical Research Council, and British Heart Foundation. He has received research funding and PET tracer from AVID Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly); has consulted for Roche, Eli Lilly, Biogen, Merck and GE; received royalties from Oxford University Press and Henry Stewart Talks; given education lectures sponsored by Eli Lilly, Biogen and GE; and served on a Data Safety Monitoring Committee for Axon Neuroscience SE. He is an associate editor for Alzheimer’s Research and Therapy and Chief Medical Officer for Alzheimer’s Research UK.
FHB has no disclosures
MC has no disclosures
DTJ receives research funding from the NIH
BCD receives research support from NIH, Alzheimer’s Drug Discovery Foundation, has been a paid consultant for Arkuda, Axovant, Lilly, Biogen, Merck, Novartis, Wave LifeSciences, performs editorial duties with payment for Elsevier (Neuroimage: Clinical and Cortex), and receives royalties from Oxford University Press and Cambridge University Press.
GDR receives research support from NIH, Alzheimer’s Association, American College of Radiology, Rainwater Charitable Foundation, Avid Radiopharmaceuticals, Eli Lilly, GE Healthcare and Life Molecular Imaging; has been a paid consultant for Axon Neurosciences, Esiai, GE Healthcare, Johnson & Johnson; and is an Associate Editor for JAMA Neurology.
MEM receives funding from the NIH (R01 AG054449), Alzheimer’s Association (AARG-17–533458), and the State of Florida (8az06, 20a22); has served as a paid consultant for AVID Radiopharmaceuticals.
References
- 1.Jack CR Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimers Dement. 2015;11:1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasa M, Gelpi E, Antonell A, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76:1720–5. [DOI] [PubMed] [Google Scholar]
- 4.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- 6.Kvello-Alme M, Brathen G, White LR, Sando SB. The Prevalence and Subtypes of Young Onset Dementia in Central Norway: A Population-Based Study. J Alzheimers Dis. 2019;69:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex. 2007;43:835–45. [DOI] [PubMed] [Google Scholar]
- 8.Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19:1401–8. [DOI] [PubMed] [Google Scholar]
- 9.Sakae N, Josephs KA, Litvan I, et al. Clinicopathologic subtype of Alzheimer’s disease presenting as corticobasal syndrome. Alzheimers Dement. 2019;15:1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schott JM, Crutch SJ, Carrasquillo MM, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138:2732–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–45. [DOI] [PubMed] [Google Scholar]
- 13.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–74. [DOI] [PubMed] [Google Scholar]
- 14.Crutch SJ, Schott JM, Rabinovici GD, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13:870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan NS, Shakespeare TJ, Lehmann M, et al. Motor features in posterior cortical atrophy and their imaging correlates. Neurobiol Aging. 2014;35:2845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schott JM, Crutch SJ. Posterior Cortical Atrophy. Continuum (Minneap Minn). 2019;25:52–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong KX, Rajdev K, Shakespeare TJ, Leff AP, Crutch SJ. Facilitating text reading in posterior cortical atrophy. Neurology. 2015;85:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong KX, Shakespeare TJ, Cash D, et al. Prominent effects and neural correlates of visual crowding in a neurodegenerative disease population. Brain. 2014;137:3284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitwell JL, Graff-Radford J, Singh TD, et al. (18)F-FDG PET in Posterior Cortical Atrophy and Dementia with Lewy Bodies. J Nucl Med. 2017;58:632–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firth NC, Primativo S, Marinescu RV, et al. Longitudinal neuroanatomical and cognitive progression of posterior cortical atrophy. Brain. 2019;142:2082–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suarez-Gonzalez A, Crutch SJ, Franco-Macias E, Gil-Neciga E. Neuropsychiatric Symptoms in Posterior Cortical Atrophy and Alzheimer Disease. J Geriatr Psychiatry Neurol. 2016;29:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-beta pathology in distinct variants of primary progressive aphasia. Ann Neurol. 2018;84:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R. Clinical, Anatomical, and Pathological Features in the Three Variants of Primary Progressive Aphasia: A Review. Front Neurol. 2018;9:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephs KA, Martin PR, Botha H, et al. [(18) F]AV-1451 tau-PET and primary progressive aphasia. Ann Neurol. 2018;83:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris JM, Saxon JA, Jones M, Snowden JS, Thompson JC. Neuropsychological differentiation of progressive aphasic disorders. J Neuropsychol. 2019;13:214–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–9. [DOI] [PubMed] [Google Scholar]
- 28.Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ. Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis. 2014;39:63–70. [DOI] [PubMed] [Google Scholar]
- 29.Woodward M, Brodaty H, Boundy K, et al. Does executive impairment define a frontal variant of Alzheimer’s disease? Int Psychogeriatr. 2010;22:1280–90. [DOI] [PubMed] [Google Scholar]
- 30.Townley RA, Graff-Radford J, Mantyh WG, et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Comm. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DT, Graff-Radford J, Lowe VJ, et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex. 2017;97:143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stopford CL, Thompson JC, Neary D, Richardson AM, Snowden JS. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex. 2012;48:429–46. [DOI] [PubMed] [Google Scholar]
- 33.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol. 2005;57:480–8. [DOI] [PubMed] [Google Scholar]
- 36.Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology. 2013;80:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140:3329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14:33–40. [DOI] [PubMed] [Google Scholar]
- 39.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. [DOI] [PubMed] [Google Scholar]
- 40.Mathew R, Bak TH, Hodges JR. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry. 2012;83:405–10. [DOI] [PubMed] [Google Scholar]
- 41.Di Stefano F, Kas A, Habert MO, et al. The phenotypical core of Alzheimer’s disease-related and nonrelated variants of the corticobasal syndrome: A systematic clinical, neuropsychological, imaging, and biomarker study. Alzheimers Dement. 2016;12:786–95. [DOI] [PubMed] [Google Scholar]
- 42.Sha SJ, Ghosh PM, Lee SE, et al. Predicting amyloid status in corticobasal syndrome using modified clinical criteria, magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. Alzheimers Res Ther. 2015;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70:327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. [DOI] [PubMed] [Google Scholar]
- 46.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips JS, Da Re F, Irwin DJ, et al. Longitudinal progression of grey matter atrophy in non-amnestic Alzheimer’s disease. Brain. 2019;142:1701–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sintini I, Graff-Radford J, Senjem ML, et al. Longitudinal neuroimaging biomarkers differ across Alzheimer’s disease phenotypes. Brain. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oboudiyat C, Gefen T, Varelas E, et al. Cerebrospinal fluid markers detect Alzheimer’s disease in nonamnestic dementia. Alzheimers Dement. 2017;13:598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skillback T, Farahmand BY, Rosen C, et al. Cerebrospinal fluid tau and amyloid-beta1–42 in patients with dementia. Brain. 2015;138:2716–31. [DOI] [PubMed] [Google Scholar]
- 51.Meyer PF, McSweeney M, Gonneaud J, Villeneuve S. AD molecular: PET amyloid imaging across the Alzheimer’s disease spectrum: From disease mechanisms to prevention. Prog Mol Biol Transl Sci. 2019;165:63–106. [DOI] [PubMed] [Google Scholar]
- 52.Pillai JA, Bonner-Jackson A, Bekris LM, Safar J, Bena J, Leverenz JB. Highly Elevated Cerebrospinal Fluid Total Tau Level Reflects Higher Likelihood of Non-Amnestic Subtype of Alzheimer’s Disease. J Alzheimers Dis. 2019;70:1051–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ossenkoppele R, Mattsson N, Teunissen CE, et al. Cerebrospinal fluid biomarkers and cerebral atrophy in distinct clinical variants of probable Alzheimer’s disease. Neurobiol Aging. 2015;36:2340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paterson RW, Toombs J, Slattery CF, et al. Dissecting IWG-2 typical and atypical Alzheimer’s disease: insights from cerebrospinal fluid analysis. J Neurol. 2015;262:2722–30. [DOI] [PubMed] [Google Scholar]
- 55.Wellington H, Paterson RW, Suarez-Gonzalez A, et al. CSF neurogranin or tau distinguish typical and atypical Alzheimer disease. Ann Clin Transl Neurol. 2018;5:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wesenhagen KEJ, Teunissen CE, Visser PJ, Tijms BM. Cerebrospinal fluid proteomics and biological heterogeneity in Alzheimer’s disease: A literature review. Crit Rev Clin Lab Sci. 2020;57:86–98. [DOI] [PubMed] [Google Scholar]
- 58.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. [DOI] [PubMed] [Google Scholar]
- 60.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. [DOI] [PubMed] [Google Scholar]
- 61.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- 62.Hanna Al-Shaikh FS, Duara R, Crook JE, et al. Selective Vulnerability of the Nucleus Basalis of Meynert Among Neuropathologic Subtypes of Alzheimer Disease. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hampel H, Mesulam MM, Cuello AC, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141:1917–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mesulam MM, Lalehzari N, Rahmani F, et al. Cortical cholinergic denervation in primary progressive aphasia with Alzheimer pathology. Neurology. 2019;92:e1580–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehmann M, Ghosh PM, Madison C, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136:844–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannini LAA, Irwin DJ, McMillan CT, et al. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology. 2017;88:2276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen C, Nolan AL, de Paula Franca Resende E, et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. 2019;138:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen RC, Lundt ES, Therneau TM, et al. Predicting Progression to Mild Cognitive Impairment. Ann Neurol. 2019;85:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Josephs KA, Whitwell JL, Tosakulwong N, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015;78:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liesinger AM, Graff-Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 2018;136:873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boon BDC, Bulk M, Jonker AJ, et al. The coarse-grained plaque: a divergent Abeta plaque-type in early-onset Alzheimer’s disease. Acta Neuropathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryan NS, Nicholas JM, Weston PSJ, et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: a case series. Lancet Neurol. 2016;15:1326–35. [DOI] [PubMed] [Google Scholar]
- 74.Rogalski E, Sridhar J, Rader B, et al. Aphasic variant of Alzheimer disease: Clinical, anatomic, and genetic features. Neurology. 2016;87:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE varepsilon4 allele. Lancet Neurol. 2011;10:280–8. [DOI] [PubMed] [Google Scholar]
- 76.Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of neuropathology and experimental neurology. 2011;70:960–9. [DOI] [PubMed] [Google Scholar]
- 80.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh H, Madison C, Baker S, Rabinovici G, Jagust W. Dynamic relationships between age, amyloid-beta deposition, and glucose metabolism link to the regional vulnerability to Alzheimer’s disease. Brain. 2016;139:2275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmqvist S, Scholl M, Strandberg O, et al. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun. 2017;8:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci. 2012;32:8767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van den Heuvel MP, Sporns O. A cross-disorder connectome landscape of brain dysconnectivity. Nat Rev Neurosci. 2019;20:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreisl WC, Lyoo CH, Liow JS, et al. Distinct patterns of increased translocator protein in posterior cortical atrophy and amnestic Alzheimer’s disease. Neurobiol Aging. 2017;51:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boon BDC, Hoozemans JJM, Lopuhaa B, et al. Neuroinflammation is increased in the parietal cortex of atypical Alzheimer’s disease. J Neuroinflammation. 2018;15:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Resende EPF, Nolan AL, Petersen C, et al. Language and spatial dysfunction in Alzheimer disease with white matter thorn-shaped astrocytes. Neurology. 2020;94:e1353–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wattmo C, Wallin AK. Early- versus late-onset Alzheimer’s disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res Ther. 2017;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dickerson BC, McGinnis SM, Xia C, et al. Approach to atypical Alzheimer’s disease and case studies of the major subtypes. CNS Spectr. 2017;22:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yong KXX, McCarthy ID, Poole T, et al. Navigational cue effects in Alzheimer’s disease and posterior cortical atrophy. Ann Clin Transl Neurol. 2018;5:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jokel R, Graham NL, Rochon E, Leonard C. Word retrieval therapies in primary progressive aphasia. Aphasiology. 2014;28:1038–68. [Google Scholar]
- 92.Suarez-Gonzalez A, Henley SM, Walton J, Crutch SJ. Posterior cortical atrophy: an atypical variant of Alzheimer disease. Psychiatr Clin North Am. 2015;38:211–20. [DOI] [PubMed] [Google Scholar]
- 93.Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement. 2019;15:292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller ZA, Rosenberg L, Santos-Santos MA, et al. Prevalence of Mathematical and Visuospatial Learning Disabilities in Patients With Posterior Cortical Atrophy. JAMA Neurol. 2018;75:728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Apostolova LG, Aisen P, Eloyan A, et al. The Longitudinal Early-onset Alzheimer’s Disease Study (LEADS): Framework and Methodology. Alzheimers Dement. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136:3461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]