Abstract
Aim of this work was to evaluate the antimicrobial activity and physical characteristics of citral microencapsulated with dextrin (Dx) by spray drying. The encapsulation was optimized using response surface methodology (RSM), maximizing yield and efficiency, considering as independent variables the citral:Dx ratio (1:5 and 1:20) and the inlet air temperature (120 and 200 °C). Yield and efficiency under optimal conditions were 71.9% and 99.9%, respectively. Antimicrobial activity against Escherichia coli, Salmonella enterica, Staphylococcus aureus and Bacillus cereus of the citral microparticles obtained under optimal conditions and of free citral was evaluated using the disk diffusion methodology. Both compounds showed a broad spectrum inhibitory effect, being Escherichia coli and Bacillus cereus the most sensitive microorganisms. The inhibition ratio varied between 55 and 75%, and the antibacterial activity was maintained after microencapsulation. The minimum inhibitory concentrations of free citral were above 0.8 mg/mL. The optimal citral microparticles showed acceptable physicochemical characteristics and broad-spectrum antimicrobial activity. Polymer and emulsifier used in microencapsulation protected the functional activity of citral, thus suggesting that these microparticles could be used in the design of antimicrobial food systems to extend the shelf life of perishable foods.
Keywords: Encapsulation, Gram-negative, Gram-positive, Morphology, Spray drying
Encapsulation; Gram-negative; Gram-positive; Morphology; Spray drying.
1. Introduction
Citral (3,7-dimethyl-2,6-octadienal) is a natural acyclic monoterpene aldehyde, composed by two geometric isomers: geranial (trans-citral, citral A) and neral (cis-citral, citral B), with antifungal, bactericide, insecticide, deodorant and expectorant activities (Maswal and Dar, 2014). Citral acts predominantly on microorganisms cell membranes by disrupting structures, causing loss of integrity and altering functions (Miron et al., 2014). Essential oils containing citral (e.g. lemongrass, lemon myrtle, lemon verbena, Litsea cubeba, lemon tea tree and melissa) have shown antimicrobial activity (Hu et al., 2019; Naik et al., 2010; Nirmal et al., 2018; Sultanbawa et al., 2009). Its isomers showed antimicrobial activity on Gram-positive, Gram-negative and post-harvest pathogens (Fancello et al., 2016; Lu et al., 2017; Saddiq and Khayyat, 2010); antimicrobial activity of encapsulated essential oils of lemongrass and lemon myrtle has been studied on Gram-positive (Enterococcus faecalis, Listeria monocytogenes and Staphylococcus aureus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella enteritidis and Salmonella typhimurium) (Cui et al., 2016; Huynh et al., 2008; Leimann et al., 2009; Liakos et al., 2016).
Due to its antimicrobial activity, in the food industry the interest on citral is increasing. However, its use is limited because of chemical instability, high hydrophobicity, volatility and susceptibility to oxidative degradation, leading to function alterations. Therefore, microencapsulation technology may help to overcome these limitations (Bakry et al., 2016; Gharsallaoui et al., 2007; Maswal and Dar, 2014) by protecting the active compounds from environment (light, oxygen, water, heat), and by controlling the release (Sutaphanit and Chitprasert, 2014). Encapsulation of citral and/or essential oils containing citral has been performed using different methods: by solvent/antisolvent method (Liakos et al., 2016), liposome-entrapped (Cui et al., 2016) and simple coacervation (Leimann et al., 2009); by oil-in-water nanoemulsion (Nirmal et al., 2018) and spray drying (Huynh et al., 2008; Sosa et al., 2014). Spray drying (SD), the most common and cheap encapsulation method (Balasubramani et al., 2013), is a method of immobilization rather than a true method of encapsulation. Of course, process and formulation optimizations are required to achieve microparticles with minimal citral on the surface, maximal encapsulation efficiency and maximal retention within the microparticles (Huynh et al., 2008).
For SD microencapsulation, the encapsulating agent plays an important role on the retention of the volatiles during the process and the shelf-life of the encapsulated powder (Jafari et al., 2008). Biopolymers used for citral encapsulation by SD are modified starch with either sucrose or trehalose (Sosa et al., 2014) and modified starch + maltodextrin and whey protein concentrate + maltodextrin (Huynh et al., 2008). Carbohydrates have been considered good encapsulating agents because they exhibit low viscosities at high solids concentration as well as good solubility, leading to high amounts of encapsulated oils (de Barros-Fernandes et al., 2014). Dextrin is a modified starch obtained by partial hydrolysis of starch, commonly used in food applications, and a well-known encapsulating agent for vegetable and marine oils (Bakry et al., 2016). However, to the best of our knowledge, citral microencapsulation with dextrin by SD has not been evaluated. The aims of this research were to optimize the spray drying encapsulation process of citral with dextrin by applying response surface methodology (RSM), to determine the antimicrobial activity of the optimized microencapsulated citral against Escherichia coli, Salmonella enterica, Staphylococcus aureus and Bacillus cereus and to evaluate the physical characteristics of the microparticles obtained under optimal conditions.
2. Materials and methods
2.1. Materials
Soy lecithin (Epikuron 145V) was supplied by Blumos Ltda. (Santiago, Chile), dextrin (Amisol® 4810) was donated by Ingredion (Perú), citral (cis and trans mixture ≥96%) and citral analytical standard (99.9%) were from Sigma-Aldrich (Germany), n-heptane (99%) was from Merck (Germany). Culture media used for the antimicrobial trials were nutrient broth (NB), plate count agar (PCA), nutrient agar (NA) and peptone bacteriological (Merck, Germany). Escherichia coli (E. coli) (reference number: ATCC® 25922™), Salmonella enterica serovar Typhimurium (S. enterica) (reference number: ATCC® 14026™), Staphylococcus aureus (S. aureus) (reference number: ATCC® 25923™) and Bacillus cereus (B. cereus) (reference number: ATCC® 14579 PK/5) were from Universidad Nacional Agraria la Molina (UNALM), Total Quality Laboratory (Lima, Perú).
2.2. Preparation of citral encapsulates
Encapsulation of citral (Ct) was performed by SD using dextrin (Dx) as encapsulating agent and soy lecithin (SL) as emulsifier. A face-center design (FCD) was applied with 12 runs (4 factorial points, 4 axial points and 4 central points). The independent variables were Ct:Dx (1:5–1:20 ratio) and inlet air temperature (IAT,120–200 °C; Table 1). The yield of SD process (Y) and encapsulation efficiency of citral (EE) were the dependent variables. All experiments were performed randomly to avoid systematic bias.
Table 1.
Experimental design conditions for the production of citral (Ct) and dextrin (Dx) emulsion microparticles by spray drying and results (mean ± standard error) of drying yield and encapsulation efficiency.
| Standard order | Independent variables |
Response variables |
||
|---|---|---|---|---|
| X1: Temperaturea (°C) | X2: Ct:Dx ratio | Y1: Yield (%) | Y2: Efficiency (%) | |
| 1 | 120 (-1) | 5 (-1) | 42.65 ± 0.65 | 99.63 ± 0.03 |
| 2 | 200 (+1) | 5 (-1) | 65.53 ± 1.56 | 99.87 ± 0.00 |
| 3 | 120 (-1) | 20 (+1) | 49.66 ± 1.23 | 97.97 ± 0.02 |
| 4 | 200 (+1) | 20 (+1) | 55.96 ± 1.34 | 97.07 ± 0.01 |
| 5 | 120 (-1) | 12.5 (0) | 35.41 ± 1.77 | 99.37 ± 0.00 |
| 6 | 200 (+1) | 12.5 (0) | 50.43 ± 0.75 | 99.25 ± 0.14 |
| 7 | 160 (0) | 5 (-1) | 70.07 ± 0.79 | 99.75 ± 0.01 |
| 8 | 160 (0) | 20 (+1) | 59.30 ± 0.86 | 97.70 ± 0.25 |
| 9 | 160 (0) | 12.5 (0) | 58.30 ± 1.18 | 99.22 ± 0.04 |
| 10 | 160 (0) | 12.5 (0) | 59.16 ± 1.20 | 99.19 ± 0.03 |
| 11 | 160 (0) | 12.5 (0) | 61.47 ± 1.19 | 99.14 ± 0.01 |
| 12 | 160 (0) | 12.5 (0) | 57.64 ± 1.16 | 99.12 ± 0.05 |
Inlet air temperature.
Infeed solution (100 g) was prepared as follows: lecithin (1 g) was dispersed in distilled water (30 g) at 50 °C using magnetic stirrer (350 rpm for 15 min). After cooling to 30 °C, citral (1 g) was added and mixed at 1500 rpm for 3 min using a Polytron homogenizer (PT-2100, Kinematica A.G, Switzerland). Dextrin (5, 12.5 or 20 g) was dispersed in distilled water (63, 55.5 or 48 g) at 50 °C and stirred for 3 h to obtain 1:5, 1:12.5 or 1:20 Ct:Dx ratios, respectively (Table 1). Citral oil-in-water emulsion was added to the Dx solution and mixed at 1500 rpm for 3 min. The resultant mixture was fed into the spray dryer (B-290, Büchi, Switzerland). The spray dryer was operated at infeed temperature of 42 ± 2.5 °C and inlet air temperature of 120, 160 or 200 ± 1 °C. The air flow, feeding rate and atomization pressure were 600 L/h, 5 mL/min and 0.14 MPa, respectively. Citral microparticles powders were stored at -20 °C in the dark until analysis.
2.3. Analysis
The encapsulation yield (Y) was determined according to the equation , where WM is the microparticle weight after SD, and WSST represents the total soluble solids in infeed solution (Sutaphanit and Chitprasert, 2014).
The EE was computed as , where CS is the citral surface calculated as . Total citral was determined by gas chromatography as follows: microparticles (0.2 g) were dispersed in hexane (2 mL), stirred for 1 min and centrifuged at 112,000 g for 1 min. The supernatant was transferred to an amber vial (2 mL) and injected into a 7890A gas chromatograph (Agilent Technologies, USA) fitted with a DB-5MS fused-silica capillary column (30 m × 0.25 mm i.d. x 0.25 μm film thickness, J&W Scientific, USA) and a flame ionization detector following the method proposed by Ruktanonchai et al. (2011). The chromatogram showed two peaks corresponding to the citral isomers, geranial and neral. Citral was quantified using a calibration curve (1–2000 μg/mL citral, R2 = 0.999). Results are expressed as mg citral/g powder.
2.4. Characterization of the citral microparticles obtained under optimal conditions
Moisture content was analysed according to method n° 925.10 (AOAC, 1997). Water activity (aw) was determined by the dew point method using a HigroLab (Rotronic, USA) at 20 ± 0.3 °C and hygroscopicity (Hg) was evaluated according to the procedure described by Cai and Corke (2000).
The outer structure of the citral microparticles was examined using scanning electron microscopy (SEM). The citral microparticles were coated with gold/palladium, using a PS 10E vacuum evaporator and analysed using a LEO 1420VP SEM (LEO Electron Microscopy Ltd., Cambridge, UK) operated at 20 kV. The scanned images were collected digitally using the EDS 7424 software (Oxford Instruments, Oxford, UK).
Particle size and size distribution were determined by light scattering using a laser diffraction particle size analyser (Mastersizer X, Malvern Instruments, Worcestershire, UK). Citral microparticles were dispersed in water and the results were expressed as volume average (D4,3).
2.5. Microbiological assays
2.5.1. Preparation of microorganism suspensions
Bacterial strains used to evaluate inhibitory activity were E. coli, S. enterica, S. aureus and B. cereus, and were prepared following the methodology proposed by Wang et al. (2009), with minimal modifications. Each strain stored in tubes in inclined position at 4 °C was transferred to Erlenmeyer flask containing NB (100 mL) and incubated at 37 °C for 18 h with constant agitation. For the determination of the colony forming units (CFU)/mL, serial dilutions up to 10−6 were prepared in 0.1% peptone water. From the last three dilutions, 0.1 mL was transferred to plates with PCA by surface seeding and incubated at 37 °C for 18 h in an oven (Binder, BD 53, Germany).
The 10−1 dilution, which had an average cell concentration of 107 CFU/mL, was used for the assessment of antimicrobial activity.
2.5.2. In-vitro antibacterial activity assay of citral microparticles
Antibacterial inhibitory activity was determined by disk diffusion method according to Wang et al. (2009), with some modifications. Filter paper discs (Whatman 2, diameter 6 mm) containing 5 μL microparticle solution (300 mg microparticles in 1 mL diluted heptane plus 1 mL distilled water) and 5 μL of pure citral mixture solution as a control, with equivalent concentrations (41 mg of citral in 1 mL of heptane diluted in 1 mL of distilled water), were applied on the plate surface with NA previously seeded with 0.1 mL test bacterial strains. The plates were incubated at 37 ± 1 °C for 18 h. The experiment was performed in quadruplicate. The diameter of the transparent inhibition zone against test microorganism was measured using a manual Vernier (Kern, Germany), and the inhibition ratio was calculated, comparing the inhibition diameter of the citral microparticles respect to citral (control), as ; where M is the inhibition zone diameter of test citral microparticles and c is the inhibition zone diameter of the pure citral mixture (control), in mm (Wang et al., 2009).
To verify that the effect of the antimicrobial agents came from citral and not from heptane (the solvent), an aliquot of pure heptane was used as a control.
2.5.3. In-vitro antibacterial activity assay of pure citral
Serial dilutions of citral in heptane (0, 0.08, 0.2, 0.4, 0.8, 2.0, 4.0, 8.0, 40.0 and 80.0 mg/mL) were prepared according to Guarda et al. (2011) and Leimann et al. (2009). The disk diffusion method was used following the procedure described above. Sterilized filter paper discs were placed on the surface of plates with NA previously seeded with the assay bacterial strains and 5 μL of serial citral dilutions were added.
2.6. Statistical analysis
The RSM experimental design matrix, data analysis, model development and optimization were studied using the Design Expert software v. 12 (Stat-Ease Inc., Minneapolis, USA). The average and standard deviations of the responses were calculated with Microsoft Excel. One-way analysis of variance (ANOVA) was performed on the data of the antibacterial trials. When significant differences were found, least significance test (LSD; p ≤ 0.05) was applied. ANOVA and LSD were performed using the software Statgraphics 15.2 (StatPoint Inc., USA, 2015).
3. Results and discussion
3.1. Optimisation by response surface methodology
Table 1 shows the results of the face centered design applied to evaluate the effect of the inlet air temperature and the formulation (Ct:Dx ratio) on the encapsulation yield and encapsulation efficiency. The Y ranged between 35.4% and 70.1% and the EE ranged from 97.1% to 99.9%, values comparable to those (Y: 60–98% and EE: higher than 85%) reported by Sutaphanit and Chitprasert (2014) for basil EO microparticles in gelatin.
The ANOVA (Table 2) evidenced that only the linear term of Ct:Dx ratio for Y and the quadratic term of the IAT for EE were not significant. The estimates of the regression coefficients of the second-order polynomial models for the two dependent variables are reported in Table 2. The yield corresponds to the ratio between the solids content in the feed solution before spray drying and the solids content after spray drying. The ANOVA showed that the IAT linear and quadratic terms were the most significant, followed by the quadratic Ct:Dx ratio. The yield model explained 90% of the variability (adjusted R2) and was represented by the following equation:
| Yield = -192.42 + 3.10 IAT - 1.66 Ct:Dx - 0.014 IAT ∗ Ct:Dx - 0.009 IAT2 + 0.143 Ct:Dx2 |
Table 2.
Estimates of the regression coefficients of the second-order polynomial models and analysis of variance (mean square, MS and significance) for drying yield and encapsulation efficiency.
| Source | df | Yield |
Efficiency |
||
|---|---|---|---|---|---|
| Coefficient | MS | Coefficient | MS | ||
| Intercept | 58.31 | 99.21 | |||
| Model | 5 | 192.49∗∗∗ | 1.680∗∗∗ | ||
| A-Temperature | 1 | 7.37 | 325.61∗∗∗ | -0.13 | 0.102∗ |
| B-Ct:Dx ratio | 1 | -2.22 | 29.61 | -1.09 | 7.070∗∗∗ |
| AB | 1 | -4.14 | 68.72∗ | -0.29 | 0.330∗∗ |
| A2 | 1 | -13.73 | 502.61∗∗∗ | 0.02 | 0.002 |
| B2 | 1 | 8.04 | 172.22∗∗ | -0.56 | 0.831∗∗∗ |
| Residual | 6 | 9.17 | 0.012 | ||
| Lack of Fit | 3 | 15.56 | 0.021 | ||
| Pure Error | 3 | 2.79 | 0.002 | ||
| R2 | 0.95 | 0.99 | |||
| Adj-R2 | 0.90 | 0.99 | |||
| Pred-R2 | 0.57 | 0.94 | |||
| C.V. % | 5.46 | 0.11 | |||
Ct, citral; Dt, dextrin; df, degrees of freedom; Adj-R2, R2 adjusted by df; Pred-R2, R2 in prediction; MS, Mean square; ∗, p ≤ 0.05; ∗∗, p ≤ 0.01; ∗∗∗, p ≤ 0.001.
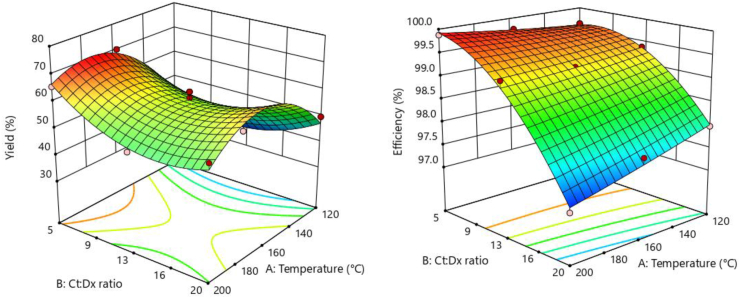
The RSM plot (Figure 1) showed that Y was higher for microparticles with lower Ct:Dx ratio and intermediate IAT. The lower Ct:Dx ratio and the higher yield of powdered microparticles could be attributed to better pulverization, smaller size and weight of the droplet inside the dryer when the solution is more diluted, favoring a better heat transfer and preventing dust from rapidly precipitating into the drying chamber (Robert et al., 2017; Vergara et al., 2014). In addition, the best yields were obtained at intermediate IATs, probably because low temperatures produce microparticles with higher humidity, helping the powder to adhere to the drying chamber and thus increasing losses (Urzúa et al., 2017). On the other hand, high temperatures could volatilize the water more quickly and result in a sample adhering to the wall of the drying chamber, reducing the yield (Cao et al., 2018). Similar results were obtained while encapsulating jambul extract in maltodextrin by spray-drying (Santhalakshmy et al., 2015). Higher yields were instead obtained by Bhandari et al. (1992) during the microencapsulation at 400 °C (IAT) of citral in maltodextrin and gum arabic, without adverse effects on the EO chemical properties, probably due to the type of encapsulating agent.
Figure 1.
Response surface plots for the production of citral (Ct) and dextrin (Dx) emulsion microparticles by spray drying: drying yield and encapsulation efficiency.
The EE of citral represents the retention percentage of the Ct-SL emulsion. The ANOVA indicated that the linear and quadratic terms of Ct:Dx ratio were the most significant on EE, followed by IAT and Ct:Dx ration interaction. The model explained 99% of the variability (adjusted R2). The quadratic regression was represented by the following equation:
| EE = +98.50 + 0.0038 IAT +0.2566 Ct:Dx - 0.0010 IAT ∗ Ct:Dx + 0.000015 IAT2 - 0.0099 Ct:Dx2 |
The RSM plot (Figure 1) showed that the EE of citral mainly increased with the decrease of the Ct:Dx ratio: the lower the Dx content, the higher the retention of the citral emulsion in the microparticle, probably as a consequence of smaller droplet size of the emulsion during spraying in the more diluted solution, favoring the increase in EE. A possible explanation for this phenomenon could be the atomization shear effect on the larger emulsion droplets, coupled with the high speed gradient and turbulence state at the atomizer "mouth", that could allow large emulsion droplets to break up into smaller droplets, causing greater loss of core material from larger emulsion droplets during spray drying (García et al., 2013; Huynh et al., 2008). Similar results were reported for garlic EO microparticles in maltodextrin (Balasubramani et al., 2013) where the highest EE was obtained at 1:6 (EO:maltodextrin) ratios. The significant IAT-Ct:Dx ratio interaction (Table 2) is evident in Figure 1 for the different behaviour of EE as a function of IAT at high and low Ct:Dx ratios. It is interesting to observe that at low Ct:Dx ratio levels, a slight EE increase occurs when IAT augments; the high temperatures could lead to a rapid formation of the crust on the drop, which allowed a greater emulsion retention and water diffusion into hot air.
The lack of fit for yield and EE was not significant indicating that the mathematical models fit the experimental data within the experimental domain.
A numerical optimization was performed to find out the best IAT and Ct:Dx ratio for maximizing Y and EE of citral microparticles. A desirability value of 0.996 was obtained for the optimal IAT of 187 °C and Ct:Dx ratio of 1:5, leading to a Y of 70.1% and an EE of 99.9%. It is important to note that the optimal conditions obtained for Ct-Dx microparticles according to the statistical design are specific for this system and may not apply when other biopolymers are used as encapsulating agents (Vergara et al., 2014). In this context, the biopolymers properties play a fundamental role in the encapsulation parameters (mainly the IAT) and the stability of the active compounds (Urzúa et al., 2017). The optimal IAT found in the present study is within the temperature range (160 and 200 °C) suggested by other authors studying the encapsulation process of different matrices such as garlic EO in maltodextrin (Balasubramani et al., 2013), gac red arils EO (Momordica conchinchinensis) in whey protein and gum arabic (Kha et al., 2014) and coffee EO in gum arabic (Frascareli et al., 2012).
3.2. Characterization of the microparticles obtained under optimal conditions
High yield (71.9%) and EE (99.9%), similar to the values predicted by the model (70.1% and 99.9%, respectively), were found for citral microparticles prepared under optimal conditions of Ct:Dx ratio (1:5) and inlet air temperature (187 °C). Under these optimal conditions and considering the EE %, a concentration of 137.56 mg citral/g microparticles was obtained.
The EE was high and superior to studies where dextrin and milk protein isolate or inulin were used as coating material (Ahn et al., 2008; García et al., 2013). The EE level obtained in this study could be attributed to the stability of the feed emulsion (Ct-SL-Dx) in the spray drying process, which play an important role in the hydrophobic molecules retention, as well as the emulsifier effect of SL. Furthermore, the high retention in the dextrin systems was attributed to the higher hydrophobicity of the polymer (García et al., 2013).
Previous studies mention that the EE of citral in spray drying is mainly influenced by the properties of the encapsulating agent (viscosity, solubility), citral:encapsulating agent ratio and temperature (Maswal and Dar, 2014; Sosa et al., 2014). Temperature, a relevant factor in citral degradation (Sosa et al., 2014), can be tolerated up to 400 °C without any negative effect on its chemical properties (Bhandari et al., 1992). In the present study, despite the high drying temperatures, the EE was high probably because of the short drying times and/or the rapid crust formation on the drop surface, which allows water diffusion while retaining EO (Gharsallaoui et al., 2007; Vergara et al., 2014).
The citral microparticles obtained under optimal conditions presented 5.3 ± 1.3% humidity, 0.2 ± 0.02 water activity, 29.7 ± 0.01 g/100 g hygroscopicity, 7.08 μm average particle size (D4,3) and particle size distribution values between 1.04 and 15.05 μm. Moisture, water activity and particle size were within the range reported for microparticles obtained by spray drying (Cai and Corke, 2000; Gharsallaoui et al., 2007). Hygroscopicity was lower than the values obtained by Frascareli et al. (2012) and Kha et al. (2014) due to the physical-chemical characteristics of the ingredients used, mainly the encapsulating agent (Bakry et al., 2016).
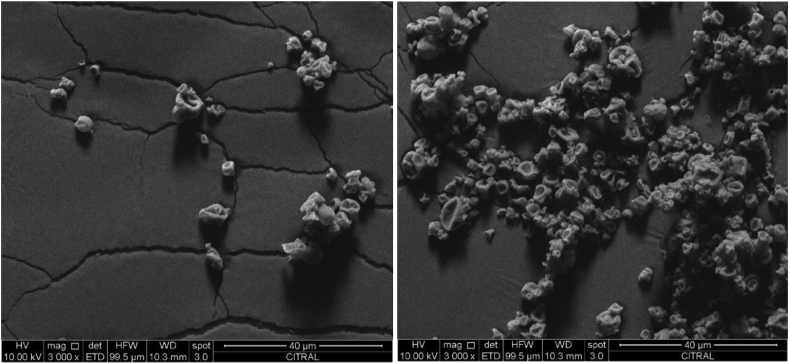
Figure 2 shows the SEM photographs of the microparticles obtained under optimal conditions. All had spherical shape with irregular and rough external surfaces and depressions, indentations or concavities, and showed a tendency to agglomeration. The depressions could be attributed to the lack of plasticizing properties of the encapsulating agent and to the contraction of the microparticles during the drying process, consequence of the high inlet air temperature. The rapid evaporation in the initial drying stage and the high pressure of the particles produce contraction (Frascareli et al., 2012). A similar morphology was observed in lemon myrtle EO microencapsulated with maltodextrin and modified starch (Huynh et al., 2008), linseed and rosemary EO with maltodextrin (de Barros-Fernandes et al., 2014), cardamom oleoresin with maltodextrin and modified starch (Krishnan et al., 2005), Haematococcus pluvialis oleoresins with capsul (Bustamante et al., 2016) and coffee EO with gum arabic (Frascareli et al., 2012), all produced by spray drying using polysaccharides as wall material (modified starch and maltodextrin). Differently, microparticles of sunflower EO in milk protein isolate and dextrin (Ahn et al., 2008) and lime EO in blend of whey protein and maltodextrin (Campelo et al., 2018) were spherical, with a homogeneous, smooth surface, free of cracks and pores, probably due to the fact that the dextrin or maltodextrin was combined with a protein-based polymer, in addition to the effect of the inlet temperature.
Figure 2.
Scanning electron microscopy photographs of citral microparticles obtained under optimal conditions (3000x). On the left, dispersed microparticles; on the right, agglomerated microparticles.
3.3. Antibacterial activity of pure citral mixture
The effect of citral on bacterial growth is shown in Table 3. Diameter means of inhibition zone were significantly different. The lowest inhibitory concentration (0.80 mg/mL) was obtained for B. cereus and the highest (4 mg/mL) for S. enterica.
Table 3.
Antibacterial effect, expressed as diameter of the inhibition zone (mm; mean ± standard error, n = 4), of pure citral mixtures at different dilutions in heptane.
| Citral concentration (mg/mL) | E. coli1 | S. enterica2 | S. aureus3 | B. cereus4 |
|---|---|---|---|---|
| 0.00 | -5 | - | - | - |
| 0.08 | - | - | - | - |
| 0.20 | - | - | - | - |
| 0.40 | - | - | - | - |
| 0.80 | - | - | - | 7.78d ± 0.31 |
| 2.00 | 8.68c ± 0.25 | - | 6.73c ± 0.21 | 8.73c ± 0.31 |
| 4.00 | 10.00b ± 0.41 | 6.65d ± 0.13 | 7.08c ± 0.27 | 9.88b ± 0.42 |
| 8.00 | 14.33a ± 0.61 | 7.73c ± 0.59 | 7.25c ± 0.17 | 10.28b ± 0.18 |
| 40.00 | 14.70a ± 0.72 | 12.93b ± 0.46 | 10.03b ± 0.62 | 13.88a ± 0.14 |
| 80.00 | 15.25a ± 0.90 | 14.05a ± 0.48 | 15.03a ± 0.32 | 14.30a ± 0.71 |
Reference numbers: 1ATCC® 25922™; 2ATCC® 14026™; 3ATCC® 25923™; 4ATCC® 14579 PK/5. 5 Dashes indicate that there was no inhibitory effect.
The bacterial strains studied exhibited a high sensitivity to low citral concentrations, stronger than those reported by other authors. For example, pure D-limonene inhibited the growth of E. coli at minimum concentrations of 25 mg/mL (Donsì et al., 2011) while pure eugenol inhibited E. coli and S. aureus at a minimum concentration of 10 mg/mL (Piletti et al., 2017). The results in Table 3 are comparable to the values reported for antimicrobial agents such as thymol, carvacrol and citrus EO against S. aureus, S. enterica, L. innocua and E coli (Fancello et al., 2016; Guarda et al., 2011). Saddiq and Khayyat (2010) concluded that citral showed greater antibacterial activity compared to industrial antibiotics such as nalidixic acid, ampicillin and nitrofurantoin.
The inhibitory effect of citral against microorganisms could be explained by its structural characteristics, as it is an α, β-unsaturated aldehyde with the carbonyl group adjacent to the α and β carbons. Due to their position, the α and β carbons are conjugated to the carbonyl group, allowing the β-carbon to become positively polarized and easily reactant with nucleophiles (nucleophilic attack) (Wuryatmo et al., 2003). According to Witz (1989), the chemical action of α, β-unsaturated aldehydes and some of their toxicological effects is based on their ability to act as direct alkylating agents. These alkylating agents are capable of covalently bind cellular nucleophilic groups, modifying cellular processes and being potentially toxic. The antimicrobial effect can also be attributed to the ability of citral to alter and penetrate the lipid and protein structure of bacterial cell wall, as recently proposed by Lu et al. (2018). This leads to protein denaturation and cell membrane destruction, followed by cytoplasmic filtration, lysis, and cell death (Saddiq and Khayyat, 2010).
3.4. Antibacterial activity of citral microparticles
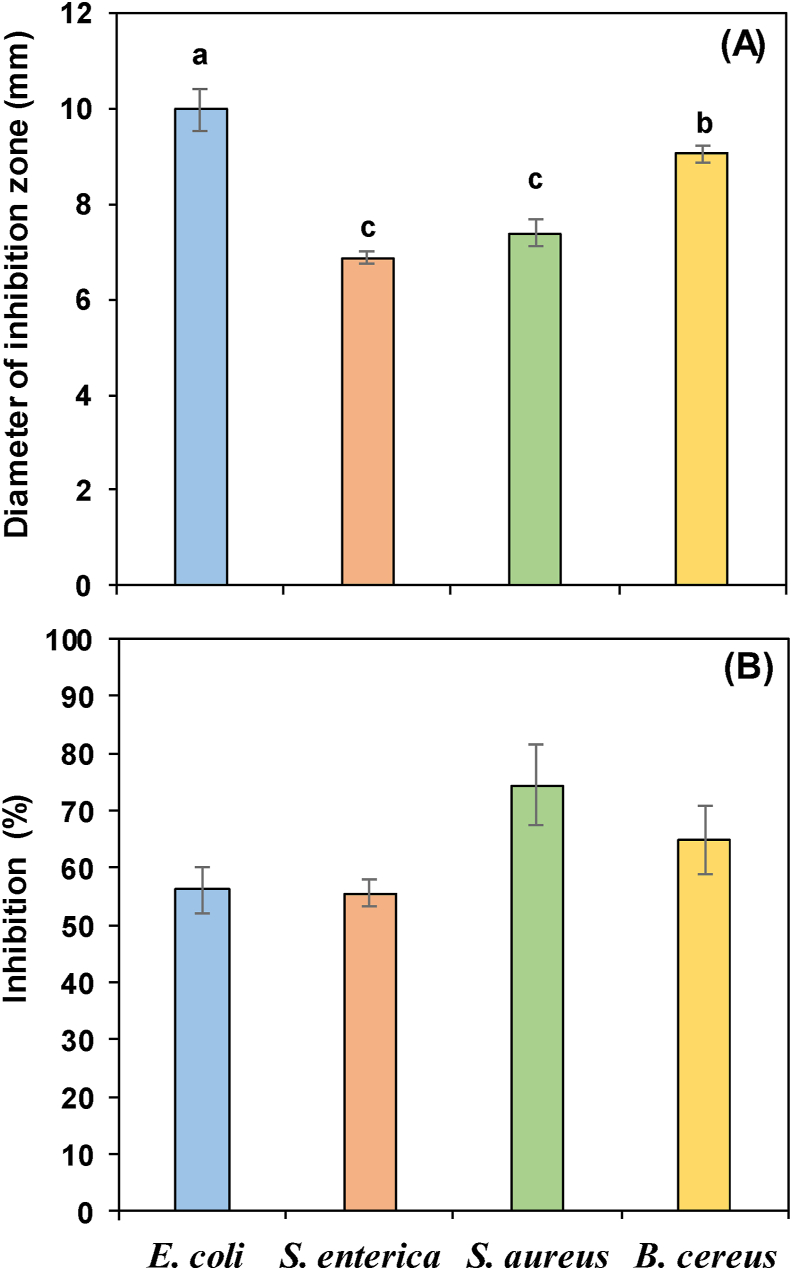
The studies carried out with solutions of pure heptane (Table 3) did not show activity against the bacterial strains under study, demonstrating that the solvent has no inhibitory effect. The inhibitory effect of citral microparticles (300 mg/mL) on the growth of E. coli and B. cereus, S. aureus and S. enterica is presented in Figure 3A. The diameter of the inhibition zone was significantly different among bacterial strains: Gram-negative E. coli and B. cereus were more sensitive to citral microparticles (9.98 and 9.05 mm, respectively) than Gram-positive S. aureus and S. enterica (7.38 and 6.88 mm, respectively).
Figure 3.
Growth inhibition of different bacteria species by citral microparticles expressed as: (A) diameter of inhibition zone at a concentration of 300 mg/mL and as: (B) inhibition percentage of the citral microparticle compared to an equivalent amount of free citral. Bars indicate standard error (n = 4). Different letters on each bar indicate statistically significant differences according to the LSD test (p < 0.05).
Citral microparticle systems would promote enhanced interaction with microbial cell membranes primarily by the following routes: increased contact surface area favours passive diffusion through the outer cell membrane and interaction with the cytoplasmic membrane (Donsì et al., 2012; Lu et al., 2018); sustained release over time of citral from the microparticles, driven by the release of citral between the wall material and the aqueous phase, prolonging its bioactivity (Majeed et al., 2016); and electrostatic interaction of the positively charged citral microparticles (through the polymer) with the negatively charged microbial cell walls increase the concentration of these oils at the action site (Chang et al., 2015). The use of micro-scale delivery systems, such as structures based on microparticles, microemulsions and liposomes, can increase passive mechanisms of cellular absorption, reducing resistance to mass transfer and increasing antimicrobial activity (Bakry et al., 2016; Donsì and Ferrari, 2016).
These results agree with previous studies of microparticles based on EO of lemongrass, cloves, thyme, palmarosa, oregano, thymol and carvacrol, which showed bactericidal properties against E. coli, L. monocytogenes, L. innocua, B. cereus, B. subtilis, Haemophilus influenzae, Neisseria gonorreae, Streptococcus pneumoniae and Vibrio cholerae (Anaya-Castro et al., 2017; Guarda et al., 2011; Salvia-Trujillo et al., 2015).
On the other hand, studies comparing the antimicrobial activity of microparticles of carvacrol, limonene and cinnamaldehyde EO, encapsulated with soy lecithin, soy protein and sucrose palmitate, against Gram-positive and Gram-negative bacteria, did not find significant differences (Donsì and Ferrari, 2016). Differently, some studies reported a higher sensitivity of Gram-positive bacteria than Gram-negatives when using microparticles of lemongrass EO (Leimann et al., 2009), Citrus limon EO (Fancello et al., 2016) and citral nanoemulsions (Lu et al., 2018). The apparently contrasting results among studies are a consequence of the antimicrobial activity dependance on the microparticle system, type and concentration of the microencapsulated active ingredients (Donsì et al., 2012; Salvia-Trujillo et al., 2015). In the present study, citral with a purity higher than 96%, microencapsulated in dextrin, was used.
The antibacterial inhibition percentage is the ratio of the diameter zone of transparent inhibition of the citral microparticles and the inhibition diameter of similar concentrations of free citral. When the inhibitory effect (inhibition ratio) of the citral microparticle was evaluated in comparison with equivalent amounts of free citral, no significant differences were obtained, and the values varied between 55.6% for S. enterica and 74.5% for S. aureus (Figure 3B).
Results showed that, even after the microencapsulation process and high temperature spray drying, citral maintained its bioactivity. The inhibition ratio of the citral microparticle was less than 100%, which could be due to the lower free mass of the microencapsulated citral compared to the equivalent amounts of free citral used in the inhibition test; during the microencapsulation process, dextrin and SL were added to citral, so the activity of microencapsulated citral was not the same as free citral. These results were lower than those reported by Leimann et al. (2009) for lemongrass EO microparticles formulated with polyvinyl alcohol, sodium sulfate and glutaraldehyde and than those reported by Wang et al. (2009) for curcumin microparticles formulated with gelatin and starch. In both studies, no emulsifiers (e.g. soy lecithin) were used. The presence of SL in the current research may be the cause of the low inhibition ratio which hinders the rapid release of citral (Donsì et al., 2011, 2012).
When carvacrol EO, D-limonene and trans-cinnamaldehyde were microencapsulated with soy lecithin and soy protein, SL was responsible for the slow release of EO in the aqueous phase, causing antimicrobial activity for 24 h; in contrast, the availability in the aqueous phase of EO microencapsulated with sugar esters, Tween 20 and glycerol monooleate EO was immediate and the bactericidal activity lasted 2 h (Donsì et al., 2012). In in vitro studies, where a rapid release of the active agent is required to avoid rapid microbial growth, the use of emulsifiers in microparticle formulation would reduce the bioactivity of the antimicrobials; however, if a bacteriostatic action is desired for a prolonged period of time to ensure a certain shelf life for a food product, the best option would probably be the use of SL, to favor a slow release and ensure a lower concentration of the antimicrobial compound in the aqueous phase, therefore prolonging its action (Donsì et al., 2011, 2012; Donsì and Ferrari, 2016). The optimal microparticles obtained in the present study could be used for the elaboration of active biofilms for the packaging of highly perishable foods.
4. Conclusion
Citral, an acyclic aldehyde with effective antimicrobial activity extracted from natural sources, was successfully microencapsulated by spray drying in a dextrin and soy lecithin matrix. The obtained microparticles showed high encapsulation efficiency and high emulsion retention (citral, SL and Dx) during the drying process, favoring citral molecules retention. Using RSM, the optimal citral microencapsulation conditions were determined with high yield and encapsulation efficiency.
The study of the inhibitory activity of optimal citral microparticles against tested strains of the foodborne pathogens E. coli, S. enterica, S. aureus, and B. cereus, suggests a broad spectrum of effects. Bactericidal activity was not significantly affected by the high temperature spray drying process, and its inhibition ratio was greater than 55%. In addition, the results of the in vitro assay of minimal inhibitory concentrations of pure citral (>0.8 mg/mL) indicated that it was effective against the bacteria strains studied, regardless of their Gram-positive or Gram-negative classification.
The citral microparticles described in this study could represent a natural antimicrobial potential for use in the design of food systems and/or active food packaging, in order to extend shelf life and ensure food safety.
Declarations
Author contribution statement
Ives Yoplac: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Luis Vargas, Paz Robert: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alyssa Hidalgo: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (PE) [179-2015-FONDECYT].
Data availability statement
Data associated with this study has been deposited at 'Repository of the National Agrarian University La Molina, Peru' under the accession number http://repositorio.lamolina.edu.pe/handle/UNALM/4157.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Cristina Vergara, Estefanía González, Inés Cea and Guibeth Morello for their contribution, technical support and suggestions.
References
- Ahn J.H., Kim Y.P., Lee Y.M., Seo E.M., Lee K.W., Kim H.S. Optimization of microencapsulation of seed oil by response surface methodology. Food Chem. 2008;107:98–105. [Google Scholar]
- Anaya-Castro M.A., Ayala-Zavala J.F., Muñoz-Castellanos L., Hernández-Ochoa L., Peydecastaing J., Durrieu V. β-Cyclodextrin inclusion complexes containing clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils: preparation, physicochemical and antimicrobial characterization. Food Packag. Shelf Life. 2017;14:96–101. [Google Scholar]
- AOAC . sixteenth ed. Association of Analytical Chemists; Washington DC, USA: 1997. Official Methods of Analysis of AOAC International. [Google Scholar]
- Bakry A.M., Abbas S., Ali B., Majeed H., Abouelwafa M.Y., Mousa A., Liang L. Microencapsulation of oils: a comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016;15:143–182. doi: 10.1111/1541-4337.12179. [DOI] [PubMed] [Google Scholar]
- Balasubramani P., Viswanathan R., Vairamani M. Response surface optimisation of process variables for microencapsulation of garlic (Allium sativum L.) oleoresin by spray drying. Biosyst. Eng. 2013;114:205–213. [Google Scholar]
- Bhandari B.R., Dumoulin E.D., Richard H.M.J., Noleau I., Lebert A.M. Flavor encapsulation by spray drying: aplication to citral and linalyl acetate. J. Food Sci. 1992;57:217–221. [Google Scholar]
- Bustamante A., Masson L., Velasco J., Del Valle J.M., Robert P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016;190:1013–1021. doi: 10.1016/j.foodchem.2015.06.062. [DOI] [PubMed] [Google Scholar]
- Cai Y.Z., Corke H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000;65:1248–1252. [Google Scholar]
- Campelo P.H., Sanches E.A., de Barros F.R.V., Botrel D.A., Borges S.V. Stability of lime essential oil microparticles produced with protein-carbohydrate blends. Food Res. Int. 2018;105:936–944. doi: 10.1016/j.foodres.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Cao X., Li N., Qi G., Sun X.S., Wang D. Effect of spray drying on the properties of camelina gum isolated from camelina seeds. Ind. Crop. Prod. 2018;117:278–285. [Google Scholar]
- Chang Y., McLandsborough L., McClements D.J. Fabrication, stability and efficacy of dual-component antimicrobial nanoemulsions: essential oil (thyme oil) and cationic surfactant (lauric arginate) Food Chem. 2015;172:298–304. doi: 10.1016/j.foodchem.2014.09.081. [DOI] [PubMed] [Google Scholar]
- Cui H.Y., Wu J., Lin L. Inhibitory effect of liposome-entrapped lemongrass oil on the growth of Listeria monocytogenes in cheese. J. Dairy Sci. 2016;99:6097–6104. doi: 10.3168/jds.2016-11133. [DOI] [PubMed] [Google Scholar]
- de Barros-Fernandes R.V., Reginaldo G., Vilela S., Alvarenga D. Effect of solids content and oil load on the microencapsulation process of rosemary essential oil. Ind. Crop. Prod. 2014;58:173–181. [Google Scholar]
- Donsì F., Annunziata M., Sessa M., Ferrari G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44:1908–1914. [Google Scholar]
- Donsì F., Annunziata M., Vincensi M., Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J. Biotechnol. 2012;159:342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Donsì F., Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Fancello F., Petretto G.L., Zara S., Sanna M.L., Addis R., Maldini M., Foddai M., Rourke J.P., Chessa M., Pintore G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;69:579–585. [Google Scholar]
- Frascareli E.C., Silva V.M., Tonon R.V., Hubinger M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012;90:413–424. [Google Scholar]
- García P., Vega J., Jimenez P., Santos J., Robert P. Alpha-tocopherol microspheres with cross-linked and acetylated inulin and their release profile in a hydrophilic model. Eur. J. Lipid Sci. Technol. 2013;115:811–819. [Google Scholar]
- Gharsallaoui A., Roudaut G., Chambin O., Voilley A., Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res. Int. 2007;40:1107–1121. [Google Scholar]
- Guarda A., Rubilar J.F., Miltz J., Galotto M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011;146:144–150. doi: 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Hu W., Li C., Dai J., Cui H., Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA) Ind. Crop. Prod. 2019;130:34–41. [Google Scholar]
- Huynh T.V., Caffin N., Dykes G.A., Bhandari B. Optimization of the microencapsulation of lemon myrtle oil using response surface methodology. Dry. Technol. 2008;26:357–368. [Google Scholar]
- Jafari S.M., Assadpoor E., He Y., Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008;26:816–835. [Google Scholar]
- Kha T.C., Nguyen M.H., Roach P.D., Stathopoulos C.E. Microencapsulation of gac oil : optimisation of spray drying conditions using response surface methodology. Powder Technol. 2014;264:298–309. [Google Scholar]
- Krishnan S., Bhosale R., Singhal R.S. Microencapsulation of cardamom oleoresin: evaluation of blends of gum Arabic, maltodextrin and a modified starch as wall materials. Carbohydr. Polym. 2005;61:95–102. [Google Scholar]
- Leimann F.V., Gonçalves O.H., Machado R.A.F., Bolzan A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater. Sci. Eng. C. 2009;29:430–436. [Google Scholar]
- Liakos I.L., D’autilia F., Garzoni A., Bonferoni C., Scarpellini A., Brunetti V., Carzino R., Bianchini P., Pompa P.P., Athanassiou A. All natural cellulose acetate-lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharm. 2016;510:508–515. doi: 10.1016/j.ijpharm.2016.01.060. [DOI] [PubMed] [Google Scholar]
- Lu W.C., Huang D.W., Wang C.C.R., Yeh C.H., Tsai J.C., Huang Y.T., Li P.H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018;26:82–89. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Huang D., Wang C.R., Yeh C. Activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2017;6:1–8. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed H., Liu F., Hategekimana J., Sharif H.R., Qi J., Ali B., Bian Y.Y., Ma J., Yokoyama W., Zhong F. Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 2016;197:75–83. doi: 10.1016/j.foodchem.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Maswal M., Dar A.A. Formulation challenges in encapsulation and delivery of citral for improved food quality. Food Hydrocolloids. 2014;37:182–195. [Google Scholar]
- Miron D., Battisti F., Silva F.K., Lana A.D., Pippi B., Casanova B., Gnoatto S., Fuentefria A., Mayorga P., Schapoval E.E.S. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Braz. J. Pharmacogn. 2014;24(6):660–667. [Google Scholar]
- Naik M.I., Fomda B.A., Jaykumar E., Bhat J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010;3:535–538. [Google Scholar]
- Nirmal N.P., Mereddy R., Li L., Sultanbawa Y. Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018;254:1–7. doi: 10.1016/j.foodchem.2018.01.173. [DOI] [PubMed] [Google Scholar]
- Piletti R., Bugiereck A.M., Pereira A.T., Gussati E., Dal Magro J., Mello J.M.M., Dalcanton F., Ternus R.Z., Soares C., Riella H.G., Fiori M.A. Microencapsulation of eugenol molecules by β-cyclodextrine as a thermal protection method of antibacterial action. Mater. Sci. Eng. C. 2017;75:259–271. doi: 10.1016/j.msec.2017.02.075. [DOI] [PubMed] [Google Scholar]
- Robert P., García P., Fredes C. Drying and preservation of polyphenols. In: Cuevas-Valenzuela J., Vergara-Salinas J.R., Pérez-Correa J.R., editors. Advances in Technologies for Producing Food-Relevant Polyphenols. CRC Press, Taylor and Francis Group; Abingdon, UK: 2017. pp. 281–302. Chapter 9. [Google Scholar]
- Ruktanonchai U., Srinuanchai W., Saesoo S., Sramala I., Puttipipatkhachorn S., Soottitantawat A. Encapsulation of citral isomers in extracted lemongrass Oil with cyclodextrins: molecular modeling and physicochemical characterizations. Biosci. Biotechnol. Biochem. 2011;75:2340–2345. doi: 10.1271/bbb.110523. [DOI] [PubMed] [Google Scholar]
- Saddiq A.A., Khayyat S.A. Chemical and antimicrobial studies of monoterpene: citral. Pestic. Biochem. Physiol. 2010;98(1):89–93. [Google Scholar]
- Salvia-Trujillo L., Rojas-Graü A., Soliva-Fortuny R., Martín-Belloso O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocolloids. 2015;43:547–556. [Google Scholar]
- Santhalakshmy S., Don Bosco S.J., Francis S., Sabeena M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015;274:37–43. [Google Scholar]
- Sosa N., Schebor C., Pérez O.E. Encapsulation of citral in formulations containing sucrose or trehalose: emulsions properties and stability. Food Bioprod. Process. 2014;92:266–274. [Google Scholar]
- Sultanbawa Y., Cusack A., Currie M., Davis C. An innovative microplate assay to facilitate the detection of antimicrobial activity in plant extracts. J. Rapid Methods Autom. Microbiol. 2009;17:519–534. [Google Scholar]
- Sutaphanit P., Chitprasert P. Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chem. 2014;150:313–320. doi: 10.1016/j.foodchem.2013.10.159. [DOI] [PubMed] [Google Scholar]
- Urzúa C., González E., Dueik V., Bouchon P., Giménez B., Robert P. Olive leaves extract encapsulated by spray-drying in vacuum fried starch–gluten doughs. Food Bioprod. Process. 2017;106:171–180. [Google Scholar]
- Vergara C., Saavedra J., Sáenz C., García P., Robert P. Microencapsulation of pulp and ultrafiltered cactus pear (Opuntia ficus-indica) extracts and betanin stability during storage. Food Chem. 2014;157:246–251. doi: 10.1016/j.foodchem.2014.02.037. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lu Z., Wu H., Lv F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009;136:71–74. doi: 10.1016/j.ijfoodmicro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Witz G. Biological interactions of α,β-unsaturated aldehydes. Free Radic. Biol. Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- Wuryatmo E., Klieber A., Scott E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003;51:2637–2640. doi: 10.1021/jf026183l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at 'Repository of the National Agrarian University La Molina, Peru' under the accession number http://repositorio.lamolina.edu.pe/handle/UNALM/4157.