Highlights
-
•
The overall survival of osteosarcoma patients in the last 15 years has improved.
-
•
The proportion of patients with metastases at the time of diagnosis unchanged.
-
•
Consultation with specialists within 4 weeks can contribute to a better prognosis.
Keywords: Osteosarcoma, Pediatric osteosarcoma, Delayed diagnosis, Symptom length, Prognostic factor
Abstract
Aims
Examine: (1) If length of symptoms (LS) of children with osteosarcoma has improved in the last 15 years (2) Is delay in diagnosis related to the presence of metastases at presentation? (3) The impact of delay in diagnosis on prognosis.
Methods
250 consecutive patients with a diagnosis of osteosarcoma of bone treated at a national bone tumor treatment center between 2004 and 2018 were studied retrospectively. Three groups comprising those diagnosed over a five-year period (Group 1: 2004–2008, Group 2: 2009–2013; Group 3: 2014–2018) were studied.
Results
There were 126 males and 124 females with a mean age 12.2 years. The median LS for all patients was eight weeks. The median LS for Group 3 was significantly shorter than that for other groups. Development of metastasis during follow-up period was significantly less in Group 3 compared to the other groups. Overall survival gradually improved over the whole study period. There was no difference in the proportion presenting with metastases at diagnosis between the three groups. The survival rates in patients with LS shorter than 4 weeks was better than those with LS longer than 4 weeks, irrespective of the study time period.
Conclusions
There has been an improvement in the LS in patients diagnosed with osteosarcoma over the last 15 years. The development of metastasis during follow-up has reduced and the overall survival in the last 15 years has improved. LS longer than 4 weeks is associated with a poorer prognosis.
1. Introduction
Osteosarcoma is the most common primary high-grade sarcoma of the bone in children [1], [2]. The most common symptom of osteosarcoma is an enlarging, painful mass [1], [2]. This symptom is usually present for a number of weeks to several months before diagnosis [1], [2]. Osteosarcoma is usually treated with a combination of chemotherapy and surgery. Before the introduction of chemotherapy, 80% of patients treated with surgery alone died of the disease [1]. However, survival improved significantly with the introduction of chemotherapy from the 1970s to the 1990s [3], [4]. Currently, the standard of care for the treatment of high-grade osteosarcoma is multiagent chemotherapy and limb salvage surgery, with a 5-year overall survival of 60–70% [1], [3]. Predictors of poor prognosis include; age, male sex, involvement of the proximal extremity or within the axial skeleton, large tumor size, metastasis at the time of diagnosis and poor response to chemotherapy [1], [5].
Delayed diagnosis of pediatric malignancy remains a problem. It is a source of remorse for physicians and parents and a leading cause of malpractice claims and litigation [6], [7]. According to recent studies, more than 80% of litigation related to sarcomas in the United States and the United Kingdom is due to a delay in diagnosis [8], [9]. A delay in diagnosis is associated with a poor outcome in several pediatric malignancies, such as, retinoblastoma, leukemia and rhabdomyosarcoma [7]. On the other hand, the impact of delayed diagnosis on outcome in osteosarcoma patients remains unclear [10]. Some reports have demonstrated that a delayed diagnosis, in which time the tumor can increase in size, can be associated with a poorer prognosis in osteosarcoma [1], [5], [11]. However, several other studies report that a delayed diagnosis does not affect prognosis. [6], [11], [12], [13], [14], [15], [16], [17] Other studies have demonstrated that the period from the onset of symptoms to diagnosis is shorter in patients with metastatic disease compared to patients with localized disease [18], [19].
The aims of the present study are to examine: (1) If length of symptoms (LS) of children with high grade osteosarcoma has improved in the last 15 years. (2) Is delay in diagnosis related to the presence of metastases at presentation? (3) The impact of delay in diagnosis on prognosis.
2. Patients and methods
2.1. Study design and patient eligibility
This study was designed as a retrospective analysis of a single institution’s experience of treating osteosarcoma. Between January 2004 and December 2018, 327 pediatric osteosarcoma cases (under 16 years old) were treated in our institute. Among them, a total of 77 cases were excluded, including low grade malignancy cases and cases with incomplete data. The remaining 250 patients were included in the study (Fig. 1). All patients were diagnosed with an osteosarcoma on histological examination by an experienced musculoskeletal pathologist. Treatment decisions were made at the supra-regional multi-disciplinary team. Chemotherapy was administered according to international protocols active at the time.
Fig. 1.
Flowchart of this study.
2.2. Patient information
Clinicopathological information including age at diagnosis, gender, year of diagnosis, involved site (upper limb, pelvis, and lower limb), involved bone, LS, metastatic status at the time of diagnosis, presence or absence of pathological fracture at the time of diagnosis, type of surgery (amputation, limb salvage, and no surgery), development of metastasis during follow-up, and status of disease at last follow-up were collected. LS was defined as a period of time between the onset of symptoms and the first review by a specialist in our institute. Three groups, comprising those diagnosed over a five-year period (Group 1: 2004–2008, Group 2: 2009–2013, Group 3: 2014–2018) were compared. In order to assess the impact of LS on overall survival, we compared patients with LS <4 weeks and <8 weeks to those patients with LS >4 weeks and >8 weeks.
2.3. Statistical analysis
Statistical analysis was performed using SPSS Statistics 24 (IBM, Armonk, NY, USA). The overall survival was defined as the time period from the date of diagnosis to that of death or the last follow-up. Survival was calculated using the Kaplan-Meier method and differences in survival were assessed by the log-rank test and Cox proportional hazard regression analysis. Categorical variables were analyzed using Chi-square or Fisher’s exact test and continuous variables were analyzed using Manne-Whitney U test. To compare continuous variables of three groups, Kruskal-Wallis test was used. The differences were considered statistically significant when P-values were less than 0.05.
3. Results
3.1. Demographics and clinical information
Demographics and clinical information for the 250 patients are shown in Table 1. There were 126 males and 124 females. Mean age was 12.2 years old and median follow-up period was 53 months (interquartile ranges (IQR) 22–100). Median LS was eight weeks (IQR 4–12). There were 86, 100 and 64 cases in Group 1, 2 and 3, respectively. Five-year overall survival for all cases was 67.4%. Surgical treatments including amputation and limb salvage surgery were performed in 237 case (94.8%). 13 cases (5.2%) had no surgical treatment. Among these 13 cases, 12 had multiple metastasis and surgery was judged not to be beneficial because of tumor progression despite chemotherapy. One other patient died because of sepsis and renal failure during 1st chemotherapy.
Table 1.
Demographics and clinical information (N = 250).
| Age, years (Mean) | 4–16 (12.2) |
| Gender | |
| Male | 126 (50.4%) |
| Female | 124 (49.6%) |
| Diagnosis year | |
| Group 1 (2004–2008) | 86 (34.4%) |
| Group 2 (2009–2013) | 100 (40.0%) |
| Group 3 (2014–2018) | 64 (25.6%) |
| Median follow-up period in months (IQR) | 55 (22–100) |
| Involved site | |
| Upper limb | 34 (13.6%) |
| Lower limb | 207 (82.8%) |
| Pelvis | 8 (3.2%) |
| Involved bone | |
| Femur | 124 (49.6%)) |
| Tibia | 71 (28.4%) |
| Humerus | 32 (12.8%) |
| Fibula | 10 (4.0%) |
| Pelvis | 8 (3.2%) |
| Radius | 2 (0.8%) |
| Soft tissue of thigh | 2 (0.8%) |
| Scapula | 1 (0.4%) |
| Median LS in weeks (IQR) | 8 (4–12) |
| Metastasis at diagnosis | |
| Yes | 73 (29.2%) |
| No | 177 (70.8%) |
| Pathological fracture at diagnosis | |
| Yes | 37 (14.8%) |
| No | 213 (85.2%) |
| Type of surgery | |
| Amputation | 58 (23.2%) |
| Limb salvage | 179 (71.6%) |
| No surgery | 13 (5.2%) |
| Metastasis during follow-up period | |
| Yes | 100 (40.0%) |
| No | 150 (60.0%) |
| State of disease at last follow-up | |
| NED | 148 (59.2%) |
| AWD | 26 (10.4%) |
| DOD | 74 (29.6%) |
| Died from chemotherapy | 2 (0.8%) |
| 5-year overall survival | 67.40% |
Abbreviations: LS, length of symptoms; IQR, interquartile ranges ;NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease
3.2. Comparison of outcomes during each five-year period
Table 2 shows the results of comparison for each five-year period studied over 15 years. The median LS for Group 3 was six weeks, significantly shorter than the eight weeks for Group 1 and 2 (p = 0.014). There was no difference in the proportion presenting with metastases at the time of diagnosis between the three groups. The overall amputation rate was 23.2% and there was no significant difference in amputation rate between the three groups. The development of metastasis during the follow-up period was significantly less in Group 3 compared to other two groups (Group 1: 42 cases (48.8%), Group 2: 41 cases (41.0%), Group 3: 17 cases (26.6%), p = 0.022). There has been a gradual improvement in 5-year overall survival (Group 1: 55.6%, Group 2: 73.2%, Group 3: 80.3%, p = 0.012).
Table 2.
Comparison of the last 15-year outcomes each five-year period.
| All case | Group 1 (2004–2008) |
Group 2 (2009–2013) |
Group 3 (2014–2018) |
p value | ||
|---|---|---|---|---|---|---|
| Patient number | 250 | 86 | 100 | 64 | ||
| Age, years (Mean) | 4–16 (12.2) | 4–16 (12.4) | 4–16 (12.3) | 4–16 (11.9) | 0.407 | |
| Gender | ||||||
| Male | 126 (50.4%) | 47 (54.7%) | 51 (51.0%) | 28 (43.8%) | 0.413 | |
| Female | 124 (49.6%) | 39 (45.3%) | 49 (49.0%) | 36 (56.2%) | ||
| Involved site | ||||||
| Upper limb | 34 (13.6%) | 7 (8.1%) | 16 (16.0%) | 12 (18.8%) | 0.112 | |
| Lower limb | 207 (82.8%) | 74 (86.1%) | 81 (81.0%) | 52 (81.2%) | ||
| Pelvis | 8 (3.2%) | 5 (5.8%) | 3 (3.0%) | 0 (0.0%) | ||
| Median LS in weeks (IQR) | 8 (4–12) | 8 (6–16) | 8 (3–12) | 6 (4–12) | 0.014 | * |
| Length of symptoms | ||||||
| ≤4 weeks | 79 (31.6%) | 20 (25.3%) | 35 (44.3%) | 24 (30.4%) | 0.114 | |
| >4 weeks | 171 (68.4%) | 66 (38.6%) | 65 (38.0%) | 40 (23.4%) | ||
| ≤8 weeks | 157 (62.8%) | 45 (28.7%) | 67 (42.6%) | 45 (28.7%) | 0.42 | * |
| >8 weeks | 93 (37.2%) | 41 (44.1%) | 33 (35.5%) | 19 (20.4%) | ||
| Metastasis at diagnosis | ||||||
| Yes | 73 (29.2%) | 25 (29.1%) | 29 (29.0%) | 19 (29.7%) | 0.995 | |
| No | 177 (70.8%) | 61 (70.9%) | 71 (71.0%) | 45 (70.3%) | ||
| Pathological fracture at diagnosis | ||||||
| Yes | 37 (14.8%) | 12 (14.0%) | 12 (12.0%) | 13 (20.3%) | 0.331 | |
| No | 213 (85.2%) | 74 (86.0%) | 88 (88.0%) | 51 (79.7%) | ||
| Type of surgery | ||||||
| Amputation | 58 (23.2%) | 16 (18.6%) | 23 (23.0%) | 19 (29.7%) | 0.15 | |
| Limb salvage | 179 (71.6%) | 62 (72.1%) | 73 (73.0%) | 44 (68.8%) | ||
| No surgery | 13 (5.2%) | 8 (9.3%) | 4 (4.0%) | 1 (1.5%) | ||
| Metastasis during follow-up period | ||||||
| Yes | 100 (40.0%) | 42 (48.8%) | 41 (41.0%) | 17 (26.6%) | 0.022 | * |
| No | 150 (60.0%) | 44 (51.2%) | 59 (59.0%) | 47 (73.4%) | ||
| 5-year OS | 67.40% | 55.60% | 73.20% | 80.30% | 0.012 | * |
Abbreviations: LS, length of symptoms; IQR, interquartile ranges; OS, overall survival.
3.3. The impact of LS on the presence of metastases at diagnosis and prognosis
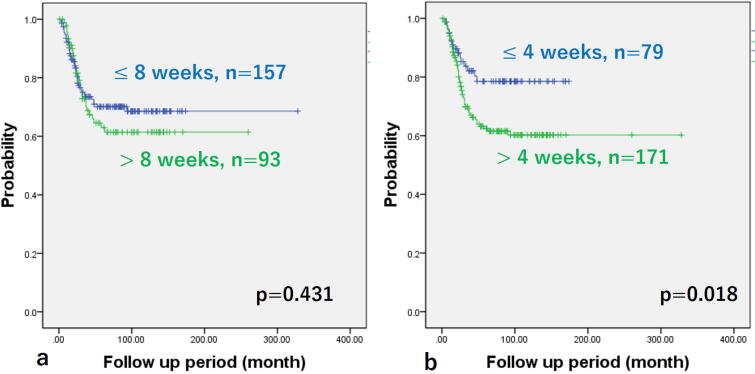
There was no difference in the LS for those patients with or without metastases at diagnosis (Fig. 2). There were no differences in overall survival between patients with LS less than 8 weeks compared to those with LS more than 8 weeks (5-year overall survival, 70.1% vs. 61.4%, p = 0.431) (Fig. 3a). However, overall survival in patients with LS less than 4 weeks were better than those with LS more than 4 weeks (5-year overall survival, 78.5% vs. 62.4%, p = 0.018) (Fig. 3b). Table 3 shows the comparison between the two groups with LS less than 4 weeks or more than 4 weeks. The mean age for those presenting with LS less than 4 weeks was 11.5 compared to mean age with those with LS more than 4 weeks was 12.6 (p = 0.016). The LS of all pelvic cases was longer than 4 weeks. Although it was not significant, the LS for pelvic cases tended to be longer than extremity cases (p = 0.06) (Fig. 4).
Fig. 2.
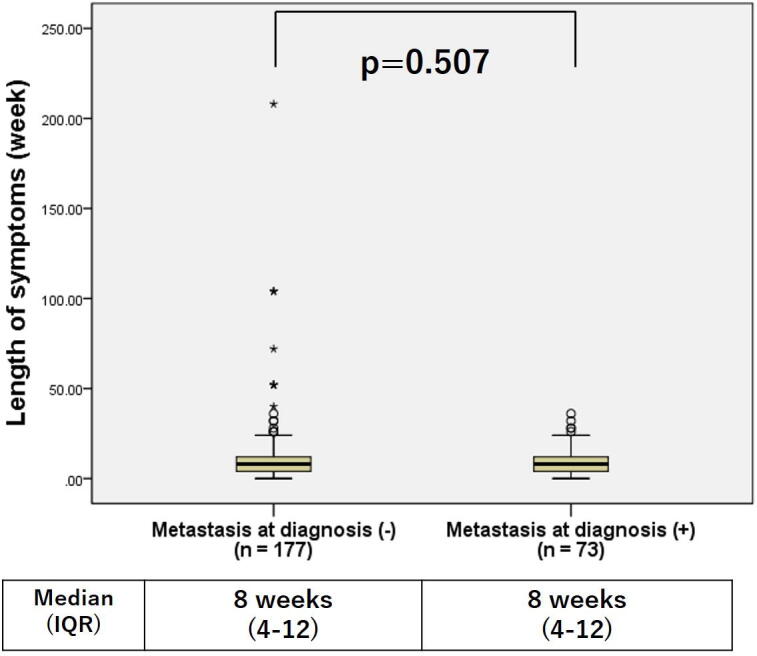
Comparison of length of symptoms (LS) grouped by presence or absence of metastasis at diagnosis. There were no significant differences in the LS of those with or without metastases at diagnosis.
Fig. 3.
Kaplan–Meier curves of time to dead of disease grouped by length of symptoms (LS). Cut-off value; a 8 weeks, b 4 weeks. There were no significant differences of survival rate between two groups divided by LS at eight weeks (a). However, the survival rates in the group with LS shorter than 4 weeks were better than those in groups with longer than 4 weeks (b).
Table 3.
Comparison of each group separating by the LS less than 4 weeks or longer.
| ≤4 weeks n = 79 | >4 weeks n = 171 | p value | |
|---|---|---|---|
| Diagnosis year (%) | |||
| Group 1 | 20 (25.3) | 66 (38.6) | 0.114 |
| Group 2 | 35 (44.3) | 65 (38.0) | |
| Group 3 | 24 (30.4) | 40 (23.4) | |
| Mean age | 11.5 | 12.6 | 0.016 |
| Gender (%) | |||
| Male | 39 (49.4) | 87 (50.9) | 0.824 |
| Female | 40 (50.6) | 84 (49.1) | |
| Site (%) | |||
| Upper limb | 14 (17.7) | 21 (12.3) | 0.089 |
| Pelvis | 0 (0) | 8 (4.7) | |
| Lower limb | 65 (82.3) | 142 (43.0) | |
| Metastasis at diagnosis (%) | |||
| Yes | 25 (31.6) | 48 (28.1) | 0.563 |
| No | 54 (68.4) | 123 (79.1) | |
| Fracture at diagnosis (%) | |||
| Yes | 15 (19.0) | 22 (12.9) | 0.205 |
| No | 64 (81.0) | 149 (87.1) | |
| Type of surgery | |||
| Amputation (%) | 17 (21.5) | 41 (24.0) | 0.694 |
| Limb salvage (%) | 59 (74.7) | 120 (70.2) | |
| No surgery (%) | 3 (3.8) | 10 (5.8) | |
| Metastasis during follow-up period (%) | |||
| Yes | 27 (34.2) | 73 (42.4) | 0.201 |
| No | 52 (65.8) | 98 (57.6) | |
Abbreviations: LS, length of symptoms.
Fig. 4.
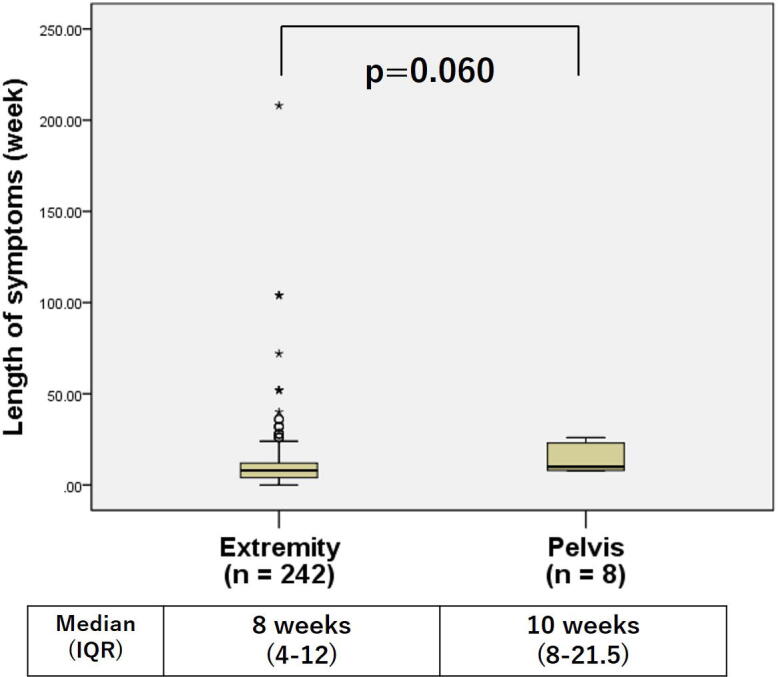
Comparison of length of symptoms (LS) grouped by involved site (Extremity or Pelvis). The LS of pelvic cases trend to longer than extremity cases (it was not significant).
3.4. Risk factor analysis of prognosis
Table 4 shows the results of risk factor analysis. The 5-year overall survival of pelvic cases was significantly lower than lower extremity cases (25% vs 70.3%, p = 0.001). In univariate analysis, six factors including patients in Group 1, pelvic cases, LS more than 4 weeks, presence of metastasis at the time of diagnosis, amputation cases and non-operative cases were significantly related to a poor prognosis. Among these six factors, five factors including patients in Group 1 (hazard ratio (HR): 1.913, 95% confidence interval (95% CI): 1.193–3.068), LS more than 4 weeks (HR: 2.103, 95% CI: 1.175–3.765), presence of metastasis at the time of diagnosis (HR: 2.240, 95% CI: 1.306–3.841), amputation cases (HR: 1.995, 95% CI: 1.153–3.454) and non-operative cases (HR: 17.96, 95% CI: 6.961–46.338) were found to correlate significantly with a poor prognosis by multivariate analysis.
Table 4.
Risk factor analysis of prognosis.
| Univariate analysis | |||||
|---|---|---|---|---|---|
| Factors | Categories | N (%) | 5y-OS (%) | p value | |
| Age | <12 years old | 90 (36.0) | 65.8 | 0.807 | |
| ≥12 years old | 160 (64.0) | 68.2 | |||
| Gender | Male | 126 (50.4) | 64.1 | 0.335 | |
| Female | 124 (49.6) | 70.9 | |||
| Diagnosis year | Group 1 | 86 (34.4%) | 55.6 | 0.019 | * |
| Group 2 | 100 (40.0%) | 73.2 | 0.423 | ||
| Group 3 | 64 (25.6%) | 80.3 | |||
| Involved site | Upper limb | 34 (13.6) | 61.5 | 0.28 | |
| Lower limb | 207 (82.8) | 70.3 | |||
| Pelvis | 8 (3.2) | 25 | 0.001 | * | |
| Length of symptoms | ≤4 weeks | 79 (31.6) | 78.5 | ||
| >4 weeks | 171 (68.4) | 62.4 | 0.018 | * | |
| Metastasis at diagnosis | Yes | 73 (29.2) | 47.5 | <0.001 | * |
| No | 177 (70.8) | 80.5 | |||
| Pathological fracture at diagnosis | Yes | 37 (14.8) | 67.5 | 0.909 | |
| No | 213 (85.2) | 67.5 | |||
| Type of surgery | Amputation | 58 (23.2) | 54.8 | 0.004 | * |
| Limb salvage | 179 (71.6) | 75.2 | |||
| No surgery | 13 (5.2) | 0 | <0.001 | * | |
| Multivariate analysis | |||||
| Factors | Hazard ratio | 95% CI | p value | ||
| Type of surgery, No surgery | 17.96 | 6.961–46.338 | less than0.001 | * | |
| Metastasis at diagnosis, Yes | 2.24 | 1.306–3.841 | 0.003 | * | |
| Type of surgery, Amputation | 1.995 | 1.153–3.454 | 0.014 | * | |
| Length of symptoms, >4 weeks | 2.103 | 1.175–3.765 | 0.012 | * | |
| Diagnosis year, 2004–2008 | 1.913 | 1.193–3.068 | 0.007 | * | |
| Involved site, Pelvis | 1.114 | 0.432–2.875 | 0.823 | ||
Abbreviations: OS, overall survival; CI, confidence interval.
4. Discussion
Numerous studies have evaluated changes in the outcomes of osteosarcoma over time [3], [4], [20], [21], [22]. Several studies have demonstrated that there has been little improvement in survival for osteosarcoma since the 1990s [3], [4], [20]. Mirabello et al. evaluated survival of osteosarcoma between 1973 and 2004 and reported that there was little improvement between 1993 and 2004 [4]. Jawad et al. reported improvement in survival since 1985 is limited to patients with high-grade disease only [20]. On the other hand, other studies report outcomes for osteosarcoma have improved in recent years [21], [22]. Hung et al. evaluated osteosarcoma cases between 1995 and 2011 [21]. They compared two groups divided by whether managed before or after 2004 and reported the survival rates of patients after 2004 were significantly higher mainly due to the effectiveness of chemotherapy [21]. Picci et al. analyzed osteosarcoma cases treated between 1982 and 2002, and reported that there was a constant improvement in survival despite the lack of new treatment modalities or new drugs [22]. In the present study, 5-year overall survival for the last 15 years was 67.4%, which is equivalent to previous reports. Especially in the last 5 years, overall survival has improved with a shortening of LS compared to the previous 10 years. Shortening of LS implies an earlier diagnosis and instigation of earlier treatment. Therefore, our results indicate that shortening of LS is considered to be one of the reasons for the improved outcomes.
Numerous studies have been reported that evaluated whether delayed diagnosis was associated with a poor prognosis in malignant disease, including osteosarcoma [6], [11], [12], [13], [14], [15], [16], [17], [19], [23]. Several reports state that the period until diagnosis does not affect the presence of metastasis at the time of diagnosis and prognosis in osteosarcoma [6], [11], [12], [13], [14], [15], [16], [17]. Lawrenz et al. evaluated 1807 bone sarcoma cases and reported that longer duration of symptom prior to diagnosis was not associated with a poorer overall survival [6]. Petrilli et al. evaluated 225 osteosarcoma patients and concluded that long duration of pre-diagnostic symptoms was not a predictive factor of advanced disease [11]. Other reports suggest that a shorter duration of symptoms was a risk factor for the presence of metastasis at the time of diagnosis and was associated with a poor prognosis [19], [23]. Bacci et al. evaluated 1071 osteosarcoma cases and reported that the interval between the onset of first symptoms and the final diagnosis was significantly shorter in patients with metastases than in patients with a localized tumor [19]. They concluded that it probably reflects a more rapid growth of the tumor [19]. Ferrari et al. reported that the short duration of symptoms until diagnosis in children with osteosarcoma was associated with a poor prognosis [23]. They concluded that the complex relations between delay in diagnosis and outcome are likely a reflection of tumor biology rather than on parental or medical factors [23]. In the present study, there were no differences in the LS for those with or without metastases at diagnosis. However, overall survival has improved with a shortening of LS. Furthermore, LS longer than 4 weeks was a predictive factor of poor prognosis. These results indicate that a delay in consultation with a specialist of more than 4 weeks may increase the risk of subsequent metastasis, which were not detected at the time of diagnosis.
Osteosarcoma of the pelvis is a particularly difficult tumor to treat [24], [25], [26]. The 5-year overall survival of pelvic osteosarcoma is reported to be less than 30% [24], [25], [26]. Wurtz et al. evaluated 68 patients who had a pelvic primary bone tumor, including osteosarcoma [15]. They concluded that patients who have a primary bone sarcoma of the pelvis often have had symptoms for a long duration that mimic those of more commonly encountered non-neoplastic musculoskeletal conditions [15]. In the present study, all eight cases of pelvic osteosarcoma had LS longer than 4 weeks. Among them, most cases had a combination of symptoms including pain around the pelvis, lower limb pain and lower limb neurological symptoms. Only 2 cases complained of a palpable mass, which is an important clinical finding when considering a musculoskeletal oncological condition, including osteosarcoma [27]. These results indicate that pelvic osteosarcoma tend to have longer symptoms because they are less likely to notice a mass.
Many previous studies about delay in diagnosis of osteosarcoma have been reported [10], [18], [28]. Most of these studies evaluated durations between the onset of symptoms and definitive diagnosis, and the results were varied [10], [18], [28]. Li et al. from Shanghai [10], Guerra et al. from Sao Paulo [28] and Bacci et al. from Rizzoli [18] reported that mean intervals between the onset of symptoms and definitive diagnosis were two months, 5.25 months and 10.5 weeks, respectively. In several studies, the details were evaluated dividing the duration between the onset of symptoms and definitive diagnosis [12], [29]. Goedhart et al. from the Netherlands evaluated 44 osteosarcoma cases dividing the duration from initial symptoms to diagnosis into four categories; between the initial symptoms and first consultation with a general practitioner (GP), between presentation to the GP’s office and presentation to a primary hospital, between presentation at a primary hospital and an oncology center, between presentation to an oncology center and definitive histopathological diagnosis. According to their data, mean duration between initial symptoms and first consultation with an oncology specialist was 17 weeks [12]. Similarly, Pan et al. evaluated 30 osteosarcoma cases around the knee joint dividing the duration from initial symptoms to diagnosis into following three categories; between the onset of symptoms and first medical consultation, between first medical consultation and radiography, between radiography and referral to specialists. According to their data, mean duration between initial symptoms and first consultation with a specialist of oncology was 15 weeks [29]. In the present study, we demonstrated that the median LS was shorter than previous studies. This difference may be due to differences in patient referral systems and medical systems, such as imaging and pathological diagnosis. Several previous studies reported that most doctor-related delays occurred at the GP’s office [12], [13]. Therefore, our findings that consultation with a specialist within four weeks of the onset of symptoms can contribute to a better prognosis is particularly important for primary and secondary care practitioners.
This study has the following limitations; many cases were excluded, other known risk factors were not evaluated such as tumor size, surgical margin and the necrosis rate after chemotherapy, and specific regimens of chemotherapy were not evaluated.
5. Conclusions
There has been an improvement in the LS at presentation to a specialized sarcoma service in patients diagnosed with high-grade osteosarcoma over the last 15 years. Furthermore, the development of metastasis during follow-up has reduced and the overall survival in the last 15 years has improved, despite the proportion of patients with metastases at the time of diagnosis remaining unchanged. We have demonstrated that LS longer than 4 weeks is associated with a poorer prognosis, even in those without measurable metastatic disease at presentation. This implies that LS is associated with the subsequent development of latent metastasis. It is encouraging that this study has demonstrated a reduction in LS for patients diagnosed with osteosarcoma over the last 15 years. This implies that physician education is highlighting the importance of early investigation and referral to specialist unit. However, it is clear that more work is needed in the general medical setting to promote a high index of suspicion if patients with bone pain and/ or swelling.
Author contributions
SY: Conception and design, Acquisition of the data, Analysis and interpretation of the data, Drafting of the article.
JC: Conception and design, Acquisition of the data, Analysis and interpretation of the data, Final approval of the article.
PC: Conception and design, Acquisition of the data, Analysis and interpretation of the data, Final approval of the article.
CT: Conception and design, Acquisition of the data, Analysis and interpretation of the data, Final approval of the article.
YK: Conception and design, Acquisition of the data, Analysis and interpretation of the data, Final approval of the article.
SE: Conception and design, Acquisition of the data, Analysis and interpretation of the data, Critical revision of the article for important intellectual content, Final approval of the article.
AA: Conception and design, Analysis and interpretation of the data, Drafting of the article, Critical revision of the article for important intellectual content, Final approval of the article.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Shinichirou Yoshida, Email: yoshidashinichirou@med.tohoku.ac.jp.
Yoichi Kaneuchi, Email: kaneuchi@fmu.ac.jp.
Scott Evans, Email: scottevans@nhs.net.
Adesegun Abudu, Email: seggy.abudu@nhs.net.
References
- 1.C.D.M Fletcher, BJA, P.C.W. Hogendoorn, F. Mertens, WHO Classification of Tumors of Soft Tissue and Bone. 4th ed. Lyon: International Agency for Research on Cancer (IARC), 2013, pp. 282–288.
- 2.Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Friebele J.C., Peck J., Pan X., Abdel-Rasoul M., Mayerson J.L. Osteosarcoma: a meta-analysis and review of the literature. Am. J. Orthop. (Belle Mead, NJ) 2015;44:547–553. [PubMed] [Google Scholar]
- 4.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K., Kotz R., Salzer-Kuntschik M., Werner M., Winkelmann W., Zoubek A., Jürgens H., Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Lawrenz J.M., Styron J.F., Parry M., Grimer R.J., Mesko N.W. Longer duration of symptoms at the time of presentation is not associated with worse survival in primary bone sarcoma. Bone Joint J. 2018;100-b:652–661. doi: 10.1302/0301-620X.100B5.BJJ-2017-1235.R1. [DOI] [PubMed] [Google Scholar]
- 7.Brasme J.-F., Morfouace M., Grill J., Martinot A., Amalberti R., Bons-Letouzey C., Chalumeau M. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol. 2012;13(10):e445–e459. doi: 10.1016/S1470-2045(12)70361-3. [DOI] [PubMed] [Google Scholar]
- 8.Harrison W.D., Sargazi N., Yin Q.i., Chandrasekar C.R. Delayed diagnosis in primary care-The main cause of sarcoma litigation in the United Kingdom. J. Surg. Oncol. 2016;113(4):361–363. doi: 10.1002/jso.24149. [DOI] [PubMed] [Google Scholar]
- 9.Mesko N.W., Mesko J.L., Gaffney L.M., Halpern J.L., Schwartz H.S., Holt G.E. Medical malpractice and sarcoma care–a thirty-three year review of case resolutions, inciting factors, and at risk physician specialties surrounding a rare diagnosis. J. Surg. Oncol. 2014;110(8):919–929. doi: 10.1002/jso.23770. [DOI] [PubMed] [Google Scholar]
- 10.Li H., Zheng S., Yu W., Huang W., Yao Y., Shen Z., Sun Y. Symptom interval of osteosarcoma around the knee joint: an analysis of 82 patients of a single institute. Eur. J. Cancer Care. 2016;25(5):849–854. doi: 10.1111/ecc.12406. [DOI] [PubMed] [Google Scholar]
- 11.Petrilli A.S., de Camargo B., Filho V.O., Bruniera P., Brunetto A.L., Jesus-Garcia R., Camargo O.P., Pena W., Péricles P., Davi A., Prospero J.D., Alves M.T.S., Oliveira C.R., Macedo C.R.D., Mendes W.L., Almeida M.T.A., Borsato M.L., dos Santos T.M., Ortega J., Consentino E. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. J. Clin. Oncol. 2006;24(7):1161–1168. doi: 10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- 12.Goedhart L.M., Gerbers J.G., Ploegmakers J.J.W., Jutte P.C. Delay in diagnosis and its effect on clinical outcome in high-grade sarcoma of bone: a referral oncological centre study. Orthopaedic Surg. 2016;8(2):122–128. doi: 10.1111/os.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal S., Roscoe J., Ryder W.D.J., Gattamaneni H.R., Eden T.O.B. Symptom interval in young people with bone cancer. Eur. J. Cancer (Oxford England) 2004;40(15):2280–2286. doi: 10.1016/j.ejca.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Yang J.Y., Cheng F.W., Wong K.C., Lee V., Leung W.K., Shing M.M., Kumta S.M., Li C.K. Initial presentation and management of osteosarcoma, and its impact on disease outcome. Hong Kong Med. J. 2009;15:434–439. [PubMed] [Google Scholar]
- 15.Wurtz L.D., Peabody T.D., Simon M.A. Delay in the diagnosis and treatment of primary bone sarcoma of the pelvis. J. Bone Joint Surg. Am. 1999;81(3):317–325. doi: 10.2106/00004623-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Rougraff B.T., Davis K., Lawrence J. Does length of symptoms before diagnosis of sarcoma affect patient survival? Clin. Orthop. Related Res. 2007;462:181–189. doi: 10.1097/BLO.0b013e3180f62608. [DOI] [PubMed] [Google Scholar]
- 17.Sneppen O., Hansen L.M. Presenting symptoms and treatment delay in osteosarcoma and Ewing's sarcoma. Acta Radiol. Oncol. 1984;23(2-3):159–162. doi: 10.3109/02841868409136005. [DOI] [PubMed] [Google Scholar]
- 18.Bacci G., Ferrari S., Longhi A., Mellano D., Giacomini S., Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86:204–206. doi: 10.1177/030089160008600305. [DOI] [PubMed] [Google Scholar]
- 19.Bacci G., Ferrari S., Longhi A., Forni C., Zavatta M., Versari M., Smith K. High-grade osteosarcoma of the extremity: differences between localized and metastatic tumors at presentation. J. Pediatric Hematol./Oncol. 2002;24(1):27–30. doi: 10.1097/00043426-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Jawad M.U., Cheung M.C., Clarke J., Koniaris L.G., Scully S.P. Osteosarcoma: improvement in survival limited to high-grade patients only. J. Cancer Res. Clin. Oncol. 2011;137(4):597–607. doi: 10.1007/s00432-010-0923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung G.Y., Yen H.J., Yen C.C., Wu P.K., Chen C.F., Chen P.C., Wu H.T., Chiou H.J., Chen W.M. Improvement in high-grade osteosarcoma survival: results from 202 patients treated at a single institution in Taiwan. Medicine. 2016;95 doi: 10.1097/MD.0000000000003420. e3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picci P., Mercuri M., Ferrari S., Alberghini M., Briccoli A., Ferrari C., Pignotti E., Bacci G. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Annal. Oncol. 2010;21(6):1366–1373. doi: 10.1093/annonc/mdp502. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari A., Lo Vullo S., Giardiello D., Veneroni L., Magni C., Clerici C.A., Chiaravalli S., Casanova M., Luksch R., Terenziani M., Spreafico F., Meazza C., Catania S., Schiavello E., Biassoni V., Podda M., Bergamaschi L., Puma N., Massimino M., Mariani L. The sooner the better? How symptom interval correlates with outcome in children and adolescents with solid tumors: regression tree analysis of the findings of a prospective study. Pediatr. Blood Cancer. 2016;63(3):479–485. doi: 10.1002/pbc.25833. [DOI] [PubMed] [Google Scholar]
- 24.Parry M.C., Laitinen M., Albergo J., Jeys L., Carter S., Gaston C.L., Sumathi V., Grimer R.J., Sumathi V., Grimer R.J. Osteosarcoma of the pelvis. Bone Joint J. 2016;98-B(4):555–563. doi: 10.1302/0301-620X.98B4.36583. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki T., Flege S., Kevric M., Lindner N., Maas R., Delling G., Schwarz R., von Hochstetter A.R., Salzer-Kuntschik M., Berdel W.E., Jürgens H., Exner G.U., Reichardt P., Mayer-Steinacker R., Ewerbeck V., Kotz R., Winkelmann W., Bielack S.S. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J. Clin. Oncol. 2003;21(2):334–341. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 26.Isakoff M.S., Barkauskas D.A., Ebb D., Morris C., Letson G.D. Poor survival for osteosarcoma of the pelvis: a report from the Children's Oncology Group. Clin. Orthop. Related Res. 2012;470:2007–2013. doi: 10.1007/s11999-012-2284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widhe B., Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J. Bone Joint Surg. Am. 2000;82(5):667–674. doi: 10.2106/00004623-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Guerra R.B., Tostes M.D., da Costa Miranda L., Pires de Camargo O., Baptista A.M., Caiero M.T., Dos Santos Machado T.M., Abadi M.D., Mendes de Oliveira C.R., Filippi R.Z. Comparative analysis between osteosarcoma and Ewing's sarcoma: evaluation of the time from onset of signs and symptoms until diagnosis. Clinics (Sao Paulo, Brazil) 2006;61(2):99–106. doi: 10.1590/s1807-59322006000200003. [DOI] [PubMed] [Google Scholar]
- 29.Pan K.L., Chan W.H., Chia Y.Y. Initial symptoms and delayed diagnosis of osteosarcoma around the knee joint. J. Orthop. Surg. (Hong Kong) 2010;18(1):55–57. doi: 10.1177/230949901001800112. [DOI] [PubMed] [Google Scholar]