Abstract
Chlorine disinfection inactivates pathogens in drinking water, but meanwhile it causes the formation of halogenated disinfection byproducts (DBPs), which may induce adverse health effects. Humans are unavoidably exposed to halogenated DBPs via tap water ingestion. Boiling of tap water has been found to significantly reduce the concentrations of halogenated DBPs. In this study, we found that compared with boiling only, adding ascorbate (vitamin C) or carbonate (baking soda) to tap water and then boiling the water further reduced the level of total organic halogen (a collective parameter for all halogenated DBPs) by up to 36% or 28%, respectively. Adding ascorbate removed the chlorine residual in tap water and thus prevented the formation of more halogenated DBPs in the boiling process. Adding carbonate elevated pH of tap water and consequently enhanced the hydrolysis (dehalogenation) of halogenated DBPs or led to the formation of more trihalomethanes that might volatilize to air during the boiling process. The comparative developmental toxicity of the DBP mixtures in the water samples was also evaluated. The results showed that adding a tiny amount of sodium ascorbate or carbonate (2.5–5.0 mg/L) to tap water followed by boiling for 5 min reduced the developmental toxicity of tap water to a substantially lower level than boiling only. The addition of sodium ascorbate or carbonate to tap water in household could be realized by preparing them in tiny pills. This study suggests simple and effective methods to reduce the adverse effects of halogenated DBPs on humans through tap water ingestion.
Keywords: Disinfection byproducts, DBPs, Ascorbate, Carbonate, Toxicity, Boiling
Graphical Abstract
1. Introduction
Chemical inactivation of pathogens is the final step to reduce microbiological contamination in drinking water, and thus to eliminate waterborne diseases such as cholera and typhoid. Chlorine has been applied for disinfecting drinking water for more than a century. However, chlorine is ineffective against certain pathogens, such as the protozoa Cryptosporidium and Giardia, and it also reacts with natural organic matter and bromide in source water to generate halogenated disinfection byproducts (DBPs). Since the discovery of trihalomethanes (THMs) in chlorine disinfected drinking water in 1970s, extensive research has been performed on the formation and control of halogenated DBPs (Jiang et al., 2020; Liu et al., 2020; Wang et al., 2020; Chen et al., 2019; Ding et al., 2019; Ersan et al., 2019; Kimura et al., 2019; Li et al., 2019; Sun et al., 2019; Xiang et al., 2019; Furst et al., 2018; He et al. 2018; Hu et al., 2018; Jiang and Zhang, 2018; Mao et al., 2018; Yan et al., 2018; Richardson and Kimura, 2017; Wu et al., 2017; Gonsior et al., 2014). Toxicological and epidemiological studies have demonstrated that some halogenated DBPs are growth inhibitory, developmentally toxic, genotoxic, carcinogenic and cytotoxic, and chlorinated drinking water is found to be associated with adverse health effects on human beings such as cancers (Li and Mitch, 2018; Cortés and Marcos, 2018; Wagner and Plewa, 2017; Li et al., 2016; Stalter et al., 2016; El-Tawil, 2016; Procházka et al., 2015; Liu et al., 2014; Sharma et al., 2014; Wang et al., 2013; Yang and Zhang, 2013). Accordingly, intake of halogenated DBPs from daily tap water ingestion has raised public health concerns.
Boiling of tap water before drinking is a common practice, especially in Asian countries. Boiling can effectively inactivate waterborne pathogenic microbes, including the chlorine resistant Cryptosporidium and Giardia (Spinks et al., 2006; Carey et al., 2004), and reduce the concentrations of halogenated DBPs in chlorinated drinking water samples (Shi et al., 2017; Ma et al., 2017; Liu et al., 2015; Pan et al., 2014; Zhang et al., 2013). The reduction of halogenated DBPs during boiling should be the net effect of the continued formation (from the reactions of chlorine residual and DBP precursors in tap water) and the thermal hydrolysis, degradation and volatilization, which could be enhanced at alkaline pH (Ding et al., 2018; Liu et al., 2015; Hua and Reckhow, 2012; Krasner and Wright, 2005).
Notably, chlorine is not only widely used as a primary disinfectant in drinking water treatment plants, it is also frequently applied to finished water to prevent microbial regrowth and maintain water quality in water distribution systems before the water reaches consumers’ faucets. According to the World Health Organization guidelines (2017) for drinking water quality, the chlorine residual in tap water could be up to 5 mg/L. In U.S. and Canada tap waters, the chlorine residual should not exceed 4 and 2 mg/L, respectively (USEPA, 2006; Health Canada, 2009). Such a chlorine residual in tap water may continue to react with organic matter in the boiling process (including heating the water from room temperature to ~100 °C) and form corresponding halogenated DBPs (Pan et al., 2014). Ascorbate (also known as vitamin C) is an essential nutrient for humans and it is a commonly used food additive in producing fruit juices, which contain around 300 mg/L vitamin C (USDA, 2019). As ascorbate is an effective antioxidant, the addition of ascorbate to tap water was expected to remove the chlorine residual and thus terminate the continued formation of halogenated DBPs in the boiling process. Sodium carbonate (also known as baking soda) is a food additive which is served as an acidity regulator, anticaking agent, raising agent, and stabilizer. Carbonate levels in soft drinks range from 2700 to more than 10,000 mg/L as CO32– (Oregon Department of Human Services, 1998). Due to the alkaline nature of sodium carbonate, the addition of it to tap water was expected to increase the solution pH, which could enhance the hydrolysis of already-formed halogenated DBPs in tap water.
In this study, two approaches (i.e., adding sodium ascorbate or carbonate to tap water followed by boiling) were proposed to further reduce the concentrations of halogenated DBPs in tap water. We aimed to investigate the combined effect of sodium ascorbate (or carbonate) and boiling on the total halogenated DBPs and developmental toxicity of tap water samples. An in vivo bioassay with the sensitive embryo-larval stages of a polychaete, Platynereis dumerilii, was employed to evaluate the comparative developmental toxicity of the DBP mixtures in tap water samples with different treatments. This species has been used in effectively evaluating the comparative toxicity of various individual DBPs and DBP mixtures in disinfected waters (Li et al., 2020; Pan et al., 2019; Han and Zhang, 2018; Jiang et al., 2017; Li et al., 2017b; Liu et al., 2017; Liu et al., 2015; Yang and Zhang, 2013).
2. Materials and methods
2.1. Chemicals and solvents
Suwannee River humic acid (SRHA, 3S101H) was purchased from the International Humic Substances Society. A free chlorine stock solution (NaOCl, ~3000 mg/L as Cl2) was prepared by diluting a commercial sodium hypochlorite solution (Allied Signal) and measured using the N,N-diethyl-p-phenylene diamine titrimetric method (APHA et al., 2012). Sodium ascorbate, sodium carbonate, methyl tert-butyl ether (MtBE, HPLC grade), and other chemicals (reagent grade or higher) and solvents used in this study were purchased from Sigma‒Aldrich. Ultrapure water (18.2 ΩM·cm) was supplied by a PALL Cascada I water purification system. Stock cultural conditions of the polychaete were maintained and the developmental toxicity bioassay was conducted according to previous studies (Liu et al., 2015; Yang and Zhang, 2013). Details are shown in Text S1.
2.2. Preparation of tap water samples
A source water sample was collected at the inlet of a drinking water treatment plant and filtered through a 0.45 μm membrane (i.e., source water I). A simulated source water sample was prepared with ultrapure water containing 3.0 mg/L SRHA as C, 90 mg/L NaHCO3 as CaCO3 and 2.0 mg/L NaBr as Br− (i.e., source water II). The characteristics of the two source waters are shown in Table 1. Before use, source water I was spiked with NaBr to 2.0 mg/L as Br− in order to maintain the same bromide level as source water II. The relatively high Br− level as reported in bromide-rich source waters (Pan and Zhang, 2013) was used to generate relatively high levels of brominated DBPs, which are of significantly higher toxicity and concern than their chlorinated counterparts (Wagner and Plewa, 2017; Li et al., 2016; Sharma et al., 2014; Yang et al., 2014; Liu and Zhang, 2014; Yang and Zhang, 2013; Richardson et al., 2007). Source waters I and II were dosed with 5.0 mg/L NaOCl as Cl2 for a contact time of 24 h to generate tap waters I and II, respectively. For better comparison, the pH values of source waters I and II were adjusted to 6.5 with 1.0 M HCl and NaOH after the chlorine addition. The chlorination was kept in darkness at room temperature (~21 °C). After 24 h, the pH and chlorine residual in each water sample were determined.
Table 1.
Characteristics of two source water samples used in this study.
| Source water | I | II |
|---|---|---|
| DOC (mg/L) | 2.45a | 3.00 |
| UV254 (cm−1) | 0.0335a | 0.155a |
| SUVA (L/mg∙m)b | 1.37 | 5.17 |
| pH | 6.87 | 8.50 |
| Alkalinity (mg/L as CaCO3) | 23.0a | 90.0 |
| Bromide (μg/L) | 26.5a | 2000 |
The dissolved organic carbon (DOC), UV absorbance at 254 nm, and bromide concentration were measured with a total organic carbon analyzer (Model TOC-Vcsh, Shimadzu), a UV/visible spectrophotometer (GE healthcare, Ultrospec 4300 pro), and an ion chromatograph (Dionex DX500), respectively; and the alkalinity was determined per the Standard Method (APHA et al., 2012).
Specific UV absorbance (SUVA) was calculated by dividing a sample’s UV254 absorbance (in cm−1) by the DOC concentration (in mg/L), and then multiplying by 100.
2.3. Boiling of tap water samples with the addition of sodium ascorbate or carbonate
To investigate the effect of sodium ascorbate on halogenated DBPs in boiled tap water, five samples (3.5 L each, samples 1–5) of tap water I were prepared, and the chlorine residual in each sample was around 3.10 mg/L as Cl2. Accordingly, the stoichiometric concentration of sodium ascorbate for completely removing the chlorine residual was 8.65 mg/L. Sample 1 was used as the control (i.e., without boiling or adding sodium ascorbate) and it was quenched immediately with 105% of the requisite stoichiometric amount of 1.0 M sodium thiosulfate (Li et al., 2017a). Samples 2–5 were added with 0, 5, 10, and 50 mg/L of sodium ascorbate and fully mixed for 1 min. The concentrations of 5, 10, and 50 mg/L represented incomplete removal, complete removal (with 116% of the requisite stoichiometric amount of chlorine residual), and excessive removal, respectively. After 1-min mixing, the pH values and chlorine residuals of samples 3–5 were measured again. Then, samples 2–5 were heated to 100 °C in open glass beakers, and kept boiling for 5 min. Previous studies have shown that boiling of tap water for 5 min significantly lowered the concentration of total halogenated DBPs and the toxicity of DBP mixtures, and it is considered as a practical measure for controlling halogenated DBPs in chlorinated tap waters (Liu et al., 2015; Pan et al., 2014; Krasner and Wright, 2005). After boiling, each water sample was quickly cooled in an ice bath to room temperature, and ultrapure water was added to compensate the water evaporation. Then, the pH of each sample was measured (Table S1); the chlorine residual in each sample was also measured and quenched with 105% of the requisite stoichiometric amount of 1.0 M sodium thiosulfate. For tap water II (in which the chlorine residual was undetectable), the preparation and boiling of samples without and with sodium ascorbate addition (i.e., samples 1–5) followed the same procedure as above.
To investigate the effect of sodium carbonate on halogenated DBPs in boiled tap water, we prepared three additional samples (3.5 L each, samples 6–8), in which 2.5, 10, and 50 mg/L of sodium carbonate were added to tap water I (or tap water II). These doses were significantly lower than those in carbonated soft drinks and commercial bottled water products (Oregon Department of Human Services, 1998). The addition of sodium carbonate increased the pH of the tap water to various levels (Table 2). Samples 6–8 were heated to 100 °C, kept boiling for 5 min, and quickly cooled in an ice bath to room temperature. The pH and chlorine residual in the boiled samples 6–8 were measured. The three samples were compared with sample 1 (i.e., without boiling or adding sodium carbonate) and sample 2 (i.e., with boiling and without adding sodium carbonate). As the chlorine residual in tap water I was relatively high, it partially remained in the boiled tap water samples with sodium carbonate (Table S2), so it was then quenched with 105% of the requisite stoichiometric amount of 1.0 M sodium thiosulfate.
Table 2.
The pH of tap water samples with different concentrations of sodium carbonate before and after boiling.
| Added sodium carbonate (mg/L) | Tap water I Before boilinga | After boilingb | Tap water II Before boilinga | After boilingb |
|---|---|---|---|---|
| 0 | 6.58 | 7.88 | 6.49 | 8.27 |
| 2.5 | 6.81 | 8.02 | 6.48 | 8.63 |
| 10 | 7.36 | 8.81 | 7.02 | 8.82 |
| 50 | 9.92 | 9.91 | 7.57 | 9.26 |
It essentially means “before heating the tap water sample”.
It essentially means “after boiling and then cooling the tap water sample to room temperature”.
All the eight samples of tap water I or II were subjected to the measurements of total halogenated DBPs and the developmental toxicity. For each sample, a 300 mL aliquot was used for the total organic halogen (TOX) analysis, and a 3000 mL aliquot was used for the developmental toxicity test with the polychaete P. dumerilii. Duplicate experiments were conducted for the whole process.
2.4. TOX measurement
TOX is a collective parameter of all halogenated DBPs in a water sample. In this study, TOX was differentiated into total organic bromine (TOBr) and total organic chlorine (TOCl), which are collective parameters for all the brominated and chlorinated DBPs in a water sample. The measurements of TOBr and TOCl were conducted in duplicate following the procedure in previous studies (Jiang et al., 2018; Liu et al., 2015). The quantitation limits for TOBr and TOCl in a water sample were 0.002 mg/L as Br and 0.002 mg/L as Cl, respectively (Liu et al., 2015).
2.5. Developmental toxicity test with the polychaete P. dumerilii
The DBP mixture in each tap water sample was extracted and concentrated via liquid-liquid extraction with MtBE and solvent evaporation following the procedure used by Liu et al. (2015). Briefly, 3000 mL of a sample was divided into three 1000 mL aliquots, and each aliquot was acidified to pH 0.5 with 70% (v/v) sulfuric acid, followed by the addition of sodium sulfate to saturation. Then, the acidified sample was extracted with 100 mL MtBE, and 70 mL of the organic layer was transferred to a rotary evaporator and concentrated to 0.5 mL. The 0.5 mL MtBE solutions obtained from three aliquots were combined (1.5 mL in total) and dried via nitrogen sparging. The solid obtained was stored at 4 °C, and it was dissolved in seawater 1 h prior to the toxicity test as a toxicity test stock solution.
The procedure for the developmental toxicity test was introduced previously (Han and Zhang, 2018; Jiang et al., 2017; Li et al., 2017b; Liu et al., 2015) and detailed in Text S1. Briefly, for each sample, different volumes of its toxicity test stock solution were transferred into a 24-well test plate and mixed with seawater containing ~150 P. dumerilii embryos at 12-h post fertilization. The total volume of tested seawater in each well was 2.0 mL, and the concentration factor of a test solution was determined as the ratio of the equivalent volume of a tap water sample before extraction to 2.0 ml seawater. Seawater with P. dumerilii embryos only was used for control, and its concentration factor was 0. All the tested embryos were developed for another 12 h. At 24-h post-fertilization, the numbers of normal and total embryos in each test solution were checked via an inverted microscope, and the normal developmental percentage was calculated. Duplicate measurements were conducted for each water sample. Afterward, a concentration factor‒response curve was obtained for the sample, and the EC50 value (i.e., the concentration factor at which the normal development percentage was half of that in the seawater control) was calculated via regression analysis using the software SigmaPlot 12 (Systat Software Inc., San Jose, CA).
3. Results and discussion
3.1. Effect of ascorbate on controlling halogenated DBPs during boiling of tap water
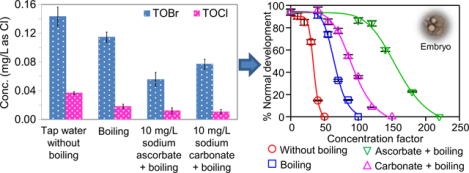
The addition of sodium ascorbate (up to 50 mg/L) did not significantly change the pH of tap water I (before the boiling process). However, boiling of the tap water resulted in a higher pH (Table S1). In tap water I (without boiling or ascorbate), the TOBr concentration was substantially higher than the TOCl concentration (Fig. 1a) because of the relatively high level of bromide in the source water. With 5 min boiling, the chlorine residual in tap water I decreased from 3.10 to 1.10 mg/L as Cl2, and the concentrations of TOBr, TOCl and TOX (the summation of TOBr and TOCl) decreased by 20.0%, 50.4% and 26.2%, respectively. These decreases were consistent with the results from previous studies (Liu et al., 2015; Pan et al., 2014). With the addition of 5 mg/L sodium ascorbate, the chlorine residual in tap water I was reduced to 1.15 mg/L as Cl2 (before the boiling process), and after boiling, it became undetectable. Adding 5 mg/L sodium ascorbate followed by boiling decreased the TOBr, TOCl and TOX concentrations in tap water I (without boiling or sodium ascorbate) by 50.2%, 62.0% and 52.6%, respectively. When the dose of sodium ascorbate increased to 10 mg/L (which completely removed the chlorine residual in the tap water before the boiling process), the TOBr, TOCl and TOX concentrations decreased by 61.2%, 68.4%, and 62.3%, respectively. The results indicated that removal of the chlorine residual before the boiling process inhibited the continued formation of halogenated DBPs at higher-than-room temperatures and thus caused lower concentrations of total halogenated DBPs in the boiled water samples. Further increasing the dose of sodium ascorbate to 50 mg/L did not significantly change the TOBr or TOCl concentration, compared with that in the boiled water sample with 10 mg/L sodium ascorbate. Accordingly, the excessive ascorbate could not further reduce TOBr or TOCl. This was confirmed by the results obtained from tap water II (Fig. 1b), which did not contain chlorine residual. Boiling for 5 min substantially decreased the TOBr, TOCl and TOX concentrations in tap water II, and the addition of sodium ascorbate (up to 50 mg/L) before the boiling process have no significant effect on their concentrations.
Fig. 1.
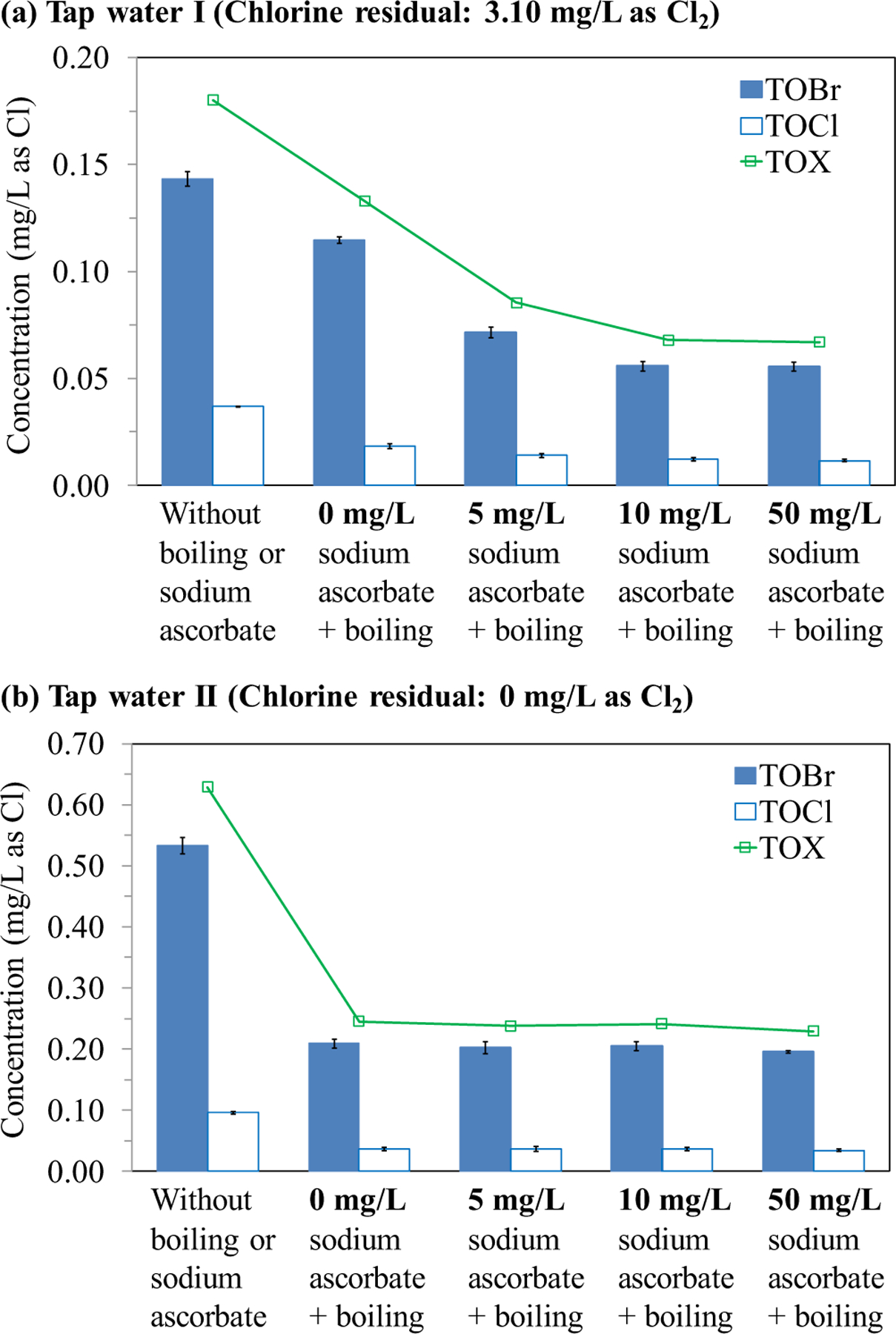
TOBr, TOCl and TOX concentrations of tap water samples without boiling or sodium ascorbate, and with the addition of different concentrations of sodium ascorbate followed by boiling: (a) tap water I, (b) tap water II. Each datum presents the mean of duplicate measurements and the difference between the mean and the measured value.
Fig. 2a shows the developmental toxicity of the DBP mixtures in different samples of tap water I. At the same concentration factor, a water sample showing a lower normal development percentage was more toxic than the sample with a higher normal development percentage. It was evident that boiling decreased the toxicity of tap water I, and more importantly, adding sodium ascorbate to the tap water and then boiling could further decrease the toxicity to a significantly lower level. The EC50 value (in terms of concentration factor) of each water sample was calculated (Table S3). A water sample with a lower EC50 value was more toxic. With the addition of 5, 10 and 50 mg/L sodium ascorbate to tap water I (before the boiling process), the EC50 value of the boiled tap water increased from 65 to 145, 155, and 176, respectively, indicating that as the dose of sodium ascorbate increased, the toxicity of the tap water decreased. The developmental toxicity index (i.e., the reciprocal of EC50 value multiplied by 1000) of the tap water was used to determine the toxicity potency (Li et al., 2017b). Compared with the boiled tap water without adding sodium ascorbate (Table S3), the addition of 5, 10 and 50 mg/L sodium ascorbate to tap water I drastically decreased the developmental toxicity of the boiled water by 55%, 58% and 63%, respectively, implying that the continued formation of some relatively toxic DBPs might be inhibited. Considering the marginal effect, the dose of 5 mg/L sodium ascorbate was suggested for mitigating the DBP-related toxicity of tap water I. It should be noted that an excessive dose of sodium ascorbate is not problematic as it adds an essential nutrient vitamin C to tap water. For the samples of tap water II, their toxicity variation was also consistent with the change of the TOX concentration, i.e., boiling detoxified the tap water, but the addition of ascorbate (5, 10, or 50 mg/L) did not further decrease the toxicity significantly (Fig. 2b and Table S4).
Fig. 2.
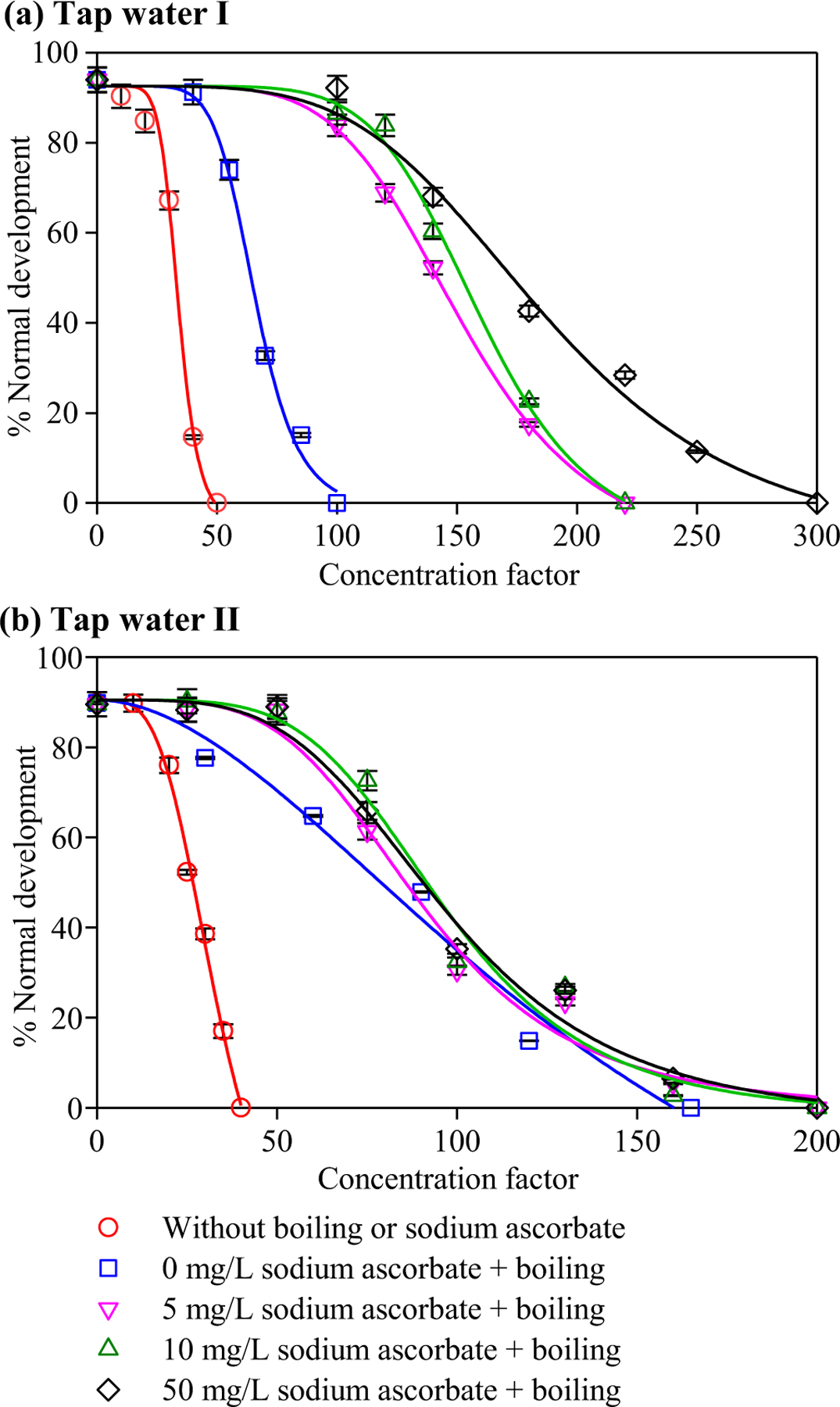
Concentration factor‒response curves of the developmental toxicity against the polychaete P. dumerilii of tap water samples without boiling or sodium ascorbate, and with the addition of different concentrations of sodium ascorbate followed by boiling: (a) tap water I, (b) tap water II. Each datum presents the mean of duplicate measurements and the difference between the mean and the measured value.
3.2. Effect of carbonate on controlling halogenated DBPs during boiling of tap water
Fig. 3a shows that adding sodium carbonate to tap water I followed by boiling further decreased the concentrations of TOBr, TOCl and TOX compared with boiling only. As shown in Table S2, considerable chlorine residuals remained in the boiled tap water samples with adding sodium carbonate (i.e., samples 6–8). Adding 0, 2.5, 10 and 50 mg/L sodium carbonate followed by boiling decreased the TOX concentration in tap water I by 26.2%, 35.9%, 51.4% and 54.1%, respectively. These decreases should be the combined results of formation, thermal decomposition and volatilization of the halogenated DBPs at higher-than-room temperatures. As shown in Table S2, different chlorine residuals were detected in the boiled samples with different doses of sodium carbonate. As the dose of sodium carbonate increased, i.e., the pH in samples of tap water I increased from 6.6 to 9.9 (Table 2), the chlorine residual in the boiled water decreased, which could be ascribed to the faster self-decay of chlorine at higher pH (Busch et al., 2019; Zhang, 2013) and thus lead to lower formation of halogenated DBPs. It has been reported that high pH favors the formation of THMs in the reactions of chlorine with various natural organic matter surrogates (Hung et al., 2017; Bond et al., 2012). THMs are a group of dominant volatile halogenated DBPs and could easily volatilize from water to air during boiling. Besides, high pH also enhances the hydrolysis (i.e., substitution of a halogen atom with a hydroxyl group) of halogenated DBPs (Abusallout et al., 2017; Pan et al., 2014). This dehalogenation process might also contribute to the further decrease of total halogenated DBPs (i.e., TOX) during heating.
Fig. 3.
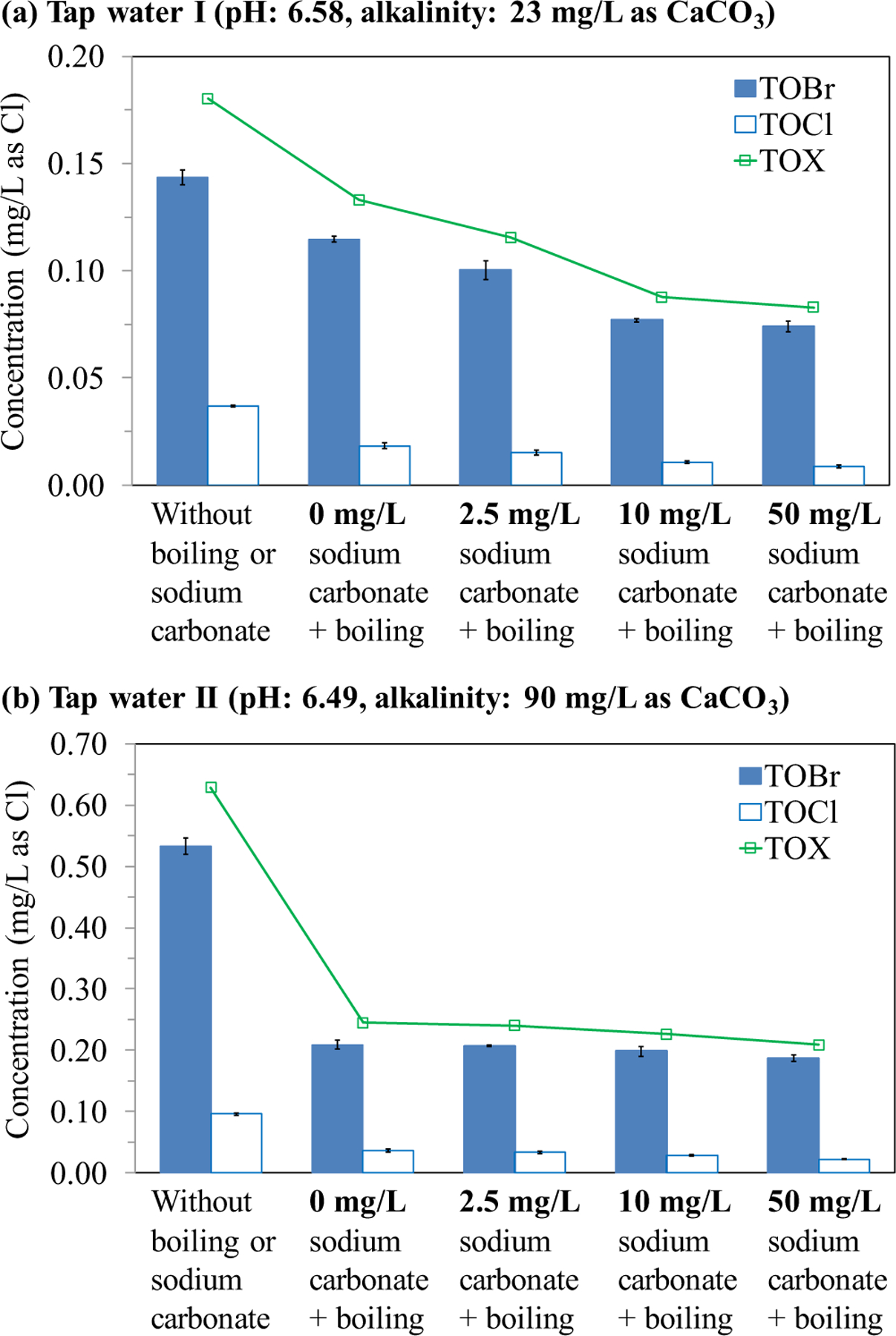
TOBr, TOCl and TOX concentrations of tap water samples without boiling or sodium carbonate, and with the addition of different concentrations of sodium carbonate followed by boiling: (a) tap water I, (b) tap water II. Each datum presents the mean of duplicate measurements and the difference between the mean and the measured value.
Fig. 4a and Table S5 show the combined effects of sodium carbonate and boiling on the developmental toxicity of tap water I. Boiling increased the EC50 value from 34 to 65 (concentration factor), and adding 2.5 mg/L sodium carbonate followed by boiling further increased the EC50 value to 89. In terms of the toxicity index, adding 2.5 mg/L sodium carbonate further reduced to the developmental toxicity of the boiled water by 26%. Higher doses of sodium carbonate (10 and 50 mg/L) slightly detoxified the boiled water (compared with 2.5 mg/L sodium carbonate), but greatly increased the pH (Table 2), which could affect the taste of water. Accordingly, the dose of sodium carbonate for controlling halogenated DBPs was suggested to be 2.5 mg/L.
Fig. 4.
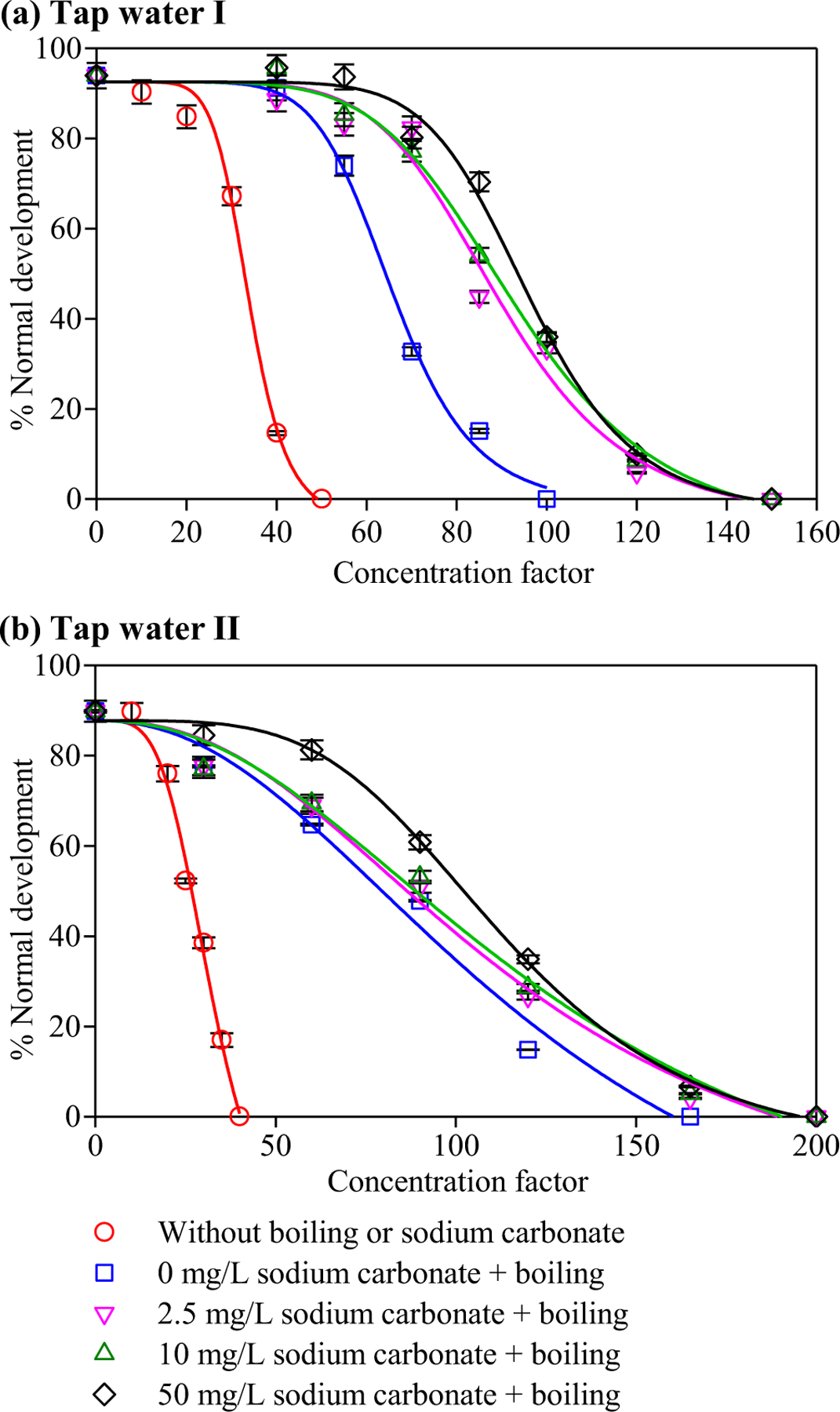
Concentration factor‒response curves of the developmental toxicity against the polychaete P. dumerilii of tap water samples without boiling or sodium carbonate, and with the addition of different concentrations of sodium carbonate followed by boiling: (a) tap water I, (b) tap water II. Each datum presents the mean of duplicate measurements and the difference between the mean and the measured value.
Compared with tap water I, tap water II showed a higher alkalinity (Table 1). Consequently, when the same dose of sodium carbonate was added, the variation of pH (from 6.5 to 7.6) in tap water II was smaller than that in tap water I (from 6.6 to 9.9, Table 2). Besides, tap water II did not contain chlorine residual and thus it might not form more halogenated DBPs in the boiling process. Consequently, the boiled samples of tap water II with different doses (from 0 to 50 mg/L) of sodium carbonate contained similar concentrations of TOBr, TOCl and TOX (Fig. 3b), indicating that sodium carbonate could not significantly reduce halogenated DBPs. The toxicity results showed that boiling significantly detoxified tap water II, i.e., it increased the EC50 value significantly from 28 to 88, whereas the addition of 2.5, 10 and 50 mg/L sodium carbonate to the tap water just slightly increased the EC50 value further to 97, 100 and 109, respectively (Fig. 4b and Table S6). The toxicity of the tap water sample with boiling only and with both boiling and adding 2.5 mg/L of sodium carbonate were reduced by 69% and 71%, respectively, indicating that adding sodium carbonate had a minor effect on reducing the developmental toxicity compared with boiling only. Accordingly, adding sodium carbonate was not as effective on detoxifying tap water II as it was on detoxifying tap water I.
4. Conclusions
Boiling effectively reduced halogenated DBPs in tap water, and more importantly, adding sodium ascorbate or carbonate to tap water before the boiling process could further reduce the overall halogenated DBPs and detoxify the tap water. Adding sodium ascorbate removed the chlorine residual in tap water and prevented the formation of more halogenated DBPs in the boiling process, and adding sodium carbonate increased the pH of tap water and consequently enhanced the hydrolysis (dehalogenation) of halogenated DBPs and favored the formation of THMs, which might volatilize to air during boiling. This study suggested a practical, simple, and promising strategy of lowering the adverse effects of halogenated DBPs on human beings via tap water ingestion. It is worth mentioning that for the convenience and accuracy of dosing the tiny amounts (2.5‒5.0 mg/L) to tap water, sodium ascorbate and sodium carbonate can be prepared in the form of tiny tablets.
Supplementary Material
Highlights.
Ascorbate prevented the formation of more halo-DBPs in tap water boiling.
Adding ascorbate and boiling was better in detoxifying tap water than boiling only.
Carbonate addition enhanced thermal hydrolysis of halo-DBPs in tap water.
Adding carbonate and boiling was better in detoxifying tap water than boiling only.
Acknowledgements
This work was funded by the Hong Kong Research Grants Council under the General Research Funds (projects 16213014 and 16212518). Dr. Jiaqi Liu was also supported by the U.S. National Institutes of Health institutional training grant T32 ES026568. The authors thank Adriaan W. C. Dorresteijn (the Johannes Gutenberg-Universität Mainz, Germany) for providing parental P. dumerilii.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article is available free of charge via the Internet at http://www.journals.elsevier.com/chemosphere.
References
- Abusallout I, Rahman S, Hua G, 2017. Effect of temperature and pH on dehalogenation of total organic chlorine, bromine and iodine in drinking water. Chemosphere 187, 11–18. [DOI] [PubMed] [Google Scholar]
- APHA, AWWA, WEF, 2012. Standard Methods for the Examination of Water and Wastewater. 22th ed., Washington, DC. [Google Scholar]
- Bond T, Goslan EH, Parsons SA, Jefferson B, 2012. A critical review of trihalomethane and haloacetic acid formation from natural organic matter surrogates. Environ. Technol. Rev 1, 93–113. [Google Scholar]
- Busch M, Simic N, Ahlberg E, 2019. Exploring the mechanism of hypochlorous acid decomposition in aqueous solutions. Phys. Chem. Chem. Phys 21, 19342–19348. [DOI] [PubMed] [Google Scholar]
- Carey CM, Lee H, Trevors JT, 2004. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocyst. Water Res. 38, 818–862. [DOI] [PubMed] [Google Scholar]
- Chen H, Tsai KP, Su Q, Chow AT, Wang J-J, 2019. Throughfall dissolved organic matter as a terrestrial disinfection byproduct precursor. ACS Earth Space Chem. 3, 1603–1613. [Google Scholar]
- Cortés C, Marcos R, 2018. Genotoxicity of disinfection byproducts and disinfected waters: a review of recent literature. Mutat. Res. Genet. Toxicol. Environ. Mutagen 831, 1–12. [DOI] [PubMed] [Google Scholar]
- Ding S, Chu W, Kranser SW, Yu Y, Feng C, Xu B, Gao N, 2018. The stability of chlorinated, brominated, and iodinated haloacetamides in drinking water. Water Res. 142, 490–500. [DOI] [PubMed] [Google Scholar]
- Ding S, Deng Y, Bond T, Fang C, Cao Z, Chu W, 2019. Disinfection byproduct formation during drinking water treatment and distribution: a review of unintended effects of engineering agents and materials. Water Res. 160, 313–329. [DOI] [PubMed] [Google Scholar]
- El-Tawil AM, 2016. Colorectal cancers and chlorinated water. World J. Gastrointest. Oncol 8, 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersan MS, Liu C, Amy G, Karanfil T, 2019. The interplay between natural organic matter and bromide on bromine substitution. Sci. Total Environ 646, 1172–1181. [DOI] [PubMed] [Google Scholar]
- Furst KE, Pecson BM, Webber BD, Mitch WA, 2018. Tradeoffs between pathogen inactivation and disinfection byproduct formation during sequential chlorine and chloramine disinfection for wastewater reuse. Water Res. 143, 579–588. [DOI] [PubMed] [Google Scholar]
- Gonsior M, Schmitt-Kopplin P, Stavklint H, Richardson SD, Hertkorn N, Bastviken D, 2014. Changes in dissolved organic matter during the treatment processes of a drinking water plant in Sweden and formation of previously unknown disinfection byproducts. Environ. Sci. Technol 48, 12714–12722. [DOI] [PubMed] [Google Scholar]
- Han J, Zhang X, 2018. Evaluating the comparative toxicity of DBP mixtures from different disinfection scenarios: a new approach by combining freeze-drying or rotoevaporation with a marine polychaete bioassay. Environ. Sci. Technol 52, 10552–10561. [DOI] [PubMed] [Google Scholar]
- He K, Okuta E, Cordero JA, Echigo S, Asada Y, Itoh S, 2018. Formation of chlorinated haloacetic acids by chlorination of low molecular weight compounds listed on pollutant release and transfer registers (PRTRs). J. Hazard. Mater 351, 98–107. [DOI] [PubMed] [Google Scholar]
- Health Canada, 2009. Guidelines for Canadian drinking water quality: guideline technical document – chlorine.
- Hu J, Chu W, Shi M, Xu B, Gao N, Ding S, 2018. Comparison of drinking water treatment processes combinations for the minimization of subsequent disinfection by-products formation during chlorination and chloramination. Chem. Eng. J 335, 352–361. [Google Scholar]
- Hua G, Reckhow DA, 2012. Effect of alkaline pH on the stability of halogenated DBPs. J. Am. Water Works Assoc 104, E107–E120. [Google Scholar]
- Hung YC, Waters BW, Yemmireddy VK, Huang C-H, 2017. pH effect on the formation of THM and HAA disinfection byproducts and potential control strategies for food processing. J. Integr. Agr 16, 2914–2923. [Google Scholar]
- Jiang J, Han J, Zhang X, 2020. Nonhalogenated aromatic DBPs in drinking water chlorination: a gap between NOM and halogenated aromatic DBPs. Environ. Sci. Technol 54, 1646–1656. [DOI] [PubMed] [Google Scholar]
- Jiang J, Li W, Zhang X, Liu J, Zhu X, 2018. A new approach to controlling halogenated DBPs by GAC adsorption of aromatic intermediates from chlorine disinfection: effects of bromide and contact time. Sep. Purif. Technol 203, 260–267. [Google Scholar]
- Jiang J, Zhang X, 2018. A smart strategy for controlling disinfection byproducts by reversing the sequence of activated carbon adsorption and chlorine disinfection. Sci. Bull 63, 1167–1169. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhang X, Zhu X, Li Y, 2017. Removal of intermediate aromatic halogenated DBPs by activated carbon adsorption: a new approach to controlling halogenated DBPs in chlorinated drinking water. Environ. Sci. Technol 51, 3435–3444. [DOI] [PubMed] [Google Scholar]
- Kimura SY, Cuthbertson AA, Byer JD, Richardson SD, 2019. The DBP exposome: development of a new method to simultaneously quantify priority disinfection by-products and comprehensively identify unknowns. Water Res. 148, 324–333. [DOI] [PubMed] [Google Scholar]
- Krasner SW, Wright JW, 2005. The effect of boiling water on disinfection by-product exposure. Water Res. 39, 855–864. [DOI] [PubMed] [Google Scholar]
- Li J, Moe B, Vemula S, Wang W, Li X-F, 2016. Emerging disinfection byproducts, halobenzoquinones: effects of isomeric structure and halogen substitution on cytotoxicity, formation of reactive oxygen species, and genotoxicity. Environ. Sci. Technol 50, 6744–6752. [DOI] [PubMed] [Google Scholar]
- Li W, Li Y, Zhang X, Han J, Zhu X, Choi KC, Jiang J, 2019. Conversion of haloacid disinfection byproducts to amino acids via ammonolysis. Chemosphere 224, 351–359. [DOI] [PubMed] [Google Scholar]
- Li X-F, Mitch WA, 2018. Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ. Sci. Technol 52, 1681–1689. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang J, Li W, Zhu X, Zhang X, 2020. Volatile DBPs contributed marginally to the developmental toxicity of drinking water DBP mixtures against Platynereis dumerilii. Chemosphere 252, 126611. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang M, Zhang X, Jiang J, Liu J, Yau CF, Graham NJ, Li X, 2017a. Two-step chlorination: a new approach to disinfection of a primary sewage effluent. Water Res. 108, 339–347. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Yang M, Liu J, Li W, Graham NJ, Li X, Yang B, 2017b. Three-step effluent chlorination increases disinfection efficiency and reduces DBP formation and toxicity. Chemosphere 168, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Liu J, Lujan H, Dhungana B, Hockaday WC, Sayes CM, Cobb GP, Sharma VK, 2020. Ferrate(VI) pretreatment before disinfection: an effective approach to controlling unsaturated and aromatic halo-disinfection byproducts in chlorinated and chloraminated drinking waters. Environ. Int 138, 105641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang X, 2014. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res. 65, 64–72. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang X, Li Y, 2015. Effect of boiling on halogenated DBPs and their developmental toxicity in real tap water. In Recent Advances in Disinfection By-Products, Karanfil T, Mitch WA, Westerhoff P, Xie YF, Eds, American Chemical Society: Washington, DC, pp 45–60. [Google Scholar]
- Liu J, Zhang X, Li Y, 2017. Photoconversion of chlorinated saline wastewater DBPs in receiving seawater is overall a detoxification process. Environ. Sci. Technol 51, 58–67. [DOI] [PubMed] [Google Scholar]
- Ma S, Gan Y, Chen B, Tang Z, Krasner S, 2017. Understanding and exploring the potentials of household water treatment methods for volatile disinfection by-products control: kinetics, mechanisms, and influencing factors. J. Hazard. Mater 321, 509–516. [DOI] [PubMed] [Google Scholar]
- Mao Y, Guo D, Yao W, Wang X, Yang H, Xie Y, Komarneni S, Yu G, Wang Y, 2018. Effects of conventional ozonation and electro-peroxone pretreatment of surface water on disinfection by-product formation during subsequent chlorination. Water Res. 130, 322–332. [DOI] [PubMed] [Google Scholar]
- Oregon Department of Human Services, 1998. Technical Bulletin, Health Effects Information: Sodium Carbonate, “Soda Ash”. Office of Environmental Public Health, Portland, Oregon. [Google Scholar]
- Pan L, Zhang X, Yang M, Han J, Jiang J, Li W, Yang B, Li X, 2019. Effects of dechlorination conditions on the developmental toxicity of a chlorinated saline primary sewage effluent: excessive dechlorination is better than not enough. Sci. Total Environ 692, 117–126. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang X, 2013. Four groups of new aromatic halogenated disinfection byproducts: effect of bromide concentration on their formation and speciation in chlorinated drinking water. Environ. Sci. Technol 47, 1265–1273. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang X, Wagner ED, Osiol J, Plewa MJ, 2014. Boiling of simulated tap water: effect on polar brominated disinfection byproducts, halogen speciation, and cytotoxicity. Environ. Sci. Technol 48, 149–156. [DOI] [PubMed] [Google Scholar]
- Procházka E, Escher BI, Plewa MJ, Leusch FD, 2015. In vitro cytotoxicity and adaptive stress responses to selected haloacetic acid and halobenzoquinone water disinfection byproducts. Chem. Res. Toxicol 28, 2059–2068. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Kimura S, 2017. Emerging environmental contaminants: challenges facing our next generation. Environ. Technol. Innovation 8, 40–56. [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM, 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res 636(1), 178–242. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Zboril R, McDonald TJ, 2014. Formation and toxicity of brominated disinfection byproducts during chlorination and chloramination of water: a review. J. Environ. Sci. Health B 49, 212–228. [DOI] [PubMed] [Google Scholar]
- Shi W, Wang L, Chen B, 2017. Kinetics, mechanisms, and influencing factors on the treatment of haloacetonitriles (HANs) in water by two household heating devices. Chemosphere 172, 278–285. [DOI] [PubMed] [Google Scholar]
- Spinks AT, Dunstan RH, Harrison T, Coombes P, Kuczera G, 2006. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res. 40, 1326–1332. [DOI] [PubMed] [Google Scholar]
- Stalter D, O’Malley E, von Gunten U, Escher BI, 2016. Fingerprinting the reactive toxicity pathways of 50 drinking water disinfection by-products. Water Res. 91, 19–30. [DOI] [PubMed] [Google Scholar]
- Sun X, Chen M, Wei D, Du Y, 2019. Research progress of disinfection and disinfection by-products in China. J. Environ. Sci 81, 52–67. [DOI] [PubMed] [Google Scholar]
- USDA, 2019. Food Data Central; Lemonade, frozen concentrate, white, prepared with water. U.S. Department of Agriculture, Beltsville, MD. https://fdc.nal.usda.gov/fdc-app.html#/food-details/173217/nutrients. Accessed on Jan 2nd, 2020. [Google Scholar]
- USEPA, 2006. National primary drinking water regulations: stage 2 disinfectants and disinfection by-products rule. Fed. Regis 71, 387–493. [Google Scholar]
- Wagner ED, Plewa MJ, 2017. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: an updated review. J. Environ. Sci 58, 64–76. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Zheng W, Wang X, Andersen ME, Pi J, He G, Qu W, 2013. Organic extract contaminants from drinking water activate Nrf2-mediated antioxidant response in a human cell line. Environ. Sci. Technol 47, 4768–4777. [DOI] [PubMed] [Google Scholar]
- Wang W, Xie Y, Tang H, 2020. The haloacetic acid leap in effluent of a biologically active carbon filter experiencing a disinfectant switch. Chemosphere 244, 125435. [DOI] [PubMed] [Google Scholar]
- WHO, 2017. Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. World Health Organization, Geneva. [PubMed] [Google Scholar]
- Wu S, Anumol T, Gandhi J, Snyder SA, 2017. Analysis of haloacetic acids, bromate, and dalapon in natural waters by ion chromatography–tandem mass spectrometry. J. Chromatogr. A 1487, 100–107. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Deng Z, Yang X, Shang C, Zhang X, 2019. Transformation of adenine and cytosine in chlorination ‒ an ESI-tqMS investigation. Chemosphere 234, 505–512. [DOI] [PubMed] [Google Scholar]
- Yan M, Roccaro P, Fabbricino M, Korshin GV, 2018. Comparison of the effects of chloramine and chlorine on the aromaticity of dissolved organic matter and yields of disinfection by-products. Chemosphere 191, 477–484. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhang X, 2013. Comparative developmental toxicity of new aromatic halogenated DBPs in a chlorinated saline sewage effluent to the marine polychaete Platynereis dumerilii. Environ. Sci. Technol 47, 10868–10876. [DOI] [PubMed] [Google Scholar]
- Yang Y, Komaki Y, Kimura SY, Hu H, Wagner ED, Mariñas BJ, Plewa MJ, 2014. Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ. Sci. Technol 48, 12362–12369. [DOI] [PubMed] [Google Scholar]
- Zhang AL, 2013. Removal of chlorine residual in tap water by boiling or adding ascorbic acid. Int. J. Eng. Res. Appl 3, 1647–1651. [Google Scholar]
- Zhang X, Yang H, Wang X, Fu J, Xie Y, 2013. Formation of disinfection by-products: effect of temperature and kinetic modeling. Chemosphere 90, 634–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


