Abstract
Marine biotoxins can accumulate in filter- feeders bivalve molluscs, that may represent a source of potential health problems being vectors of toxins, that are transferred to humans through their consumption. Harmful Algal Blooms impact on aquaculture may give also economic losses due to temporary closures of contaminated shellfish harvest and marketing. The presence of toxic algae for Paralytic Shellfish Poisoning (PSP), with recurrent toxic blooms of dinoflagellates, such as several Alexandrium species, been known since 2000 in the waters of an Ionian bay of Sicily, the Syracuse harbour, where shellfish farms are located. Our previous works reported in this area the positivity for PSP toxin in mussels (Mytilus galloprovincialis) with saxitoxin concentrations above the limit of the law and the simultaneous presence of toxic species of the genus Alexandrium in the waters. This work reports new recent episodes of algal blooms of Alexandrium minutum in the waters of the Syracuse harbour and PSP toxin contamination in farmed mussels, with values beyond the limits established by law, with the consequent immediate closure of the production area. PSP toxicity was detected with the MBA (Mouse Bioassay) with the confirm carried out with Lawrence method to quantify the total saxitoxin equivalents and characterize the toxic profile. Regular application of the implemented health plan is very important in order to prevent any risk and protect consumer health.
Key words: Alexandrium minutum, PSP toxin profile, Sicily, Shellfish farms
Introduction
Harmful algal blooms (HAB) are natural phenomena carried out by the overgrowth of marine phytoplankton. Over the last decades the occurrence and intensity of HAB appear to be increasing on a global scale due to rising ocean temperatures and growing coastal eutrophication (Visciano et al., 2016). Bloom impact on aquaculture may be dramatic, with economic losses due to temporary harvest closures of contaminated shellfish, and risks for human health (Ade et al., 2003; Masò et al., 2006). It is known that live bivalve molluscs, in relation to their filtering capacity, can concentrate dangerous toxins produced by different species of microalgae and therefore represent a risk for humans due to their consumption, even after cooking; the presence of toxins in bivalve molluscs beyond the limits established by law, prohibits the harvesting and marketing in order to protect public health (Reg. (EC) 853/2004).
Along many coastal localities of the Mediterranean Sea, such as worldwide, the species most responsible for Harmful Algal Bloom (HAB) events belong to the dinoflagellate group. In particular, several species of the Alexandrium genus has been intensively studies as a result of its toxigenic blooms. In fact, these species are able to produce potent toxic compounds among which saxitoxin (STX) and gonyautoxin (GTX) and so they are the most important in terms of impact on humans (Anderson et al., 2002; EFSA, 2009). Other species such as Gymnodinium catenatum in the Mediterranean Sea can be present and also reported along the Italian coasts (Dell’Aversano et al., 2019). Saxitoxin and its analogues (paralytic shellfish toxins, PSTs) are neurotoxic alkaloids mainly responsible for paralytic shellfish poisoning (PSP), one of the most serious bio-intoxications (Batoréu et al., 2005). More than 30 different STX analogues have been identified of which STX, NeoSTX, GTX1 and dc-STX are known as the most toxic ones (EFSA, 2009). Humans can contract PSP by ingesting seafood, mainly shellfish that have accumulated PSTs in their tissues. The member states of the European Union must ban live bivalve molluscs harvesting if PSTs exceed the limit of 800 μg STXeq kg−1 in any edible part (Reg. (EC) 853/2004). The monitoring of biotoxins in shellfish and health effects due to their consumption are important tasks for seafood control, because marine biotoxins may cause serious diseases in humans.
There are more reports of PSP toxic dinoflagellate occurrence than of PSP shellfish contamination in the Mediterranean waters: in Italy, the presence of PST-producing species such as A. minutum Halim and A. pacificum Litaker (ex A. catenella (Whedon and Kofoid), Balech), is a phenomenon so far limited to some specific sites (Giacobbe et al., 2012, 2014, Ciminiello et al., 2014; Bazzoni et al., 2019). Blooms of A. minutum since 1994 are reported along the Emilia-Romagna coast (NW Adriatic Sea) (Honsell et al., 1996), since 2001 by the Friuli Venezia Giulia coast (NE Adriatic Sea) (Milandri et al., 2008), and since 2002 from Olbia gulf in Sardinia often in concomitance to the presence of A. pacificum in seawater (Lorenzoni et al., 2013). Along the Sardinian coasts the toxic species A. tamarense has also been isolated (Luglie’ et al., 2017). Bivalve molluscs collected from the same geographical areas were sometimes found contaminated by PST with some records above the EU regulatory limit, although no human poisoning was reported simultaneously.
In Sicily, the presence of toxic algae for PSP (Paralytic Shellfish Poisoning), with recurrent blooms of A. minutum, has been known since 2000 in the waters of an Ionian bay, the Syracuse harbour (Vila et al., 2005) in which are located shellfish aquaculture practices, in waters classified as “Area B”. Our previous works reported in this area the positivity for PSP toxin in mussels (Mytilus galloprovincialis) with saxitoxin concentrations above the limit of the law, with closure and marketing blocks of farming areas (Costa et al., 2007): the simultaneous presence of toxic species A. minutum in the waters in the same area was reported (Costa et al., 2007; Milandri et al., 2008). Subsequentely, we are found for the first time the species A. pacificum, supposing a recent introduction in this Ionian site, and its co-occurrence with A. minutum in the same bay of Sicily in May 2012: no PSP toxins was found in shellfish at that time (Giacobbe et al., 2014). However, a high PST contamination in mussels, in concomitance with a mixed bloom of A. minutum and A. pacificum has been reported in spring summer 2015 to 2017 in the waters of the Syracuse bay (Dell’Aversano et al., 2019). In recent years, in Sicily, the veterinary services of the healthcare companies at the provincial level have implemented monitoring plans, in particular in the eastern coast, where the production or relaying plants for mussels are located. Following the presence and wide distribution of potentially toxic HAS along the Ionian coast and, occasionally, the toxicity in the mussels raised in the bay of Syracuse, the veterinary services of Syracuse have started a regular monitoring program of the classified production areas since 2019, coordinated by the Sicilian Region Health Prevention Department.
This work reports new recent episodes of Alexandrium algal blooms belonging to the toxic species A. minutum, which occurred in 2019 in the waters of the Syracuse harbour and high levels of contamination by PSP toxins in mussels farmed in the bay.
Materials and Methods
Study site and sample collection
The study was conducted from January to December 2019 in two sampling points inside of the Syracuse harbour, on the Ionian coast of Sicily, in which are located shellfish aquaculture practices, in waters classified as “Area B” (Figure 1). According to the Monitoring and surveillance Program for live bivalve molluscs, regularly implemented since January 2019 by the Syracuse Veterinary Department, the mussel samples (Mytilus galloprovincialis) were collected weekly to detect lipophilic (Diarrhetic Shellfish Poisoning DSP) and hydrophilic toxins (PSP and Amnesic Shellfish Poisoning ASP); on the same dates, but starting from February, the water samples were collected monthly for the presence of potentially toxic Harmful Algal Species (HAS). In addition, the sampling frequency increased when the mussel toxicity exceeded the legal limits.
Water samples
During 2019, 17 water samples were taken at three depths of the bay of Syracuse and therefore, a total of 51 water samples were sent to NRL on Marine Biotoxins for the microscopic examination of phytoplankton.
The samples (1 L) were taken manually in clean polyethylene (PE) bottles at a depth of 1, 7, 14 m from the water surface and fixed with Lugol’s iodine solution in order to preserve them for further analysis, carried out at NRL on Marine Biotoxins (Cesenatico). Using the Utermöhl’s method (1958) in accordance with the EU reference method BS EN ISO 15204:2006, the PSTsproducing microalgal species were counted using settling chambers (25-50 mL) under an inverted Nikon microscope (Eclipse Ti- U, objectives: 20x CFI planapo, 40x CFI planapo and 100x CFI planfluor oil) equipped with digital cameras (Nikon DSFi2) and NIS-Elements imaging software. The morphology of fixed cells was analysed also with an Ultraviolet 100 W Mercury lamp. Plate patterns were studied following Balech (1995) after staining with Calcofluor white (Fluorescent Brightener 28, Sigma, Steinheim, Germany) and observation under UV epifluorescence (the UV filter arrangement was for 330-380 nm excitation and 420 nm emission wavelength) (Fritz and Triemer, 1985). Microalgal abundance was expressed as cell numbers per liter (cells/L). The count of Alexandrium species by Utermöhl’s method has quantitative detection limit of 120 cells/L for subsample of 25 mL and a level of significance of 0.05 or 60 cell/L for subsample of 50 mL at the same level of significance, such as described in the BS EN 15204:2006.
Mussel samples
A total of 50 shellfish samples from January to December 2019, were examined for toxins analysis. Particularly, PSP toxicity was detected by biological method (MBA) according to the AOAC Official Method 959.08, 2005, and confirmed with Lawrence method (AOAC 2005.06) to more deeply investigate the toxic profile and total STX equivalents.
Preparation of test sample
The molluscs were cleaned with fresh water and opened by cutting the adductor muscles; the meat was removed and transferred to sieve, letting drain for 5 min: 200g of mussel samples were then weighed of which an aliquot of 100g were used for mouse bioassay and the residual quantity stored in the freezer at -20°C for any subsequent confirmation.
Mouse bioassay
In accordance with AOAC 959.08 (2005), PSP toxins were extracted from shellfish tissue. An aliquot of 100 g from edible portion of sample was homogenized (Sterilmixer 12 PBI) and extracted with 100 ml of HCl 0.1 M; pH was adjusted around 3.0 and the mixture was boiled for 5 minutes and let cool to room temperature. The pH was again adjusted by addition of HCl 0.5 M or NaOH 0.1 M.; the mixture was dilute to 200 ml and centrifuge at 3000 r.p.m for 5 minutes. The supernatant (acid extract) was separated and 1 ml was used for intraperitoneally inoculation into 2 mice (Swiss mice with 19-21 g of body weight) and symptoms after the injection were observed and lethal time reported. According to AOAC 959.08 method, the test was considered positive when the mice died within 60 minutes. The death time was used to suspect the level of toxin present: the death of mice in a few minutes with the presence of characteristic neurologic and respiratory symptoms, can represents an alarm for high concentrations of toxin in the sample examined (EFSA, 2009). In case of positive biological tests, the result of the suspected presence of PSP toxins was immediately notified to the Veterinary Department of Syracuse for the adoption of the temporary and precautionary suspension of live bivalve molluscs harvest.
Figure 1.
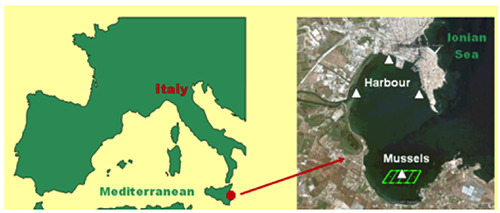
Study area in the Ionian Sea of Sicily: harbour and mussels sites.
Furthermore, after telephone contact, the preserved aliquot of bivalve molluscs, positive for PSP mouse test, was quickly sent to the NRL in Cesenatico by a fast courier, for confirmation, determine total STX equivalents and investigate the toxic profile.
Chemical analysis by LC-FLD
The preserved aliquots of the MBA samples were analysed by Liquid Chromatography with Fluorescence Detection (LC-FLD) for confirmation and characterization of PSP toxins, in accordance with AOAC Official Method 2005.06 (AOAC, 2005).
Since PSP toxins do not exhibit natural ultraviolet absorption or fluorescence they must therefore be oxidised into fluorescent derivatives before detection by FLD. This process consists in the oxidation of toxins into iminopurine derivatives before separation and determination by LC-FLD (precolumn oxidation). The method involves the following steps: i) acid duplicate extraction of shellfish homogenates with 1% acid acetic solution (the first extraction with heating); ii) cleanup using a solid phase extraction (SPE) 500 mg C18 cartridge (Supelclean, Supelco); iii) an aliquot of C18-cleaned extract is added to a SPE ion exchange cartridge (SPE-COOH) (Bakerbond, J.T. Baker) being sequentially eluted to obtain three individual fractions (fraction 1 to 3); iv) aliquots of C18 and SPE-COOH extracts are used for oxidation of PSTs with periodate and peroxide oxidants depending on the type of toxin (Nhydroxylated or non N-hydroxylated). The LC analysis was carried out by Agilent system, UHPLC Infinity 1290 II consisted of a binary pump (1290 High Speed), autosampler, column oven, and 1260 Series spectra fluorescence detector. The Agilent Chemstation_software (OpenLAB) performed data acquisition and peak integration. Separation was performed using a reversed-phase column Supelcosil LC18 (150x4.6mm id, 5 μm, SUPELCO) equipped with a security guard column C18 (Phenomenex). Column was kept in a column oven at 30° C. Detection wavelengths were set at 340 nm for excitation and 395 nm for emission. The mobile phase gradient used to elute the PST oxidation products consisted of 2 mobile phases (A = 0.1 M ammonium formate, pH 6 and B = 0.1 M ammonium formate in 10% methanol, pH 6) under the following conditions: 0 – 5% B in the first 5 minutes, 5 – 70% B for the next 8 min, 70% B for the next 2 min, 70 – 0% B for the next 4 min, and 100% mobile phase A was used for 5 min before next injection. The flow rate was 1 mL/min. The injection volumes were 25 mL and 50 mL, for the oxidation products of peroxide and periodate reaction, respectively.
The concentrations for each toxin or epimeric pair (GTX1+4, GTX2+3, C1+2, dcGTX2+3 and C3+4) were quantified with linear calibration curves, achieved using PST certified references standards. To calculate total sample toxicity in terms of saxitoxin equivalence (STXeq.), Toxicity Equivalence Factors (TEFs) were applied for each toxin. For epimeric pairs, the highest TEF is used in order to always obtain the over-estimation in toxicity.
The limit of quantification (LOQ) of the analytical method for each toxin (STX, GTX1+4, GTX2+3, GTX5, C1+2, neoSTX, dcSTX, dcGTX2+3) was in the range of 0.135-1.035 pmol/LC. The limit of detection (LOD) was assumed to be one-third of the limit of quantification.
Ritention times (RT) and number of oxidation products were used to identify PSP toxins by comparing the sample with standard substances. It is necessary carry out sample blanks to exclude chromatographics peaks corresponding to the presence of natural fluorescent compounds.
Solvents were HPLC and all chemicals were analytical grade.
Standards used for this study were purchased from the Institute for marine Bioscinces, National research Council of Canada, Halifax, Nova Scotia, Canada: NRC-CRM-STX, NRC-CRM-dcSTX, NRC-CRM-GTX2,3, NRC-CRM-GTX1,4, NRC-CRM-NEO, NRC-CRM-GTX5, NRC-CRM dcGTX2,3, NRC-CRM-C1,2, NRC-CRM-dcNEO.
Results
Plankton producing toxins
The monitoring of Harmful Algal Species, including toxic dinoflagellates producing PST showed in the late winter and spring of the year 2019, the presence of the species Alexandrium minutum Halim in the waters of the Syracuse bay (Figure 2). In particular, the microscopical analysis of seawater samples collected from February to March, revealed the presence of A. minutum with values between 60 and 5400 cell/L in the two sites: the highest number was reported on 19 March (Table 1). From April to May, water samples collected monthly at the same sites continued to show the presence of A. minutum cells, with value between 180 and 1820 cell/L, but PSP toxin was undetectable by mouse assay in the mussel sampled weekly. The water samples were negative in summer, while in November and December was revealed the species A. minutum with values lower than 100 cells/L: also in this case the MBA was negative.
Table 1.
Mouse bioassay (MBA) and PSP toxicity (μg eq STX/kg e.p.) in mussels and toxic algal specie A. minutum(cell/L) during positive events.
| Sampling date | Sp | MBA | Death time (min) | PSP toxins μg eq STX/kg e.p | A. minutum (cell/L) dept -1, -7, -14m |
|---|---|---|---|---|---|
| 28/01/19 | 1 | Positive | 2’ | 8.139 | n.s. |
| 12/02/19 | 1 | n.d. | - | 4.885 | n.s. |
| 14/02/19 | 1 | Positive | 4’ | 5.310 | n.s. |
| 19/02/19 | 1 | Positive | 4’ | 5.277 | <60, 780, <60 |
| 21/02/19 | 1 | Positive | 4’ | 5.647 | 60, 1300, 100 |
| 28/02/19 | 1 | Positive | 20’ | 366* | 340, 1680, 60 |
| 06/03/19 | 1 | Positive | 8’ | 948 | 120, 1200, <60 |
| 06/03/19 | 1 | Positive | 10’ | 723* | 400, 1760, 320 |
| 11/03/19 | 2 | Positive | 20’ | 308* | n.s. |
| 11/03/19 | 1 | Negative | >60 | <200 | |
| 19/03/19 | 2 | Positive | 7’ | 1180 | <60, 5400, 440 |
| 19/03/19 | 1 | Negative | >60 | <200 | |
| 25/0319 | 1 | Negative | >60 | 293* | |
| 25/03/19 | 2 | Positive | 12’ | 548* | <60, 180, <60 |
Sp: Sampling point; MBA: Mouse Bioassay; n.d.: not done; n.s.: not sampled.*PSP toxins not overcoming the legal limit.
During the recurrent blooms of A. minutum in these waters, other harmful species that do not produce PSP have been simultaneously detected, e.g.: the dinoflagellates Lingulodinium polyedra (F.Stein) J.D.Dodge, Dinophysis acuminata Clap. & J.Lachm. and the diatoms belonging to Pseudo-nitzschia H.Perag. genus.
Mouse bioassay
Between late January and March a total of ten mussel samples were positive at the MBA for PSP detection: the short interval of survival time of the mouse during the test of some examined sample and the presence of characteristic symptoms referable to the neurotoxins after the inoculation of the extract, made to suspect the presence of high values of PSP biotoxins (Table 1).
Chemical analysis by LC-FLD
PSTs (Paralytic shellfish toxins) analysis conducted by LC-FLD had revealed a maximum of toxicity of 8,139 μg STX 2HCl eq./Kg e.p. (Table 1); the chromatograms related to this sample (ID 430/19) are reported in Figures 3 and 4.
The PSTs chemical method confirmed the presence of toxins in all mussel samples positive for the biological test of which seven samples exceeded the regulatory legal limit of 800 μg STXeq kg.
The PSP toxins profile in all contaminated mussel samples revealed the presence of the following toxins in decreasing order of molar percentage of total content: GTX1+4 (from 44 to 100%), GTX2+3 (9-49%), C3+C4 (0-14%), C1+2 (0-11%), STX (0-7%), GTX5 (0-5%), neoSTX (0-3% with a single value of 30%); the toxins dcGTX2+3, GTX6 and dcSTX were practically absent. Table 2 and Figure 5 report the toxin profiles (%) in mussels’ samples in Syracuse bay during the positive events.
Figure 2.

Alexandrium minutum from Syracuse Bay (Bar= 20 μm).
Discussion
In the last years the presence of algal toxins in the aquatic environment has attracted great interest, with increasing geographical spread in marine waters of toxic dinoflagellates producing Harmful Algal Blooms (HAB) influenced by the climate change pressures (Anderson D, 2014).
The harbour of Syracuse is a critical area in Sicily which is subject to outbreaks of various dinoflagellate species with a high risk of shellfish contamination. Recurrent blooms of the PSP producer A. minutum occur particularly every spring with even greenish-brown spots in the waters.
In March 2001, a bloom of 4.6x106 cells/L was observed near the area where mussels (Mytilus galloprovincialis) are present in suspended culture (Giacobbe et al., 2006). Similar events also occurred between 2002 and 2005 with maximum values of 1.2x106 cells/L in April 2003 inside the harbour while, as in most of the study years, the cells densities were lower in the mussel area (Vila et al., 2005). HPLC analysis of phytoplankton and mussels performed in 2001 during A. minutum blooms indicated the presence of only gonyautoxins (GTX 1 and GTX 4 respectively) plus a minimum percentage of saxitoxins (STX) in 2003 event (Giacobbe et al., 2006).
Furthermore, in the period February- March 2007 we reported positive results of PSP biotoxins that exceed the legal limit, on mussels collected in the same areas of the Syracuse Bay as confirmed by the analysis in HPLC (AOAC 2005.06), with a maximum toxicity of 921 μg eq STX/kg e.p. and prevalence of gonyautoxins GTX 1.4 on February 28, 2007 (Costa et al., 2007; Milandri et al., 2008). A. minutum reached the maximum density of 520 cell/L in March 2007 (Milandri et al., 2010). The HAB monitoring conducted on a monthly basis from October 2010 to October 2013 in the same Ionian bay, showed interestingly the contemporary presence of the specie Alexandrium pacificum Litaker, 2014 (former A. catenella (Whedon & Kof.) Balech, 1985), no usually found in the phytoplankton community in the Syracuse site (Giacobbe et al, 2014). We did not find toxin contamination in mussels farmed in the bay, despite the fact that shellfish plant being located close to the bloom area (Giacobbe et al., 2012, 2014). This study which including biological and chemical methods, algal toxins analyses, molecular and toxicity assays, was conducted in the framework of a research programme promoted by the Italian Ministry of Health (RF-IZI-2008-1139874) More recently, Dell’Aversano et al (2019), have been reported higher cell concentrations (106 cells/L) of A. minutum and A. pacificum in the spring of 2017 in most of the sampling points of the Syracuse harbour with water discolorations. These authors reported different levels of toxicity in mussels sampled in from 2015-2017, with also high levels of PSP toxins, reporting in general that the mussels were found to vary greatly in total toxicity, depending on the geographical location.
Figure 3.
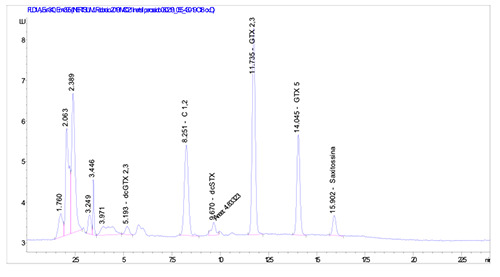
Chromatogram obtained by HPLC-FLD with pre-column peroxide oxidation for ID sample 430/19.
Figure 4.
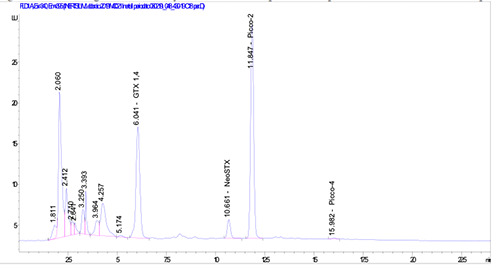
Chromatogram obtained by HPLC-FLD with pre-column periodate oxidation for ID sample 430/19.
In this our study, performed during 2019, the most concentrated sample, taken at the end of January 2019, was found to contain 8.139 μgSTX/ kg p.e; PSP values were above the regulatory limit, with positivity to the biological method, until the beginning of March. The toxic profile revealed the presence of gonyautoxins (mainly GTX1.4, GTX2.3), group C toxins (C1.2, C3.4) and a minimum percentage of GTX5 and saxitoxins (STX, neoSTX). In addition, the algae bloom of the PSP producer has been identified in the phytoplankton community as A. minutum, in water samples taken later as the water withdrawal plan started in mid-February.
The monitoring of biotoxins and harmful phytoplankton in mussel farm areas permits to give an early warning and, if needed, an immediate closing of the shellfish farm, preventing risk of intoxication. Subsequent analysis, at the end of March, resulted negative and this allowed the reopening of the classified area.
Numerous reports document the incidence of PSP toxicity in the Ionian area of Sicily particularly in the spring and often with higher cell concentrations of Alexandrium species.
In our research, positivity presence has been reported in the study area in winter, as well as earlier in February 2007 so it seems that Alexandrium species may have expanded their presence regarding time. In the case of blooms, the maximum values reported were 5.4x103 cells/ L in March 2019 and in general the values were not always clearly correlated to the toxicity of the mollusks.
These facts indicate how difficult it is to investigate any possible correlation between PSP toxins in shellfish, and the occurrence of toxic species, although the presence of PSP toxin seems to coincide with the highest Alexandrium abundance detected during the study (Table 1). Anyway, in some cases there was Alexandrium presence in April and May 2019 but the toxin was absent. The results of this study are in agreement with statistical previous long-term studies carried out. This unclear correlation is also confirmed by other authors (Bazzoni et al., 2016). It is not predictable when a bloom of dinoflagellates will develop; neither is the population density a predictable factor. Climatic and environmental conditions such as changes in salinity, rising water temperature, and increased nutrients and sunlight trigger cyst germination to a vegetative stage that enables rapid reproduction (Anderson et al., 2012).
Figure 5.
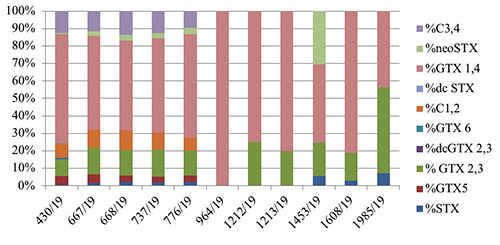
Toxin profiles (%) in mussels samples in Syracuse bay during the positive events.
Table 2.
Percentage of each PST (Paralytic Shellfish Toxins) type contribution to total concentration (μg eq STX/kg e.p) determined in mussels by LC-FLD during positive events.
| ID sample | Sampling date | %STX | %GTX5 | % GTX 2,3 | %dcGTX 2,3 | %GTX 6 | %C 1,2 | %dc STX | %GTX 1,4 | %neo STX | %C 3,4 | STXeq μg STX HCl/Kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 430/19 | 28/01/19 | 0.49 | 5.13 | 9.4 | 0.39 | 0.82 | 8.25 | 0.2 | 62.11 | 0.56 | 12.65 | 8.139 |
| 667/19 | 12/02/19 | 1.60 | 4.85 | 15.44 | 10.28 | 53.57 | 2.77 | 11.50 | 4.885 | |||
| 668/19 | 14/02/19 | 2.27 | 3.72 | 14.59 | 11.22 | 51.41 | 3.10 | 13.70 | 5.310 | |||
| 737/19 | 19/02/19 | 1.85 | 3.35 | 15.45 | 9.60 | 54.25 | 3.00 | 12.50 | 5.277 | |||
| 776/19 | 21/02/19 | 2.09 | 3.69 | 14.24 | 7.47 | 59.41 | 3.44 | 9.66 | 5.647 | |||
| 964/19 | 28/02/19 | 100 | 366 | |||||||||
| 1212/19 | 06/03/19 | 25.09 | 74.91 | 948 | ||||||||
| 1213/19 | 06/03/19 | 19.91 | 80.09 | 723 | ||||||||
| 1453/19 | 11/03/19 | 5.62 | 19.10 | 44.94 | 30.34 | 308 | ||||||
| 1608/19 | 19/03/19 | 2.78 | 16.01 | 81.21 | 1.180 | |||||||
| 1985/19 | 25/03/19 | 7.10 | 49.18 | 43.72 | 548 |
Conclusions
The problem of toxic algae blooms, and recurring in various zones of the Italian coast, locations of shellfish, has long been known: the resulting health risk is subject to continuous attention due to the current national and European regulations that impose systematic monitoring of water and live bivalve molluscs to detect respectively the possible presence of toxin-producing plankton and biotoxins in production areas, as well as measures of early closure of fishing areas when accumulated to levels above legal limits. The area of the Syracuse harbour is more suitable to the study, in terms of area at risk due to the presence of various phytoplankton species that producing HAB with recurrent blooms of PSP producer Alexandrium; therefore, it is a critical area in Sicily and it is necessary to monitor it regularly.
It is also important to better understand the relationship between shellfish toxicity and potentially toxic HAS densities. The algal cells can sometimes also have a density of a few hundred cells/L that lead to the accumulation of toxin above the regulatory level, or the total content of toxins can increase at the end of a flowering therefore the risk of shellfish toxicity may persist even when the abundance of cells is lower. We believe that monitoring of this area should extend throughout the year, considering also the climatic impact and other factors (biological, physical, physiological) that can help clarify the relationship between potentially toxic HAS and toxic phenomena in bivalve molluscs.
Funding Statement
Funding: This work was supported by the Italian Ministry of Health, Research Project IZS SI 15/16 RC.
References
- Ade P, Funari E, Poletti R, 2003. Il rischio sanitario associato alle tossine di alghe marine. Ann Ist Super Sanità 39:53-68. [PubMed] [Google Scholar]
- Anderson D, Glibert P, Burkholder J, 2002. Harmful algal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries 25:704-26. [Google Scholar]
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M, 2012. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14:10–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, 2014. HABs in a changing world: a perspective on harmful algal blooms, their impacts and research and management in a dynamic era of climactic and environmental change. Harmful Algae 2012: 3–17. [PMC free article] [PubMed] [Google Scholar]
- AOAC Official Method 959.08, 2005. Paralytic Shellfish Poison Biological Method Chapter 49: 80-82. [Google Scholar]
- AOAC, 2005. Officinal methods of analysis. 18th ed. Association of Official Analytical Chemists ed., Gaithersburg MD, USA. [Google Scholar]
- Balech E, 1995. The genus Alexandrium Halim (Dinoflagellata). Sherkin Island Marine Station, Sherkin Island, Co. Cork, Ireland, 151. [Google Scholar]
- Batoréu MCC, Dias E, Pereira P, Franca S, 2005. Risk of human exposure to paralytic toxins of algal origin. Environ Toxicol Pharmacol 19:401-6. [DOI] [PubMed] [Google Scholar]
- Bazzoni AM, Mudadu AG, Lorenzoni G, Arras I, Lugliè A, Vivaldi B., Cicotelli V, Sanna G, Tedde G, Ledda S, Alesso E, Marongiu E, Virgilio S, 2016. Occurrence of harmful algal species and shellfish toxicity in Sardinia (Italy). Ital J Food Safety 5:194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni AM, Cangini M, Mudadu AG, Lorenzoni G, Arras I, Sanna G, Pino F, Milandri A, Virgilio S. 2019. Recent findings of paralytic shellfish toxin linked to genus Alexandrium Halim in Mediterranean mollusc production areas. Toxicon 174:48-56. [DOI] [PubMed] [Google Scholar]
- BS EN ISO 15204:2006. Water quality. Guidance standard on the enumeration of phytoplankton using inverted microscopy (Utermoehl technique). [Google Scholar]
- Ciminiello P, Dell’Aversano C, Forino M, Tartaglione L, 2014. Marine Toxins in Italy: The More You Look, the More You Find. Eur J Org Chem 7:1357-69. [Google Scholar]
- Costa A, Di Noto AM, Russo Alesi EM, Alio V, Milandri A, Pompei M, Poletti R, Giacobbe MG, Caracappa S, 2007. Presenza di biotossine algali del tipo PSP (Paralytic Shellfish Poison) in mitili allevati nel porto grande di Siracusa, Sicilia. In: Proceedings of the LXI Convegno Nazionale SISVeT, Salsomaggiore Terme 2007:375-6. [Google Scholar]
- Dell’Aversano C, Tartaglione L, Polito G, Dean K, Giacobbe M, Casabianca S, Capellacci S, Penna A., Turner AD, 2019. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 215:881-92. [DOI] [PubMed] [Google Scholar]
- EFSA, 2009. Marine biotoxins in shellfish- Saxitoxin group. EFSA J 1019:1-76. [Google Scholar]
- European Commission, 2004. Regulation of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs, 853/2004/EC. In: Official Journal, L 139, 30/04/2004. [Google Scholar]
- Fritz L, Triemer RE, 1985. A rapid simple technique utilizing calcofluor white M2R for the visualization of dinoflagellate thecal plates. J Phycol 21:662-4. [Google Scholar]
- Giacobbe MG, Maso M, Milandri A, Penna A, Poletti R, 2006. Plankton toxicity and shellfish contamination by phycotoxins in a new Mediterranean locality Henshilwood K., Deegan B., McMahon T., Cusack C., Keaveney S., Silke J., O’ Cinneide M., Lyons D., Hess P. (eds), Molluscan Shellfish Safety. Marine Institute Pubblication, Galway: 206-214. [Google Scholar]
- Giacobbe MG, Costa A, Cangemi E, Penna A, Borzì S, Rabito A, 2012. Blooms of the dinoflagellate Alexandrium in an Ionian bay hosting shellfish aquaculture: risks for PSP toxicity. In: Proceedings of the AQUA 2012 Prague: 1229. [Google Scholar]
- Giacobbe MG, Costa A, Gangemi E, Penna A, Casabianca S, Borzì S, Riccardi E, Milandri A, 2014. Occurrence and toxicity of the PSP-Dinoflagellate Alexandrium catenella in an Ionian bay of Sicily. Biol Mar Mediterr 21:119-20. [Google Scholar]
- Honsell G, Poletti R, Pompei M, Sidari L, Milandri A, Casadei C, Viviani R, 1996. Alexandrium minutum Halim and PSP contamination in northern Adriatic Sea (Mediterranean Sea). In: Yasomoto T, Oshima T, Fukuyo Y. (Eds) Harmful and Toxic Algae. IOC of UNESCO: 77-80. [Google Scholar]
- Lorenzoni G, Arras I, Sanna G, Delogu P, Mudadu A, Piras A, Mura A, Marongiu E, Virgilio S, 2013. Paralytic shellfish poison algal biotoxins: Sardinia report 2002-2011 and non-compliance management. Ital J Food Safety 2:104-8. [Google Scholar]
- Lugliè A, Giacobbe MG, Riccardi E, Bruno M, Pigozzi S, Mariani MA, Satta CT, Stacca D, Bazzoni AM, Caddeo T, Farina P, Padedda MB, Pulina S, Sechi N, Milandri A, 2017. Paralytic Shellfish Toxins and Cyanotoxins in the Mediterranean: New Data from Sardinia and Sicily (Italy). Microorganisms 5: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masó M., Garcés E, 2006. Harmful microalgae blooms (HAB): Problematic and conditions that induce them. Mar Pollut Bull 53:620-30. [DOI] [PubMed] [Google Scholar]
- Milandri A, Cangini M, Costa A, Giacobbe MG, Poletti R, Pompei M, Riccardi E, Rubini S, Virgilio S, Pigozzi S, 2008. Caratterizzazione delle tossine PSP (Paralytic Shellfish Poisoning) in mitili raccolti in differenti aree marine italiane (Article in Italian). Biol Mar Mediterr 15:38-41. [Google Scholar]
- Milandri A, Ceredi A, Poletti R, Pompei M, Pigozzi S, Riccardi E, Rubini S, Scordella G, Bresolin R, Virgilio S, Tedde T, Lorenzoni G, Mancuso R, Peruzzu T, Soro B, Costa A, Giacobbe MG, 2010. Paralytic shellfish poisoning toxins in mussels Mytilus galloprovincialis from italian coasts. In: Proceedings of the Aquaculture Europe Porto: 1014-5. [Google Scholar]
- Vila M, Giacobbe MG, Masó M, Gangemi E, Penna A, Sampedro N, Azzaro F, Camp J, Galluzzi L, 2005. A comparative study on recurrent blooms of Alexandrium minutum in two Mediterranean harbours. Harmful Algae 4: 673-695. [Google Scholar]
- Visciano P, Schirone M, Berti M, Milandri A, Tofalo R, Suzzi G, 2016. Marine biotoxins: occurrence, toxicity, regulatory limits and reference methods. Front Microbiol 7:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]


