Abstract
Beef burgers are meat preparations with easy perishability. To ensure a longer shelflife, the Regulation EU 1129/11 allows the use of some additives. However, healthconscious consumers prefer products which do not contain synthetic substances. Aim of the present study was to evaluate the effect of Red Beetroot (Beta vulgaris) integration on Black Angus made burgers shelf life. Red beet was prepared as powder and added to meat mixture as the same or in water solution. The study was split into 2 trials to assess the extract activity also in burgers vacuum-packaged stored. Burgers were analysed (up to 9 days at 4°C) in terms of sensory properties, microbiological profile, pH, aw and lipid oxidation (TBARS). At the end of storage, treated samples showed the highest values of redness and the lowest content of malondialdehyde, probably due to antioxidant properties of red beet towards myoglobin and lipid oxidation processes. Moreover, results highlighted that Red Beetroot activities were dose-dependent and intensified if dissolved in water. The aw values did not appear to be conditioned by extract integrations, unlike the pH that was lower in treated samples than control ones. Microbiological analyses identified beetroot as a potential antimicrobial substance, especially in high concentration. In conclusion, Beta vulgaris extract could be proposed as natural compound exploitable in beef burgers to preserve qualities and extend their shelf-life.
Key words: Antimicrobial, lipid oxidation, colour, plant, vacuum
Introduction
Meat preparations are highly perishable foods and require protection to maintain their quality (Karimian et al., 2019). The main cause of deterioration is oxidative stress, due to uneven generation of free radicals, reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Falowo et al., 2014). Lipid and myoglobin oxidation are closely related: they reduce meat quality and accelerate surface discolouration. Recently, many authors’ attention has been captured by the challenge to preserve product quality and extend shelf life of meat preparations (Theivendran et al., 2006; Kerry et al., 2006; Peruzy et al., 2019; Gogliettino et al., 2020). Hamburger is one of the most common fast food in the world and is growing in popularity especially because of its convenience. The Reg. EU 1129/11 allows the use of lactates, ascorbates and citrates for meat preparation, according to quantum satis principle. Several studies and recent reports investigated the use of natural compounds to reduce microbial growth and oxidation processes (Lee et al., 2012). For instance, antimicrobial activity of different proteins extracts from L. europeaus yellow berries was proved against two Gram-positive bacteria, L. monocytogenes and the methicillinresistant S. aureus (Ambrosio et al., 2020). Likewise, several authors have demonstrated that rosemary (Rosmarinus officinalis) gives an important contribution to meats oxidative stability (Anastasio et al., 2014).
The Red beet (Beta vulgaris) contains bioactive phytochemicals, including polyphenols, flavonoids and other functional antioxidant compounds. Moreover, it is a source of water-soluble pigments known as betalains (62.04-118.92 mg in 100 g of vegetable, Stagnari et al., 2014), which can be split in two classes, betacyanins (red) and betaxanthins (yellow), and might be adopted as a natural food colorant. Beetroot peel carries the main portion (54%) of these compounds and the 50% of total phenolic content (Kujala et al., 2000).
Betanin is the major betalain fraction in red beetroot and the only approved for use as a natural red colourant in food. Since there are few studies concerning the application of red beet to food processing (Burri et al., 2019; Hwang et al., 2017; Martínez et al., 2006; Rey et al., 2004; Smaldone et al., 2017; Sucu et al., 2018), aim of the present study has been to evaluate the effect of Red Beetroot (Beta vulgaris) integration (water solution and powder) on Black Angus burgers shelf life, vacuum packaged or not.
Materials and Methods
Experimental design
Different meat cuts from Black Angus (Scotland) were placed in a conditioned refrigerator and dry-aged for 30 days. Beetroot was collected from a local market and, after cooking under vacuum for 1 hour at 100 degrees and prompt cooling, its pulp was extracted and dried. The concentration of betanin was not determined.
The experimental design was split into 2 trials to compare the shelf life of products packed under aerobic conditions (ap) and vacuum packed (vp).
Minced meat was mixed with NaCl (10 g kg-1), pepper (2 g kg-1) and: (i) beetroot powder 0.8% (B1ap and D1vp), (ii) beetroot water solution 2% (D2vp), 5% (B2ap and D3vp) and 10% (B3ap); the percentages refer to final product concentrations. Hamburgers with no additions were used as controls (Cap and Cvp). A preliminary study identified extract concentrations to employ in burgers, fitting the percentages on a specific condition. As a consequence, the combined effect of vacuum packing with beetroot extract allowed to reduce extracts doses under 10%.
Each portion was homogenized manually for 15 min. Burger samples for each trial were prepared using hamburger forming machine to obtain a standard size and weight (approximately 200 g, with a diameter of 12 cm and 1.5 cm of thickness). Samples were kept at refrigeration temperature (4±1°C) for 24 h before experimental study and throughout the experiment.
Analyses were performed in laboratories of the Department of Veterinary Medicine and Animal Productions of the University of Naples Federico II in triplicate and at different times: T0 (1 day), T1 (6 days) and T2 (9 days), from production.
For each sampling time n. 4 hamburger per type were tested. All determinations were done in triplicate. Sampling times were selected taking into account the results of preliminary shelf-life studies (data not published).
Sensory evaluation
Sensory evaluation was assessed by a five-members untrained panel (Altieri et al., 2005) for colour, odour, texture, drip loss and general appearance. The control samples were used as standard. Panelist scored each sample with a 5-point scale, on which 5 meant the most and 0 the least liked.
Colourimetric analysis
Sample’s colour was described in terms of Lightness (L*), redness (a*) and yellowness (b*) space values (CIE L*a*b*) (Smaldone et al., 2019). The measurements were carried out on burger surface exposed to air by using a Konica Minolta CR300 colourimeter (Minolta, Osaka, Japan). Colour variation was monitored for 9 days and expressed as the mean value of three random readings.
Physical-chemical analyses
The physical and chemical variations of treated and control beef burgers were monitored during the whole storage. pH and aw were measured with a digital pH-meter (Crison-Micro TT 2022, Crison Instruments, Barcelona) and with Aqualab 4 TE (Decagon Devices Inc., USA), respectively. Fat alteration index was evaluated by Thiobarbituric acid (TBA) test (AOAC, 2000), and TBA reactive substances were reported as malondialdehyde concentration (mg/Kg).
Microbiological analyses
For microbiological analyses, 10 g of each sample and 90 mL (1:10 (W/W)) of sterilized Peptone Water (PW, CM0009, OXOID, Basingstoke, UK) were placed in a sterile stomacher bag and homogenized for three minutes at 230 rpm using a peristaltic homogenizer (BagMixer®400 P, Interscience, Saint Nom, France). Ten-fold serial dilutions of each homogenate were plated on the surface of appropriate media in Petri dishes.
The viable counts of the following micro-organisms were carried out using procedures validated by the UNI CEI EN ISO/IEC 17025 European Standard: (i) total aerobic bacterial counts (TACs) on Plate Count Agar (PCA; Oxoid) incubated at 30°C for 48/72-h, according to ISO 4833-2:2013; (ii) Enterobacteriaceae (EB) on Violet Red Bile Glucose Agar (VRBG, Oxoid) incubated at 37°C for 24-h, according to ISO 21528-2:2017; (iii) total coliforms on Violet Red Bile Lactose Agar (VRBL, Biotec), according to ISO 4832/91; (iv) Lactic Acid Bacteria on MRS agar with Tween 80 (Biolife), incubated at 30°C for 72-h, according to ISO 15214:1998; (v) Pseudomonas spp. on Pseudomonas Agar Base with CFC supplement (Biotec) incubated at 25°C for 48-h, according to ISO 13720:2010; (vi) yeasts and moulds on Dichloran Rose-Bengal Chloramphenicol Agar (DRBC, Oxoid) incubated at 25°C for 120/168-hours, according to ISO 21527-1.
Statistical analyses
Physicochemical and microbiological data were statistically analysed with generalized linear mixed model of SPSS version 26 (IBM Analytics, Armonk, NY, USA). Statistical significance was set at 0.05. All results are expressed as means and standard error (±).
Results and Discussion
Sensory evaluation
Results regarding sensory evaluation are shown in Figure 1. Drip loss and texture measurements appear to overlap among samples, not showing any difference. On the contrary, panelists pointed out differences in colour, odour, and general appearance between burgers treated and control ones. Indeed, they appreciated the positive beetroot influence on these parameters, since the addition of the vegetable seemed to reduce the browning of the meat and because of beetroot extract is rich in betanin. Nevertheless, the high concentration of this red pigment could make beef burgers unpleasant for the consumers (10% water solution, B3ap). Likewise, the vegetable provided a certain odour which appears to be appreciated by panelists when used in low doses. Beef burger D2v (treated with combined treatments of vacuum and Beetroot water solution 2%) obtained the best score.
Figure 1.
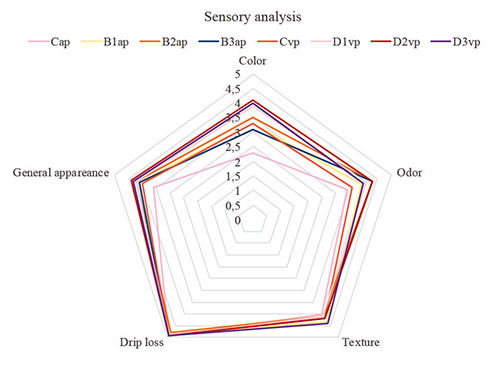
Influence of Different Percentages and Formulations of Red Beetroot on Sensory Panel Scores of Beef Burger at the end of storage. ap: packed under aerobic condition; vp: vacuum packaging; C: no addition, B1 and D1: with 0,8% Red Beetroot (RBR) in powder; D2: with 2% RBR in water solution; B2 and D3: with 5% RBR in water solution; B3: with 10% RBR in water solution.
Colourimetric analysis
As shown in Figure 2A-C, the addition of beetroot, both as powder and water solution, modified the L*, a* and b* values of burger, in comparison with the respective controls.
In particular, L*(brightness) values showed a continued decline during whole storage time for all treated samples, especially significant in the burgers’ group stored in aerobic condition. At T2 L *showed the lowest value of 20.53 in burger treated with beetroot solution (10%). Conversely, in Cap and Cvp lightness always kept around 47.82±0.54 and 42.53±1.37, respectively.
As displayed in Figure 2B, at each time B3ap, D1vp and D3vp showed the highest a* values, with a deeper difference after 6 days of storage if compared to Cap and Cvp. Controls strongly evidenced a decreasing trend of redness, probably due to microbial spoilage and the consequent increased of pH values (Borch et al., 1996). These results suggest that red beet is very effective on the increasing of the redness value and a* value increased with the growing amount of vegetable extracts addition, as demonstrated by several authors in studies with Turkish fermented beef sausage (Sucu et al., 2018) and fresh pork sausage (Martínez et al., 2006). The betalain content of natural compound could justify the phenomena. However, in all samples the redness parameter decreased over storage time, probably caused by pigment degradation as reported by Jin et al., 2014.
Contrariwise, the yellowness appeared to be negatively correlated to beetroot integrations, displaying low b* values into samples treated with high doses of the extract. Besides, the downward trend arose in both trials, regardless of the package typology adopted.
It is worth to note that the intensity of beetroot effect was higher in burgers packaged in aerobic condition than the other ones. The difference among the two types of packaging could be a consequence of a higher oxygen concentration around beef burgers stored under the aerobic condition that could penetrate meat samples making them brighter (Orkusz et al., 2017). For the same reason, the beetroot effect on redness was less evident on vacuum packed burgers.
Overall, the integration of beetroot appeared to decrease brightness and increase redness of burgers, making doses evaluation necessary to obtain the desired result. For this reason, the best formulation which displayed the most stable trend up to 9 days of storage was D2vp.
Physical-chemical analyses
In Table 1 are reported the pH values of burgers. At the first sampling time, all samples showed similar pH values, especially among vacuum-packaged ones. After 6 and 9 days of storage, in both experimental conditions control samples presented a slower downward trend than treated ones.
Overall, pH trends appeared to be considerably influenced by the concentration of extract and the package typology, registering the lowest pH values in D3vp burgers at ninth day. Indeed, the addition of beetroot clearly caused a fast decrease of pH in all treated samples, displaying a dose-depended effect. However, our results were not in agreement with the literature’s data, highlighting inconstant pH behaviour in response to beetroot adding. For instance, Lee et al. (2012) reported that red beet extract added to pork patties did not significantly influence the pH values.
Table 1.
Growth of total mesophilic bacteria (TAB), Coliform bacteria, and Pseudomonas spp. (Log CFU/g) and pH trends of beef burger, evaluated up to 9 days of storage at 4±1°C.
| DaYs | Mean ± SE | ||||
|---|---|---|---|---|---|
| Cap | B1ap | B2ap | B3ap | ||
| pH | 1 | 5.83±0.02X,AA | 6.02±0.03X,,B | 5.86±0.03X,A | 5.96±0.05X,B |
| 6 | 5.63±0.02Y,A | 5.38±0.03Y,B | 5.08±0.02Y,C | 5.06±0.03Y,C | |
| 9 | 5.71±0.02Z,A | 5.33±0.03Y,B | 4.89±0.02Z,C | 4.86±0.03Z,C | |
| TABs | 1 | 6.66±0.15X,A | 7.74±0.19X,B | 6.74±0.18X,A | 8.00±0.17X,B |
| 6 | 7.71±0.15Y,A | 8.24±0.18X,B | 7.73±0.17Y,A | 7.95±0.14X | |
| 9 | 8.91±0.18Z | 8.91±0.16Y | 8.74±0.14Z | 8.74±0.20Y | |
| Pseudomonas spp. | 1 | 5.00±0.23X,A | 6.93±0.15X,B | 5.04±0.15X,A | 6.32±0.16X,C |
| 6 | 6.80±0.20Y,A | 8.75±0.18Y,B | 8.04±0.14Y,C | 6.74±0.20X,A | |
| 9 | 9.16±0.16Z,A | 9.22±0.18Y,A | 8.86±0.15Z,A | 7.96±0.29Y,B | |
| Coliforms | 1 | 3.44±0.20A | 3.74±0.19X,A | 4.74±0.20B | 4.82±0.21B |
| 6 | 4.91±0.19aA | 7.02±0.16Y,B | 6.21±0.16aC | 5.56±0.19bAC | |
| 9 | 6.10±0.24 | 6.00±0.29Z | 6.22±0.19 | 5.96±0.20 | |
| Cvp | D1vp | D2vp | D3vp | ||
| pH | 1 | 6.01±0.02X | 6.03±0.04X | 6.01±0.03X | 6.01±0.03X |
| 6 | 5.75±0.02Y,A | 5.44±0.04Y,B | 5.36±0.03Y,B | 5.25±0.03Y,C | |
| 9 | 5.75±0.03Y,A | 5.44±0.02Y,B | 5.19±0.03Z,C | 5.09±0.02Z,D | |
| TABs | 1 | 7.41±0.17 | 7.24±0.18X | 7.56±0.23 | 7.56±0.23X |
| 6 | 7.64±0.24 | 7.76±0.16Y | 7.52±0.16 | 7.82±0.16X | |
| 9 | 7.63±0.19A | 7.60±0.31A | 7.59±0.21A | 5.96±0.22Y,B | |
| Pseudomonas spp. | 1 | 6.02±0.22X,A | 5.37±0.14X,BA | 6.01±0.15B | 6.02±0.34 |
| 6 | 6.10±0.15X,A | 5.51±0.17X,B | 5.76±0.16 | 5.86±0.16 | |
| 9 | 4.96±0.16Y,A | 6.10±0.15YY,B | 6.26±0.20B | 6.10±0.23B | |
| Coliforms | 1 | 4.89±0.15X,A | 4.55±0.24X | 4.79±0.19X,A | 4.22±0.17X,BB |
| 6 | 4.10±0.19Y,A | 4.07±0.17X,A | 3.31±0.25Y,B | 3.59±0.15Y,B | |
| 9 | 6.68±0.29Z,A | 6.49±0.18Y,A | 5.56±0.21Z,B | 6.07±0.19Z | |
ap: packed under aerobic condition; vp: vacuum packaging; C: no addition, B1 and D1: with 0,8% Red Beetroot (RBR) in powder; D2: with 2% RBR in water solution; B2 and D3: with 5% RBR in water solution; B3: with 10% RBR in water solution. Statistical analysis was performed comparing experimental groups at each sampling time and within experimental group along shelf-life period. Different superscript uppercase letters indicate a significant difference at p < 0.01. Different superscript lowercase letters indicate a significant difference at p<0.05. a-dMean values in the same row (different samples on the same sampling time) with different letter presented significant differences. x-yMean values in the same column (same batch in different weeks) with different letter presented significant differences.
Although, it is worth to note that beetroot effect on pH did not appear in the first 24 hours, probably because of a timedependent antimicrobial activity of the extract. This hypothesis could justify the pH decrease as a consequence of organic acids’ production by Gram-positive bacteria, as proposed by Hwang et al., (2017) in lowsalt frankfurters. Nevertheless, mesophilic aerobic acid lactic bacteria investigated showed similar levels in all samples, underlining no significant differences correlated to the beetroot adding.
Water activity values were comparable for all samples; they showed a progressive and slow decrease from 24 h till the end of the storage (from 0.980±0.001 to 0.970±0.005).
The effect of antioxidant addition on TBARS levels is shown in Figure 3A-B. Packaging, extract doses and storage time significantly affected fat degradation index and the lowest concentrations of malondialdehyde were detected at the end of storage in D2vp and D3vp. Jin et al. (2014) reported that the addition of low percentages would not be effective to slow down lipid oxidation despite betalains content. Moreover, the synergic antioxidant effect of plant extract and vacuum packaging was confirmed by Corbo et al. (2008). Martinez et al. (2006) highlighted the connection between pigment oxidation and lipid oxidation: in fact, catalytic species involved in such process are generated through myoglobin oxidation. In our study too, malondialdehyde concentrations changed slower than colour (a*) in a time-dependent manner (Figures 2 and 3).
Microbiological analyses
Table 1 displays the microbial counts at each sampling time. Data showed significant differences among samples of the first trial for TABs after one day from production. Nevertheless, high levels of mesophilic bacteria were detected in all samples. In particular, TABs overcame the limit of 6.7 Log CFU/g (5x106 CFU/g) fixed by the European regulation (Reg. EC 2073/2005) for minced meat at the end of the manufacturing process. This data should be referred to uncorrected manipulation during burger preparation.
Overall, TABs of B1ap and B3ap showed slower microbial growth than the control. On the other hand, TAB levels of vacuum products remained constant during the whole experiment, except D3vp with the highest integration of beetroot water solution that seems to induce an important decrease at 9 days of storage (Table 1). Indeed, mesophilic bacteria counts of burgers D3vp significantly differed to other ones of remaining samples at the end of the trial.
Concerning Total Coliforms, levels registered suggest possible contamination during processing. Trends almost overlapped among the two trials and beetroot extracts showed a clear antimicrobial dose-dependent effect against this bacteria population. Data pointed out the significant influence of beetroot concentration on microbial growth, above all evident at sixth day of sampling.
Figure 2.
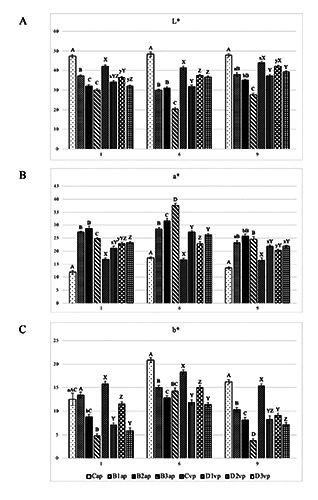
Colour values L* (A), a* (B), and b* (C) of burgers with beetroot extract addition compared to controls. Colour parameters were measured in burger surface up to 9 days of storage at 4 ± 1°C. ap: packed under aerobic condition; vp: vacuum packaging; C: no addition, B1 and D1: with 0,8% Red Beetroot (RBR) in powder; D2: with 2% RBR in water solution; B2 and D3: with 5% RBR in water solution; B3: with 10% RBR in water solution. Statistical analysis was performed comparing experimental groups at each sampling time. Different superscript uppercase letters indicate a significant difference at p<0.01. Different superscript lowercase letters indicate a significant difference at p<0.05. a-dMean values referred to ap samples with different letter presented significant differences. x-yMean values referred to vp samples with different letter presented significant differences.
As well, the addition of natural compound influenced counts of Pseudomonas spp. when burgers were packaged under aerobic condition. Beetroot effect appeared to be connected with dose and storage time, and B2ap and B3ap were identified as burgers with the lowest levels at T2, despite their starting contaminations were the highest.
Counts of Enterobacteriaceae, Lactic Acid Bacteria, yeasts and moulds were not influenced by beetroot integration in each trial during storage, reaching in all samples at T2 values of 6.2-6.7, 7.6-8.1 and 5.2-5.8 Log (CFU/g), respectively.
Conclusions
Results collected in the present study confer to beetroot (Beta vulgaris) a potential role as a burger shelf-life extender. Analytical determinations appeared to be effective for mapping antioxidant and antimicrobial capacities of vegetable extracts in different concentrations and formulations over time. Red beet slightly decreased lipid oxidation due to its betalains content. Besides, it improved the colour properties of fresh meat and the general appearance, especially in association with vacuum packaging. Concerning antimicrobial properties, an influence on TABs growth was recorded when the beetroot was used at high concentrations.
Overall, data pointed out that beetroot formulation and dose, and packaging typology are variables which intensely influence the effect on burgers. For instance, the integration of high doses could negatively reflect on general appearance, making burgers undesirable for consumers. In the same way, extracts in water solution seem to be more efficacy than powder which could remain “trapped” in the matrix and be poorly bioavailable. For these reasons, the best choice could be represented by D2vp.
The choice of a natural compound in place of synthetic additives could improve the quality characteristics and meet the consumers’ needs who are frequently demanding natural products.
There are many varieties of plants, like Beta vulgaris, with a high content of phenolic compounds and antioxidant capacities that could be introduced in the agro-food processing industry to enhance products with a low commercial cost.
Figure 3.
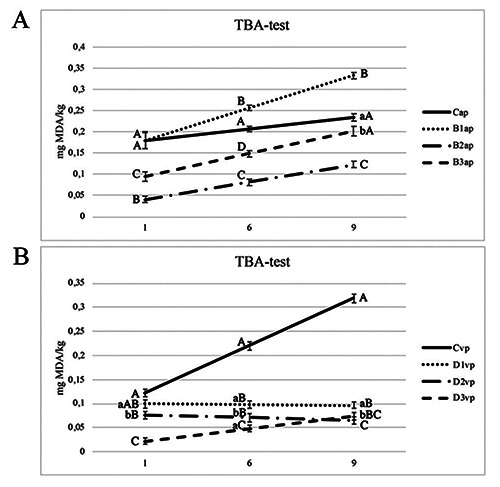
2-thiobabituric acid reactive substance analysis of beef burgers with Red Beetroot extract integration during cold storage at 4°C for 9 d. ap: packed under aerobic condition; vp: vacuum packaging; C: no addition, B1 and D1: with 0,8% Red Beetroot (RBR) in powder; D2: with 2% RBR in water solution; B2 and D3: with 5% RBR in water solution; B3: with 10% RBR in water solution. Statistical analysis was performed comparing experimental groups at each sampling time. Different superscript uppercase letters indicate a significant difference at p<0.01. Different superscript lowercase letters indicate a significant difference at p<0.05.
Acknowledgement
The authors thank Mr Gerardo Buono and Mr Michele Sgamato of “La Fattoria del Campiglione” who provided expertise that greatly assisted the research.
Funding Statement
Funding: None.
References
- Altieri C, Speranza B, Del Nobile MA, Sinigaglia M, 2005. Suitability of bifidobacteria and thymol as biopreservatives in extending the shelf life of fresh packed plaice fillets. J Appl Microbiol 99:1294-302. [DOI] [PubMed] [Google Scholar]
- Ambrosio RL, Gratino L, Mirino S, Cocca E, Pollio A, Anastasio A, Palmieri G, Balestrieri M, Genovese A, 2020. The bactericidal activity of protein extracts from Loranthus europaeus berries: a natural resource of bioactive compounds. Antibiotics 9:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio A, Marrone R, Chirollo C, Smaldone G, Attouchi M, Adamo P, Sadok S, Pepe T, 2014. Swordfish steaks vacuum-packed with Rosmarinus officinalis. Ital J Food Sci 26:390-7. [Google Scholar]
- AOAC, 2000. Official Methods of Analysis, 17th ed. Method 976.18. Association of Official Analytical Chemists, Gaithersburg, MD, USA. [Google Scholar]
- Borch E, Kant-Muermans M-L, Blixt Y, 1996. Bacterial spoilage of meat and cured meat products. Int J Food Microbiol 33:103-20. [DOI] [PubMed] [Google Scholar]
- Burri SCM, Granheimer K, Rémy M, Ekholm A, Håkansson Å, Rumpunen K, Tornberg E. Lipid Oxidation Inhibition Capacity of 11 Plant Materials and Extracts Evaluated in Highly Oxidised Cooked Meatballs. Foods, 8, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo MR, Speranza B, Filippone A, Granatiero S, Conte A, Sinigaglia M, Del Nobile MA, 2008. Study on the synergic effect of natural compounds on the microbial quality decay of packed fish hamburger. Int J Food Microbiol 127:261-7. [DOI] [PubMed] [Google Scholar]
- Falowo AB, Fayemi PO, Muchenje V, 2014. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res Int 64:171-81. [DOI] [PubMed] [Google Scholar]
- Gogliettino M, Balestrieri M, Ambrosio RL, Anastasio A, Smaldone G, Proroga YTR, Moretta R, Rea I, De Stefano L, Agrillo B, Palmieri G, 2020. Extending the Shelf-Life of Meat and Dairy Products via PET-Modified Packaging Activated with the Antimicrobial Peptide MTP1. Front Microbiol 10:2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang KE, Kim TK, Kim HW, Oh NS, Kim YB, Jeon KH, Choi YS, 2017. Effect of fermented red beet extracts on the shelf stability of lowsalt frankfurters. Food Sci Biotechnol 26:929-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SK, Choi JS, Moon SS, Jeong JY, Kim GD, 2014. The assessment of red beet as a natural colorant, and evaluation of quality properties of emulsified pork sausage Containing red beet powder during cold storage. Korean J Food Sci Ann 34:472-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian Z, Tabatabaee Bafroee AS, Sharifan A, 2019. Physico-mechanical and antimicrobial properties of isolated soy protein film incorporated with peppermint essential oil on raw hamburger. J Agr Sci Technol 21:1145-59. [Google Scholar]
- Kerry JP, O’Grady MN, Hogan SA, 2006. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci 74:113-30. [DOI] [PubMed] [Google Scholar]
- Kujala TS, Loponen JM, Klika KD, Pihlaja K, 2000. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agr Food Chem 48:5338-42. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chin KB, 2012. Evaluation of antioxidant activities of red beet extracts, and physicochemical and microbial changes of ground pork patties containing red beet extracts during refrigerated storage. Korean J Food Sci Ann 32:497-503. [Google Scholar]
- Marrone R, Balestrieri A, Pepe T, Vollano L, Murru N, D’Occhio MJ, Anastasio A, 2014. Physicochemical composition, fatty acid profile and cholesterol content of “Pecorino Carmasciano” cheese, a traditional Italian dairy product. J Food Compos Anal 36:85-9. [Google Scholar]
- Martínez L, Cilla I, Beltrán JA, Roncalés P, 2006. Antioxidant effect of rosemary, borage, green tea, pu-erh tea and ascorbic acid on fresh pork sausages packaged in modified atmosphere. Influence of the presence of sodium chloride. J Sci Food Agric 86:1298-307. [Google Scholar]
- Martínez L, Cilla I, Beltrán JA, Roncalés P, 2006. Comparative effect of red yeast rice (Monascus purpureus), red beet root (Beta vulgaris) and betanin (E-162) on colour and consumer acceptability of fresh pork sausages packaged in a modified atmosphere. Sci Food Agric 86:500-8. [Google Scholar]
- Martínez L, Cilla I, Beltran JA, Roncalés P, 2006. Combined effect of modified atmosphere packaging and addition of rosemary (Rosmarinus officinalis), ascorbic acid, red beet root (Beta vulgaris), and sodium lactate and their mixtures on the stability of fresh pork sausages. J Agr Food Chem 54:4674-80. [DOI] [PubMed] [Google Scholar]
- Mozaffari NAS, Shabani S, Bayat M, Hosseini SE, 2014. Antibacterial effect of garlic aqueous extract on staphylococcus aureus in hamburger. Jundishapur J Microb 7:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkusz A, Haraf G, Okruszek A, Weren ´ska- Sudnik M, 2017. Lipid oxidation and color changes of goose meat stored under vacuum and modified atmosphere conditions. Poultry Science 96:731-7. [DOI] [PubMed] [Google Scholar]
- Peruzy MF, Murru N, Yu Z, Cnockaert M, Joossens M, Proroga YTR, Houf K, 2019. Determination of the microbiological contamination in minced pork by culture dependent and 16S amplicon sequencing analysis. Int J Food Microbiol, 290:27-35. [DOI] [PubMed] [Google Scholar]
- Rey AI, Hopia A, Kivikari R, Kahkonen M, 2005. Use of natural food/plant extracts: Cloudberry (Rubus Chamaemorus), beetroot (Beta Vulgaris “Vulgaris”) or willow herb (Epilobium angustifolium) to reduce lipid oxidation of cooked pork patties. Food Sci Tech 38:363-70. [Google Scholar]
- Smaldone G, Marrone R, Vollano L, Peruzy MF, Barone C, Ambrosio RL, Anastasio A, 2019. Microbiological, rheological and physical-chemical characteristics of bovine meat subjected to a prolonged ageing period. Ital J Food Saf 8:8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaldone G, Marrone R, Zottola T, Vollano L, Grossi G, Cortesi ML, 2017. Formulation and shelf-life of fish burgers served to preschool children. Ital J Food Saf 6:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagnari F, Galieni A, Speca S, Pisante M, 2014. Water stress effects on growth, yield and quality traits of red beet. Sci Hortic 165:13-22. [Google Scholar]
- Sucu C, Turp GY, 2018. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci 140:158-66. [DOI] [PubMed] [Google Scholar]
- Theivendran S, Hettiarachchy NS, Johnson MG, 2006. Inhibition of Listeria monocytogenes by nisin combined with grape seed extract or green tea extract in soy protein film coated on turkey frankfurters. J Food Sci 71. [Google Scholar]


