Abstract
Celastrol, a triterpenoid derived from traditional Chinese medicinal plants, has anti-inflammatory, anti-oxidant, and anti-cancer activities. Celastrol has shown preventive/therapeutic effects in experimental models of several chronic diseases. These include chronic inflammatory and autoimmune diseases (e.g., rheumatoid arthritis, multiple sclerosis, systemic lupus erythematous, inflammatory bowel disease, and psoriasis), neurodegenerative disorders (e.g., Alzheimer’s disease, Parkinson’s disease, and Amyotrophic lateral sclerosis), atherosclerosis, obesity, Type 2 diabetes, and cancer. Celastrol modulates intricate cellular pathways and networks associated with disease pathology, and it interrupts or redirects the aberrant cellular and molecular events so as to limit disease progression and to facilitate recovery, where feasible. The major cell signaling pathways modulated by celastrol include the NF-kB pathway, MAPK pathway, JAK/STAT pathway, PI3K/Akt/mTOR pathway, and anti-oxidant defense mechanisms. Furthermore, celastrol modulates cell proliferation, apoptosis, proteasome activity, heat-shock protein responses, innate and adaptive immune responses, angiogenesis, and bone remodeling. Current understanding of the mechanisms of action of celastrol and information about its disease-modulating activities in experimental models have set the stage for testing celastrol in clinical studies as a therapeutic agent for several chronic human diseases.
Keywords: Celastrol, Inflammation, Autoimmune diseases, Neurodegenerative diseases, Metabolic disorders, Immune modulation, Natural products, Traditional Chinese medicine
INTRODUCTION
Celastrol is a bioactive component of several traditional Chinese medicinal plants including Tripterygium wilfordii (Thunder God Vine), Celastrus orbiculatus, Celastrus aculeatus Celastrus reglii, Celastrus scandens, and others that belong to the Celastraceae family [1–5]. The extracts of the root, bark and stem of some of these plants have long been used in China and other Asian countries for the treatment of a wide range of chronic inflammatory disorders, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and allergies [1–5]. In this article, we describe the diverse molecular and cellular pathways modulated by celastrol, with emphasis on chronic inflammatory, autoimmune, neurodegenerative, and metabolic diseases [6–12]. Celastrol also possesses anti-cancer activity [13–15,5]. A summary of the anti-cancer mechanisms employed by celastrol is also presented at the end.
PHYSICO-CHEMICAL PROPERTIES OF CELASTROL
Celastrol is a pentacyclic triterpene (Figure 1) that belongs to a small class of organic compounds called quinone methides. It has a molecular weight of 450.6 and its molecular formula is C29H38O4. It is a pale brown to orange red crystalline powder, and its melting point is between 219–2300 C. Celastrol has maximum UV/visible absorption spectra at 253 and 424 nm. It is sparingly soluble in water, but is soluble in nonpolar solvents such as dimethylsulfoxide (DMSO) and ethanol. Celastrol is an electrophilic compound and it can react with nucleophilic thiol groups of cysteine residues of a variety of proteins to form adducts or induce other modifications within those proteins [16,17,6,18]. Apparently, this is one of the mechanisms by which celastrol can affect biological functions of proteins. Celastrol is also known as tripterine/tripterin, but the name celastrol is commonly used.
Figure 1. Molecular structure of celastrol.
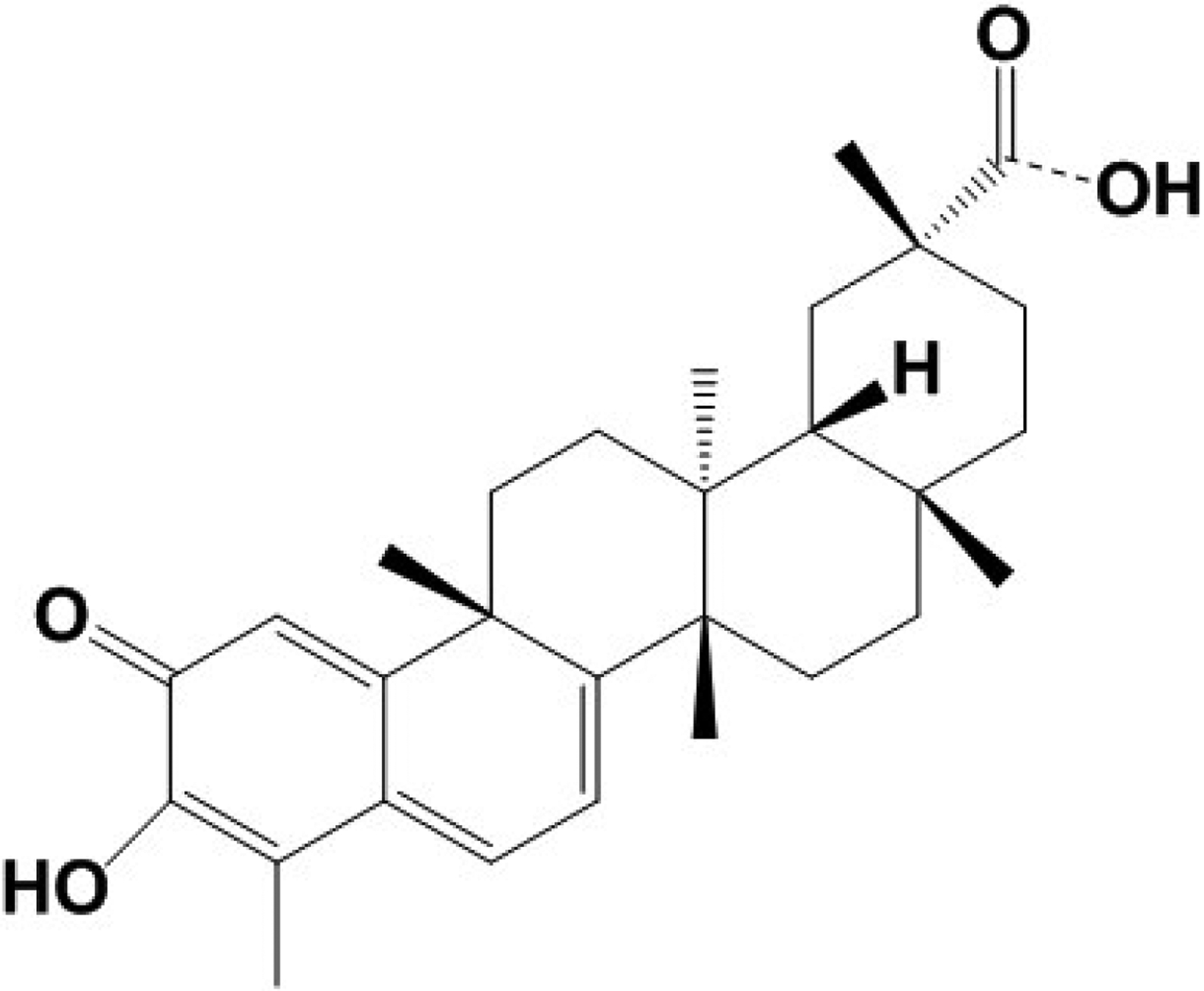
Celastrol is a pentacyclic triterpenoid with a molecular weight 450.6 and molecular formula C29H38O4. It belongs to a small class of organic compounds known as quinone methides. Celastrol has an acidic group at one end and a phenolic quinone at the other end.
CELASTROL CONTROLS INFLAMMATION AND OTHER PATHOLOGICAL PROCESSES IN ANIMAL MODELS OF CHRONIC DISEASES
Celastrol has been shown to be beneficial in various chronic disease conditions in studies in animal models of immune-mediated diseases, neurodegenerative diseases, and others. The preventive/therapeutic potential of celastrol in various in vivo and in vitro models of these diseases is summarized in Table 1. Also mentioned therein are the cell signaling pathways as well as cellular and molecular targets of celastrol in various disease processes. The details of these and other mechanisms of action of celastrol are described below in separate sections. In addition, celastrol has potent anti-cancer activity. The mechanisms underlying the anti-cancer activity of celastrol are summarized below in a separate section.
Table 1.
Celastrol-induced prevention/treatment of chronic diseases of diverse etiology
| Type of disease | Experimental model(s) | Targets/mechanisms of celastrol action | References |
|---|---|---|---|
| A. Inflammatory and Autoimmune Diseases | |||
| Rheumatoid Arthritis (RA) | Rat AA, Rat/Mouse CIA, Human RA-FLS | Modulates pro-inflammatory cytokines, chemokines, and T helper 17 (Th17)/T regulatory cell balance. Inhibits RA-FLS invasion and apoptosis. Decreases production of antibodies to the disease-related antigens and anti-cyclic citrullinated peptides (aCCP) antibodies. Also inhibits osteoclast differentiation, and reduces cartilage and bone damage. | [8, 9, 19–21, 88, 118, 137–140] |
| Multiple sclerosis (MS) | Rat/mouse experimental autoimmune encephalomyelitis (EAE) | Modulates Th17 responses, shifts Th1 response towards Th2, decreases TNFα but increases IL-10. Inhibits NF-κBexpression, nitrites levels, and expression of Toll-like receptor (TLR)2. | [10, 22] |
| Systemic lupus erythematosus (SLE) (or Lupus) | BW F1 mice, BALB/c mice (chromatin injection) | Decreases transforming growth factor (TGF)-β, renal collagen type IV, urine protein excretion, and serum autoantibodies. | [23–25] |
| Ulcerative colitis | DSS-induced colitis in mice | Modulates oxidative stress, inflammatory cytokines, and intestinal homeostasis. | [11] |
| Psoriasis | HACaT keratinocytes | Inhibits NF-κB expression and induces apoptosis through caspase-3 activation | [141] |
| Hypersensitivity (e.g., Asthma and skin inflammation) | Ovalbumin/allergen-induced airway inflammation, phorbol myristate acetate-induced skin inflammation, and skin hypersensitivity to dinitrochlorobenzene in mice | Downregulates the expression of stem cell factor (SCF) in fibroblasts, and inhibits the production of histamine and eotaxin in mast cells. Inhibits antibody responses and immunoglobulin Fc epsilon receptor I signaling. Regulates the balance between isoforms of MMPs (MMP-2/9) and TIMPs (TIMP-1/2). | [26–29] |
| B. Neurodegenerative Diseases | |||
| Parkinson’s disease (PD) | MPTP mouse model, SH-SY5Y cells | Modulates pro-inflammatory cytokines, prevents the generation of ROS, lipid peroxidation, and mitochondrial membrane potential. Protects against cellular injury and apoptotic cells death. | [12, 31, 34] |
| Alzheimer’s disease | Transgenic mouse model | Inhibits pro-inflammatory cytokines and expression of MHC-II molecules. Induces neuroprotective heat-shock proteins (Hsps). Lowers oxidative stress and regulates BACE-1 expression level via an NF-κB-dependent mechanism. | [30, 32, 33, 36, 37] |
| Amyotrophic lateral sclerosis (ALS) | G93A SOD1 transgenic mouse model | Blocks neuronal cell death, reduces TNF-α, iNOS, CD40, and GFAP immunoreactivity. | [35] |
| Gaucher disease (GD) | GD fibroblasts | Modulates molecular chaperones and increases glucocerebrosidase activity. | [38] |
| Age-related macular degeneration (AMD) | Human retinal pigment epithelial cells (ARPE-19 cells) | Reduces IL-6 and inhibits NF-κB. | [39] |
| C. Atherosclerotic and Metabolic Diseases | |||
| Atherosclerosis | Apo-E-deficient mice, Rabbit carotid atherosclerosis model, and human platelets | Inhibits lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) and generation of ROS. Reduces serum level of low density lipoproteins and VEGF expression. Inhibits platelet aggregation by reducing the expression of P-selectin and glycoprotein IIb/IIIa on platelets. | [40, 41, 142] |
| Obesity | Hyperleptinemic diet-induced obese mice | Reduces food intake, increases energy expenditure, leading to weight loss by increasing leptin sensitivity. | [42] |
| Type 2 diabetes | The db/db mouse | Acting on the liver, adipose tissue, and kidney, it Inhibits NF-kB, reduces insulin resistance, improves abnormal lipid metabolism and oxidative stress, reduces pro-inflammatory cytokines, and limits renal injury. | [43] |
AA, Adjuvant-induced arthritis; CIA, Collagen-induced arthritis; DSS, Dextran sulfate sodium; FLS, Fibroblasts-like synoviocytes; Hsp, Heat-shock proteins; MMPs, Matrix metalloproteases; TIMPs, Tissue inhibitor of matrix metalloproteases; MPTP, 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridin;, ROS, Reactive oxygen species; MHC-II, Major histocompatibility complex class II; BACE1, Beta-site APP-cleaving enzyme 1; iNOS, Inducible nitric oxide synthase; GFAP, Glial fibrillary acidic protein; p-JNK, Phospho c-Jun N-terminal kinase; NF-κB, Nuclear factor-kappa B; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
Inflammatory, autoimmune, and allergic diseases
For rheumatoid arthritis (RA), using the rat adjuvant arthritis (AA) model, mouse collagen-induced arthritis (CIA) model and fibroblast-like synoviocytes from RA patients (RA-FLS) culture model, celastrol has been shown to reduce the severity of clinical and histopathological features of arthritis, as well as to modulate the production of pro-inflammatory cytokines and chemokines [8,9,19], to re-set the T helper 17 (Th17)/T regulatory (Treg) balance to facilitate the suppression of arthritis [20], and to afford protection against bone damage [8,9,19] (Table 1A). Celastrol also inhibits RA-FLS invasion and protects against bone and cartilage damage [21]. For multiple sclerosis (MS), celastrol is shown to modulate Th17 responses, to shift Th1 responses towards Th2, and to increase the production of anti-inflammatory cytokines in the experimental autoimmune encephalomyelitis (EAE) model of MS [22,10]. For systemic lupus erythematosus (SLE) (also known as lupus), celastrol treatment decreases transforming growth factor (TGF)-β production, urine protein excretion, and serum autoantibody levels in BW F1 and BALB/c mouse models of SLE [23–25]. For ulcerative colitis, using the mouse dextran sulphate sodium (DSS)-induced colitis model, celastrol has been shown to modulate oxidative stress, inflammatory cytokines and intestinal homeostasis [11]. For asthma and other hypersensitivity reactions, celastrol inhibits histamine and eotaxin production and other mediators involved in hypersensitivity reactions [26–29]. The main mediators and pathways targeted by celastrol in above-mentioned diseases are described in Table 1A.
Neurodegenerative disorders
The effects of celastrol on MS, a neurological disease of autoimmune origin, have been described above. For other neurological diseases such as Parkinson’s disease, Alzheimer’s disease and amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease), celastrol has been shown to modulate pro-inflammatory cytokine production, to prevent the generation of reactive oxygen species (ROS), to limit oxidative damage, to protect against cell death, and to regulate heat-shock proteins (Hsps), as observed in mouse models and in vitro models of these diseases (Table 1B) [30–34,12,35–37]. For Gaucher disease (GD), celastrol modulates molecular chaperones and increases glucocerebrosidase activity in the GD fibroblasts model [38]. Celastrol is also known to modulate age-related macular degeneration [39].
Atherosclerotic, Metabolic, and Infectious diseases
Celastrol can inhibit platelet activation [40], prevent atherosclerotic plaque size in apo E-deficient mice [41], and decreased ratio of the plaque area and the arterial wall cross-section area in a rabbit model of carotid atherosclerosis [41], thus revealing the anti-atherosclerosis effect of this natural triterpene (Table 1C). Recently, celastrol has been reported to be a leptin sensitizer, whose effects are manifest as reduced intake of food, increased energy expenditure, and weight loss, and thereby it may potentially serve as an anti-obesity agent [42]. Celastrol is also effective in improving insulin resistance and limiting renal injury in a mouse model of Type 2 diabetes [43]. Furthermore, celastrol can modulate human immunodeficiency virus (HIV) 1- transactivator of transcription (Tat)-induced inflammatory responses in astrocytes in vitro by inhibiting the activation of JNK, MAPK, AP-1, and NF-kB as well as the expression of pro-inflammatory chemokines (e.g., CXCL10, IL-18, and monocyte chemotactic protein-1 (MCP-1)) and adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1)/vascular cell adhesion molecule-1 (VCAM-1) [44]. Celastrol has also been reported to target defined functional components of pathogens such as the HIV-Tat [18] to inhibit the transcription and replication of that virus, and the enzyme enoyl-acyl carrier protein reductase of Plasmodium falciparum [45], which is a drug target for this malaria parasite. However, because of the limited scope of this article, we have not elaborated further on direct effects of celastrol on various pathogens.
CELASTROL MODULATES CELL SIGNALING PATHWAYS OF INFLAMMATION
Inflammation involves interplays among a variety of cellular, molecular and biochemical mediators that are activated/induced in response to different pro-inflammatory stimuli. These mediators comprise diverse pathways (Figure 2) that are described below. Inflammation is associated with multiple diseases, including chronic inflammatory diseases, autoimmune diseases, obesity, and cancer. Celastrol controls inflammation by targeting one or many of these pathways depending on the underlying disease process.
Figure 2. Cell signaling pathways and other disease-related processes modulated by celastrol for the control of chronic diseases.
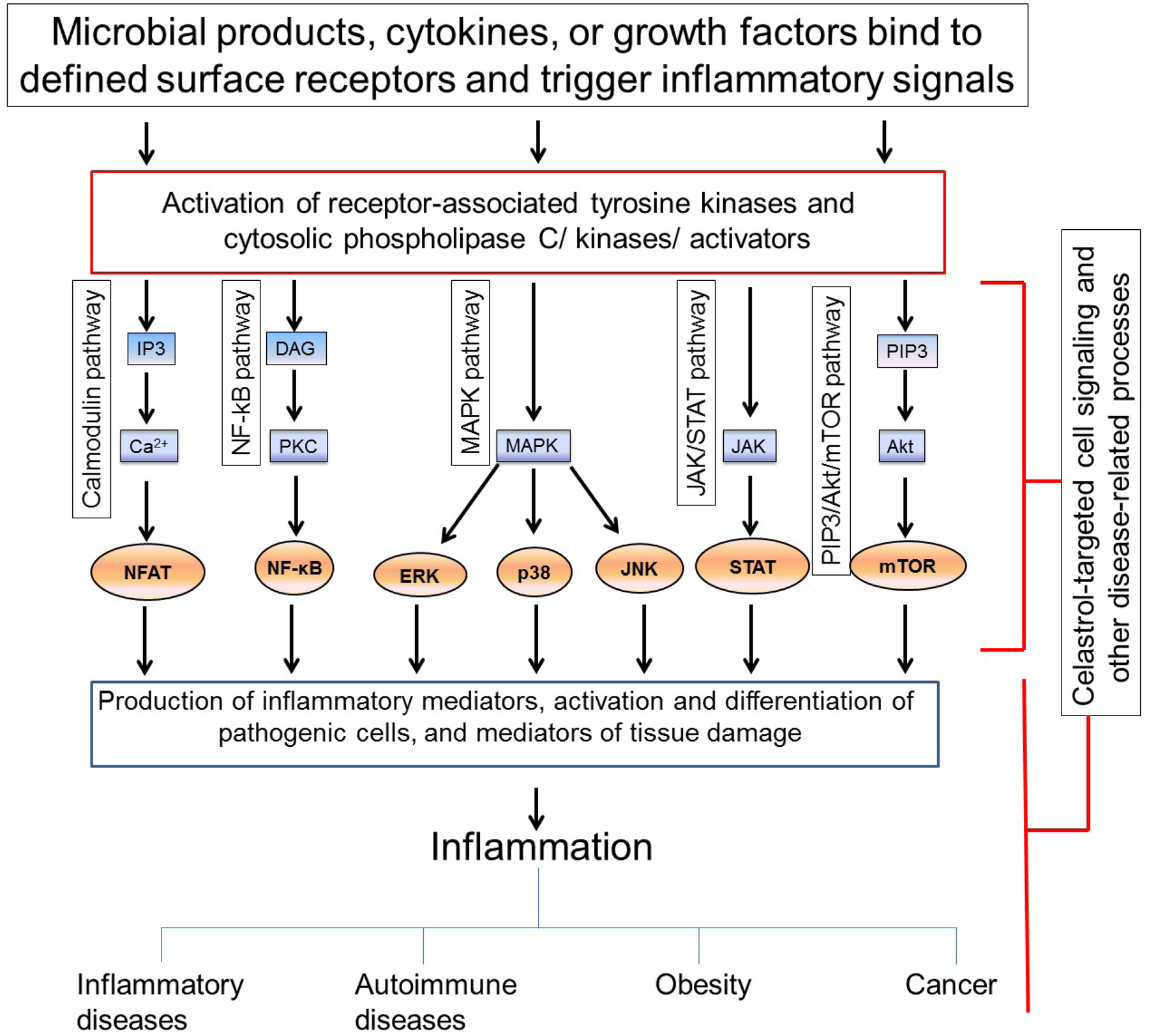
A schematic representation of the cell signaling pathways that are regulated by celastrol. These pathways are activated in response to diverse stimuli. Celastrol is known to target one or more of these pathways leading to the suppression of inflammation associated with various chronic diseases including inflammatory diseases, autoimmune diseases, obesity, and cancer. Besides cell signaling, celastrol modulates other disease-related processes such as angiogenesis, heat-shock protein responses, proteasome activity, etc. to limit disease progression and to facilitate recovery, where feasible.
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway is a central regulator of inflammation. A broad range of pro-inflammatory stimuli including cytokines, growth factors, and microbial products activate the I-kappa B kinase (IKK) complex consisting of IKK1, IKK2 and NF-κB essential modulator (NEMO). Activated IKK complex phosphorylates I-kappa B (IκB), which leads to ubiquitination and proteasomal degradation of IκB. The degradation of IκB in turn activates NF-κB, which then translocates to the nucleus, where it binds to DNA and regulates the expression of several target genes. NF-kB activation enhances the production of pro-inflammatory cytokines (e.g., interleukin-1 β (IL-1β), IL-6 and tumor necrosis factor α (TNFα)) and other inflammatory mediators (e.g., matrix metalloproteinases (MMPs) and inducible nitric oxide synthase (iNOS)) [46,47], without much effect on the induction of anti-inflammatory cytokines (e.g., IL-10 or IL-1 receptor antagonist (IL-1Ra)) [48]. Celastrol inhibits NF-κB activation and regulates NF-κB-regulated gene expression. It has been suggested that celastrol targets cysteine 179 in IKK [16] and blocks IKK activity as well as the degradation and phosphorylation of IκB [21,49,16]. This in turn blocks the activation of NF-κB and its nuclear translocation.
Mitogen activated protein kinase (MAPK) pathway is another important signal transduction pathway besides NF-κB pathway that is critical for immune-mediated inflammatory responses [50]. MAPKs are a family of serine/threonine protein kinases [51]. Three well-defined members of this family are the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, and they are activated by pro-inflammatory stimuli, including cytokines [50]. ERK signal transduction pathway leads to the activation of transcription factors c-Jun, c-Fos, and activating transcription factor 2 (ATF-2), whereas JNK activation leads to the activation of c-Jun and ATF-2. Furthermore, the p38 MAPK-mediated processes involve the participation of transcription factors cAMP response element-binding protein (CREB) and ATF-2 [50,52,53]. Celastrol selectively regulates the MAPK pathway. It inhibits the phosphorylation of JNK and ERK in various models of inflammation and arthritis [27,54,8]. However, activation of JNK by celastrol has also been observed in another system [55]. For p38 MAPK, one report stated that phosphorylation of p38 MAPK is unaffected by celastrol [56], whereas another [57] indicated that celastrol can activate p38 MAPK; the latter effect has been associated with the anti-metastatic activity of celastrol against cancer.
The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway is a common signaling cascade for many cytokines [58] [59]. JAKs are known to associate with the cytoplasmic domain of cytokine receptors for interferon (IFN)-α/β, IFNγ, IL-2, IL-4, IL-6, IL-10, IL-12/23 and others [58]. In general, the binding of these cytokines to their respective receptors phosphorylates JAK, which then leads to the phosphorylation of STATs. Activated STATs dimerize and translocate to the nucleus, where they bind to promoter regions of cytokine-responsive genes and thereby activate gene transcription [58]. Different STATs are involved in the differentiation of naïve T cells into particular T cell subsets (Th1, Th2, Th17 and Treg). In our studies, we have shown that celastrol inhibits STAT3 activation and suppress IL-17 expression as well as Th17 differentiation in the rat AA model [20,8]. In models of cancer such as multiple myeloma and hepatocellular carcinoma, constitutive STAT3 activation plays a role in cell proliferation, survival, and metabolism, and thereby to the disease process. Celastrol is shown to inhibit STAT3-mediated cell proliferation [60,61]. It involved inhibition of activation of upstream JAK1 and JAK2. However, effects of celastrol on other JAKs and STATs remain to be determined.
Another signaling pathway affected by celastrol is phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, which is involved in immune-mediated diseases and cancer [62–67]. Altered activation of the PI3K/Akt/mTOR pathway is observed in many human tumors, and it regulates the proliferation, differentiation, metabolism and survival of cancer cells [68]. Furthermore, there is an association between the accumulation of hypoxia-inducible factor-1 (HIF-1) and amplified PI3K/Akt/mTOR pathway signaling [69]. HIF-1 is a transcription factor highly expressed under hypoxic conditions and regulates cell survival response to hypoxia and cancer [63,70,71]. Inhibitors of HIF-1 have been tried for cancer therapy [72]. Celastrol can inhibit both PI3 activity as well as HIF-1 [63,66]. Celastrol inhibited HIF-1 activity in various cancer cell lines by decreasing the accumulation of HIF-1 and preventing the expression of HIF-1 target genes. Furthermore, the accumulation of HIF-1 by celastrol is correlated with inhibition of the phosphorylation of mTOR, ribosomal protein S6 kinase (p70S6K), eukaryotic initiation factor 4E (eIF4E) and ERK [63]. Contrary to the above, celastrol is also reported to induce HIF-1 accumulation through the induction of ROS and Akt/p70S6K signaling, and promote transcription of HIF target genes [70]. Therefore, additional studies are needed to clearly understand the role of celastrol in the regulation of mTOR and HIF-1.
Celastrol possesses anti-oxidant activity. Oxidative stress is one of the mediators of inflammation [73]. Oxidative stress builds up with the generation of high levels of reactive free radicals, such as reactive oxygen species (ROS; e.g., chemically reactive molecules derived from O2 mainly O2− (superoxide anions), H2O2 (hydrogen peroxide) and OH• (hydroxyl radicals)) and reactive nitrogen species (RNS; e.g., radicals derived from nitrogen and oxygen, particularly nitric oxide (NO)) in the cell [74,75]. Celastrol is shown to inhibit lipid peroxidation in rat liver mitochondria by direct radical scavenging [76] as well as neutralizing oxygen radicals [77]. Celastrol also enhanced the antioxidant defense system and offered protection against bleomycin-induced pulmonary fibrosis in rats by restoring antioxidant enzymes such as hemoxygenase-1 (HO-1), glutathione-S-transferase (GSTs) and nicotinamide adenine dinucleotide phosphate (H) (NADP(H)): quinine oxidoreductase (NQO1) via the NF-E2-related factor-2 (Nrf2) pathway [78]. Similarly, celastrol decreased obesity-induced oxidative stress by increasing antioxidant enzymes and inhibiting NADH oxidase and ROS [79]. Defense against oxidant system by celastrol has also been attributed to decreased expression of iNOS and NO production [30,34], and the blocking of reactive thiols [17]. In contrast to the above, celastrol has been reported to induce ROS accumulation and to initiate apoptosis through the down-regulation on HSP90 in tumor cells [80]. Similarly, in osteosarcoma, celastrol caused G2/M phase arrest, and induced apoptosis and autophagy via the ROS/JNK signaling pathway [81].
CELASTROL HAS ANTI-ANGIOGENIC ACTIVITY AND PROTECTS AGAINST ENDOTHELIAL BARRIER DYSFUNCTION
Angiogenesis, the formation of new blood vessels, is a hallmark of cancer [82–84]. However, autoimmune diseases such as RA are also characterized by angiogenesis in the target organ, the inflamed joints [85,86]. Accordingly, anti-angiogenic therapy has been considered for both these categories of disorders [87,86]. As discussed above, celastrol treatment inhibits the progression of autoimmune arthritis in experimental models of AA and CIA [20,9,8,88]. Similarly, celastrol suppresses tumor growth, for example, in mouse models and in vitro models of human prostate cancer [89,90]. Interestingly, celastrol has been shown to inhibit angiogenesis, both in vitro and in vivo [91,83], and to inhibit vascular endothelial growth factor (VEGF)-induced Akt/mTOR/p70S6K signaling [83]. Furthermore, celastrol can inhibit hypoxia-mediated angiogenesis, which involves inhibition of HIF-1α and its downstream genes such as VEGF [92]. Celastrol’s ability to inhibit Hsp90 was also implicated in reduced HIF-1α in this process. In another study, celastrol was shown to inhibit vasculogenesis by decreasing VEGF secretion, adhesion of endothelial cells to the extracellular matrix (ECM) and their subsequent migration, and tubule formation [93]. Inhibition of Akt/endothelial nitric oxide synthase (eNOS) signaling was implicated in this process. Celastrol has also been reported to inhibit lipopolysaccharide (LPS)-induced angiogenesis, which involved suppression of Toll like receptor- 4 (TLR-4)-mediated NF-kB activation [94], and to inhibit angiogenesis via suppression of VEGFR-1 and VEGFR-2 expression [95].
In RA, vascular endothelial cell physiology is relevant not only for angiogenesis but also for immune cell interaction and migration through blood vessels, as well as for maintaining a healthy endothelial barrier. In this regard, celastrol has been shown to inhibit the expression of cytokine-induced adhesion molecules such as ICAM-1 and VCAM-1 [96], and to prevent endothelial barrier dysfunction by inhibiting endogenous peroxynitrite formation in endothelial cells exposed to pro-inflammatory stimuli [97]. The latter effect involved inhibition of JAK-2-dependent iNOS and NADPH oxidase type 1 (Nox-1) induction.
CELASTROL-INDUCED MODULATION OF HEAT-SHOCK RESPONSE AND ITS POTENTIAL THERAPEUTIC APPLICATIONS IN CHRONIC DISEASES
Heat-shock proteins (Hsps), also known as Stress proteins, can be induced by heat and other types of stimuli that cause cellular stress [98,99]. These are highly conserved proteins evolutionarily. Hsps can be categorized into different families based on their molecular mass (kD), for example, Hsp110, Hsp90, Hsp70, Hsp60, Hsp40, and small Hsps. One of the major functions of Hsps is to protect cells from damage under different types of stressful conditions [98,99]. Acting as chaperones, Hsps bind to cellular proteins, ensure proper folding of cellular proteins, attempt to repair defective proteins, and protect them against denaturation and other types of damage. Hsps are also involved in signal transduction and apoptosis. Thus, heat-shock response is cytoprotective under situations that otherwise would promote apoptosis of cells. Stress-inducible heat-shock transcription factor-1 (HSF1) plays an important regulatory role in response to environmental stress and pathophysiological conditions.
Dysregulation of cellular stress pathways and protein folding can lead to intracellular accumulation of protein aggregates, which is turn can induce tissue pathology [100,101]. Many pathophysiological conditions including neurodegenerative disorders (e.g., Alzheimer’s disease, Parkinson’s disease), cardiovascular diseases, cancer, diabetes, and aging are associated with accumulation of misfolded and/or aggregated proteins within certain tissues, attributable in part to defective cellular stress response pathways [32,17,102,100,101]. In addition, inflammation and oxidant damage also contribute to the pathogenic processes in these disorders. Accordingly, pharmacological agents that possess anti-inflammatory and anti-oxidant activities, and can reset these defective pathways are increasingly being sought [32,17,102].
Celastrol has been shown to have a cytoprotective effect in response to stress-induced cell death. Celastrol can induce the expression of various Hsps, for example, Hsp70, Hsp40, and Hsp27, by activation of HSF1 and these Hsps might contribute to its cytoprotective effect [103]. Celastrol’s anti-oxidant attributes can also contribute to its cytoprotective effects. As described under cell signaling, the anti-oxidant response involves transcription factors Nrf-2 and Atf4, and celastrol has been shown to activate both these transcription factors [17]. In one study, celastrol’s cytoprotective effect was shown to be mediated via induction of Hsp32 (also known as heme oxygenase-1; HO-1) [104]. This induction of Hsp32 was mediated via Nrf-2 instead of HSF-1, and Hsp32 in turn inhibited pro-apoptotic JNK. Besides the induction of Hsps mentioned above, inhibition of Hsp90 has been shown to have a therapeutic effect in certain neurodegenerative diseases; the latter effect is attributable to selective proteasomal degradation of Hsp90 client proteins [100]. Importantly, celastrol is an inhibitor of Hsp90 [105,106], and it can modulate several nuclear transcription factors that are Hsp90 clients, including the aryl hydrocarbon receptor (AhR) [105],[106]. The involvement of celastrol-induced inhibition of Hsp90 and its anti-cancer effect is discussed below.
ANTI-CANCER ACTIVITY OF CELASTROL
Celastrol is known to have anti-cancer and anti-metastatic activities [107,82,83,15,108]. In addition, celastrol is shown to enhance the therapeutic efficacy of other anti-cancer drugs when used with them, and to potentiate the beneficial effects of radiotherapy [109,110]. The major processes involved in these activities and affected by celastrol include, the inhibition of cellular proliferation, induction of apoptosis, prevention of malignant tissue invasion, and blockade of angiogenesis [82,7,111,15]. Celastrol can inhibit cell proliferation and induce apoptosis via multiple actions. These include, potentiation of TNF-induced apoptosis via suppression of the NF-kB pathway [4]; downregulation of cytokines such as IL-6, which is an inducer of cell proliferation [112]; activation of caspases [113–115]; inhibition of the expression of anti-apoptotic proteins such as cellular inhibitor of apoptosis protein 1 and 2 (cIAP1 and cIAP2), cellular FLICE-inhibitory protein (FLIP), and B-cell lymphoma 2 (Bcl-2) [4,114]; induction of cell cycle arrest [81,116]; and downregulation of cell survival proteins coupled with upregulation of death receptors [117]. Furthermore, celastrol inhibits adhesion, migration and invasion of tumor cells via reduced expression of specific integrins, as well as reduced MMP activity [118–120]. In addition, as described above, celastrol can suppress angiogenesis [63,83].
Two additional mechanisms contribute to the anti-cancer effects of celastrol, namely inhibition of Hsp90 and proteasome inhibition. In regard to Hsp90, celastrol directly binds to the C-terminal domain of Hsp90 inducing oligomerization, and it interferes with specific biological functions through modulation of Hsp90-associated nuclear transcription factors [121,106]. In addition, celastrol has been shown to inhibit Hsp90 in thiol-related reactions[116], and to downregulate Hsp90 client proteins via inhibition of enzymes of mitochondrial complexes and accumulation of ROS [80]. Furthermore, celastrol-induced inhibition of Hsp90 contributes to HIF-1α inhibition and cell cycle arrest [92]. As far as the proteasome is concerned, celastrol has been shown to inhibit proteasome function by targeting its chymotrypsin-like activity. This in turn results in accumulation of ubiquitinated proteins and some of the known proteasomal substrates such as cell cycle-regulating proteins and others (IkBa, Bax and p27, etc.) leading to inhibition of cell proliferation coupled with induction of apoptosis [122,123]. Accordingly, proteasomal inhibition has also been exploited in cancer therapy [124,123]. Inhibition of NF-kB activation may contribute to the beneficial effect of proteasomal inhibition therapy. In addition, NF-kB inhibitors combined with standard chemotherapy drugs might be of benefit in the chronic inflammatory stage of tumor progression [125]. However, this aspect of cancer therapy needs further evaluation.
Another beneficial effect of celastrol in cancer relates to its ability to remodel bone by relatively reducing osteoclastic activity, while maintaining or increasing osteoblastic activity. Metastasis of cancer to the bone may cause osteolysis, which involves increased bone resorption. In regard to bone remodeling, the receptor activator of nuclear factor-κB ligand (RANKL) promotes the proliferation and differentiation of osteoclasts, whereas osteoprotegerin (OPG) secreted by the osteoblasts is a soluble decoy receptor for RANKL, and it serves as a natural inhibitor of osteoclast activation. In a study on an osteolytic bone metastasis model, celastrol suppressed trabecular bone loss, reduced the number and size of osteolytic bone lesions, osteoclast number, and bone resorption [126]. In another study on human osteosarcoma cells, celastrol caused G2/M phase cell cycle arrest, and induced apoptosis and autophagy [81]. These observations in cancer studies are supported by the results of our study in rat AA model [9]. Celastrol protected against bone and cartilage damage by regulating pro-inflammatory cytokines, inhibiting RANKL, increasing RANKL/OPG ratio, inhibiting the secretion of matrix-degrading enzymes such as MMPs, and reducing the number of osteoclasts without much effect on osteoblasts [9].
USE OF CELASTROL-CONTAINING NATURAL PRODUCTS FOR THE TREATMENT OF CHRONIC INFLAMMATORY AND AUTOIMMUNE DISEASES IN HUMANS.
Celastrol is present in several plants belonging to the celastraceae family. Some of these plants have been used in traditional Chinese medicine (TCM) for several decades/centuries as medicinal herbs for the treatment of a wide range of chronic inflammatory disorders. For example, the extracts of the root, bark and stem of Tripterygium wilfordii (Thunder God Vine), Celastrus orbiculatus, Celastrus aculeatus and some other members of the Celastraceae family have been used for the treatment of RA, SLE, and other disorders [5,1,3,2,127]. However, a large part of this information is based on folklores as well as documented descriptions of the use of these herbal products in old archived literature. Limited available information is derived from studies on small numbers of patients and/or scientifically-controlled randomized clinical studies on the use of T. wilfordii in chronic inflammatory and autoimmune diseases such as RA, juvenile RA, ankylosing spondylitis, and SLE [5,128–132]. Of these, the most reliable clinical studies have been performed using T. wilfordii in patients with RA. The efficacy of T. wilfordii extract against RA was compared with that of two of the mainstream anti-arthritic drugs, namely sulphasalazine and methotrexate. Interestingly, T. wilfordii extract reduced the severity of RA as assessed by well-established criteria, and the efficacy of T. wilfordii was comparable to, or better than, that of sulphasalazine/ methotrexate [128,133–136]. Furthermore, the combination of T. wilfordii and methotrexate was better than methotrexate alone. However, the toxicity profile of this natural product needs further assessment before T. wilfordii is approved for therapeutic purposes.
CONCLUDING REMARKS
Celastrol, a natural triterpene, has anti-inflammatory, anti-oxidant, and anti-cancer activities. Besides targeting multiple cell signaling pathways, celastrol modulates several other pathophysiological processes involved in chronic inflammatory diseases, autoimmune diseases, and cancer. Most of this information on celastrol is based on in vitro model systems in the laboratory and preclinical studies in animal models of human diseases. These studies have also offered mechanistic insights into the use of celastrol-containing herbal extracts from celastraceae family of plants for the treatment of some of these disorders in the traditional systems of medicine. Taken together, this knowledge has encouraged the clinical testing of T. wilfordii and related herbal preparations. In particular, the testing of T. wilfordii in RA patients has shown promising results. It is hoped that in the near future, T. wilfordii and similar other natural products might be approved for use in mainstream therapy as adjuncts for, or in place of, conventional allopathic drugs for RA and some other chronic diseases. This would be a significant contribution to the therapeutic arsenal against several chronic debilitating human diseases.
ACKNOWLEDGEMENTS
We thank Dr. Hua Yu, Dr. Brian Astry, Dr. Siddaraju Nanjundaiah, Dr. Rajesh Rajaiah, and Dr. Li Tong for their contribution to the original studies based on celastrol as well as for their helpful discussions. This work was supported by National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM) grant number AT004321.
REFERENCES
- 1.Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH, Lee JJ (2002) Antiinflammatory constituents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production. J Nat Prod 65 (1):89–91 [DOI] [PubMed] [Google Scholar]
- 2.Luo DQ, Wang H, Tian X, Shao HJ, Liu JK (2005) Antifungal properties of pristimerin and celastrol isolated from Celastrus hypoleucus. Pest Manag Sci 61 (1):85–90. doi: 10.1002/ps.953 [DOI] [PubMed] [Google Scholar]
- 3.Tong L, Moudgil KD (2007) Celastrus aculeatus Merr. suppresses the induction and progression of autoimmune arthritis by modulating immune response to heat-shock protein 65. Arthritis Res Ther 9 (4):R70. doi: 10.1186/ar2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi G, Ahn KS, Pandey MK, Aggarwal BB (2007) Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood 109 (7):2727–2735. doi: 10.1182/blood-2006-10-050807 [DOI] [PubMed] [Google Scholar]
- 5.Wong KF, Yuan Y, Luk JM (2012) Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin Exp Pharmacol Physiol 39 (3):311–320. doi: 10.1111/j.1440-1681.2011.05586.x [DOI] [PubMed] [Google Scholar]
- 6.Salminen A, Lehtonen M, Paimela T, Kaarniranta K (2010) Celastrol: Molecular targets of Thunder God Vine. Biochem Biophys Res Commun 394 (3):439–442. doi: 10.1016/j.bbrc.2010.03.050 [DOI] [PubMed] [Google Scholar]
- 7.Kannaiyan R, Shanmugam MK, Sethi G (2011) Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303 (1):9–20. doi: 10.1016/j.canlet.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 8.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD (2011) Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem 286 (17):15138–15146. doi: 10.1074/jbc.M111.226365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanjundaiah SM, Venkatesha SH, Yu H, Tong L, Stains JP, Moudgil KD (2012) Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J Biol Chem 287 (26):22216–22226. doi: 10.1074/jbc.M112.356816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Cao L, Xu LM, Cao FF, Peng B, Zhang X, Shen YF, Uzan G, Zhang DH (2015) Celastrol Ameliorates EAE Induction by Suppressing Pathogenic T Cell Responses in the Peripheral and Central Nervous Systems. J Neuroimmune Pharmacol. doi: 10.1007/s11481-015-9598-9 [DOI] [PubMed] [Google Scholar]
- 11.Shaker ME, Ashamallah SA, Houssen ME (2014) Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chem Biol Interact 210:26–33. doi: 10.1016/j.cbi.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 12.Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B (2009) Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci 10:109. doi: 10.1186/1471-2202-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Ma L, Zhou GB (2011) The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules 16 (6):5283–5297. doi: 10.3390/molecules16065283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbas Bukhari SN, Jantan I, Seyed MA (2015) Effects of Plants and Isolates of Celastraceae Family on Cancer Pathways. Anticancer Agents Med Chem [DOI] [PubMed] [Google Scholar]
- 15.Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB (2010) Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel) 2 (10):2428–2466. doi: 10.3390/toxins2102428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, Hong YS, Lee JJ (2006) Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol 72 (10):1311–1321. doi: 10.1016/j.bcp.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Trott A, West JD, Klaic L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA (2008) Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell 19 (3):1104–1112. doi: 10.1091/mbc.E07-10-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan V, Ravindra KC, Chiaro C, Cary D, Aggarwal BB, Henderson AJ, Prabhu KS (2011) Celastrol inhibits Tat-mediated human immunodeficiency virus (HIV) transcription and replication. Journal of molecular biology 410 (5):972–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascao R, Vidal B, Raquel H, Neves-Costa A, Figueiredo N, Gupta V, Fonseca JE, Moita LF (2012) Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun Rev 11 (12):856–862. doi: 10.1016/j.autrev.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Astry B, Venkatesha SH, Laurence A, Christensen-Quick A, Garzino-Demo A, Frieman MB, O’Shea JJ, Moudgil KD (2015) Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol 157 (2):228–238. doi: 10.1016/j.clim.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Liu D, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, Hisamitsu T, Liu Y (2013) Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-kappaB-mediated matrix metalloproteinase-9 expression. PLoS One 8 (7):e68905. doi: 10.1371/journal.pone.0068905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdin AA, Hasby EA (2014) Modulatory effect of celastrol on Th1/Th2 cytokines profile, TLR2 and CD3+ T-lymphocyte expression in a relapsing-remitting model of multiple sclerosis in rats. Eur J Pharmacol 742:102–112. doi: 10.1016/j.ejphar.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zhang YY, Huang XY, Sun YN, Jia YF, Li D (2005) Beneficial effect of tripterine on systemic lupus erythematosus induced by active chromatin in BALB/c mice. Eur J Pharmacol 512 (2–3):231–237. doi: 10.1016/j.ejphar.2005.02.030 [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Wu Z (2002) [The effect of tripterine in prevention of glomerulosclerosis in lupus nephritis mice]. Zhonghua Nei Ke Za Zhi 41 (5):317–321 [PubMed] [Google Scholar]
- 25.Xu X, Zhong J, Wu Z, Fang Y, Xu C (2007) Effects of tripterine on mRNA expression of TGF-beta1 and collagen IV expression in BW F1 mice. Cell Biochem Funct 25 (5):501–507. doi: 10.1002/cbf.1338 [DOI] [PubMed] [Google Scholar]
- 26.Kim DY, Park JW, Jeoung D, Ro JY (2009) Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol 612 (1–3):98–105. doi: 10.1016/j.ejphar.2009.03.078 [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Kim K, Lee H, Han S, Lee YS, Choe J, Kim YM, Hahn JH, Ro JY, Jeoung D (2009) Celastrol binds to ERK and inhibits FcepsilonRI signaling to exert an anti-allergic effect. Eur J Pharmacol 612 (1–3):131–142. doi: 10.1016/j.ejphar.2009.03.071 [DOI] [PubMed] [Google Scholar]
- 28.Liu RL, Liu ZL, Li Q, Qiu ZM, Lu HJ, Yang ZM, Hong GC (2004) [The experimental study on the inhibitory effect of tripterine on airway inflammation in asthmatic mice]. Zhonghua Jie He He Hu Xi Za Zhi 27 (3):165–168 [PubMed] [Google Scholar]
- 29.Zhang LX, Yu FK, Zheng QY, Fang Z, Pan DJ (1990) [Immunosuppressive and antiinflammatory activities of tripterine]. Yao Xue Xue Bao 25 (8):573–577 [PubMed] [Google Scholar]
- 30.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C (2001) Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Progress in neuro-psychopharmacology & biological psychiatry 25 (7):1341–1357 [DOI] [PubMed] [Google Scholar]
- 31.Choi BS, Kim H, Lee HJ, Sapkota K, Park SE, Kim S, Kim SJ (2014) Celastrol from ‘Thunder God Vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson’s disease. Neurochem Res 39 (1):84–96. doi: 10.1007/s11064-013-1193-y [DOI] [PubMed] [Google Scholar]
- 32.Chow AM, Brown IR (2007) Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones 12 (3):237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow AM, Tang DW, Hanif A, Brown IR (2014) Localization of heat shock proteins in cerebral cortical cultures following induction by celastrol. Cell Stress Chaperones 19 (6):845–851. doi: 10.1007/s12192-014-0508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleren C, Calingasan NY, Chen J, Beal MF (2005) Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. Journal of neurochemistry 94 (4):995–1004. doi: 10.1111/j.1471-4159.2005.03253.x [DOI] [PubMed] [Google Scholar]
- 35.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF (2005) Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis 2 (5):246–254. doi: 10.1159/000090364 [DOI] [PubMed] [Google Scholar]
- 36.Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, March A, Ait-Ghezala G, Mullan MJ (2010) Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation 7:17. doi: 10.1186/1742-2094-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabuchi H, Konishi M, Saito N, Kato M, Mimura M (2014) Reverse Fox test for detecting visuospatial dysfunction corresponding to parietal hypoperfusion in mild Alzheimer’s disease. Am J Alzheimers Dis Other Demen 29 (2):177–182. doi: 10.1177/1533317513511291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, Swallows CL, Zhang C, Lu J, Xiao H, Brady RO, Zhuang Z (2014) Celastrol increases glucocerebrosidase activity in Gaucher disease by modulating molecular chaperones. Proc Natl Acad Sci U S A 111 (1):249–254. doi: 10.1073/pnas.1321341111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paimela T, Hyttinen JM, Viiri J, Ryhanen T, Karjalainen RO, Salminen A, Kaarniranta K (2011) Celastrol regulates innate immunity response via NF-kappaB and Hsp70 in human retinal pigment epithelial cells. Pharmacol Res 64 (5):501–508. doi: 10.1016/j.phrs.2011.05.027 [DOI] [PubMed] [Google Scholar]
- 40.Hu H, Straub A, Tian Z, Bassler N, Cheng J, Peter K (2009) Celastrol, a triterpene extracted from Tripterygium wilfordii Hook F, inhibits platelet activation. J Cardiovasc Pharmacol 54 (3):240–245. doi: 10.1097/FJC.0b013e3181b21472 [DOI] [PubMed] [Google Scholar]
- 41.Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y, Meng G, Xie L, Wang J, Xiao Y, Shan L, Zhou S, Wei L, Ferro A, Ji Y (2013) Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One 8 (6):e65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U (2015) Treatment of obesity with celastrol. Cell 161 (5):999–1011. doi: 10.1016/j.cell.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JE, Lee MH, Nam DH, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Han SY, Han KH, Han JY, Cha DR (2013) Celastrol, an NF-kappaB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One 8 (4):e62068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youn GS, Kwon DJ, Ju SM, Rhim H, Bae YS, Choi SY, Park J (2014) Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol Appl Pharmacol 280 (1):42–52. doi: 10.1016/j.taap.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 45.Tallorin L, Durrant JD, Nguyen QG, McCammon JA, Burkart MD (2014) Celastrol inhibits Plasmodium falciparum enoyl-acyl carrier protein reductase. Bioorganic & medicinal chemistry 22 (21):6053–6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmonds RE, Foxwell BM (2008) Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 47 (5):584–590. doi: 10.1093/rheumatology/kem298 [DOI] [PubMed] [Google Scholar]
- 47.Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107 (1):7–11. doi: 10.1172/JCI11830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bondeson J, Foxwell B, Brennan F, Feldmann M (1999) Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci U S A 96 (10):5668–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ju SM, Youn GS, Cho YS, Choi SY, Park J (2015) Celastrol ameliorates cytokine toxicity and pro-inflammatory immune responses by suppressing NF-kappaB activation in RINm5F beta cells. BMB Rep 48 (3):172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hommes DW, Peppelenbosch MP, van Deventer SJ (2003) Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 52 (1):144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaminska B (2005) MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754 (1–2):253–262. doi: 10.1016/j.bbapap.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 52.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ (1996) FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J 15 (17):4629–4642 [PMC free article] [PubMed] [Google Scholar]
- 53.Pierrat B, Correia JS, Mary JL, Tomas-Zuber M, Lesslauer W (1998) RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38alpha mitogen-activated protein kinase (p38alphaMAPK). J Biol Chem 273 (45):29661–29671 [DOI] [PubMed] [Google Scholar]
- 54.Jung HW, Chung YS, Kim YS, Park YK (2007) Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp Mol Med 39 (6):715–721. doi: 10.1038/emm.2007.78 [DOI] [PubMed] [Google Scholar]
- 55.Kannaiyan R, Manu KA, Chen L, Li F, Rajendran P, Subramaniam A, Lam P, Kumar AP, Sethi G (2011) Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis 16 (10):1028–1041 [DOI] [PubMed] [Google Scholar]
- 56.Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, Kim JK (2009) Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J Clin Invest 39 (9):819–827. doi: 10.1111/j.1365-2362.2009.02186.x [DOI] [PubMed] [Google Scholar]
- 57.Zhu H, Liu XW, Cai TY, Cao J, Tu CX, Lu W, He QJ, Yang B (2010) Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J Pharmacol Exp Ther 334 (2):489–499. doi: 10.1124/jpet.110.165654 [DOI] [PubMed] [Google Scholar]
- 58.Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3 (11):900–911. doi: 10.1038/nri1226 [DOI] [PubMed] [Google Scholar]
- 59.Darnell JE Jr. (1997) STATs and gene regulation. Science 277 (5332):1630–1635 [DOI] [PubMed] [Google Scholar]
- 60.Kannaiyan R, Hay HS, Rajendran P, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Chng WJ, Sethi G (2011) Celastrol inhibits proliferation and induces chemosensitization through down-regulation of NF-kappaB and STAT3 regulated gene products in multiple myeloma cells. Br J Pharmacol 164 (5):1506–1521. doi: 10.1111/j.1476-5381.2011.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajendran P, Li F, Shanmugam MK, Kannaiyan R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, Luk JM, Sethi G (2012) Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of STAT3/JAK2 signaling cascade in vitro and in vivo. Cancer Prev Res (Phila) 5 (4):631–643. doi: 10.1158/1940-6207.CAPR-11-0420 [DOI] [PubMed] [Google Scholar]
- 62.Chen S, Gu C, Xu C, Zhang J, Xu Y, Ren Q, Guo M, Huang S, Chen L (2014) Celastrol prevents cadmium-induced neuronal cell death via targeting JNK and PTEN-Akt/mTOR network. Journal of neurochemistry 128 (2):256–266. doi: 10.1111/jnc.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma J, Han LZ, Liang H, Mi C, Shi H, Lee JJ, Jin X (2014) Celastrol inhibits the HIF-1alpha pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep 32 (1):235–242. doi: 10.3892/or.2014.3211 [DOI] [PubMed] [Google Scholar]
- 64.Mabuchi S, Kuroda H, Takahashi R, Sasano T (2015) The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol 137 (1):173–179. doi: 10.1016/j.ygyno.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 65.Sha M, Ye J, Zhang LX, Luan ZY, Chen YB, Huang JX (2014) Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-kappaB signaling pathway. Pharmacology 93 (1–2):39–46. doi: 10.1159/000357683 [DOI] [PubMed] [Google Scholar]
- 66.Shrivastava S, Jeengar MK, Reddy VS, Reddy GB, Naidu VG (2015) Anticancer effect of celastrol on human triple negative breast cancer: Possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol Pathol 98 (3):313–327. doi: 10.1016/j.yexmp.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 67.Zhao J, Sun Y, Shi P, Dong JN, Zuo LG, Wang HG, Gong JF, Li Y, Gu LL, Li N, Li JS, Zhu WM (2015) Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int Immunopharmacol 26 (1):221–228. doi: 10.1016/j.intimp.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 68.Polivka J Jr., Janku F (2014) Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther 142 (2):164–175. doi: 10.1016/j.pharmthera.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 69.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22 (20):7004–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han X, Sun S, Zhao M, Cheng X, Chen G, Lin S, Guan Y, Yu X (2014) Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1alpha protein synthesis. PLoS One 9 (11):e112470. doi: 10.1371/journal.pone.0112470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29 (10):2570–2581. doi: 10.1128/MCB.00166-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onnis B, Rapisarda A, Melillo G (2009) Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med 13 (9A):2780–2786. doi: 10.1111/j.1582-4934.2009.00876.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holmstrom KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15 (6):411–421. doi: 10.1038/nrm3801 [DOI] [PubMed] [Google Scholar]
- 74.Ye ZW, Zhang J, Townsend DM, Tew KD (2015) Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim Biophys Acta 1850 (8):1607–1621. doi: 10.1016/j.bbagen.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halliwell B (1987) Free radicals and metal ions in health and disease. Proc Nutr Soc 46 (1):13–26 [DOI] [PubMed] [Google Scholar]
- 76.Sassa H, Takaishi Y, Terada H (1990) The triterpene celastrol as a very potent inhibitor of lipid peroxidation in mitochondria. Biochem Biophys Res Commun 172 (2):890–897 [DOI] [PubMed] [Google Scholar]
- 77.Sassa H, Kogure K, Takaishi Y, Terada H (1994) Structural basis of potent antiperoxidation activity of the triterpene celastrol in mitochondria: effect of negative membrane surface charge on lipid peroxidation. Free Radic Biol Med 17 (3):201–207 [DOI] [PubMed] [Google Scholar]
- 78.Divya TDV, Soumyakrisnan S, Sureshkumar S, Sudandiran G. (2016) Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chemico-Biological Interactions:Epub Ahead of Print [DOI] [PubMed] [Google Scholar]
- 79.Wang C, Shi C, Yang X, Yang M, Sun H, Wang C (2014) Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur J Pharmacol 744:52–58. doi: 10.1016/j.ejphar.2014.09.043 [DOI] [PubMed] [Google Scholar]
- 80.Chen G, Zhang X, Zhao M, Wang Y, Cheng X, Wang D, Xu Y, Du Z, Yu X (2011) Celastrol targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent cytotoxicity in tumor cells. BMC Cancer 11:170. doi: 10.1186/1471-2407-11-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie T, Zhang N, Ye ZM (2015) Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study. Cell Death Dis 6:e1604. doi: 10.1038/cddis.2014.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29 (3):405–434. doi: 10.1007/s10555-010-9235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pang X, Yi Z, Zhang J, Lu B, Sung B, Qu W, Aggarwal BB, Liu M (2010) Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res 70 (5):1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan KA, Bicknell R (2015) Anti-angiogenic alternatives to VEGF blockade. Clinical & experimental metastasis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szekanecz Z, Besenyei T, Paragh G, Koch AE (2009) Angiogenesis in rheumatoid arthritis. Autoimmunity 42 (7):563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koch AE (2000) The role of angiogenesis in rheumatoid arthritis: recent developments. Ann Rheum Dis 59 Suppl 1:i65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Husein B, Abdalla M, Trepte M, Deremer DL, Somanath PR (2012) Antiangiogenic therapy for cancer: an update. Pharmacotherapy 32 (12):1095–1111. doi: 10.1002/phar.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gan K, Xu L, Feng X, Zhang Q, Wang F, Zhang M, Tan W (2015) Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int Immunopharmacol 24 (2):239–246. doi: 10.1016/j.intimp.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 89.Dai Y, Desano J, Tang W, Meng X, Meng Y, Burstein E, Lawrence TS, Xu L (2010) Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS One 5 (12):e14153. doi: 10.1371/journal.pone.0014153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ji N, Li J, Wei Z, Kong F, Jin H, Chen X, Li Y, Deng Y (2015) Effect of celastrol on growth inhibition of prostate cancer cells through the regulation of hERG channel in vitro. Biomed Res Int 2015:308475. doi: 10.1155/2015/308475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou YX, Huang YL (2009) Antiangiogenic effect of celastrol on the growth of human glioma: an in vitro and in vivo study. Chin Med J (Engl) 122 (14):1666–1673 [PubMed] [Google Scholar]
- 92.Huang L, Zhang Z, Zhang S, Ren J, Zhang R, Zeng H, Li Q, Wu G (2011) Inhibitory action of Celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1alpha pathway. Int J Mol Med 27 (3):407–415 [DOI] [PubMed] [Google Scholar]
- 93.Huang S, Tang Y, Cai X, Peng X, Liu X, Zhang L, Xiang Y, Wang D, Wang X, Pan T (2012) Celastrol inhibits vasculogenesis by suppressing the VEGF-induced functional activity of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun 423 (3):467–472 [DOI] [PubMed] [Google Scholar]
- 94.Ni H, Zhao W, Kong X, Li H, Ouyang J (2013) Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing TLR4-triggered nuclear factor-kappa B activation. Acta Haematol 131 (2):102–111 [DOI] [PubMed] [Google Scholar]
- 95.Huang Y, Zhou Y, Fan Y, Zhou D (2008) Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett 264 (1):101–106. doi: 10.1016/j.canlet.2008.01.043 [DOI] [PubMed] [Google Scholar]
- 96.Zhang DH, Marconi A, Xu LM, Yang CX, Sun GW, Feng XL, Ling CQ, Qin WZ, Uzan G, d’Alessio P (2006) Tripterine inhibits the expression of adhesion molecules in activated endothelial cells. J Leukoc Biol 80 (2):309–319. doi: 10.1189/jlb.1005611 [DOI] [PubMed] [Google Scholar]
- 97.Wu F, Han M, Wilson JX (2009) Tripterine prevents endothelial barrier dysfunction by inhibiting endogenous peroxynitrite formation. Br J Pharmacol 157 (6):1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677 [DOI] [PubMed] [Google Scholar]
- 99.Rajaiah R, Moudgil KD (2009) Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev 8 (5):388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adachi H, Katsuno M, Waza M, Minamiyama M, Tanaka F, Sobue G (2009) Heat shock proteins in neurodegenerative diseases: pathogenic roles and therapeutic implications. Int J Hyperthermia 25 (8):647–654 [DOI] [PubMed] [Google Scholar]
- 101.Arawaka S, Machiya Y, Kato T (2010) Heat shock proteins as suppressors of accumulation of toxic prefibrillar intermediates and misfolded proteins in neurodegenerative diseases. Curr Pharm Biotechnol 11 (2):158–166 [DOI] [PubMed] [Google Scholar]
- 102.Muchowski PJ, Wacker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6 (1):11–22 [DOI] [PubMed] [Google Scholar]
- 103.Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI (2004) Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem 279 (53):56053–56060 [DOI] [PubMed] [Google Scholar]
- 104.Francis SP, Kramarenko II, Brandon CS, Lee FS, Baker TG, Cunningham LL (2011) Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32. Cell Death Dis 2:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hughes D, Guttenplan JB, Marcus CB, Subbaramaiah K, Dannenberg AJ (2008) Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation. Cancer Prev Res (Phila) 1 (6):485–493 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Zhang D, Xu L, Cao F, Wei T, Yang C, Uzan G, Peng B (2010) Celastrol regulates multiple nuclear transcription factors belonging to HSP90’s clients in a dose- and cell type-dependent way. Cell Stress Chaperones 15 (6):939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petronelli A, Pannitteri G, Testa U (2009) Triterpenoids as new promising anticancer drugs. Anticancer Drugs 20 (10):880–892. doi: 10.1097/CAD.0b013e328330fd90 [DOI] [PubMed] [Google Scholar]
- 108.Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, Chaturvedi MM, Aggarwal BB (2010) Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. Journal of molecular medicine (Berlin, Germany) 88 (12):1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng L, Fu Y, Zhuang L, Gai R, Ma J, Lou J, Zhu H, He Q, Yang B (2014) Simultaneous NF-kappaB inhibition and E-cadherin upregulation mediate mutually synergistic anticancer activity of celastrol and SAHA in vitro and in vivo. Int J Cancer 135 (7):1721–1732. doi: 10.1002/ijc.28810 [DOI] [PubMed] [Google Scholar]
- 110.Dai Y, DeSano JT, Meng Y, Ji Q, Ljungman M, Lawrence TS, Xu L (2009) Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys 74 (4):1217–1225. doi: 10.1016/j.ijrobp.2009.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li-Weber M (2013) Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett 332 (2):304–312. doi: 10.1016/j.canlet.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 112.Chiang KC, Tsui KH, Chung LC, Yeh CN, Chen WT, Chang PL, Juang HH (2014) Celastrol blocks interleukin-6 gene expression via downregulation of NF-kappaB in prostate carcinoma cells. PLoS One 9 (3):e93151. doi: 10.1371/journal.pone.0093151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu L, Shi W, Deshmukh RR, Long J, Cheng X, Ji W, Zeng G, Chen X, Zhang Y, Dou QP (2014) Tumor necrosis factor-alpha sensitizes breast cancer cells to natural products with proteasome-inhibitory activity leading to apoptosis. PLoS One 9 (11):e113783. doi: 10.1371/journal.pone.0113783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mi C, Shi H, Ma J, Han LZ, Lee JJ, Jin X (2014) Celastrol induces the apoptosis of breast cancer cells and inhibits their invasion via downregulation of MMP-9. Oncol Rep 32 (6):2527–2532. doi: 10.3892/or.2014.3535 [DOI] [PubMed] [Google Scholar]
- 115.Yang HS, Kim JY, Lee JH, Lee BW, Park KH, Shim KH, Lee MK, Seo KI (2011) Celastrol isolated from Tripterygium regelii induces apoptosis through both caspase-dependent and -independent pathways in human breast cancer cells. Food Chem Toxicol 49 (2):527–532. doi: 10.1016/j.fct.2010.11.044 [DOI] [PubMed] [Google Scholar]
- 116.Peng B, Xu L, Cao F, Wei T, Yang C, Uzan G, Zhang D (2010) HSP90 inhibitor, celastrol, arrests human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way. Mol Cancer 9:79. doi: 10.1186/1476-4598-9-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sung B, Park B, Yadav VR, Aggarwal BB (2010) Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem 285 (15):11498–11507 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Li GQ, Liu D, Zhang Y, Qian YY, Zhu YD, Guo SY, Sunagawa M, Hisamitsu T, Liu YQ (2013) Anti-invasive effects of celastrol in hypoxia-induced fibroblast-like synoviocyte through suppressing of HIF-1alpha/CXCR4 signaling pathway. Int Immunopharmacol 17 (4):1028–1036. doi: 10.1016/j.intimp.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 119.Xu J, Wu CL, Huang J (2015) [Effect of celastrol in inhibiting metastasis of lung cancer cells by influencing Akt signaling pathway and expressing integrins]. Zhongguo Zhong Yao Za Zhi 40 (6):1129–1133 [PubMed] [Google Scholar]
- 120.Xu J, Wu CL (2015) [Anti-metastasis of celastrol on esophageal cancer cells and its mechanism]. Sheng Li Xue Bao 67 (3):341–347 [PubMed] [Google Scholar]
- 121.Zanphorlin LM, Alves FR, Ramos CH (2014) The effect of celastrol, a triterpene with antitumorigenic activity, on conformational and functional aspects of the human 90kDa heat shock protein Hsp90alpha, a chaperone implicated in the stabilization of the tumor phenotype. Biochim Biophys Acta 1840 (10):3145–3152. doi: 10.1016/j.bbagen.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 122.Wang WB, Feng LX, Yue QX, Wu WY, Guan SH, Jiang BH, Yang M, Liu X, Guo DA (2012) Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J Cell Physiol 227 (5):2196–2206 [DOI] [PubMed] [Google Scholar]
- 123.Yang H, Landis-Piwowar KR, Chen D, Milacic V, Dou QP (2008) Natural compounds with proteasome inhibitory activity for cancer prevention and treatment. Curr Protein Pept Sci 9 (3):227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dou QP, Zonder JA (2014) Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Current cancer drug targets 14 (6):517–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoesel B, Schmid JA (2013) The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH (2009) Pharmacologic inhibitors of IkappaB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol Cancer Ther 8 (8):2339–2347. doi: 10.1158/1535-7163.MCT-09-0133 [DOI] [PubMed] [Google Scholar]
- 127.Wang KW, Mao JS, Tai YP, Pan YJ (2006) Novel skeleton terpenes from Celastrus hypoleucus with anti-tumor activities. Bioorg Med Chem Lett 16 (8):2274–2277. doi: 10.1016/j.bmcl.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 128.Tao X, Cush JJ, Garret M, Lipsky PE (2001) A phase I study of ethyl acetate extract of the chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol 28 (10):2160–2167 [PubMed] [Google Scholar]
- 129.Zhang W, Shi Q, Zhao LD, Li Y, Tang FL, Zhang FC, Zhang X (2010) The safety and effectiveness of a chloroform/methanol extract of Tripterygium wilfordii Hook F (T2) plus methotrexate in treating rheumatoid arthritis. J Clin Rheumatol 16 (8):375–378. doi: 10.1097/RHU.0b013e3181fe8ad1 [DOI] [PubMed] [Google Scholar]
- 130.Ji W, Li J, Lin Y, Song YN, Zhang M, Ke Y, Ren Y, Deng X, Zhang J, Huang F, Yu D (2010) Report of 12 cases of ankylosing spondylitis patients treated with Tripterygium wilfordii. Clin Rheumatol 29 (9):1067–1072. doi: 10.1007/s10067-010-1497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao ZG, Zang AC, Bai RX (1986) Radix Tripterygium Wilfordii Hook F in rheumatoid arthritis, ankylosing spondylitis and juvenile rheumatoid arthritis. Chin Med J (Engl) 99 (4):317–320 [PubMed] [Google Scholar]
- 132.Patavino T, Brady DM (2001) Natural medicine and nutritional therapy as an alternative treatment in systemic lupus erythematosus. Altern Med Rev 6 (5):460–471 [PubMed] [Google Scholar]
- 133.Tao X, Younger J, Fan FZ, Wang B, Lipsky PE (2002) Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis and rheumatism 46 (7):1735–1743 [DOI] [PubMed] [Google Scholar]
- 134.Goldbach-Mansky R, Wilson M, Fleischmann R, Olsen N, Silverfield J, Kempf P, Kivitz A, Sherrer Y, Pucino F, Csako G, Costello R, Pham TH, Snyder C, van der Heijde D, Tao X, Wesley R, Lipsky PE (2009) Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Annals of internal medicine 151 (4):229–240, W249–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lv QW, Zhang W, Shi Q, Zheng WJ, Li X, Chen H, Wu QJ, Jiang WL, Li HB, Gong L, Wei W, Liu H, Liu AJ, Jin HT, Wang JX, Liu XM, Li ZB, Liu B, Shen M, Wang Q, Wu XN, Liang D, Yin YF, Fei YY, Su JM, Zhao LD, Jiang Y, Li J, Tang FL, Zhang FC, Lipsky PE, Zhang X (2014) Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204807 [DOI] [PubMed] [Google Scholar]
- 136.Moudgil KD, Berman BM (2014) Traditional Chinese medicine: potential for clinical treatment of rheumatoid arthritis. Expert review of clinical immunology 10 (7):819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H, Zhang YY, Tan HW, Jia YF, Li D (2008) Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol 118 (3):479–484. doi: 10.1016/j.jep.2008.05.028 [DOI] [PubMed] [Google Scholar]
- 138.Li H, Jia YF, Pan Y, Pan DJ, Li D, Zhang LX (1997) Effect of tripterine on collagen-induced arthritis in rats. Zhongguo Yao Li Xue Bao 18 (3):270–273 [PubMed] [Google Scholar]
- 139.Xu Z, Wu G, Wei X, Chen X, Wang Y, Chen L (2013) Celastrol induced DNA damage, cell cycle arrest, and apoptosis in human rheumatoid fibroblast-like synovial cells. Am J Chin Med 41 (3):615–628. doi: 10.1142/S0192415X13500432 [DOI] [PubMed] [Google Scholar]
- 140.Li GQ, Zhang Y, Liu D, Qian YY, Zhang H, Guo SY, Sunagawa M, Hisamitsu T, Liu YQ (2012) Celastrol inhibits interleukin-17A-stimulated rheumatoid fibroblast-like synoviocyte migration and invasion through suppression of NF-kappaB-mediated matrix metalloproteinase-9 expression. Int Immunopharmacol 14 (4):422–431. doi: 10.1016/j.intimp.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 141.Zhou LL, Lin ZX, Fung KP, Cheng CH, Che CT, Zhao M, Wu SH, Zuo Z (2011) Celastrol-induced apoptosis in human HaCaT keratinocytes involves the inhibition of NF-kappaB activity. Eur J Pharmacol 670 (2–3):399–408. doi: 10.1016/j.ejphar.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 142.Zhu F, Li C, Jin XP, Weng SX, Fan LL, Zheng Z, Li WL, Wang F, Wang WF, Hu XF, Lv CL, Liu P (2014) Celastrol may have an anti-atherosclerosis effect in a rabbit experimental carotid atherosclerosis model. International journal of clinical and experimental medicine 7 (7):1684–1691 [PMC free article] [PubMed] [Google Scholar]


