Abstract
Background & aims
Given reports of changes in dietary habits during covid-19 lockdown, our aim was to assess weight changes, over a 3-month Covid-19 national lockdown in a cohort of NAFLD-HIV patients on a dietary intervention trial.
Methods
After NAFLD screening in an outpatient Infectious Diseases Clinic, NAFLD patients were randomly allocated to general dietary recommendations (SC group) or to a structured dietary intervention based on the Mediterranean diet (intervention group). During lockdown, follow-up consultations in the intervention group were done by video and/or phone. After 3 months of lockdown, all patients (intervention and SC group) consented to a telephone interview which aimed to characterize eating habits and lifestyle changes and evaluate stress and depression. Biochemical data when available, was compared between the peri-period of confinement.
Results
One hundred and twelve patients were screened. From the 55 NAFDL identified, 27 were allocated to dietary intervention and 28 to SC and were followed before lockdown for a mean period of 5.0 ± 1.5 months in which SC group gained a median of 0.65 kg vs. a median loss of 1.5 kg in the intervention group (p < 0.001). During lockdown, 93.3% of patients in the SC group referred that “diet got worse” vs. 6.7% in the intervention group p < 0.01), and 35.3% vs. 15.7% (p = 0.014) reported increase in appetite, respectively. Both groups gained weight, SC group vs. 0.7 ± 1.7 kg in the intervention group, p < 0.001). Higher weight gain was associated with changes in the dietary pattern (3.8 ± 2.1 kg vs. 2.0 ± 1.3 kg in “no change in dietary pattern”; p = 0.002). Glucose blood levels increased after lockdown in the SC group, with a mean increase of 15 mg/dl (p = 0.023). The remaining metabolic parameters remained unchanged.
Conclusion
The maintenance of dietary intervention, using telemedicine, can mitigate the adverse change in dietary habits and physical activity pattern, preventing a substantial increase in body weight.
Keywords: Dietary intervention, Covid-19 lockdown, NAFLD, Nutrition
1. Introduction
The pandemic caused by SARS-Cov 2 has originated an unprecedent global lockdown, with every nation struggling with their own challenges and repercussions in social well-being [1]. Currently there are still some countries in this situation. From previous experiences, mandatory quarantines can result in psychological distress, generating depressive symptoms, irritability, anger, and insomnia [2,3].
Stressful events are recognized as triggers for alterations in eating patterns [2]. An increase in staying at home time could lead to a higher intake of alcohol and frequent snacking of comfort food and processed foods [1,2,[4], [5], [6]]. An increase in a sedentary behaviour motivated by social isolation and decreased outdoor time, further contribute to variations in body weight [1,2,7]. Recent studies have demonstrated that during lockdown, there was a significant reduction in physical activity and a change in dietary habits [2,6,8]. In particular, lockdown associates with an increase in dietary intake [6] mostly based on ultra-processed foods [5], comfort food [4,6] and drinking, which has been described as a way of dealing with anxiety and distress [3,4]. Additionally, changes in dietary habits may be a consequence of a lower access to grocery stores/supermarkets [9]. This change in dietary habits seems to promote weight gain and the development of obesity and eating disorders [1,2,4,7]. In fact, previous studies showed that patients already obese tend to gain a significant amount of weigh during lockdown [7,10]. Also, the disruption of circadian rhythms can lead to metabolic abnormalities, such as insulin resistance [2].
Non-alcoholic fatty liver disease (NAFLD) is a multisystemic disease that occurs in association with metabolic syndrome and in the absence of significant alcohol intake [[11], [12], [13]]. Currently there is no pharmacological treatment available, and lifestyle changes are the cornerstone of treatment of NAFLD [13,14]. In the last decade, there is a growing interest in NAFLD in patients living with human immunodeficiency virus (PLHIV). However, data on metabolic characteristics of this population and the efficacy of dietary intervention in PLHIV is scarce [[15], [16], [17], [18]].
Given that an increased compliance to dietary guidelines can possible mitigate metabolic disturbances [1], our aim was to assess the impact of a dietary intervention, on weight variation during a 3-month Covid-19 national lockdown in a cohort of NAFLD-HIV patients undergoing a dietary intervention trial, as well as the impact on metabolic data.
2. Materials and methods
2.1. Patients
Patients recruited from an outpatient Infectious Diseases Clinic were screened for NAFLD. The diagnosis was based on the presence of liver steatosis on ultrasound, after exclusion of significant alcohol intake (defined as more than 20 g per day in women and 30 g per day in men), and of other liver diseases, namely chronic hepatitis B or C, primary biliary cholangitis, autoimmune hepatitis, primary sclerosing cholangitis, Wilson's disease, hemochromatosis or α1-antitripsin deficiency. Patients were also excluded if there were under medication with potentially steatogenic drugs such as steroids, high dose estrogen, tamoxifen, methotrexate, or amiodarone within six months of enrolment, and if there were an history of gastrointestinal bypass surgery or segmental small bowel resection. The severity of liver disease was evaluated by non-invasive scores of fibrosis, FIB-4, as well as transient hepatic elastography (Fibroscan©) [19,20].
The study was approved by the local Human Ethics Committee and written informed consent was obtained from all participants.
2.2. Lifestyle counselling
NAFLD patients were consecutively and randomly allocated 1:1 to general dietary recommendations or to a structured dietary intervention that included general dietary recommendations and a nutritional plan. General recommendations, based on Mediterranean diet, consisted in the delivery of written resources containing healthy eating guidelines and tips. The structured dietary intervention included these and a detailed nutritional plan aiming at caloric restriction and weight loss, based on the Mediterranean diet and was individualized according to nutritional needs, with a caloric deficit of 500 kcal, and taking in to account the presence of other dietary restrictions alongside NAFLD, and the personal preferences and habits of the patient. With the nutritional plan, information on portion size, culinary specifications and exchange tips were provided. Other specifications of dietary advice included alcohol abstinence, and a limitation in total fruit content of the diet when the intake was above 5 units/day. Regarding physical activity, the aim was to stimulate an increase in baseline physical activity, with an incremental approach of exercise in everyday life. The intervention was conducted at baseline, 1 month, 3 months and 6 months by a single dietitian, all of them before lockdown. During appointments, adherence to recommendations was evaluated using PREDIMED, with maximum score of 14 [21,22]. All patients (SC and intervention group) had their weight monitored at 1, 3 and 6 months after baseline.
2.3. Clinical assessment and laboratorial tests
Anthropometric data were collected with participants wearing light clothes and bare foot. Weight was measured using a calibrated scale and height was assessed using a stadiometer. Waist circumference (WC) was measured at halfway between the inferior rib and the iliac crest. Body mass index (BMI) was defined as an individual's weight in kilograms divided by the square of height in meters (kg/m2). Body fat mass (BFM, %) was assessed using a single frequency analyser (OMNRON BF350).
Clinical data including liver biochemistry, abdominal ultrasound and transient hepatic elastography, were collected. FIB-4 score was calculated.
2.4. Lockdown assessment
Given the restrictions to clinical visits in the hospital, during the 3 months confinement, follow-up consultations in the intervention group were done by video and/or phone and included a review of dietary advice. Weight was measured using the patient's household equipment, with a measurement of error, using a standardized measure, conducted on the first video contact. Body composition during the confinement was unavailable in the participants' households.
After 3 months of lockdown, all patients (intervention and SC group) consented to a telephone interview which aimed to characterize eating habits and lifestyle changes during COVID-19 lockdown, using methodology previously published [23]. Also, four questions, previously validated for the Portuguese population, were included to characterize stress (“I found myself getting agitated”; “I found it difficult to relax”, “I had a hard time calming down”) and depression (“I felt discouraged and melancholic”) and asked the participants to define during this period, if this sentence applied or not to them. Questions were scored from zero to three (0-did not apply to me; 3-applied most of the times) [24].
Clinical data when available, was compared between the peri-period of confinement.
2.5. Statistical analysis
To determine a significant difference in both groups for α = 0.05 and a power of 0.8 we estimated a minimum sample of 21 participants in each group. Continuous variables were summarized as mean and standard deviation (mean ± SD) and categorical variables were summarized using frequency and percentage. Parametric and non-parametric tests were applied based on the different variable distribution. Chi-square was used for independence of categorical variables and Student T-Test or ANOVA, were used for continuous variables. Multiple comparisons adjusted with the Bonferroni correction. For each analysis, unadjusted and adjusted odds ratio (OR) and the corresponding 95% confidence interval (CI) and p-value were reported. For the analysis of peri-period of lockdown pairwise comparisons were used. The significance level was set at 5%. All statistical analyses were performed with IBM® SPSS® software, version 24.0.
3. Results
From the 148 HIV-positive patients referred for screening from the Infectious Disease Outpatient Clinic, 27 refused to participate in this study; 8 were excluded prior to screening due to the presence of co-infection with hepatitis C, 1 patient had an hospital admission, 2 patients had alcohol-associated liver disease, 3 patients had an opportunistic infection after referral, and 1 patient was lost to follow up. After screening, 15 additional patients were excluded due to the presence of previously undiagnosed alcohol-associated liver disease (in 9) and co-infection with viral hepatitis (in 6).
Ninety-seven patients were enrolled, mostly males (n = 72, 74.2%), Caucasian (n = 81, 83.5%) with a mean age of 54.6 ± 12.6 years and with a mean time since HIV diagnosis of 17.9 ± 8.3 years; 83.5% (n = 82) had T-CD4+≥ 500 cel/mm3; all presented undetectable viral load. As for metabolic risk factors: 17.5% (n = 17) had type 2 diabetes mellitus, 42.3% (n = 42) had hypertension, 45.4% (n = 44) had dyslipidaemia and 82.5% (n = 61) were overweight (BMI≥25 kg/m2) (Table 1 ). Regarding alcohol intake, 57.7% (n = 52) did not consume any alcoholic beverages.
Table 1.
Baseline patient characteristics.
| Non NAFLD Patients (n = 42) |
NAFLD Patients (n = 55) |
p-value | NAFLD Standard of Care Group (n = 28) |
NAFLD Intervention Group (n = 27) |
p-value | |
|---|---|---|---|---|---|---|
| Sex (%) | ||||||
| Male | 76.2 (32) | 72.7 (40) | >0,05 | 71.4 (20) | 74.1 (20) | >0,05 |
| Female | 23.8 (10) | 27.3 (15) | 28.6 (8) | 25.9 (7) | ||
| Age (years) | 55.1 ± 13.7 | 54.2 ± 10.9 | >0,05 | 55.9 ± 11.7 | 52.6 ± 9.9 | >0,05 |
| Weight (kg) | 71.5 ± 12.0 | 78.3 ± 10.4 | 0.04 | 77.8 ± 9.9 | 78.9 ± 11.1 | >0,05 |
| BMI (kg/m2) | 24.7 ± 3.4 | 27.9 ± 4.4 | 0.001 | 28.1 ± 4.0 | 27.7 ± 4.9 | >0,05 |
| BMI category (%) | ||||||
| Normal (18.5–24.9 kg/m2) | 47.6 (20) | 29.1 (16) | 0.01 | 25.0 (7) | 33.3 (9) | >0,05 |
| Overweight (25–29.9 kg/m2) | 47.6 (20) | 43.6 (24) | 46.4 (13) | 40.7 (11) | ||
| Obesity (≥30 kg/m2) | 4.8 (2) | 27.3 (15) | 28.6 (8) | 25.9 (7) | ||
| Waist circumference (cm) | 91.3 ± 9.9 | 98.3 ± 10.2 | 0.005 | 98.1 ± 10.6 | 98.5 ± 9.9 | >0,05 |
| AST | 23.3 ± 7.1 | 24.1 ± 9.1 | >0,05 | 23.5 ± 7.7 | 24.7 ± 10.5 | >0,05 |
| % above threshold | 16.7 (7) | 10.9 (6) | >0,05 | 7.1 (2) | 14.8 (4) | |
| ALT | 25.4 ± 9.8 | 30.9 ± 10.1 | >0,05 | 28.8 ± 16.1 | 33.0 ± 19.8 | >0,05 |
| % above threshold | 16.7 (7) | 27.3 (15) | >0,05 | 28.6 (8) | 25.9 (7) | |
| HIV diagnosis time (years) | 17.9 ± 9.2 | 18.0 ± 7.6 | >0,05 | 17.2 ± 7.1 | 18.4 ± 8.1 | >0,05 |
| T-CD4+cell ≥500 cel/mm3 | 73.8 (31) | 90.9 (50) | 0.025 | 82.1 (23) | 100 (27) | 0.021 |
| Type 2 diabetes mellitus (%) | 19.0 (8) | 15.4 (9) | >0,05 | 17.9 (5) | 14.8 (4) | >0,05 |
| High blood pressure (%) | 40.5 (17) | 43.6 (24) | >0.05 | 53.6 (15) | 33.3 (9) | >0,05 |
| Dyslipidaemia (%) | 35.7 (15) | 52.7 (29) | 0.095 | 50.0 (14) | 55.6 (15) | >0,05 |
At baseline screening, 56.7% (n = 55) had hepatic steatosis on abdominal ultrasound, 12.4% (n = 13) and 22.7% (n = 22) had increased serum levels of AST and ALT, respectively; 78.4% (n = 76) presented non-invasive score FIB4 with low probability of liver fibrosis.
NAFLD was associated with higher BMI, with BMI>30 kg/m2 in 88.2% of patients in the NAFLD group, vs. 11.8% in non-NAFLD patients; p = 0.005. However, NAFLD did not associate with metabolic syndrome comorbidities (Table 1).
FIB-4 presented low probability for liver fibrosis in 87.3% (n = 48). Regarding Fibroscan©: 63.6% (n = 35) had no evidence of fibrosis, 27.3% (n = 15) had a moderate degree of fibrosis (F2–F3) and 9.1% (n = 5) had severe fibrosis (F4).
A subset of patients with NAFLD were randomly assigned to dietary intervention (n = 27) or standard of care – SC (n = 28). This subset of patients did not differ in baseline characteristics to the global cohort (Table 1).
3.1. Anthropometric evolution before the lockdown period
From the 55 NAFLD patients, 2 did not comply with regular appointments during face-to-face follow up, 1 was diagnosed with anal cancer and 1 with an opportunistic disease (Kaposi's Sarcoma) and were excluded from the analysis.
Patients were followed for a mean period of 5.0 ± 1.5 months before lockdown. Globally, 51% of patients lost weight, with a mean weight loss of 1.7 ± 1.4 kg. We witness that the standard of care (SC) group gained a median of 0.65 kg vs. a median loss of 1.5 kg in the intervention group (p < 0.001).
3.2. Lockdown period
At the beginning of the lockdown period, mean BMI was not significantly different between SC group and intervention group (28.3 ± 0.85 kg/m2 vs. 26.8 ± 0.88 kg/m2; p = 0.139).
After the 3 month-lockdown, on the telephone interview, 41.2% (n = 21) of patients reported that there was no change in their professional situation, but 35.5% (n = 18) changed to telework and 23.5% (n = 12) became unemployed or experienced a significant reduction in household budget due to lay-off. This demographic characteristic did not differ between SC and intervention group (p = 0.835).
As for psychological impact, stress was reported in a significant number of patients, with 90.2% (n = 46) referring agitation, 88.2% (n = 45) difficulty in relaxing and 92.2% (n = 47) difficulty in calming down. Feelings associated with depression were reported by 62.6% (n = 28) patients.
There were no differences between groups. A positive score in the depression question was associated with higher weight gain during lockdown (2.6 ± 1.8 kg vs. 0.7 ± 1.9 kg, p = 0.003).
Even though dietary and lifestyle behaviours changed in both groups, with a decrease in physical activity in 62.7% (n = 32) and changes in dietary pattern in 52.9% (n = 27), they were more dramatic in the SC group as compared to the intervention group.
As such, 93.3% of patients in the SC group referred that “diet got worse” vs. 6.7% in the intervention group p < 0.01), and 35.3% vs. 15.7% (p = 0.014) reported increase in appetite, respectively. The SC group also presented a more prevalent increase in snack frequency during the day (41.2 vs. 19.6% in the intervention group, p = 0.02).
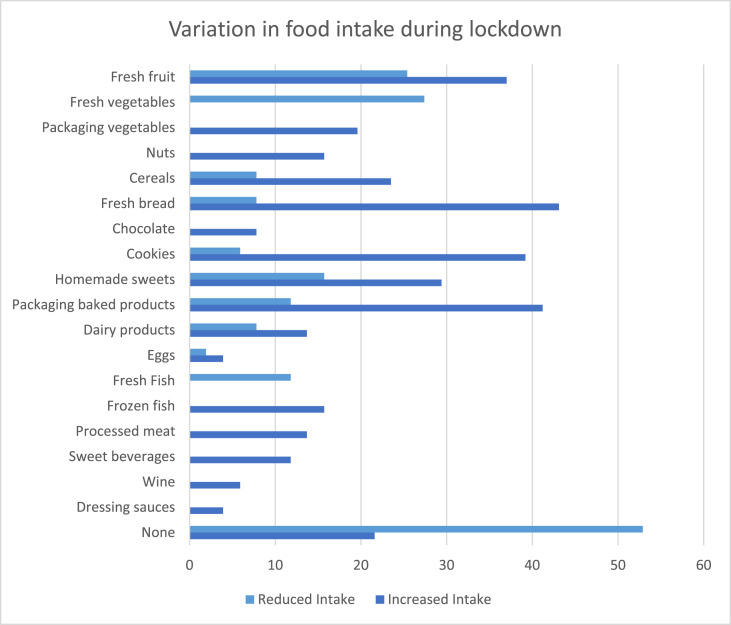
Mean Mediterranean diet score during lockdown was 7.4 ± 2.8 points, with patients on the intervention group presenting higher scores (9.2 ± 1.9 vs. 5.6 ± 2.4 points in the STD group, p < 0.01). Higher adherence to Mediterranean diet was associated with lower weight gain (r = −0,397, p = 0.004). Changes in dietary pattern during lockdown reported by patients are represent in Fig. 1 .
Fig. 1.
Variation in food intake during lockdown in overall patients.
As for physical activity, more patients in SC group, reported a decrease in activity compared with the intervention group (73.1%, n = 19, vs 52%, n = 13 respectively; p = 0.009).
Changes in dietary pattern did not associate with the presence of T2DM, dyslipidaemia, degree of fibrosis, liver enzymes, previous weight loss before lockdown or changes in professional situation. Of note, BMI and hypertension at baseline were predictors of changes in the dietary pattern. Patients with hypertension had a higher likelihood of changing their dietary pattern (OR = 1.995 CI95% [1.005–3.961]; p = 0.031) as well as patients with BMI above 25 kg/m2 (OR = 2.188 CI95% [1.276–3.750]; p = 0.007). However, in subgroup analysis, BMI above 25 kg/m2 remained a predictor of dietary change only in SC group (OR = 2.714 CI95% [1.119–6.586]; p = 0.036).
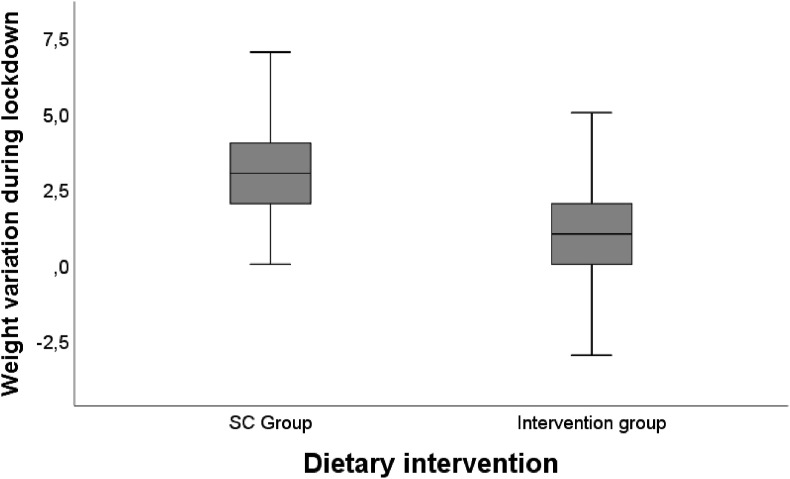
During lockdown, both groups gained weight, which was significantly higher in the SC group (3.1 ± 1.6 kg vs. 0.7 ± 1.7 kg in the intervention group, p < 0.001) (Fig. 2 ).
Fig. 2.
Weight variation during lockdown between groups (SC vs. nutritional intervention).
Weight gain was higher in patients that reported “more appetite (2.4 ± 2.1 kg vs. “no change” 0.7 ± 1.3 kg; p = 0.003). Comparing BMI category at the end of lockdown with BMI category of the last face-to-face evaluation, we witnessed worsening in the BMI category in 15.7% (n = 8) patients, with no differences between groups. However, mean BMI at the end of lockdown was significantly higher in the SC group compared with the intervention group (29.5 kg/m2 vs. 27.0 kg/m2, p = 0.035).
Weight gain during the intervention period was significantly correlated with weight gain during lockdown (r = 0.4999, p < 0.001). A decrease in physical activity during lockdown was also associated with weight gain in both groups, more pronounced in the SC group (3.6 ± 1.5 kg vs. “no change” 1.6 ± 0.9 kg; p = 0.015) compared with the intervention group (1.6 ± 1.5 kg vs. “no change” 0.8 ± 1.5 kg; p = 0.008). Variation of weight during lockdown was also associated with changes in the dietary pattern, but only in SC group, with patients presenting higher weigh gain (3.8 ± 2.1 kg) vs. “no change in dietary pattern” (2.0 ± 1.3 kg; p = 0.002).
Neither metabolic comorbidities, degree of fibrosis or liver enzyme alterations, or previous weight loss before lockdown were associated with weight variation during lockdown.
Immediately after the end of national lockdown, 12 patients in the STD group and 13 patients in the intervention group had laboratory data available. Blood glucose levels increased after lockdown in the SC group, with a mean increase of 15 mg/dl (95 ± 15.4 vs. 110 ± 31.0 mg/dL, p = 0.023). The remaining metabolic parameters remained unchanged (Table 2 ).
Table 2.
Patient characteristics before and after lockdown.
| NAFLD SC Group (n = 28) |
p-valueb | NAFLD Intervention Group (n = 27) |
p-valueb | |
|---|---|---|---|---|
| Weight (kg) | ||||
| Before lockdown | 79.1 ± 10.7 | <0.001 | 77.0 ± 11.7 | 0.099 |
| After lockdown | 82.2 ± 11.4 | 77.6 ± 12.3 | ||
| BMI (kg/m2) | ||||
| Before lockdown | 28.3 ± 4.4 | <0.001 | 26.8 ± 4.4 | 0.094 |
| After lockdown | 29.5 ± 4.7 | 27.0 ± 4.6 | ||
| BMI Obesity category (%) | ||||
| Before lockdown | 30.8 | >0.05 | 24 | >0.05 |
| After lockdown | 34.6 | 28 | ||
| Glycemiaa | ||||
| Before lockdown | 95.7 ± 15.4 | 0.023 | 94.6 ± 13.7 | >0.05 |
| After lockdown | 110.2 ± 31.0 | 97.6 ± 10.4 | ||
| Triglyceridesa | ||||
| Before lockdown | 174.8 ± 56.5 | >0.05 | 148.9 ± 58.5 | >0.05 |
| After lockdown | 201.0 ± 83.2 | 126.2 ± 44.4 | ||
| Total Cholesterola | ||||
| Before lockdown | 220.6 ± 49.8 | >0.05 | 216.6 ± 41.3 | >0.05 |
| After lockdown | 214.8 ± 63.6 | 214.6 ± 45.7 | ||
| HDL cholesterola | ||||
| Before lockdown | 52.9 ± 16.3 | >0.05 | 52.2 ± 11.8 | >0.05 |
| After lockdown | 50.3 ± 16.9 | 53.4 ± 9.4 | ||
| FIB-4 low probability of fibrosisa (%) | ||||
| Before lockdown | 80.8 | >0.05 | 100 | 0.038 |
| After lockdown | 91.7 | 69.2 | ||
Values presented as % of patients or mean ± SD; BMI: Body Massa Index; AST: Aspartate transaminase, ALT: Alanine aminotransferase; NAFDL: non-alcoholic fatty liver disease; HIV: human immunodeficiency virus.
Available from 12 patients in the STD group and 13 patients in the intervention group.
Reported comparison between before and after lockdown.
In the SC group, triglyceride levels after lockdown were directly correlated with a positive variation in weight during lockdown (r = 0.675 p = 0.016).
4. Discussion
During Covid-19 lockdown each individual had a sole responsibility to ensure an adequate diet to maintain a healthy weight and preserve overall health. This study demonstrates that a population of NAFDL patients with HIV, underwent an increase in their body weight during a period of forced confinement. However, to the best of our knowledge this is the first published study that demonstrates that this weight gain under these circumstances can be mitigated by telemedicine, namely dietary intervention.
This is an important achievement, since for NAFLD patients, an increase in body weight may lead to progression of the disease [14] and in HIV patients, weight gain has been associated with the onset of cardiovascular disease [25].
Recently published studies documented an increase in body weight during lockdown, around 3 kg. In our study SC group presented a mean weigh gain similar to other observational studies [8,26,27] (around 3 kg), and higher than the weight gain on the intervention group (less than 1 kg). Also, given the recent reports describing an increase in alcohol consumption during lockdown [28] in patients with hepatic disease, dietary intervention can improve adherence to alcohol abstinence. Experiencing mood disorders, with reports of increased anxiety and/or stress seemed to have an impact on weight gain, consistent with observational cross-sectional studies [6,29].
Also, a change in dietary habits during lockdown was demonstrated in our study, with patients referring an increase in snack and processed food intake, as documented in other observational cohort studies [3,6,9,29,30]. However, this change in dietary pattern was less pronounced in the intervention group, who maintained higher adherence to the Mediterranean pattern, and who had less than 10% of patients reporting a worse dietary pattern.
Physical activity decreased in this group of patients, and it has been reported that physical active people experienced a reduction in their physical activity time during lockdown [8,29]. However, more patients in the intervention group maintained their physical activity pattern.
The aggregate data showed that patients on the intervention group presented less weight gain and lower alterations in dietary habits and were able to maintain their physical activity pattern during lockdown. Our study suggests a beneficial effect of regular dietary follow up by telemedicine in promoting healthy lifestyle and weight control during lockdown.
This study has some limitation. Given that this was an ongoing dietary trial, the number of patients allocated to both groups was small and the Fibroscan® that was planned for 6 months after the beginning of the intervention was not performed, due to national and local constraints. The maintenance of these constraints also prevented a new Fibroscan and biochemical data collection in some patients.
In conclusion, this study shows the impact on body weight, dietary habits, physical activity, and mood of a national lockdown in a group of NAFLD-HIV patients and demonstrates that the maintenance of dietary intervention, using telemedicine, can mitigate the change in dietary habits and physical activity pattern, preventing a substantial increase in body weight. Given the recent data on the pandemic evolution this data reinforces the need to maintain health services, namely nutritional appointments, in future lockdowns, to guarantee adherence to dietary recommendations.
Acknowledgments
All authors contributed to study design. SP was responsible for data analysis. All authors were responsible for preparation and critical review of the manuscript. The authors declare no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.King A.J., Burke L.M., Halson S.L., Hawley J.A. The challenge of maintaining metabolic health during a global pandemic. Sports Med. 2020;50(7):1233–1241. doi: 10.1007/s40279-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidor A., Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12(6) doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muscogiuri G., Barrea L., Savastano S., Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74(6):850–851. doi: 10.1038/s41430-020-0635-2. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matias T., Dominski F.H., Marks D.F. Human needs in COVID-19 isolation. J Health Psychol. 2020;25:871–882. doi: 10.1177/1359105320925149. [DOI] [PubMed] [Google Scholar]
- 5.Ruíz-Roso M.B., de Carvalho Padilha P., Matilla-Escalante D.C., Brun P., Ulloa N., Acevedo-Correa D. Changes of physical activity and ultra-processed food consumption in adolescents from different countries during covid-19 pandemic: an observational study. Nutrients. 2020;12(8):1–13. doi: 10.3390/nu12082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Renzo L., Gualtieri P., Cinelli G., Bigioni G., Soldati L., Attinà A. Psychological aspects and eating habits during covid-19 home confinement: results of ehlc-covid-19 Italian online survey. Nutrients. 2020;12(7):1–14. doi: 10.3390/nu12072152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellegrini M., Ponzo V., Rosato R., Scumaci E., Goitre I., Benso A. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):1–11. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-de-Quel Ó., Suárez-Iglesias D., López-Flores M., Pérez C.A. Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: a longitudinal study. Appetite. 2021;158(November 2020):1–6. doi: 10.1016/j.appet.2020.105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao A., Li Z., Ke Y., Huo S., Ma Y., Zhang Y. Dietary diversity among Chinese residents during the COVID-19 outbreak and its associated factors. Nutrients. 2020;12(6):1–13. doi: 10.3390/nu12061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Đogaš Z., Kalcina L.L., Dodig I.P., Demirović S., Madirazza K., Valić M. The effect of COVID-19 lockdown on lifestyle and mood in Croatian general population: a cross-sectional study. Croat Med J. 2020;61(4):309–318. doi: 10.3325/cmj.2020.61.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Loomba R., Sanyal A.J. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Mundi M.S., Velapati S., Patel J., Kellogg T.A., Abu Dayyeh B.K., Hurt R.T. Evolution of NAFLD and its management. Nutr Clin Pract. 2020;35(1):72–84. doi: 10.1002/ncp.10449. [DOI] [PubMed] [Google Scholar]
- 15.Guaraldi G., Squillace N., Stentarelli C., Orlando G., D'Amico R., Ligabue G. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 16.Lemoine M., Serfaty L., Capeau J. From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: diagnosis and management. Curr Opin Infect Dis. 2012;25(1):10–16. doi: 10.1097/QCO.0b013e32834ef599. [DOI] [PubMed] [Google Scholar]
- 17.Tafesh Z.H., Verna E.C. Managing nonalcoholic fatty liver disease in patients living with HIV. Curr Opin Infect Dis. 2017;30(1):12–20. doi: 10.1097/QCO.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 18.Verna E.C. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with HIV. Lancet Gastroenterol Hepatol. 2017;2(3):211–223. doi: 10.1016/S2468-1253(16)30120-0. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Gómez M., Cortez-Pinto H. Detecting liver fat from viscoelasticity: how good is CAP in clinical practice? The need for universal cut-offs. J Hepatol. 2017;66(5):886–887. doi: 10.1016/j.jhep.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Marchesini G., Day C.P., Dufour J.F., Canbay A., Nobili V., Ratziu V. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-González M.A., García-Arellano A., Toledo E., Salas-Salvadó J., Buil-Cosiales P., Corella D. A 14-item mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-González M.A., Bastarrika G. Mediterranean diet as the ideal model for preventing non-alcoholic fatty liver disease (NAFLD) Hepatobiliary Surg Nutr. 2020;9(3):379–381. doi: 10.21037/hbsn.2019.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attinà A., Cinelli G. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18(1):1–15. doi: 10.1186/s12967-020-02399-5. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pais-Ribeiro J., Honrado A., Leal I. Contribution to the adaptation study of the Portuguese adaptation of the lovibond and lovibond depression anxiety stress scales (EADS) with 21 items. Psicol Saúde Doenças. 2004;5(2):229–239. [Google Scholar]
- 25.Bailin S.S., Gabriel C.L., Wanjalla C.N., Koethe J.R. Obesity and weight gain in persons with HIV. Curr HIV AIDS Rep. 2020;17(2):138–150. doi: 10.1007/s11904-020-00483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Luis Román D.A., Izaola O., Primo Martín D., Gómez Hoyos E., Torres Torres B., López Gómez J.J. Effect of lockdown for COVID-19 on self-reported body weight gain in a sample of obese patients. Nutr Hosp. 2020;37(6):1232–1237. doi: 10.20960/nh.03307. [DOI] [PubMed] [Google Scholar]
- 27.Zachary Z., Brianna F., Brianna L., Garrett P., Jade W., Alyssa D. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14(3):210–216. doi: 10.1016/j.orcp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Lancet Gastroenterology, Hepatology Drinking alone: COVID-19, lockdown, and alcohol-related harm. Lancet Gastroenterol Hepatol. 2020;5:625. doi: 10.1016/S2468-1253(20)30159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giacalone D., Frøst M.B., Rodriguez-Péres C. Reported changes in dietary habits during the Covid-19 lockdown in the Danish population: the Danish COVIDiet study. Front Nutr. 2020;7(592112):1–8. doi: 10.3389/fnut.2020.592112. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh A., Arora B., Gupta R., Anoop S., Misra A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab Syndrome Clin Res Rev. 2020;14(5):917–920. doi: 10.1016/j.dsx.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]