Abstract
Background
With the continuance of the global COVID-19 pandemic, cardiovascular disease (CVD) and cardiac injury have been suggested to be risk factors for severe COVID-19.
Objective
The aim is to evaluate the mortality risks associated with CVD and cardiac injury among hospitalized COVID-19 patients, especially in subgroups of populations in different countries.
Methods
A comprehensive systematic literature search was performed using 9 databases from November 1, 2019 to November 9, 2020. Meta-analyses were performed for CVD and cardiac injury between non-survivors and survivors of COVID-19.
Results
Although the prevalence of CVD in different populations was different, hospitalized COVID-19 patients with CVD were at a higher risk of fatal outcomes (OR = 2.72; 95% CI 2.35–3.16) than those without CVD. Separate meta-analyses of populations in four different countries also reached a similar conclusion that CVD was associated with an increase in mortality. Cardiac injury was common among hospitalized COVID-19 patients. Patients with cardiac injury had a significantly higher mortality risk than those without cardiac injury (OR = 13.25; 95% CI: 8.56–20.52).
Conclusions
Patients' CVD history and biomarkers of cardiac injury should be taken into consideration during the hospital stay and incorporated into the routine laboratory panel for COVID-19.
Keywords: Cardiovascular diseases, Cardiac injury, COVID-19, Mortality, Meta analysis
1. Introduction
Coronavirus disease 2019 (COVID-19), a recently discovered disease, has broken through the limits of national borders and circulated rapidly worldwide. Considering the speed of its spread and disease outcomes on an international scale, it was announced as a pandemic by the World Health Organization (WHO) on March 11, 2020. As of March 19, 2021, 223 countries or regions were involved, more than 120.9 million confirmed cases were diagnosed, and these numbers have continued to increase [1]. The global mortality rate of COVID-19 is approximately 2.2% [1], while the mortality rate of hospitalized COVID-19 cases is commonly over 10.0% [[2], [3], [4]]. This is because hospitalized patients tend to have a more serious condition and generally require more effective and special therapies than those in isolation at home. Facing the current severe epidemic situation with limited medical resources, it is urgent to identify significant risk factors leading to death and then take targeted measures to reduce the fatal risk of hospitalized patients, which can also improve the utilization of medical resources.
The Society of Cardiology [5] and the American College of Cardiology [6] strongly recommend careful cardiac assessment of symptomatic COVID-19 cases. Some primary studies also mentioned two potentially major cardiac risk factors that might be related to the severity of COVID-19 infection [[2], [3], [4]]. One risk factor is baseline cardiovascular disease (CVD), which is defined as a group of disorders of the heart and blood vessels, such as coronary heart disease, peripheral arterial disease, and congenital heart disease [7], occurring before COVID-19 infection and causing more serious clinical outcomes. The other risk factor is acute cardiac injury, as assessed by increased serum levels of high-sensitivity cardiac troponin I (hs-cTnI) or troponin T (hs-cTnT) [8]. Acute cardiac injury has been observed as a common complication of COVID-19 infection and could lead to an increase in severe cardiac events among critically ill patients with COVID-19, including cardiac arrest, arrhythmia, and acute myocardial infarction [9]. Furthermore, several meta-analyses also attempted to assess the combined prognostic effect of CVD or acute cardiac injury on COVID-19, but these estimates varied [10,11]. This might be due to the difference in data sources, sample size and definitions of keywords. For example, the study population was mainly from China, the number of articles included in the meta-analysis was small, or hypertension was regarded as a kind of CVD, which is inconsistent with the WHO's definition of CVD [7].
Therefore, we wanted to more comprehensively assess and compare the pooled effect of two risk factors (baseline CVD and acute cardiac injury) on the fatal outcomes of hospitalized COVID-19 patients worldwide. We were also interested in analysis of populations in different countries separately, the analysis of which is lacking. In this study, we selected hospitalized COVID-19 patients as the target group, chose a one-year time span and retrieved 3651 articles from 9 databases to perform meta-analysis. In particular, our study further assessed and compared the association of baseline CVD and COVID-19 mortality in populations from four countries.
2. Methods
2.1. Inclusion criteria
Research articles fulfilling the following criteria were included: (1) the published original research studies used quantitative methods to report the number of confirmed cases of COVID-19 with baseline CVD or acute cardiac injury among survivors and non-survivors from 2019 to 2020, or studies reported the mortality rate of these cases when the total number of survival and death cases was known; (2) according to the WHO, CVD is defined as a group of disorders of the heart and blood vessels, including coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, and deep vein thrombosis and pulmonary embolism[7], (3) acute cardiac injury is assessed by increased serum levels of high-sensitivity cardiac troponin I (hs-cTnI) or troponin T (hs-cTnT)[8], (4) randomized controlled trials (RCTs), cohort studies, case-control studies, and cross-sectional studies were included.
2.2. Exclusion criteria
Research articles fulfilling the following criteria were excluded: (1) data that were duplicated in more than one study, and the least recent or the one with a smaller number of cases would be excluded; (2) articles that investigated factors related to cardiac-related infection and other factors (e.g., diabetes) but did not report them separately; (3) cases that were enrolled in studies without a diagnosis of COVID-19 confirmed by real-time reverse-transcriptase polymerase chain reaction (RT-PCR); (4) the research object of the articles was a special population that may affect the meta-analysis results, such as pregnant women or recipients of kidney transplants; (5) the study enrolled cases with unnecessary limitation, for example, patients who presented to the emergency department (ED) with COVID-19 infection were excluded; (6) patients that were not admitted to hospitals.
2.3. Search strategy
The meta-analysis was conducted based on the preferred reporting items for systematic reviews and PRISMA checklist [12] (Supplementary fig. 1). A comprehensive systematic literature search was performed in various online databases, including PubMed, Scopus, Web of Science, Cochrane Library databases, WanFang Data, WeiPu Data, CNKI, China CDC and USA CDC, from November 1, 2019 to November 9, 2020. The key words are described in Table 1. All references of the included articles were hand-searched and browsed to identify more potentially eligible studies. No ethics committee approval was mandatory.
The following are the supplementary data related to this article.Supplementary Fig. 1.
PRISMA 2009 checklist.
Table 1.
The key words used in the meta-analysis
| Section | Key words |
|---|---|
| Disease | “COVID-19” OR “2019-nCoV” OR “2019 novel coronavirus” OR “coronavirus 2019” OR “SARS-CoV-2” |
| Risk factor | “cardiovascular disease” OR “CVD” OR “cardiac injury” OR “coronary heart disease” OR “cardiomyopathy” OR “heart failure” |
| Outcome | “fatal” OR “mortality” OR “death “ |
2.4. Research selection and meta-analysis
The articles that fulfilled the search criteria were carefully assessed by title, abstract and full text independently by two investigators (Jiali Long and Yefei Luo) to select eligible ones for meta-analysis. The articles written in Chinese were translated to English by a medical professional fluent in both Chinese and English. Two investigators collected and sorted characteristics of eligible articles into tables, including authors, published year, country, study type, sample size, mortality rate, age, and prevalence of CVD/cardiac injury. Disagreement between the investigators was solved through discussion and consensus of all authors. Later, meta-analyses were performed using Review Manager 5.3 with odds ratio (OR) as a principal effect measure. I2 acted as a measure of heterogeneity for each meta-analysis. In meta-analysis, if P < 0.05 or I2 value>50%, the studies are considered heterogeneous, and a random effects model is used; if P > 0.05 and I2 value<50%, there is no significant evidence to consider the studies as heterogeneous, and a random effects model is used. Subgroup analyses will be performed if more than 2 countries have 3 or more articles.
2.5. Bias assessment
Firstly, we used Newcastle-Ottawa Scale (NOS) to assess the methodological quality of the included articles. NOS judges individual articles on three broad perspectives: “Selection of study groups”, “Comparability of study groups”, and “Ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively”. Therefore, NOS can quantitatively assess bias of articles such as selection bias and information bias. The quality assessment of articles ranges from low scores (0–4) to moderate scores (5–6) to high scores (7–9), representing three different levels of study quality. When the score is higher, the article is less likely to be biased. Later, we performed a symmetrical shape and regression-based Harbord's test for all outcomes to assess whether there was publication bias or small-study effect. We also conducted a sensitivity analysis in which we tried to remove one study at a time in a meta-analysis, re-estimated the combined effect size, and compared it with the results of the meta-analysis before this exclusion. If the results before and after do not change substantially, it suggests that a single study has no obvious effect on heterogeneity.
3. Results
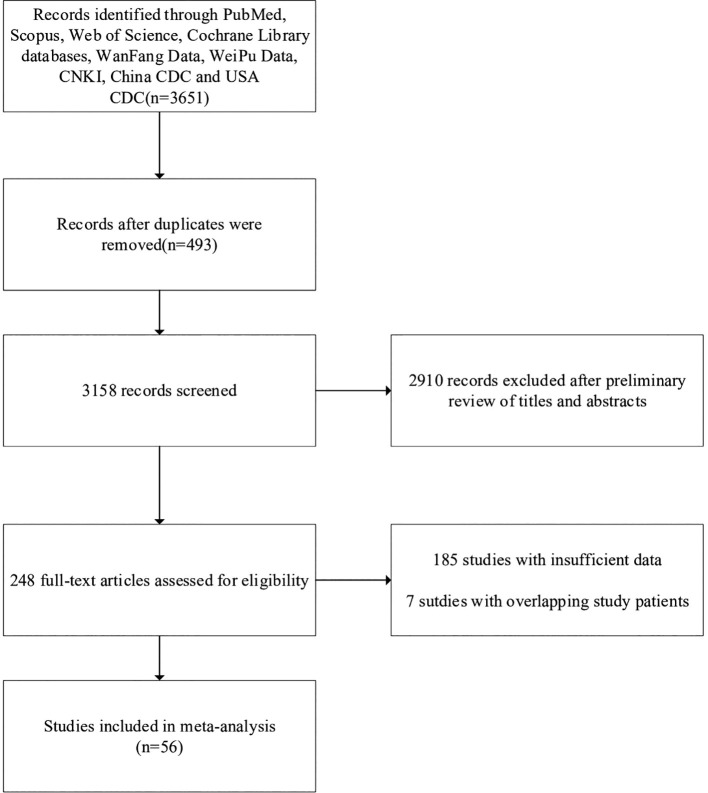
The search process yielded a total of 3651 articles (Fig. 1 ). 493 articles were excluded because of duplication, and 2910 articles were further excluded after a preliminary review of titles and abstracts, resulting in 248 studies. Among these articles, 7 articles overlapped study data with other articles, and 185 articles met the other exclusion criteria. Finally, 56 articles remained [[2], [3], [4],9,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]].
Fig. 1.
Flow diagram for selection of articles for inclusion in later analysis.
3.1. Basic characteristics of included studies
3.1.1. General information
Fifty-six articles were included in the analysis, with available data on 52,301 hospitalized COVID-19 patients from 13 countries, 17.8% of whom died. Among these articles, 3 articles provided data on both CVD and cardiac injury, while the other 44 articles and 9 articles only mentioned information on CVD and cardiac injury, respectively (Fig. 2 ).
Fig. 2.
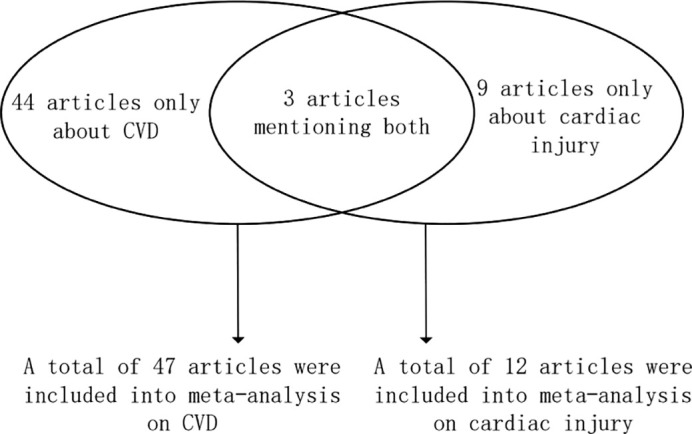
The number and distribution of articles used for meta-analysis on CVD and cardiac injury.
3.1.2. CVD
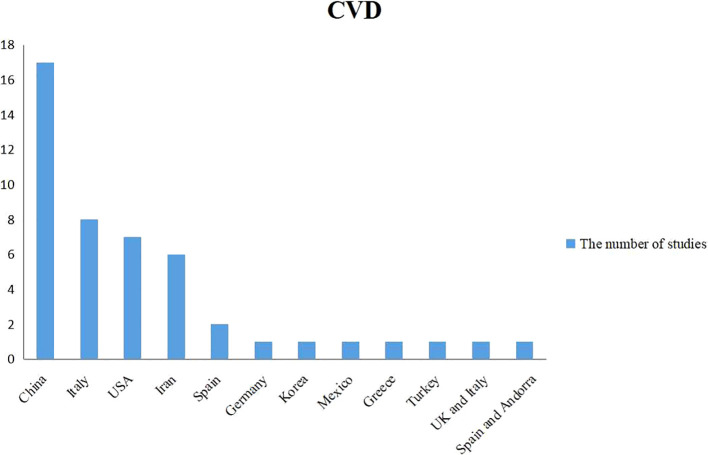
Forty-seven studies [[2], [3], [4],9,[13], [14], [15],17,19,[21], [22], [23],[25], [26], [27], [28],[30], [31], [32], [33],[35], [36], [37], [38], [39], [40], [41],[43], [44], [45], [46], [47], [48],[50], [51], [52], [53], [54], [55], [56], [57],[59], [60], [61], [62], [63], [64]] reported data about CVD involving research populations from 12 countries, of which China, Italy, the USA, and Iran had 3 or more articles (Table 2 , Fig. 3 ). A total of 49,285 cases were included in the analysis, with a 18.5% (9137/49,285) mortality rate. The overall prevalence of baseline CVD in survivors was 7.1% (2836/40,148), while the rate in non-survivors was 18.4% (1684/9137). Furthermore, the prevalence of CVD in China, Italy, the USA, and Iran was 10.2%, 15.8%, 9.0% and 9.5%, respectively.
Table 2.
The information of 47 studies included in the meta-analysis of CVD to fatal COVID-19
| No. | Author (year studied) | Country | Study type | Sample size (N) | Mortality rate | Age (yrs) | Non-survivors |
Survivors |
NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | CVDs | N | CVDs | ||||||||
| 1 | Abbasi, B. (2020) | Iran | A retrospective cohort study | 262 | 21.4% | 58 (43–67#) | 56 | 34 (60.7%¶) | 206 | 44 (21.4%) | 6 |
| 2 | Aladağ, N. (2020) | Turkey | A retrospective cohort study | 50 | 30.0% | 64.80 (50.3–79.3) | 15 | 7 (46.67%) | 35 | 25 (71.43%) | 6 |
| 3 | Alamdari, N. M. (2020) | Iran | A large-scale retrospective cross-sectional study | 459 | 13.7% | 61.8 (49.9–73.7) | 63 | 29 (46.0%) | 396 | 156 (39.4%) | 5 |
| 4 | Alvarez-Garcia, J. (2020) | USA | A retrospective cohort study | 6439 | 25.8% | 63.5 (45.9–81.1) | 1664 | 169 (10.2%) | 4775 | 253 (5.3%) | 8 |
| 5 | An, W. (2020) | China | A retrospective cohort study | 110 | 10.0% | – | 11 | 1 (9.1%) | 99 | 4 (4.0%) | 7 |
| 6 | Bursi, F. (2020) | Italy | A retrospective cohort study | 49 | 32.7% | 65.7 (53.1–78.3) | 16 | 6 (37.5%) | 33 | 5 (15.2%) | 7 |
| 7 | Cao, Y. (2020) | China | A two-centre retrospective case control study | 101 | 34.7% | 56.6 (41.5–71.7) | 35 | 11 (31.4%) | 66 | 10 (15.2%) | 7 |
| 8 | Carrillo-Vega, M. F. (2020) | Mexico | A descriptive cohort study | 9946 | 9.7% | 48.2 (33.8–62.5) | 963 | 64 (6.6%) | 8983 | 246 (6.3%) | 6 |
| 9 | Carter, B. (2020) | UK and Italy | A multi-centre international observational cohort study | 1559 | 27.1% | 74.0 (61.0–83.0) | 422 | 132 (31.3%) | 1137 | 213 (18.7%) | 7 |
| 10 | Chen, F. F. (2020) | China | A retrospective cohort study | 681 | 15.3% | 65.0 (54.0–72.0) | 104 | 25 (24.0%) | 577 | 55 (9.5%) | 5 |
| 11 | Chen, R. (2020) | China | A retrospective cohort study | 1590 | 3.1% | 69 (51–86) | 50 | 8 (16.0%) | 1540 | 51 (3.3%) | 5 |
| 12 | Chilimuri, S. (2020) | USA | A retrospective cohort study | 375 | 42.7% | 63.0 (52.0–72.0) | 160 | 38 (23.8%) | 215 | 24 (11.2%) | 5 |
| 13 | Ciardullo, S. (2020) | Italy | A single-centre retrospective cohort study | 373 | 38.1% | 72 (58–86) | 142 | 65 (45.8%) | 231 | 75 (32.5%) | 5 |
| 14 | Ciceri, F. (2020) | Italy | An observational cohort study | 410 | 23.2% | 65 (56–75) | 95 | 25 (26.3%) | 315 | 26 (8.3%) | 7 |
| 15 | Cipriani, A. (2020) | Italy | A single-centre observational cohort study | 109 | 18.3% | 71 (60–81) | 20 | 9 (45.0%) | 89 | 9 (10.1%) | 6 |
| 16 | Di Castelnuovo, A. (2020) | Italy | A national retrospective observational cohort study | 3762 | 17.5% | – | 665 | 165 (24.8%) | 3097 | 266 (8.6%) | 7 |
| 17 | Eslami, V. (2020) | Iran | A single-centre prospective cohort study | 87 | 14.9% | 54.6 (39.3–69.9) | 13 | 4 (30.8%) | 74 | 11 (14.9%) | 7 |
| 18 | Farré, N. (2020) | Spain | A single-centre cohort study | 623 | 11.9% | – | 74 | 15 (20.3%) | 549 | 18 (3.3%) | 7 |
| 19 | Ferrando, C. (2020) | Spain and Andorra | A multi-centre prospective observational and cohort study | 663 | 30.6% | 64 (56–72) | 203 | 6 (3.0%) | 460 | 3 (0.7%) | 7 |
| 20 | Grasselli, G. (2020) | Italy | A retrospective observational cohort study | 1715 | 53.4% | 64 (56–70) | 915 | 224 (24.5%) | 800 | 88 (11.0%) | 7 |
| 21 | Guo, T. (2020) | China | A single-centre retrospective observational cohort study | 187 | 23.0% | 58.5 (43.8–73.2) | 43 | 29 (67.4%) | 144 | 37 (86.0%) | 7 |
| 22 | Gupta, S. (2020) | USA | A multi-centre cohort study | 2215 | 35.4% | 62 (51–71) | 784 | 158 (20.2%) | 1431 | 130 (9.1%) | 7 |
| 23 | Halvatsiotis, P. (2020) | Greece | A multi-centre retrospective corss-sectional study | 86 | 28.9% | 65.5 (56–73) | 26 | 4 (15.4%) | 60 | 14 (23.3%) | 7 |
| 24 | Harmouch, F. (2020) | USA | A retrospective cohort study | 560 | 14.5% | 63 (39–87) | 81 | 19 (23.5%) | 479 | 35 (7.3%) | 5 |
| 25 | He, X. W. (2020) | China | A single-centre retrospective cohort study | 54 | 48.1% | 68.0 (59.8–74.3) | 26 | 5 (19.2) | 28 | 3 (10.7%) | 8 |
| 26 | He, Y. (2020) | China | A cohort study | 336 | 39.6% | 65 (50–77) | 133 | 36 (27.1%) | 203 | 23 (11.3%) | 6 |
| 27 | Homayounieh, F. (2020) | Iran | A retrospective cohort study | 75 | 20.0% | – | 15 | 5 (33.3%) | 60 | 13 (21.7%) | 6 |
| 28 | Hwang, J. M. (2020) | Korea | A retrospective cohort study | 103 | 25.2% | 67.6 (52.3–82.9) | 26 | 6 (23.1%) | 77 | 6 (7.8%) | 6 |
| 29 | Inciardi, R. M. (2020) | Italy | A cohort study | 99 | 26.3% | 67 (55–79) | 26 | 19 (73.1%) | 73 | 34 (46.6%) | 8 |
| 30 | Lanza, G. A. (2020) | Italy | A retrospective cohort study | 324 | 13.6% | 65.9 (50.7–81.1) | 44 | 17 (38.6%) | 280 | 50 (17.9%) | 5 |
| 31 | Li, C. (2020) | China | A retrospective cohort study | 2068 | 8.8% | 63 (51–70) | 183 | 28 (15.3%) | 1885 | 154 (8.2%) | 5 |
| 32 | Li, J. (2020) | China | A retrospective cohort study | 74 | 18.9% | 66 (55–72) | 14 | 4 (28.6%) | 60 | 2 (3.3%) | 6 |
| 33 | Lu, J. (2020) | China | A retrospective cohort study | 20 | 50.0% | 69.8 (57.8–81.8) | 10 | 2 (20.0%) | 10 | 2 (20.0%) | 6 |
| 34 | McCullough, S. A. (2020) | USA | A retrospective observational cohort study | 756 | 11.9% | 63.3 (47.3–79.3) | 90 | 16 (17.8%) | 666 | 39 (5.9%) | 5 |
| 35 | Nikpouraghdam, M. (2020) | Iran | A retrospective cohort study | 2964 | 8.1% | 55.50 (40.4–70.7) | 239 | 4 (1.7%) | 2725 | 33 (1.2%) | 6 |
| 36 | Pan, F. (2020) | China | A case-control study | 124 | 71.8% | 68 (61–75) | 89 | 13 (14.6%) | 35 | 6 (17.1%) | 5 |
| 37 | Peterson, E. (2020) | USA | A single-centre retrospective cohort study | 355 | 22.5% | – | 80 | 24 (30.0%) | 275 | 53 (19.3%) | 8 |
| 38 | Rastad, H. (2020) | Iran | A retrospective cohort study | 2957 | 10.2% | 54.8 (37.9–71.7) | 301 | 54 (17.9%) | 2656 | 260 (9.8%) | 5 |
| 39 | Rath, D. (2020) | Germany | A consecutive prospective cohort study | 123 | 13.0% | 68 (53–83) | 16 | 6 (37.5%) | 107 | 22 (20.6%) | 7 |
| 40 | Rey, J. R. (2020) | Spain | A retrospective cohort study | 3080 | 20.3% | 62.3 (42.0–82.6) | 626 | 74 (11.8%) | 2454 | 78 (3.2%) | 7 |
| 41 | Shi, S. (2020) | China | A retrospective cohort study | 671 | 9.2% | 63 (50–72) | 62 | 21 (33.9%) | 609 | 39 (6.4%) | 5 |
| 42 | Sun, J. H. (2020) | China | A retrospective cohort study | 939 | 11.7% | 62 (51–70) | 110 | 8 (7.3%) | 829 | 57 (6.9%) | 6 |
| 43 | Twigg, H. L. (2020) | USA | A cohort study | 242 | 19.8% | 59.6 (44.1–75.1) | 48 | 9 (18.8%) | 194 | 19 (9.8%) | 6 |
| 44 | Xu, J. (2020) | China | A multicenter retrospective cohort study | 239 | 61.5% | 62.5 (49.2–75.8) | 147 | 21 (14.3%) | 92 | 14 (15.2%) | 6 |
| 45 | Zhang, J. (2020) | China | A retrospective cohort study | 541 | 9.8% | – | 53 | 32 (60.4%) | 488 | 112 (23.0%) | 6 |
| 46 | Zhao, Y. (2020) | China | A single-centre retrospective case control study | 539 | 23.2% | 58 (43–69) | 125 | 20 (16.0%) | 414 | 17 (4.1%) | 6 |
| 47 | Zhou, F. (2020) | China | A multi-centre retrospective cohort study | 191 | 28.3% | 56·0 (46·0–67·0) | 54 | 13 (24.1%) | 137 | 2 (1.5%) | 5 |
#The numbers in our study were accurate to one digit after the decimal point, but some of the numbers were only displayed as integers in a few primary articles included.
¶The percentage of cases in each group was marked in the bracket.
Fig. 3.
National distribution of the study population included in the meta-analysis of CVD.
3.1.3. Cardiac injury
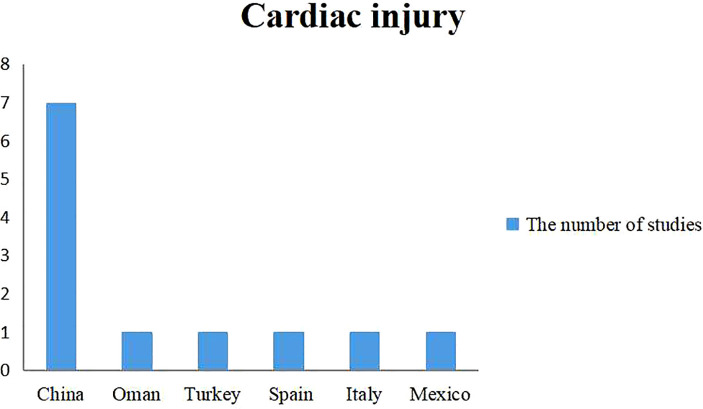
The relationship between cardiac injury and mortality was described in 12 studies involving 6 countries (Table 3 , Fig. 4 , 16, 18, 20, 24, 29, 34, 36, 40, 42, 49, 58, 64]. Cardiac injury was a common condition among hospitalized COVID-19 patients, ranging from 17.3% to 60.7%. Due to the limited number of articles included, there was no subgroup analysis on populations in different countries.
Table 3.
The information of 12 studies included in the meta-analysis of acute cardiac injury to fatal COVID-19
| No. | Author (year studied) |
Country | Study type | Sample size (N) | Mortality rate | Age (yrs) | Non-survivors |
Survivors |
NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Cardiac injury | N | Cardiac injury | ||||||||
| 1 | Al-Wahaibi, K. (2020) | Oman | A retrospective cohort study | 143 | 16.8% | 49.4 (34.0–64.7) | 24 | 16 (66.7%) | 119 | 15 (12.6%) | 6 |
| 2 | Barman, H. A. (2020) | Turkey | A multi-centre retrospective cohort study | 607 | 17.0% | – | 103 | 64 (17.0%) | 504 | 86 (17.1%) | 7 |
| 3 | Calvo-Fernández, A. (2020) | Spain | A cohort study | 872 | 9.2% | 62.3 (44.2–80.4) | 80 | 66 (82.5%) | 792 | 159 (20.1%) | 9 |
| 4 | Chen, L. (2020) | China | A single-centre observational cohort study | 63 | 27.0% | 53 (43–65) | 17 | 12 (70.6%) | 46 | 11 (23.9%) | 8 |
| 5 | Deng, Y. (2020) | China | A retrospective case control study | 225 | 48.4% | – | 109 | 65 (59.6%) | 116 | 1 (0.9%) | 6 |
| 6 | Ferrante, G. (2020) | Italy | A single-centre cohort study | 332 | 20.5% | 66.9 (55.4–75.5) | 68 | 50 (73.5%) | 264 | 73 (27.7%) | 8 |
| 7 | Guo, T. (2020) | China | A single-centre retrospective observational cohort study | 187 | 23.0% | 58.5 (43.8–73.2) | 43 | 31 (72.1%) | 144 | 21 (14.6%) | 7 |
| 8 | He, X. W. (2020) | China | A single-centre retrospective cohort study | 54 | 48.1% | 68.0 (59.8–74.3) | 26 | 18 (69.2%) | 28 | 6 (21.4%) | 8 |
| 9 | Heberto, A. B. (2020) | Mexico | A multi-centre prospective observational cohort study | 254 | 35.0% | 53.8 (41.1–66.5) | 89 | 46 (51.7%) | 165 | 27 (16.4%) | 9 |
| 10 | Lu, Q. (2020) | China | A retrospective cohort study | 56 | 39.3% | 71.5 (56.3–85.8) | 22 | 20 (90.1%) | 34 | 14 (41.2%) | 7 |
| 11 | Shi, S. (2020) | China | A single-centre retrospective cohort study | 416 | 13.7% | 64 (21–95) | 57 | 42 (73.4%) | 359 | 40 (11.1%) | 5 |
| 12 | Zhou, F. (2020) | China | A multi-centre retrospective cohort study | 191 | 28.3% | 56·0 (46·0–67·0) | 54 | 32 (59.3%) | 137 | 1 (0.7%) | 5 |
Fig. 4.
National distribution of the study population included in the meta-analysis of cardiac injury.
3.2. Methodological quality of included studies
The quality of the searched articles was assessed using NOS, and the scoring was performed as described in Table 2 and Table 3. Because the searched articles were not of bad quality, these studies were all used for subsequent meta-analysis. (1) CVD: The searched articles about CVD were assessed ranging from moderate quality (5–6 scores) to high quality (7–8 scores), and none of the studies scored less than 5. The average score of the included studies was 6.2, with a standard deviation (SD) of 1.1. Some studies scored lower on the “Comparability of study groups” part of the scale because these studies mainly focused on the analysis of research results without sufficient description of the characteristics of the cohort. (2) Cardiac injury: Most articles regarding cardiac injury were of high quality, with an average score of 7.1 and an SD of 1.1. None of the studies scored less than 5. Two studies received full scores.
3.3. Meta-analysis
3.3.1. CVD
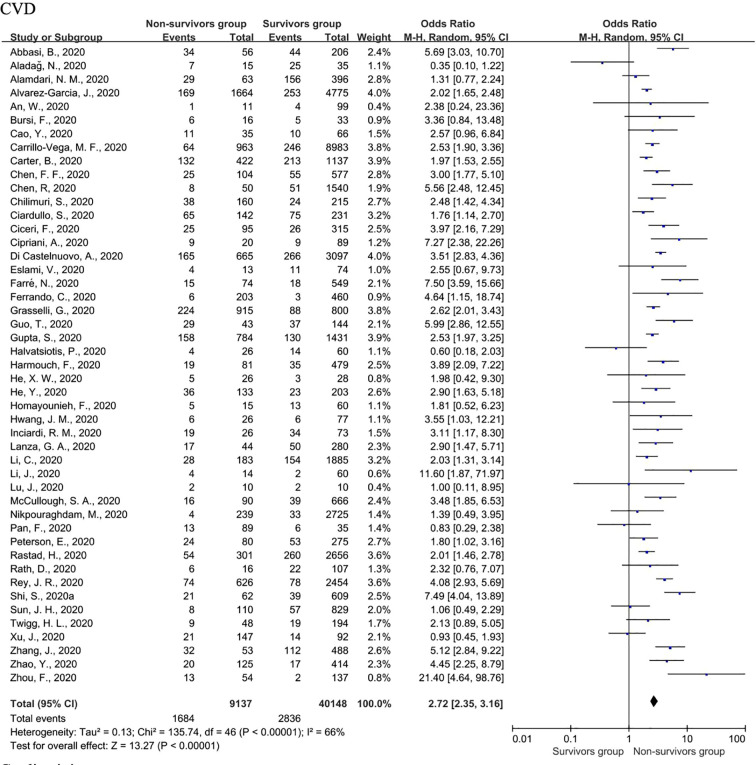
The main individual studies included in the analysis revealed that patients who died had a higher ratio of baseline CVD, with quite a few studies showing a neutral effect. The pooled meta-analysis showed that baseline CVD increased the fatal risk of hospitalized COVID-19 patients (OR = 2.72; 95% CI 2.35–3.16; I2 = 66%, P < 0.00001) (Fig. 5 ). An I2 value of 66% suggested that the included studies might be heterogeneous. For this reason, we used a random effect model for the meta-analysis. we conducted a sensitivity analysis, and the results before and after did not change substantially, which excluded the influence of the inclusion of a single study on heterogeneity, indicating that the meta-analysis results were relatively robust. Furthermore, we used a random effect model for the meta-analysis, which is considered more robust for studies with possible heterogeneity. Furthermore, we considered whether the heterogeneity was due to the differences in the research subjects in different countries and conducted a subgroup analysis as shown below.
Fig. 5.
Forest plots of the association of CVD with fatal COVID-19.
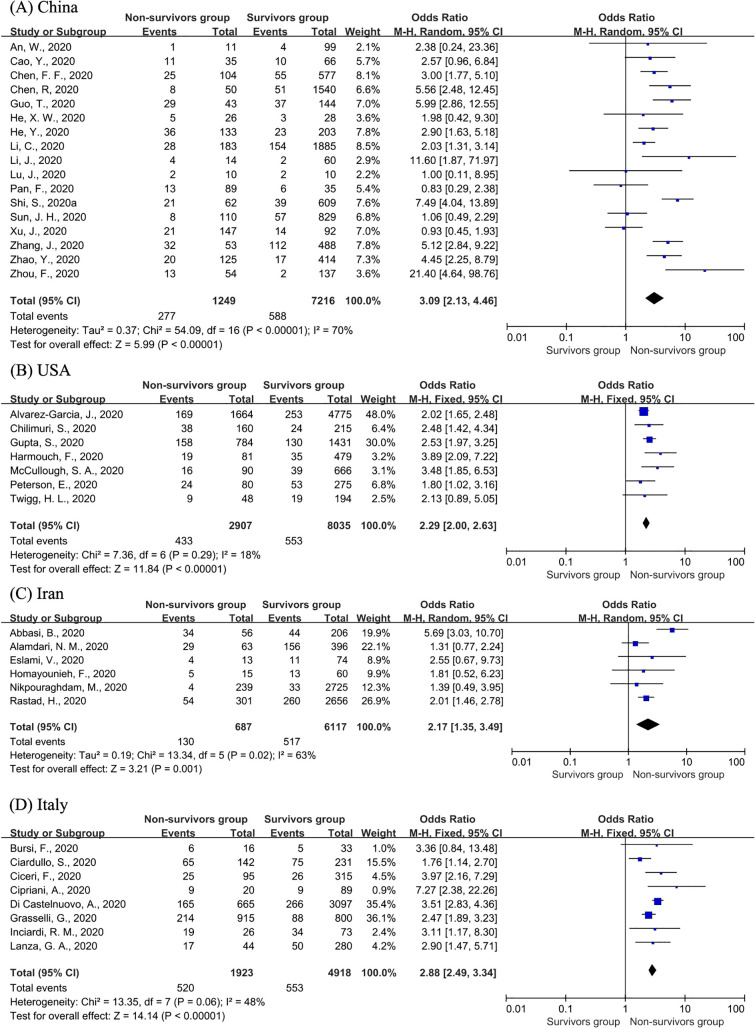
We further analysed the risk of CVD on fatal outcomes in populations of different countries. A total of four countries had three or more articles included in the analysis, namely, China, the USA, Italy and Iran (Fig. 6 ). The OR values of these four countries were all significant, fluctuating up and down the global OR value. The risks of CVD in China (OR = 3.09; 95% CI 2.13–4.46; I2 = 70%, P < 0.00001) and Italy (OR = 2.88; 95% CI 2.49–3.34; I2 = 48%, P < 0.00001) were higher than the global value, while those of the USA (OR = 2.29; 95% CI 2.00–2.63; I2 = 18%, P < 0.00001) and Iran (OR = 2.17; 95% CI 1.35–3.49; I2 = 63%, P < 0.00001) were lower than the global value. In addition, the heterogeneity of the meta-analysis in Italy, the USA and Iran was smaller than the total heterogeneity. Finally, based on the I2 value, we used a fixed effect model for the population of the USA and Italy, and a random effect model for the population in the other two countries.
Fig. 6.
Forest plots of the association of CVD in China (A), the USA (B), Iran (C) and Italy (D) with fatal COVID-19.
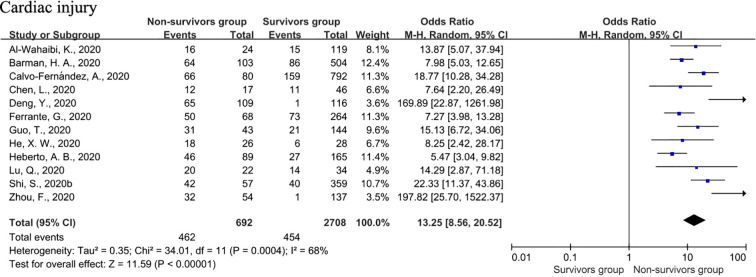
3.3.2. Cardiac injury
A random-effect model was used in the analysis of cardiac injury. Cardiac injury was found to be strongly associated with an increased risk of a fatal outcome (OR = 13.25; 95% CI: 8.56–20.52; I2 = 68%, P < 0.00001) (Fig. 7 ). All included articles pointed to the strengthening effect of cardiac injury with fatal results. We conducted a sensitivity analysis again, and a similar conclusion was reached. The influence of the inclusion of a single study on heterogeneity was excluded, implying that the results of the meta-analysis were relatively reliable.
Fig. 7.
Forest plots of the association of cardiac injury with fatal COVID-19.
3.3.3. Publication Bias
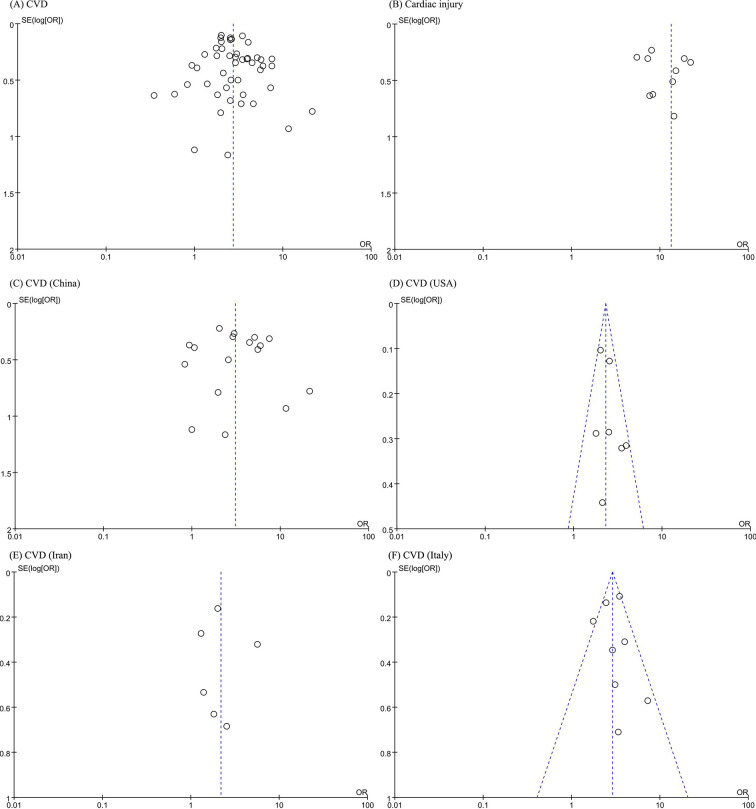
The funnel-plot analysis showed a symmetrical shape for all outcomes (CVD (8A), cardiac injury (8B), and CVD in China (8C), the USA (8D), Iran (8E) and Italy (8F)), indicating no possible publication bias (Fig. 8 ). Regression-based Harbord's test showed no indication of small-study effects (Table 4 ), which ruled out the possible heterogeneity to a certain extent. Therefore, we believe that the differences in the inclusion and data collection of research subjects in different countries may be a potential source of heterogeneity, and the results could be considered credible.
Fig. 8.
Funnel-plot analysis of CVD (A), cardiac injury (B), and CVD in China (C), the USA (D), Iran (E) and Italy (F).
Table 4.
Regression-based Harbord's test for all associations analysed
| Number of studies included | P value for small-study effects | |
|---|---|---|
| CVD | 47 | 0.176 |
| Cardiac injury | 12 | 0.812 |
| CVD (China) | 17 | 0.244 |
| CVD (USA) | 7 | 0.218 |
| CVD (Italy) | 8 | 0.85 |
| CVD (Iran) | 6 | 0.98 |
4. Discussion
So far, many countries including Italy, Spain, Germany, the USA and the UK have reported that that they are in trouble. On one hand, their hospitals are facing an overload of ICU and a shortage of ICU nurses and protective equipment. On the other hand, there are a large number of COVID-19 patients in these countries who need emergency treatment. Due to the rapid deterioration of COVID-19, these patients will face death threats if they cannot get emergency treatment in time such as being admitted to ICU for continuous monitoring and respiratory assistance. This contradiction has greatly affected the normal operation of the hospitals and the treatment of COVID-19 patients. By identifying the risk factors associated with mortality, and then intervening in time, the proportion of patients who get worse and then die will decrease, thereby reducing the need for first aid and saving limited medical resources.
4.1. Main findings
This meta-analysis of 56 studies systematically assessed the impact of baseline CVD and acute cardiac injury on the fatal risk of hospitalized COVID-19 patients. This analysis included more global data with a clear definition of the research objects, carefully compared the duplication of study data and then deleted the repetitive studies as appropriate to reduce bias. In particular, our study further assessed and compared the risk value of CVD in populations from four countries. In this study, we found that (1) CVD: from a global perspective, the overall mortality of hospitalized COVID-19 patients was high, and patients with baseline CVD were at a higher risk of fatal outcomes than those without CVD. In addition, further analysis of the population in four countries reached a similar conclusion as above, again highlighting the importance of CVD. In addition, the CIs of OR values in different countries on fatal outcomes overlapped; thus, there was no significant evidence to prove that the risk of CVD on adverse outcomes in a single country was significantly higher than those in other countries. (2) Cardiac injury: cardiac injury was obviously common among hospitalized COVID-19 patients, ranging from 17.3% to 60.7%. Patients with cardiac injury had a thirteen-fold higher mortality risk than those without cardiac injury, suggesting that cardiac injury can act as a more powerful prognostic predictor of COVID-19 than CVD.
4.2. Pathogenic mechanism of study results
The adverse effect of CVD and acute cardiac injury on COVID-19 mortality may be due to the pathogenic mechanism of diseases. Inflammation plays important roles in diseases; that is, inflammatory activation mediates the vulnerability of patients with CVD to COVID-19 infection, and the systemic inflammatory response syndrome caused by COVID-19 promotes the development of cardiac injury, which may worsen the condition of COVID-19 patients and even aggravate mortality. CVD is defined as a group of disorders of the heart and blood vessels [7]. Patients with CVD often have chronic inflammation since inflammatory activation acts as an essential contributor to CVD development and progression. For example, the elevated serum inflammatory factors tumour necrosis factor-α (TNF-α) and interleukin-1 beta (IL-1β) might induce a decrease in myocardial contractility and cause left heart dysfunction and dilation [65], while an increased co-expression of interleukin-9 (IL-9) and interleukin-17 (IL-17) showed a putative pro-inflammatory role in tissue inflammation and chronicity of tissue damage in giant cell arteritis and atherosclerosis [66,67]. On the other hand, inflammation is a common characteristic of COVID-19 infection and is important for its development. Increased serum inflammatory factors (e.g., IL-9, IL-1β, TNF-α) and decreased lymphocytes were detected in COVID-19 patients, especially in critically ill patients [62]. The changes in serum inflammatory factors in COVID-19 infection and CVD overlap. Besides, decreased lymphocytes were implicated in the progression and destabilization of atherosclerosis [68]. The virus itself also seemed to mediate myocardial damage [69]. This evidence explains why the cardiovascular system and heart are involved in this respiratory disease, and patients who have a group of disorders of the heart and blood vessels seem to be more vulnerable to COVID-19 and to have a more severe clinical course and prognosis. On the other hand, an increasing number of studies have shown that systemic inflammatory response syndrome (including cytokine storm, immune cell imbalance and uncontrolled inflammation) caused by COVID-19 plays an important role in the development of cardiac injury. For example, cardiac injury markers were elevated simultaneously with inflammatory factors (amino-terminal B-type natriuretic peptide, D-dimers, procalcitonin, IL-6) in patients with cardiac injury, and the inflammatory factors were higher than those of patients without cardiac injury, suggesting that patients suffering from cardiac injury had more severe systemic inflammation [45,49]. Furthermore, severe cardiac events (e.g., myocardial failure) induced by cardiac injury were correlated with COVID-19-induced pulmonary distress and elevation of pulmonary artery pressure, which promoted the occurrence of acute respiratory distress syndrome (ARDS), a common cause of deterioration and mortality in COVID-19 patients [55]. This was supported by evidence that patients with ARDS had a high incidence of elevated cardiac injury biomarkers, which was also independently associated with an increase in 60-day mortality and organ failure [70]. Therefore, cardiac injury is common in COVID-19 infection and increases the risk of deterioration and even mortality.
4.3. Recommendations for clinical practice
Our study indicated that CVD and acute cardiac injury were associated with an increase in mortality of hospitalized COVID-19 patients. Deterioration of the condition will increase the need for emergency rescue in hospitalized patients, such as Intensive Care Unit (ICU) admission. In order to better treat patients and save limited medical resources, we suggest that inquiry of CVD history and monitoring of biochemical predictors of cardiac injury could be taken into consideration during the hospital stay, especially within the first week of admission for critically ill patients. Li et al. [9] described that the median levels of cardiac markers and inflammatory markers peaked on the 3rd day and then gradually decreased between the 4th and 7th days of hospitalization in critically ill survivors, while the levels of these markers continued to rise among non-survivors. In the case of rapid development of the disease, the test results can remind medical staff of the risks faced by COVID-19 patients and provide evidence-based support for the optimal use of therapies, such as risk stratification or consideration of the use of drugs and/or cooperation with other examinations, ultimately delaying the deterioration of the disease and promoting the recovery of COVID-19 patients. For example, Fried, J.A. et al. described that if the patient had cardiomyopathy and prolonged baseline QT, the use of hydroxychloroquine and azithromycin might be affected [71]. Besides, the susceptibility of people with CVD to COVID-19 infection requires people to pay special attention to personal protection to prevent COVID-19.
4.4. Limitations
Although we performed the above studies, which specifically analysed the data of different countries and made some suggestions for clinical practice based on the results, further discussion was limited by some weaknesses. (1) RCTs that met the inclusion criteria but not the exclusion criteria were not searched. If there are RCT data, the persuasiveness of the meta-analysis results will be further improved. However, we have fully discussed the 56 articles searched and included in the analysis above based on attention to article quality. NOS assessed the methodological quality of all 56 articles and proved that their quality was sufficient for meta-analysis. The results of sensitivity analysis, funnel-plot analysis, and regression-based Harbord's test indicated the credibility of the meta-analysis results. In addition, the meta-analysis results were biologically reasonable without conflict with known biological knowledge on pathogenic mechanism, which further proved the credibility of the results of this article. (2) Some people wondered whether cardiac injury might be secondary to baseline CVD but occur before COVID-19 infection and whether the development of cardiac injury may be prompted by the stronger inflammation induced by the interaction between CVD and COVID-19 infection. However, Heberto, A. B. et al. once indirectly rejected this speculation since they found no statistically significant difference in the prevalence of baseline CVD among COVID-19 cases with and without cardiac injury [42]. The current data still fails to explain the relationship between the two risk factors. It will be interesting to compare cardiac biomarkers before and after COVID-19 infection to determine the context of the two risk factors in future studies.
5. Conclusion
In brief, both CVD and cardiac injury are associated with increasing mortality among hospitalized COVID-19 patients. Therefore, inquiry of patients' CVD history should be taken into consideration, and biomarkers of cardiac injury could be incorporated into the routine laboratory panel for COVID-19 during the hospital stay.
Data availability
All data analysed during this study are included within the manuscript and its additional file.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81473034), General Guidance Project of Guangzhou Health and Family Planning Technology (Grant No. 20181A011050).
Authors' contributions
All authors met PRISMA guidelines for authorship and provided analysis interpretation, critical review, revision, and final approval of the article. Jiali Long, Yefei Luo and Yuehong Wei contributed to the concept/design, data collection, analysis and writing manuscript of the study. Chaojun Xie and Jun Yuan aided in the revision and publication management. The first three authors, two corresponding authors contributed equally to this manuscript, separately.
Declaration of Competing Interest
All authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 81473034), General Guidance Project of Guangzhou Health and Family Planning Technology (Grant No. 20181A011050).
References
- 1.WHO Coronavirus Disease (COVID-19) Pandemic. 2021. https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019
- 2.Abbasi B., Akhavan R., Ghamari Khameneh A., Zandi B., Farrokh D., Pezeshki Rad M., et al. Evaluation of the relationship between inpatient COVID-19 mortality and chest CT severity score. Am. J. Emerg. Med. 2020;S0735-6757(20):30851–30852. doi: 10.1016/j.ajem.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter B., Collins J.T., Barlow-Pay F., Rickard F., Bruce E., Verduri A., et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-nosocomial study (COVID in older PEople) J Hosp Infect. 2020;106(2):376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciceri F., Castagna A., Rovere-Querini P., De Cobelli F., Ruggeri A., Galli L., et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardiology ESo: ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. 2020. https://wwwescardioorg/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
- 6.Oren O.K.S., Gluckman T.J., Gersh B.J., Blumenthal R.S. Coronavirus Disease 2019 (COVID-19): Epidemiology, Clinical Spectrum and Implications for the Cardiovascular Clinician. 2020. https://wwwaccorg/latest-in-cardiology/articles/2020/04/06/11/08/covid-19-epidemiology-clinical-spectrum-and-implications-for-the-cv-clinician
- 7.WHO Cardiovascular Diseases (CVDs) 2021. https://wwwwhoint/health-topics/cardiovascular-diseases#tab=tab_1
- 8.Thygesen K., Fau Alpert J.S., Fau Alpert Js, Jaffe A.S., Fau Jaffe As, Chaitman B.R., et al. 2018. Fourth Universal Definition of Myocardial Infarction. [Google Scholar]
- 9.Li C., Jiang J., Wang F., Zhou N., Veronese G., Moslehi J.J., et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell. Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Vol. 8. Arch Acad Emerg Med.; 2020. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis; p. e35. [PMC free article] [PubMed] [Google Scholar]
- 11.Parohan M., Yaghoubi S., Seraji A. Vol. 9. Eur Heart J Acute Cardiovasc Care; 2020. Cardiac injury is Associated with Severe Outcome and Death in Patients with Coronavirus Disease 2019 (COVID-19) infection: A Systematic Review and Meta-Analysis of Observational Studies; pp. 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Fau Tetzlaff J., Fau Tetzlaff J., Altman D.G. Vol. 6. PLoS Med.; 2020. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement; p. e1000097. [PMC free article] [PubMed] [Google Scholar]
- 13.Aladağ N., Atabey R.D. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID-19 patients. Acta Cardiol. 2020;4:1–8. doi: 10.1080/00015385.2020.1810914. [DOI] [PubMed] [Google Scholar]
- 14.Alamdari N.M., Afaghi S., Rahimi F.S., Tarki F.E., Tavana S., Zali A., et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral Center in Iran. Tohoku J. Exp. Med. 2020;252(1):73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Garcia J., Lee S., Gupta A., Cagliostro M., Joshi A.A., Rivas-Lasarte M., et al. Prognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19. J. Am. Coll. Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Wahaibi K., Al-Wahshi Y., Mohamed Elfadil O. Myocardial Injury Is Associated with Higher Morbidity and Mortality in Patients with 2019 Novel Coronavirus Disease (COVID-19) SN Compr Clin Med. 2020:1–7. doi: 10.1007/s42399-020-00569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An W., Xia F., Chen M., Yang P., Fang S.S., Ly L., et al. Analysis of clinical features of 11 death cases caused by COVID-19. The J Pract Med. 2020;36:1125–1130. [Google Scholar]
- 18.Barman H.A., Atici A., Sahin I., Alici G., Aktas Tekin E. Baycan Ö F, Ozturk F, Oflar E, Tugrul S, Yavuz MB, et al: prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron. Artery Dis. 2020;10:1097. doi: 10.1097/MCA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bursi F., Santangelo G., Sansalone D., Valli F., Vella A.M., Toriello F., et al. Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography. 2020;37:2029–2039. doi: 10.1111/echo.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo-Fernández A., Izquierdo A., Subirana I., Farré N., Vila J., Durán X., et al. Markers of myocardial injury in the prediction of short-term COVID-19 prognosis. Rev Esp Cardiol (Engl Ed) 2020 doi: 10.1016/j.recesp.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y., Han X., Gu J., Li Y., Liu J., Alwalid O., et al. Prognostic value of baseline clinical and HRCT findings in 101 patients with severe COVID-19 in Wuhan, China. Sci. Rep. 2020;10(1):17543. doi: 10.1038/s41598-020-74497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrillo-Vega M.F., Salinas-Escudero G., García-Peña C., Gutiérrez-Robledo L.M., Parra-Rodríguez L. Early estimation of the risk factors for hospitalization and mortality by COVID-19 in Mexico. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F.F., Zhong M., Liu Y., Zhang Y., Zhang K., Su D.Z., et al. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J. Crit. Care. 2020;60:32–37. doi: 10.1016/j.jcrc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L., Feng Y., Tang J., Hu W., Zhao P., Guo X., et al. Surface electrocardiographic characteristics in coronavirus disease 2019: repolarization abnormalities associated with cardiac involvement. ESC Heart Fail. 2020;7(6):4408–4415. doi: 10.1002/ehf2.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 From a Nationwide Analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chilimuri S., Sun H., Alemam A., Mantri N., Shehi E., Tejada J., et al. Predictors of mortality in adults admitted with COVID-19: retrospective cohort Study from New York City. West. J. Emerg. Med. 2020;21(4):779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciardullo S., Zerbini F., Perra S., Muraca E., Cannistraci R., Lauriola M., et al. Impact of diabetes on COVID-19-related in-hospital mortality: a retrospective study from Northern Italy. J. Endocrinol. Investig. 2020;44(4):843–850. doi: 10.1007/s40618-020-01382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipriani A., Capone F., Donato F., Molinari L., Ceccato D., Saller A., et al. Cardiac injury and mortality in patients with Coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern. Emerg. Med. 2021;16(2):419–427. doi: 10.1007/s11739-020-02495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Castelnuovo A., Bonaccio M., Costanzo S., Gialluisi A., Antinori A., Berselli N., et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST study. Nutr. Metab. Cardiovasc. Dis. 2020;30(11):1899–1913. doi: 10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eslami V., Abrishami A., Zarei E., Khalili N., Baharvand Z., Sanei-Taheri M. The Association of CT-measured Cardiac Indices with Lung Involvement and Clinical Outcome in Patients with COVID-19. Acad. Radiol. 2021;28(1):8–17. doi: 10.1016/j.acra.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farré N., Mojón D., Llagostera M., Belarte-Tornero L.C., Calvo-Fernández A., Vallés E., et al. Prolonged QT Interval in SARS-CoV-2 Infection: Prevalence and Prognosis. J. Clin. Med. 2020;9(9):2712. doi: 10.3390/jcm9092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrando C., Mellado-Artigas R., Gea A., Arruti E., Aldecoa C., Bordell A., et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study. Rev. Esp. Anestesiol. Reanim. 2020;67(8):425–437. doi: 10.1016/j.redar.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrante G., Fazzari F., Cozzi O., Maurina M., Bragato R., D’Orazio F., et al. Risk factors for myocardial injury and death in patients with COVID-19: insights from a cohort study with chest computed tomography. Cardiovasc. Res. 2020;116(14):2239–2246. doi: 10.1093/cvr/cvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. 180(10) JAMA Intern Med.; 2020. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy; pp. 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020;180(11):1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halvatsiotis P., Kotanidou A., Tzannis K., Jahaj E., Magira E., Theodorakopoulou M., et al. Demographic and clinical features of critically ill patients with COVID-19 in Greece: the burden of diabetes and obesity. Diabetes Res. Clin. Pract. 2020;166:108331. doi: 10.1016/j.diabres.2020.108331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harmouch F., Shah K., Hippen J.T., Kumar A., Goel H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J. Med. Virol. 2021;93(2):973–982. doi: 10.1002/jmv.26347. [DOI] [PubMed] [Google Scholar]
- 40.He X.W., Lai J.S., Cheng J., Wang M.W., Liu Y.J., Xiao Z.C., et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(6):456–460. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 41.He Y., Xie M., Zhao J., Liu X. Clinical characteristics and outcomes of patients with severe COVID-19 and chronic obstructive pulmonary disease (COPD) Med. Sci. Monit. 2020;26:e927212. doi: 10.12659/MSM.927212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heberto A.B., Carlos P.C.J., Antonio C.R.J., Patricia P.P., Enrique T.R., Danira M.P.J., et al. Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19) Int J Cardiol Heart Vasc. 2020;30:100638. doi: 10.1016/j.ijcha.2020.100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homayounieh F., Zhang E.W., Babaei R., Karimi Mobin H., Sharifian M., Mohseni I., et al. Clinical and imaging features predict mortality in COVID-19 infection in Iran. PLoS One. 2020;15(9):e0239519. doi: 10.1371/journal.pone.0239519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang J.M., Kim J.H., Park J.S., Chang M.C., Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol. Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in northern Italy. Eur. Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanza G.A., De Vita A., Ravenna S.E., D’Aiello A., Covino M., Franceschi F., et al. Electrocardiographic findings at presentation and clinical outcome in patients with SARS-CoV-2 infection. Europace. 2021;23(1):123–129. doi: 10.1093/europace/euaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J., Xu G., Yu H., Peng X., Luo Y., Cao C. Clinical Characteristics and Outcomes of 74 Patients With Severe or Critical COVID-19. Am J Med Sci. 2020;360(3):229–235. doi: 10.1016/j.amjms.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J., Zhang Y., Cheng G., He J., Wu F., Hu H., et al. Clinical characteristics and outcomes of adult critically ill patients with COVID-19 in Honghu, Hubei Province. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(6):778–785. doi: 10.12122/j.issn.1673-4254.2020.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Q., Wang J.L., Zhang B., Li Z.G., Kong D.N., Xiong S.Q., et al. Characteristics of myocardial injury in severe and critical coronavirus disease 2019 patients and its effect on prognosis. Acad J Second Military Med Univ. 2020;41:596–603. [Google Scholar]
- 50.McCullough S.A., Goyal P., Krishnan U., Choi J.J., Safford M.M., Okin P.M. Electrocardiographic findings in coronavirus Disease-19: insights on mortality and underlying myocardial processes. J. Card. Fail. 2020;26(7):626–632. doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikpouraghdam M., Jalali Farahani A., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H., et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study. J. Clin. Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan F., Yang L., Li Y., Liang B., Li L., Ye T., et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int. J. Med. Sci. 2020;17(9):1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson E., Lo K.B., DeJoy R., Salacup G., Pelayo J., Bhargav R., et al. The relationship between coronary artery disease and clinical outcomes in COVID-19: a single-center retrospective analysis. Coron. Artery Dis. 2020 doi: 10.1097/MCA.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 54.Rastad H., Karim H., Ejtahed H.S., Tajbakhsh R., Noorisepehr M., Babaei M., et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol. Metab. Syndr. 2020;12:57. doi: 10.1186/s13098-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rath D., Petersen-Uribe Á., Avdiu A., Witzel K., Jaeger P., Zdanyte M., et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin. Res. Cardiol. 2020;109(21):1491–1499. doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rey J.R., Caro-Codón J., Rosillo S.O., Iniesta Á.M., Castrejón-Castrejón S., Marco-Clement I., et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur. J. Heart Fail. 2020;22(12):2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun J.H., Hx W., Chen C.Y., Wang Y., Zeng H.S. Risk factors of deaths in patients with COVID-19: a multivariate logistic regression analysis. J Intern Intensive Med. 2020;26:364–368. [Google Scholar]
- 60.Twigg H.L., 3rd, Khan S.H., Perkins A.J., Roberts S., Sears C., Rahman O., et al. Mortality rates in a diverse cohort of mechanically ventilated patients with novel coronavirus in the urban Midwest. Crit Care Explor. 2020;2(8):e0187. doi: 10.1097/CCE.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J., Yang X., Yang L., Zou X., Wang Y., Wu Y., et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit. Care. 2020;24(1):394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J., Lu S., Wang X., Jia X., Li J., Lei H., et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart. 2020;106(15):1148–1153. doi: 10.1136/heartjnl-2020-316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y., Nie H.X., Hu K., Wu X.J., Zhang Y.T., Wang M.M., et al. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect Dis Poverty. 2020;9(1):108. doi: 10.1186/s40249-020-00723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mann D.L. 91(11) Circ Res.; 2002. Inflammatory Mediators and the Failing Heart: Past, Present, and the Foreseeable Future; pp. 988–998. [DOI] [PubMed] [Google Scholar]
- 66.Ciccia F., Rizzo A., Guggino G., Cavazza A., Alessandro R., Maugeri R., et al. 54(9) Rheumatology (Oxford).; 2015. Difference in the Expression of IL-9 and IL-17 Correlates with Different Histological Pattern of Vascular Wall Injury in Giant Cell Arteritis; pp. 1596–1604. [DOI] [PubMed] [Google Scholar]
- 67.Moaaz M.A.-O., Lotfy H. 28(4) Vascular.; 2020. Changes and Significance of T Helper-9 Cells and Interleukin-9 in Patients with Atherosclerotic Chronic Lower Limb Ischemia: Effect on IL-17 Release; pp. 378–389. [DOI] [PubMed] [Google Scholar]
- 68.Wada H., Dohi T., Miyauchi K., Shitara J., Endo H., Doi S., et al. Vol. 265. Atherosclerosis.; 2017. Pre-Procedural Neutrophil-to-Lymphocyte Ratio and Long-Term Cardiac Outcomes after Percutaneous Coronary Intervention for Stable Coronary Artery Disease; pp. 35–40. [DOI] [PubMed] [Google Scholar]
- 69.Oudit G.Y., Kassiri Z., Fau Jiang C., Fau Jiang C., Liu P.P., SM Liu Pp Fau Poutanen, et al. 39(7) Eur J Clin Invest.; 2009. SARS-Coronavirus Modulation of Myocardial ACE2 Expression and Inflammation in Patients with SARS; pp. 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bajwa E.K., Boyce P.d., Januzzi J.L., Gong M.N., Thompson B.T., Christiani D.C., et al. 35(11) Crit Care Med.; 2007. Biomarker Evidence of Myocardial Cell Injury is Associated with Mortality in Acute Respiratory Distress Syndrome; pp. 2484–2490. [DOI] [PubMed] [Google Scholar]
- 71.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., et al. 141(23) Circulation.; 2020. The Variety of Cardiovascular Presentations of COVID-19; pp. 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analysed during this study are included within the manuscript and its additional file.