Abstract
Background
The relationship between obesity and prevalent high blood pressure in older adults has predominantly been estimated using categorical measures of body mass index (BMI) and waist circumference (WC), masking the shape of the dose-response relationship. We aimed to examine the precise relationship of BMI, WC with high blood pressure and to assess the appropriate level of BMI and WC for high blood pressure.
Methods
We examined data for 126,123 individuals in Xinzheng city aged ≥60 years from a population based study from January to December 2019. Logistic regression and restricted cubic spline models were applied to assess the relationship and the appropriate level of BMI and WC for high blood pressure. An additive interaction analysis was used to test synergistic effects between a higher BMI and WC for high blood pressure.
Results
The full-adjusted odds ratios (ORs) with 95% confidence intervals (CIs) of an increase of 1 kg/m2 in BMI and 1 cm in WC for high blood pressure were 1.084 (1.080–1.087) and 1.026(1.024–1.027), respectively. Multivariable adjusted restricted cubic spline analyses showed the nonlinear relationships of BMI and WC with high blood pressure in both men and women (all P < 0.001). The risk of high blood pressure increased steeply with increasing BMI from ≥25 kg/m2 and WC ≥ 88 cm or 86 cm for males and females, respectively. And we observed a significant additive interaction between a higher BMI and WC such that the prevalence of high blood pressure was significantly enhanced.
Conclusion
These findings suggest increased high blood pressure prevalence in the older adults with increased BMI and WC. BMI ≤ 25 kg/m2 and WC ≤ 88 cm or 86 cm for males and females may be the best suggestion with regard to primary prevention of high blood pressure in older adults.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02154-5.
Keywords: High blood pressure, Body mass index, Waist circumference, Restricted cubic spline, Additive interaction
Background
Hypertension is a substantial public health burden [1] and a strong modifiable risk factor for cardiovascular disease [2], which has become one of the leading causes of global mortality, accounting for 9.4 million deaths each year [3, 4]. From 2010 to 2017, the weighted prevalence of hypertension increased by 23.4% and is increasing with aging in China [4]. Obesity is one of the modifiable risk factors associated with cardiovascular disease [5–7], which is most generally assessed by body mass index (BMI) and waist circumference (WC). Commonly, BMI is used as a substitute for general obesity because of its strong correlation with gold standard body fat [8–10], and WC is regarded as a better index for evaluating abdominal obesity [11].
Accumulating evidence suggests a potential link between obesity-related high blood pressure (HBP) [12–14], but controversy exists about the degree of the associations between two anthropometric indicators and the risk of HBP. And information about the relationship between BMI, WC and HBP is limited in older adults. In particular, few studies have explored the dose–response relationship and investigated the interaction between BMI and WC. In addition, higher BMI and WC increase the risk of HBP alone, but their interrelations with an increased risk of HBP remain uncertain.
Thus, in this study, we aimed to assess the independent association of BMI and WC with HBP as well as their possible additive interactions on the risk of HBP using a large and contemporary population in central China and to explore the appropriate level of BMI and WC for the older adults.
Methods
Study population
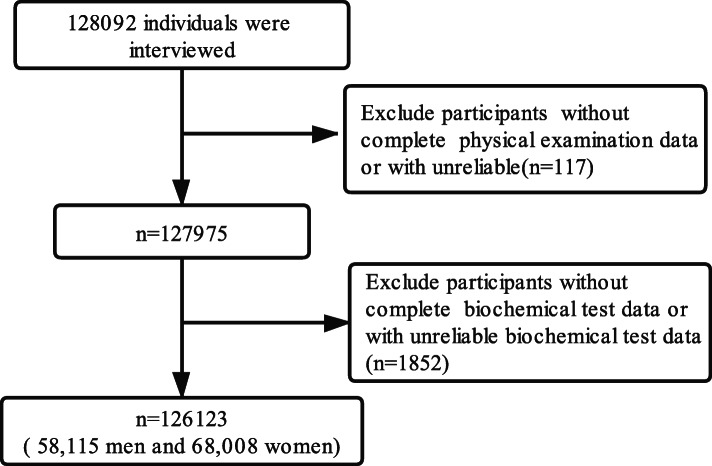
The study population consisted of participants of a comprehensive health check-up program conducted at fourteen medical examination centers (Supplementary 1). Generally, the Central People’s Government of China demands that residents aged ≥60 years participate in health examinations annually to promote good health by enabling early detection of chronic diseases and associated risk factors. The study collected clinical, demographic, and lifestyle information from all participants by face-to-face interviews, physical examinations and blood biochemical examinations. Cross-sectional study data from Xinzheng from January to December 2019 were combined for analyses. For this study, 1969 people were excluded from the current study due to missing physical examination data (n = 117) or biochemical test data (n = 1852). Finally, we had data for 58,115 men and 68,008 women resident ≥60 years of age, who were enrolled to assess the association between BMI, WC and HBP (Fig. 1). Written informed consent was obtained from each participant before data collection. The research ethics committee of Zheng Zhou University approved the current study methodology, protocol, and procedures. (Reference Number: ZZUIRB2019–019).
Fig. 1.
Flow diagram of the selection of eligible participants
Exposure
Height, weight, and WC were measured twice by trained nurses following rigorous protocols. Body height was measured without shoes with a stadiometer, and body weight was measured with participants in light clothing and without shoes by electronic scales. WC was measured with gentle breathing at the midpoint between the lowest rib and the iliac crest to the nearest 0.1 cm. BMI was calculated as weight (kg) divided by the square of height (m). BMI was categorized into quintiles (Male: < 22.20, 22.20 ~ 24.02, 24.02 ~ 25.73, 25.73 ~ 27.78, ≥27.78 Female: < 22.43, 22.43 ~ 24.42, 24.4 ~ 26.27, 26.27 ~ 28.58, ≥28.58). And WC was categorized into quintiles (Male:< 81,81 ~ 86,86 ~ 90,90 ~ 96,≥96Female:< 79,79 ~ 84,84 ~ 89,89 ~ 95,≥95).
Outcome
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were collected according to the World Health Organization definition [15]. After sitting quietly for five minutes, certified nurses measured each participant’s seated blood pressure three times using a mercury sphygmomanometer (Omron HEM-7125, Kyoto, Japan). We calculated the means of SBP and DBP. Diagnosis of HBP was defined as SBP ≥ 140 mmHg/or DBP ≥90 mmHg or use of antihypertensive medication within 2 weeks [15].
Covariates
Information on demographic characteristics (age, sex, marital status, and place of residence) and behavioral measures (smoking, physical exercise and alcohol consumption) were obtained by a standardized questionnaire, which was in strict accordance with the National Standards for Basic Public Health Services (2011). Marital status was categorized as unmarried, married, divorced and death of a spouse. Smoking status was defined as current smoker, former smokers, or never smoker [16]; Alcohol consumption and physical exercise status were categorized as never, once in a while, more than once a week and every day. Place of residence (rural and urban) was defined according to country-specific definitions. Stroke was defined as sudden onset of a focal, non-convulsive neurological deficit persisting longer than 24 h. Diagnosis of psychotic illnesses was met Diagnostic and Statistical Manual of Mental Disorders (DSM − 5) criteria for a diagnosis within the spectrum of primary psychotic illnesses. Diagnosis of cancer based on the International Classification of Diseases. Overnight fasting blood samples were collected into vacuum tubes for assessing serum levels of blood glucose using standard methods.
Statistical analysis
Continuous data were expressed as the mean ± standard deviation (SD) and categorical data as the number (percentage) for quintiles of BMI or WC, using different values for men and women. Comparisons of the basic characteristics of the quintiles were performed with the χ2test, ANOVA, and Kruskal-Wallis test.
Logistic regression models were used to estimate the adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for BMI, WC and HBP, taking the quintile with the lowest baseline BMI or WC values as the reference. ORs and 95% CIs for HBP were estimated for each group with adjustment of multiple confounders. One obesity parameter was introduced at a time in each model to avoid the collinear effect. Model 1 was unadjusted. Model 2 was adjusted for sex and age. Model 3 was adjusted for Model 2 and marital status, alcohol consumption, smoking, physical activity, place of residence, cancer, stroke, psychotic illness and blood glucose. A linear trend test was performed by modeling the median value of each exposure category as a continuous variable in the models. Fully adjusted restricted cubic spline analyses were used to characterize the dose-response association and explore the potential linear or nolinear relationship of BMI, and WC with HBP. The knots were placed at the 5th, 25th, 50th, 75th and 95th percentiles. The test result for overall association was checked first. If the test for overall association was significant, the test result for nonlinearity and linearity were checked, and the P-value for non-linear association < 0.05 indicated a significant result indicating the linear association. We also evaluated the additive interaction between BMI and WC for HBP with BMI and WC analyzed as continuous variable in two categories [(BMI: BMI < 25 and BMI ≥ 25. WC: WC < 102 for males, WC < 88 for females and WC ≥ 102 for males, WC ≥ 88 for females) or (WHO proposed cut-off points [17])]. We applied three indicators to evaluate the additive interaction: relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP) or synergy index (S). RERI > 0, AP > 0, or S > 1 was regarded as a significant additive interaction.
Statistical analyses involved the use of SAS V .9.1 (SAS Institute) and R × 64 4.0.0.All reported P values were two-sided, with P < 0.05 considered statistically significant.
Results
Basic demographic characteristics
A total of 126,123 participants were eligible for inclusion in this study. The mean (SD) age was 70.29 (6.94) years. The basic demographic characteristics of the study population according to BMI and WC quintiles are shown in Tables 1 and 2. The prevalence of HBP and stroke, levels of alcohol consumption, physical exercise and blood glucose showed a significant progressive increase from the quintile with the lowest BMI to the quintile with the highest BMI. This was also the case for the WC quintiles.
Table 1.
Basic demographic characteristics of Subjects According to BMI Quintiles
| Characteristics | First Quintile | Second Quintile | Third Quintile | Fourth Quintile | Fifth Quintile | P for trend |
|---|---|---|---|---|---|---|
| Male | BMI < 22.20 | 22.20 ≤ BMI < 24.02 | 24.02 ≤ BMI < 25.73 | 25.73 ≤ BMI < 27.78 | BMI ≥ 27.78 | |
| Female | BMI < 22.43 | 22.43 ≤ BMI < 24.42 | 24.42 ≤ BMI < 26.27 | 26.27 ≤ BMI < 28.58 | BMI ≥ 28.58 | |
| N0. | 25,164 | 25,236 | 25,166 | 25,268 | 25,289 | |
| High blood pressure | < 0.001 | |||||
| no | 13,812 (54.89) | 12,206 (48.37) | 11,346 (45.08) | 10,508 (41.59) | 9205 (36.4) | |
| yes | 11,352 (45.11) | 13,030 (51.63) | 13,820 (54.92) | 14,760 (58.41) | 16,084 (63.6) | |
| Cancer | 0.005 | |||||
| no | 25,137 (99.89) | 25,215 (99.92) | 25,146 (99.92) | 25,246 (99.91) | 25,282 (99.97) | |
| yes | 27 (0.11) | 21 (0.08) | 20 (0.08) | 22 (0.09) | 7 (0.03) | |
| Stroke | 0.003 | |||||
| no | 25,036 (99.49) | 25,099 (99.46) | 25,056 (99.56) | 25,163 (99.58) | 25,194 (99.62) | |
| yes | 128 (0.51) | 137 (0.54) | 110 (0.44) | 105 (0.42) | 95 (0.38) | |
| Psychotic illness | 0.009 | |||||
| no | 24,982 (99.28) | 25,107 (99.49) | 25,027 (99.45) | 25,177 (99.64) | 25,136 (99.39) | |
| yes | 182 (0.72) | 129 (0.51) | 139 (0.55) | 91 (0.36) | 153 (0.61) | |
| Age, years | 72.08 ± 7.75 | 70.54 ± 7.17 | 69.96 ± 6.66 | 69.59 ± 6.49 | 69.29 ± 6.17 | < 0.001 |
| sex,% | 0.981 | |||||
| men | 11,619 (46.17) | 11,584 (45.9) | 11,612 (46.14) | 11,642 (46.07) | 11,658 (46.1) | |
| women | 13,545 (53.83) | 13,652 (54.1) | 13,554 (53.86) | 13,626 (53.93) | 13,631 (53.9) | |
| Marital status, % | < 0.001 | |||||
| unmarried | 558 (2.22) | 432 (1.71) | 341 (1.36) | 285 (1.13) | 246 (0.97) | |
| married | 19,847 (78.87) | 20,968 (83.09) | 21,315 (84.7) | 21,686 (85.82) | 21,916 (86.66) | |
| divorced | 135 (0.54) | 91 (0.36) | 124 (0.49) | 92 (0.36) | 83 (0.33) | |
| death of a spouse | 4624 (18.38) | 3745 (14.84) | 3386 (13.45) | 3205 (12.68) | 3044 (12.04) | |
| Smoking, % | < 0.001 | |||||
| Never smokers | 20,950 (83.25) | 21,506 (85.22) | 21,557 (85.66) | 21,879 (86.59) | 22,079 (87.31) | |
| Former smokers | 537 (2.13) | 591 (2.34) | 611 (2.43) | 623 (2.47) | 678 (2.68) | |
| Current smokers | 3677 (14.61) | 3139 (12.44) | 2998 (11.91) | 2766 (10.95) | 2532 (10.01) | |
| Alcohol consumption, % | < 0.001 | |||||
| Never | 23,681 (94.11) | 23,651 (93.72) | 23,364 (92.84) | 23,379 (92.52) | 23,296 (92.12) | |
| Once in a while | 906 (3.6) | 998 (3.95) | 1113 (4.42) | 1129 (4.47) | 1215 (4.8) | |
| More than once a week | 243 (0.97) | 277 (1.1) | 305 (1.21) | 350 (1.39) | 352 (1.39) | |
| Every day | 334 (1.33) | 310 (1.23) | 384 (1.53) | 410 (1.62) | 426 (1.68) | |
| Physical exercise, % | < 0.001 | |||||
| Never | 16,555 (65.79) | 15,881 (62.93) | 15,014 (59.66) | 14,897 (58.96) | 15,290 (60.46) | |
| Once in a while | 863 (3.43) | 912 (3.61) | 943 (3.75) | 998 (3.95) | 966 (3.82) | |
| More than once a week | 1576 (6.26) | 1809 (7.17) | 1937 (7.7) | 1914 (7.57) | 1830 (7.24) | |
| Every day | 6170 (24.52) | 6634 (26.29) | 7272 (28.9) | 7459 (29.52) | 7203 (28.48) | |
| Residence | < 0.001 | |||||
| Rural areas | 21,838 (86.78) | 20,703 (82.04) | 20,322 (80.75) | 20,314 (80.39) | 20,365 (80.53) | |
| Urban areas | 3326 (13.22) | 4533 (17.96) | 4844 (19.25) | 4954 (19.61) | 4924 (19.47) | |
| Blood glucose, mmol/L | 5.44 ± 1.8 | 5.68 ± 1.86 | 5.82 ± 1.91 | 5.91 ± 1.91 | 6.04 ± 1.95 | < 0.001 |
Data are shown as mean ± SD or n (%). BMI body mass index, Residence, area of residence; Blood glucose, Fasting plasma glucose
Table 2.
Basic demographic characteristics of Subjects According to WC Quintiles
| Characteristics | First Quintile | Second Quintile | Third Quintile | Fourth Quintile | Fifth Quintile | P for trend |
|---|---|---|---|---|---|---|
| Male | WC < 81 | 81 ≤ WC < 86 | 86 ≤ WC < 90 | 90 ≤ WC < 96 | WC ≥ 96 | |
| Female | WC < 79 | 79 ≤ WC < 84 | 84 ≤ WC < 89 | 89 ≤ WC < 95 | WC ≥ 95 | |
| N0. | 23,098 | 23,498 | 24,971 | 26,736 | 27,820 | |
| High blood pressure | < 0.001 | |||||
| no | 12,346 (53.45) | 11,421 (48.6) | 11,219 (44.93) | 11,539 (43.16) | 10,552 (37.93) | |
| yes | 10,752 (46.55) | 12,077 (51.4) | 13,752 (55.07) | 15,197 (56.84) | 17,268 (62.07) | |
| Age, years | 71.29 ± 7.65 | 70.48 ± 7.15 | 69.96 ± 6.79 | 69.86 ± 6.57 | 70.01 ± 6.51 | < 0.001 |
| sex,% | 0.001 | |||||
| men | 10,850 (46.97) | 10,887 (46.33) | 10,190 (40.81) | 13,076 (48.91) | 13,112 (47.13) | |
| women | 12,248 (53.03) | 12,611 (53.67) | 14,781 (59.19) | 13,660 (51.09) | 14,708 (52.87) | |
| Cancer | 0.076 | |||||
| no | 23,075 (99.9) | 23,479 (99.92) | 24,949 (99.91) | 26,720 (99.94) | 27,803 (99.94) | |
| yes | 23 (0.1) | 19 (0.08) | 22 (0.09) | 16 (0.06) | 17 (0.06) | |
| Stroke | 0.008 | |||||
| no | 22,974 (99.46) | 23,375 (99.48) | 24,869 (99.59) | 26,623 (99.58) | 27,707 (99.59) | |
| yes | 124 (0.54) | 123 (0.52) | 102 (0.41) | 113 (0.42) | 113 (0.41) | |
| Psychotic illness | 0.091 | |||||
| no | 22,937 (99.3) | 23,384 (99.51) | 24,835 (99.46) | 26,613 (99.54) | 27,660 (99.42) | |
| yes | 161 (0.7) | 114 (0.49) | 136 (0.54) | 123 (0.46) | 160 (0.58) | |
| Marital status, % | < 0.001 | |||||
| unmarried | 546 (2.36) | 427 (1.82) | 312 (1.25) | 289 (1.08) | 288 (1.04) | |
| married | 18,446 (79.86) | 19,404 (82.58) | 21,106 (84.52) | 22,908 (85.68) | 23,868 (85.79) | |
| divorced | 120 (0.52) | 99 (0.42) | 106 (0.42) | 103 (0.39) | 97 (0.35) | |
| death of a spouse | 3986 (17.26) | 3568 (15.18) | 3447 (13.8) | 3436 (12.85) | 3567 (12.82) | |
| Smoking, % | < 0.001 | |||||
| Never smokers | 19,441 (84.17) | 19,920 (84.77) | 21,755 (87.12) | 22,749 (85.09) | 24,106 (86.65) | |
| Former smokers | 469 (2.03) | 534 (2.27) | 555 (2.22) | 748 (2.8) | 734 (2.64) | |
| Current smokers | 3188 (13.8) | 3044 (12.95) | 2661 (10.66) | 3239 (12.11) | 2980 (10.71) | |
| Alcohol consumption, % | < 0.001 | |||||
| Never | 21,794 (94.35) | 22,082 (93.97) | 23,500 (94.11) | 24,555 (91.84) | 25,440 (91.45) | |
| Once in a while | 799 (3.46) | 913 (3.89) | 927 (3.71) | 1322 (4.94) | 1400 (5.03) | |
| More than once a week | 208 (0.9) | 217 (0.92) | 233 (0.93) | 406 (1.52) | 463 (1.66) | |
| Every day | 297 (1.29) | 286 (1.22) | 311 (1.25) | 453 (1.69) | 517 (1.86) | |
| Physical exercise, % | < 0.001 | |||||
| Never | 14,960 (64.77) | 14,768 (62.85) | 15,405 (61.69) | 15,830 (59.21) | 16,674 (59.94) | |
| Once in a while | 808 (3.5) | 835 (3.55) | 996 (3.99) | 998 (3.73) | 1045 (3.76) | |
| More than once a week | 1507 (6.52) | 1790 (7.62) | 1871 (7.49) | 1962 (7.34) | 1936 (6.96) | |
| Every day | 5823 (25.21) | 6105 (25.98) | 6699 (26.83) | 7946 (29.72) | 8165 (29.35) | |
| Residence | < 0.001 | |||||
| Rural areas | 20,201 (87.46) | 20,096 (85.52) | 20,773 (83.19) | 21,110 (78.96) | 21,362 (76.79) | |
| Urban areas | 2897 (12.54) | 3402 (14.48) | 4198 (16.81) | 5626 (21.04) | 6458 (23.21) | |
| Blood glucose, mmol/L | 5.44 ± 1.71 | 5.64 ± 1.81 | 5.76 ± 1.88 | 5.9 ± 1.97 | 6.08 ± 2.01 | < 0.001 |
Data are shown as mean ± SD or n (%). WC waist circumference; Residence, area of residence; Blood glucose, Fasting plasma glucose
OR and 95% confidence intervals (CIs) for HBP according to BMI and WC
Table 3 presents the results from the logistic regression that estimated the association between the levels of BMI, WC and HBP. The multivariable adjusted OR (95% CI) per 1 kg/m2 increase in BMI was 1.084 (1.08 to 1.087). In all three models, the ORs for HBP increased significantly with increasing BMI quintiles (P for trend < 0.01). In Model 3, the multivariate-adjusted OR (95% CIs) for HBP with the highest BMI quintile group compared with the lowest quintile group was 2.300(2.217 to 2.386). The multivariable adjusted OR (95% CI) per 1 cm increase in WC was 1.025 (1.024 to 1.027). The highest quintile group had a greater HBP prevalence than the other quintile group, and the crude ORs (95% CIs) for HBP compared with the lowest quintile group was 1.879 (1.814 to 1.947). After adjustment for sex, age, marital status, alcohol consumption, smoking, physical activity, place of residence and blood glucose, the ORs were enhanced, and the multivariate-adjusted ORs (95% CIs) for HBP with the highest WC quintile group compared with the lowest quintile group was 1.977(1.906 to 2.050).
Table 3.
Association between BMI, WC and HBP
| Characteristics | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR(95%CI) | OR(95%CI) | OR(95%CI) | |
| BMI,per1kg/m2 change | 1.077 (1.074,1.08) | 1.085 (1.081,1.088) | 1.084 (1.080,1.087) |
| BMI group | |||
| Q1 | 1.000(ref) | 1.000(ref) | 1.000(ref) |
| Q2 | 1.299 (1.254,1.345) | 1.361 (1.313,1.41) | 1.363 (1.316,1.413) |
| Q3 | 1.482 (1.431,1.535) | 1.581 (1.525,1.638) | 1.580 (1.524,1.637) |
| Q4 | 1.709 (1.65,1.77) | 1.844 (1.779,1.911) | 1.835 (1.770,1.903) |
| Q5 | 2.126 (2.051,2.203) | 2.317 (2.235,2.403) | 2.300 (2.217,2.386) |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
| WC,per1 cm change | 1.022 (1.021,1.024) | 1.025 (1.024,1.026) | 1.026 (1.024,1.027) |
| WC group | |||
| Q1 | 1.000(ref) | 1.000(ref) | 1.000(ref) |
| Q2 | 1.214 (1.171,1.259) | 1.239 (1.195,1.286) | 1.237 (1.192,1.283) |
| Q3 | 1.408 (1.358,1.459) | 1.443 (1.391,1.496) | 1.445 (1.394,1.499) |
| Q4 | 1.512 (1.46,1.567) | 1.577 (1.522,1.635) | 1.593 (1.537,1.652) |
| Q5 | 1.879 (1.814,1.947) | 1.95 (1.881,2.021) | 1.977 (1.906,2.050) |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
BMI body mass index, WC waist circumference. Model1: unadjusted. Model2: adjusted for sex and age. Model3 adjusted for Model2 and marital status, alcohol consumption, smoking, physical activity, place of residence,cancer, stroke, psychotic illness and blood glucose. Linear trend test was performed by modeling the median value of each exposure category as a continuous variable in the models
Dose response analysis
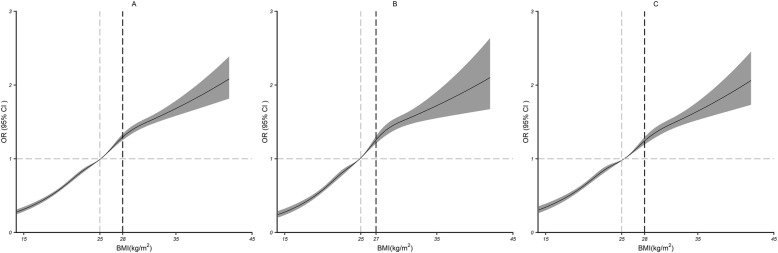
Multivariable adjusted restricted cubic spline analyses showed the nonlinear relationships of BMI with HBP (all P < 0.001; Fig. 2a). The risk of HBP increased with increasing BMI. As BMI increased, the ORs increased from 0.31(0.28 to 0.35) to 2.08(1.82 to 2.39) in the 15–42 kg/m2 range. As a result, the ORs were inversely associated with HBP when BMI was below 25 kg/m2, but presented a significant risk effect above this value. Subgroup analyses on men-women did not show significant differences (Fig. 2b; c).
Fig. 2.
Dose–response relationship between BMI and HBP. BMI and HBP outcomes in the total study population (a), male study population (b) and female study population (c). The associations were adjusted for age, marital status, alcohol consumption, smoking, physical activity, area, cancer, stroke, psychotic illness and blood glucose. The solid lines and gray areas represent the estimated ORs and their 95% CIs. The gray dashed lines represent the corresponding BMI when the OR is 1, and the black dashed lines represent the point where the slope suddenly decreases the most, corresponding to the BMI
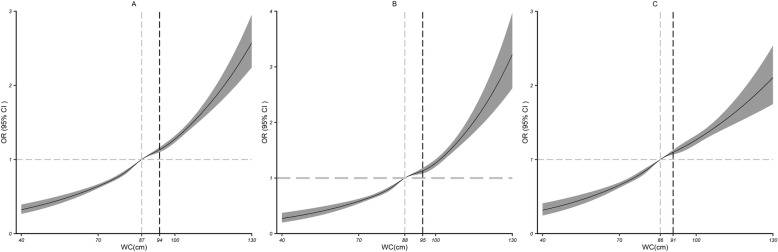
The results of the dose-response relationship analysis between WC and HBP are shown in Fig. 3a. A nonlinear association (all P < 0.001) between WC and HBP was detected. As WC increased, the ORs increased from 0.32(0.27 to 0.39) to 2.57(2.24 to 2.95) in the 40–130 cm range. When stratified by sex, the ORs increased from 0.27(0.20 to 0.37) to 3.22(2.62 to 3.97) in the male population. With an increase in WC, when WC was over 88 cm and 86 cm for males and females, respectively, WC was more steeply positively associated with the risk of HBP (Fig. 3b; c).
Fig. 3.
Dose–response relationship between WC and HBP. WC and HBP outcomes in the total study population (a), male study population (b) and female study population (c). The associations were adjusted for age, marital status, alcohol consumption, smoking, physical activity, area, cancer, stroke, psychotic illness and blood glucose (serum levels of glucose). The solid lines and gray areas represent the estimated ORs and their 95% CIs. The gray dashed lines represent the corresponding WC when the OR is 1, and the black dashed lines represent the WC corresponding to the point where the slope changes the most
Additive interaction analysis
Table 4 presents the results from additive interaction analysis. We observed a significant additive interaction between higher BMI and WC such that the prevalence of HBP increased (RERI = 1.28, 95% CI: 1.13–1.43; AP = 0.43, 95% CI: 0.41–0.45; S = 2.88, 95% CI: 2.79–2.97). If BMI < 25.0 kg/m2 and WC < 88 cm for males and WC < 86 cm for females were used as the reference, BMI ≥ 25 alone and WC ≥ 88 for males or WC ≥ 86 for females alone were both associated with increased risks of HBP. The copresence of both factors greatly enhanced the adjusted ORs of higher BMI alone 1.476(1.418 to 1.536) and higher WC alone 1.230(1.186 to 1.275) to 1.729(1.685 to 1.775) for HBP, with significant additive interactions. When BMI and WC were classified by the proposed cut-off points [17] on the waist circumference continuum (BMI: 25 kg/m2, WC: 88 cm for females and 102 cm for males), there was still significant additive interaction (RERI = 1.68, 95% CI: 1.48–1.87; AP = 0.48, 95% CI: 0.46–0.50; S = 3.00, 95% CI: 2.91–3.10).
Table 4.
Additive interaction analysis of BMI and WC for HBP
| Characteristics | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR(95%CI) | OR(95%CI) | OR(95%CI) | |
| Additive interaction models1 | |||
| BMI < 25 and WC < 88/102 | 1.000(ref) | 1.000(ref) | 1.000(ref) |
| BMI ≥ 25 and WC < 88/102 | 1.459 (1.42,1.5) | 1.546 (1.503,1.589) | 1.517 (1.476,1.56) |
| BMI < 25 and WC ≥ 88/102 | 1.377 (1.304,1.453) | 1.286 (1.216,1.36) | 1.301 (1.232,1.375) |
| BMI ≥ 25 and WC ≥ 88/102 | 1.749 (1.699,1.799) | 1.757 (1.705,1.811) | 1.771 (1.719,1.823) |
| Additive interaction models2 | |||
| BMI < 25 and WC < 86/88 | 1.000(ref) | 1.000(ref) | 1.000(ref) |
| BMI ≥ 25 and WC < 86/88 | 1.469 (1.412,1.528) | 1.512 (1.453,1.574) | 1.476 (1.418,1.536) |
| BMI < 25 and WC ≥ 86/88 | 1.215 (1.172,1.26) | 1.214 (1.171,1.259) | 1.230 (1.186,1.275) |
| BMI ≥ 25 and WC ≥ 86/88 | 1.662 (1.62,1.705) | 1.727 (1.683,1.772) | 1.729 (1.685,1.775) |
BMI body mass index, WC waist circumference. Model 1: unadjusted. Model 2: adjusted for sex and age. Model 3 adjusted for Model 2 and marital status, alcohol consumption, smoking, physical activity, place of residence, cancer, stroke, psychotic illness and blood glucose
Discussion
In this study, the prevalence of HBP increased with rising BMI and WC in the older population of Xinzheng, China, in males and females and in the entire study population. These associations remained significant after adjustment for multiple factors and restricted cubic spline analysis showed clear dose-response relationships. At the same time, this study conducted an additive interaction analysis, which concluded a significant additive interaction between BMI and WC such that the prevalence of HBP increased. To some extent, this study provides a better understanding of the association of anthropometric indicators of obesity with HBP rather than focusing on individual indices, which would be more enlightening for HBP prevention.
Our study confirmed that there was a nonlinear dose-response relationship between BMI and the risk of HBP, which was similar to previous studies [18, 19]. However, a prospective study including 1412 subjects provided evidence that an increase in BMI is associated with a linearly increased adjusted risk of developing conditions with high HBP risk [20], possibly because of estimating the relationship using categorical measures of BMI, masking the shape of the dose-response relationship. Furthermore, in our large sample (n = 126,123), BMI below 25 kg/m2 was regarded as a healthy weight for the older adults in terms of HBP prevalence. However, our proposed appropriate level for HBP were higher than those in some previous studies [21], which could be due to differences in the age range. The average age we sampled was much higher because BMI is greater in older populations, which might be a reason for the inconsistent findings, and when one of the studies stratified data by age (< 50 and ≥ 50 years), the appropriate level for the older group was <25 kg/m2 for men and women [22]. Contrary to the three studies above, the NHANES study suggested 27 kg/m2 as the value [23], but there may be innate or cultural differences between the U.S. and Asia, including dietary habits, macronutrient content, and physical activity habits. Given the marked variations in different world regions, countries and populations within countries, the use of unified range may underestimate or overestimate the health hazards [24]; thus, it is of great interest to determine the appropriate level for cardiovascular disease risks.
For WC, the relationship with HBP was reported by most studies [25, 26]. Most studies have found a positive association between WC and HBP [27], whereas a null association was found in a prospective study from European populations [26]. The low participation rate and the relatively high study drop-out rate may bias the result. We highlighted the increased risk of HBP when WC was over 88 cm and 86 cm for males and females, respectively. Our proposed WC appropriate level is higher than those reported by M Gus et al. [28], and increasing WC over time could be the cause of the difference. The worldwide upward trend in obesity has been dramatic; from 2013 to 2018, the mean WC increased from 82 cm to 86.3 cm for men and from 79.1 cm to 83.4 cm for older women [29]. However, lower appropriate level were suggested by previous studies [30, 31], and ethnic and racial differences might explain the discrepancy between different studies.
To our knowledge, the present study is the first to report a synergistic effect of higher BMI and WC on the risk of HBP in the aged. In other words, the copresence of higher BMI and WC greatly increased the risk of HBP, more than the summation of the risks due to exposure to either of them. In a cohort of 17,803 pregnant Chinese women, the copresence of a higher BMI and WC interacts to further increase the risk of gestational diabetes mellitus [32]. In addition, RISKESDAS research showed that only when general obesity or overweight coexisted with central obesity was the prevalence of HBP significantly increased [13]. This finding supported the stable relation between excess body fat and blood pressure. It is generally believed that the increased body mass would raise blood volume and cardiac output and then lead to the inadequate vasodilatation while the increased activity of the sympathetic nervous system, abnormal rennin-angiotensin-aldosterone relation and insulin resistance would arouse defects in the control of vascular resistance. These adverse vascular responses may dominate the development of obesity-associated HBP [33, 34]. Besides, natriuretic peptides and inflammatory adipokines have an active metabolic role on adipocytes, the deficit in natriuretic peptides and inflammatory adipokines may contribute to hypertension in obesity [35].
Several additional points warrant discussion. First, the findings of this cross-sectional study are not conclusive evidence of a causal relation of WC and BMI with HBP. And the measurement of blood pressure is not taking into account home or 24 h blood pressure levels. Thus, we must be cautious in interpreting the present results, and further studies are needed to clarify our findings. Second, as the study data come from the Chinese middle area among the older population, our proposed appropriate level for the indices are only valid for this population. Third, selection of the appropriate levels for BMI and WC for HBP was based on visual checking of the shapes of the OR curves. The true appropriate level of BMI and WC for HBP remained arbitrary and might deviate slightly from the selected values. However, to the best of our knowledge, our study is the first to explore the association between anthropometric Indicators of obesity and HBP among older people with a large sample size in central China, and it is of practical significance to improve relevant research. Second, this study is based on a comprehensive health check-up program, which not only contains data related to physical measurements and disease, but also includes information on demographic characteristics, physical activity, daily living habits and some blood biochemical tests, therefore, we can make full use of this information for a more comprehensive and reliable analysis.
Conclusion
Understanding the association between BMI, WC and risks of HBP is very important because various interpretations can lead to conflicting recommendations of ideal BMIs and WCs among the older adults. The results of this study revealed that being as lean as possible within the normal range may be a best suggestion in reducing the risks of HBP. However, further cohort studies and replication studies in Chinese and other populations are needed before the results can be used in clinical practice to detect high-risk older adults for early intervention.
Supplementary Information
Acknowledgements
The investigators thank the dedicated participants and all research staff of the study.
Abbreviations
- BMI
Body mass index
- HBP
High blood pressure
- WC
Waist Circumference
- ANOVA
Analysis of variance
- OR
Odds ratio
- CI
Confidence intervals
- RCS
Restricted cubic spline
- CHS centers
Community health service centers
Authors’ contributions
WLZ: Conceptualization, Methodology, Software, Formal analysis, Data Curation, Writing - Original Draft. KH: Validation, Data Curation, Investigation, Formal analysis. HZ & XQH: Data Curation, Investigation. CYY & XYZ: Funding acquisition. SHS: Funding acquisition, Project administration, Supervision, Revising it critically for important intellectual content, final approval of the version to be submitted. All authors read and approved the final manuscript.
Funding
This study was supported by National Key Research and Development Program “Research on prevention and control of major chronic non-communicable diseases” of China (Grant NO: 2017YFC1307705). The funding body did not play any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
This data set is still being used for analysis. Please contact the corresponding author regarding access to the full dataset.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from each participant before data collection. The research ethics committee of Zheng Zhou University approved the current study methodology, protocol, and procedures. (Reference Number: ZZUIRB2019–019).
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaborators GBDRF Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386(9995):801–812. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao R, On behalf of the China Hypertension Survey Investigators* Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. 2018;137(22):2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 5.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 6.González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3(1):17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Bi Y, Ning G. Curbing the obesity epidemic in China. Lancet Diabetes Endocrinol. 2016;4(6):470–471. doi: 10.1016/S2213-8587(16)30007-9. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Ioannidis JPA, Doehner W. Flawed methods and inappropriate conclusions for health policy on overweight and obesity: the global BMI mortality collaboration meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(1):9–13. doi: 10.1002/jcsm.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, Pfäffle R, Kiess W, Körner A. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379(14):1303–1312. doi: 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- 10.Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ. 2018;360:k1274. doi: 10.1136/bmj.k1274. [DOI] [PubMed] [Google Scholar]
- 11.Seo DC, Choe S, Torabi MR. Is waist circumference ≥102/88cm better than body mass index ≥30 to predict hypertension and diabetes development regardless of gender, age group, and race/ethnicity? Meta-analysis. Prev Med. 2017;97:100–108. doi: 10.1016/j.ypmed.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Liang X, Zheng S, Wang Y, Lu W. Association of Body Fat Mass and Fat Distribution With the Incidence of Hypertension in a Population-Based Chinese Cohort: A 22-Year Follow-Up. J Am Heart Assoc. 2018;7(6):e007153. 10.1161/JAHA.117.007153. [DOI] [PMC free article] [PubMed]
- 13.Nurdiantami Y, Watanabe K, Tanaka E, Pradono J, Anme T. Association of general and central obesity with hypertension. Clin Nutr. 2018;37(4):1259–1263. doi: 10.1016/j.clnu.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Xu RY, Zhou YQ, Zhang XM, Wan YP, Gao X. Body mass index, waist circumference, body fat mass, and risk of developing hypertension in normal-weight children and adolescents. Nutr Metab Cardiovasc Dis. 2018;28(10):1061–1066. doi: 10.1016/j.numecd.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. Seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and nutrition examination survey. J Periodontol. 2000;71(5):743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 17.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 18.Qu Y, Niu H, Li L, Li M, Yan S, Li M, Jiang S, Ma X, Li B, Wu H. Analysis of dose-response relationship between BMI and hypertension in northeastern China using restricted cubic spline functions. Sci Rep. 2019;9(1):18208. doi: 10.1038/s41598-019-54827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayedi A, Rashidy-Pour A, Khorshidi M, Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes Rev. 2018;19(5):654–667. doi: 10.1111/obr.12656. [DOI] [PubMed] [Google Scholar]
- 20.Bombelli M, Facchetti R, Sega R, Carugo S, Fodri D, Brambilla G, Giannattasio C, Grassi G, Mancia G. Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension. 2011;58(6):1029–1035. doi: 10.1161/HYPERTENSIONAHA.111.175125. [DOI] [PubMed] [Google Scholar]
- 21.Hossain FB, Adhikary G, Chowdhury AB, Shawon MSR. Association between body mass index (BMI) and hypertension in south Asian population: evidence from nationally-representative surveys. Clin Hypertens. 2019;25(1):28. doi: 10.1186/s40885-019-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng RN, Zhao C, Wang C, Niu YC, Li K, Guo FC, Li ST, Sun CH, Li Y. BMI is strongly associated with hypertension, and waist circumference is strongly associated with type 2 diabetes and dyslipidemia, in northern Chinese adults. J Epidemiol. 2012;22(4):317–323. doi: 10.2188/jea.JE20110120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwabara M, Kuwabara R, Niwa K, Hisatome I, Smits G, Roncal-Jimenez CA, MacLean PS, Yracheta JM, Ohno M, Lanaspa MA et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients. 2018;10(8):1011. 10.3390/nu10081011. [DOI] [PMC free article] [PubMed]
- 24.Jiang M, Zou Y, Xin Q, Cai Y, Wang Y, Qin X, Ma D. Dose-response relationship between body mass index and risks of all-cause mortality and disability among the elderly: a systematic review and meta-analysis. Clin Nutr. 2019;38(4):1511–1523. doi: 10.1016/j.clnu.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Luz RH, Barbosa AR, d'Orsi E. Waist circumference, body mass index and waist-height ratio: are two indices better than one for identifying hypertension risk in older adults? Prev Med. 2016;93:76–81. doi: 10.1016/j.ypmed.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Seven E, Thuesen BH, Linneberg A, Jeppesen JL. Abdominal adiposity distribution quantified by ultrasound imaging and incident hypertension in a general population. Hypertension. 2016;68(5):1115–1122. doi: 10.1161/HYPERTENSIONAHA.116.07306. [DOI] [PubMed] [Google Scholar]
- 27.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham offspring study. Prev Med. 1987;16(2):235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 28.Gus M, Cichelero FT, Moreira CM, Escobar GF, Moreira LB, Wiehe M, Fuchs SC, Fuchs FD. Waist circumference cut-off values to predict the incidence of hypertension: an estimation from a Brazilian population-based cohort. Nutr Metab Cardiovasc Dis. 2009;19(1):15–19. doi: 10.1016/j.numecd.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Bragg F, Tang K, Guo Y, Iona A, Du H, Holmes MV, Bian Z, Kartsonaki C, Chen Y, Yang L, et al. Associations of general and central adiposity with incident diabetes in Chinese men and women. Diabetes Care. 2018;41(3):494–502. doi: 10.2337/dc17-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khader Y, Batieha A, Jaddou H, El-Khateeb M, Ajlouni K. The performance of anthropometric measures to predict diabetes mellitus and hypertension among adults in Jordan. BMC Public Health. 2019;19(1):1416. doi: 10.1186/s12889-019-7801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramezankhani A, Ehteshami-Afshar S, Hasheminia M, Hajebrahimi MA, Azizi F, Hadaegh F. Optimum cutoff values of anthropometric indices of obesity for predicting hypertension: more than one decades of follow-up in an Iranian population. J Hum Hypertens. 2018;32(12):838–848. doi: 10.1038/s41371-018-0093-7. [DOI] [PubMed] [Google Scholar]
- 32.Han Q, Shao P, Leng J, Zhang C, Li W, Liu G, Zhang Y, Li Y, Li Z, Ren Y, Chan JCN, Yang X. Interactions between general and central obesity in predicting gestational diabetes mellitus in Chinese pregnant women: a prospective population-based study in Tianjin, China. J Diabetes. 2018;10(1):59–67. doi: 10.1111/1753-0407.12558. [DOI] [PubMed] [Google Scholar]
- 33.Aroor AR, Jia G, Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2018;314(3):R387–R398. doi: 10.1152/ajpregu.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asghar A, Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017;315:18–26. doi: 10.1016/j.cellimm.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Sarzani R, Bordicchia M, Spannella F, Dessì-Fulgheri P, Fedecostante M. Hypertensive heart disease and obesity: a complex interaction between hemodynamic and not hemodynamic factors. High Blood Press Cardiovasc Prev. 2014;21(2):81–87. doi: 10.1007/s40292-014-0054-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This data set is still being used for analysis. Please contact the corresponding author regarding access to the full dataset.