Abstract
Loss of skeletal muscle and inferior muscle quality are associated with poor prognosis in patients undergoing preoperative treatment for pancreatic cancer, so maintaining skeletal muscle health before surgery may help accelerate patients’ functional recovery and improve their quality of life following surgery. While exercise helps maintain or increase skeletal muscle in individuals undergoing cancer treatment, its efficacy during pancreatic cancer treatment is unclear. Accordingly, in this study we compared changes in skeletal muscle quantity (skeletal muscle index [SMI]) and quality (skeletal muscle density [SMD]) during preoperative pancreatic cancer treatment in participants in a home-based exercise program (EP) and a historical cohort of patients who received the usual care (UC) with no formal exercise programming. Recommendations for the EP cohort included both aerobic and resistance exercise. We assessed changes in SMI and SMD using computed tomography scans administered at treatment planning (T0, prior to EP enrollment) and preoperative restaging (T1) for 33 EP and 64 UC patients and compared changes between groups. The UC patients had statistically significant SMI decreases from T0 to T1 (−1.4 ± 3.8 cm2/m2; p = .005), while the EP patients did not (0.2 ± 3.2 cm2/m2; p = .7). The SMI loss was significantly worse for the UC than for the EP patients (p = .03). Neither group demonstrated statistically significant changes in SMD from T0 to T1, nor did the groups differ in the amount of change in SMD. An adjusted linear regression model demonstrated that EP participation was significantly associated with better SMI maintenance (p = .02). These results suggest that participation in a home-based EP during preoperative treatment may help improve skeletal muscle health and clinical and quality of life outcomes for pancreatic cancer survivors.
Keywords: prehabilitation, skeletal muscle index, skeletal muscle density, body composition, chemotherapy
Introduction
Pancreatic cancer is a leading cause of cancer mortality in the US, but survival rates are improving, particularly among individuals who undergo potentially curative surgery.1 Recent studies have shown that nearly one-third of individuals with surgically resectable tumors survive 5 years following pancreatectomy.2,3 Individuals diagnosed with pancreatic cancer tend to be older adults, who frequently present with loss of skeletal muscle as a sequela of both their age and disease.4 Chemotherapy and (chemo)radiation are increasingly administered prior to surgery for localized pancreatic cancer, and these may contribute to further physiologic and nutritional deficiencies that accelerate loss of skeletal muscle.5-7 Low skeletal muscle mass and loss of skeletal muscle during the administration of preoperative therapy have been associated with poor postsurgical outcomes and short durations of survival.8-10 Skeletal muscle maintenance is thus important to pancreatic cancer survivorship and represents a critical target for intervention.
Exercise programs—particularly those that incorporate resistance training—are effective in reversing, preventing, or mitigating skeletal muscle loss.11 Interventions prior to surgery, known collectively as exercise prehabilitation, have helped patients maintain and improve perioperative physical functioning and performance status.12-15 Previous work, mostly conducted among individuals with more common cancers, has suggested that exercise improves physical functioning and body composition and maintains or increases skeletal muscle mass.16-20 In studies of patients with colorectal cancer, multimodal exercise prehabilitation featuring both aerobic and resistance exercise training improved functional exercise capacity after surgery.21,22
Current evidence linking exercise prehabilitation with counteracting muscle loss is limited, especially among individuals with less prevalent cancers, such as pancreatic cancer.23 The physiological underpinnings by which exercise—and particularly strengthening exercise—mitigates muscle loss mechanistically through anabolic processes are clear,24 but it is important for behavioral research studies to examine perioperative changes in skeletal muscle with and without exercise intervention. Skeletal muscle can be assessed for quantity (ie, mass) and quality (ie, radiodensity or the absence of fatty infiltration), and both have demonstrated independent associations with fitness and clinical outcomes among cancer survivors.25,26 Therefore, a thorough examination of skeletal muscle changes in the preoperative pancreatic cancer context should involve both quantity and quality.
We previously demonstrated the benefits associated with a home-based exercise program during preoperative pancreatic cancer treatment.27,28 Participants enrolled in the program were encouraged to perform at least 60 minutes of moderate-intensity aerobic exercise and at least 60 minutes of resistance exercise using resistance tubes per week. Participants maintained functional fitness, physical functioning, and health-related quality of life throughout their treatment.27,28 The purpose of this study was to compare preoperative changes in skeletal muscle mass between participants in this home-based, aerobic, and strengthening exercise program and a historical population of similar patients who received no formal exercise prescription. We hypothesized that patients who enrolled in the study and participated in home-based exercise would better maintain muscle quantity and quality than those who did not.
Methods
Participants
Historically, 20% to 80% of patients with localized pancreatic cancer of any clinical or radiographic stage and who are initially treated nonoperatively undergo pancreatectomy.29-31 Accordingly, to make the results of this study widely applicable to patients undergoing pancreatectomy, all of the patients we included (1) had a presenting diagnosis of localized pancreatic cancer, (2) received chemotherapy and/or chemoradiation as the first line of therapy, (3) underwent pancreatectomy following chemotherapy and/or chemoradiation, and (4) had abdominal computed tomography (CT) scans from their treatment planning (T0) and preoperative (T1) visits.
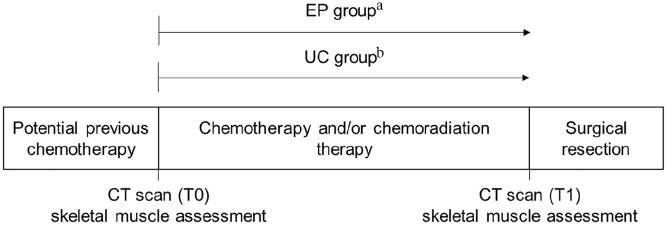
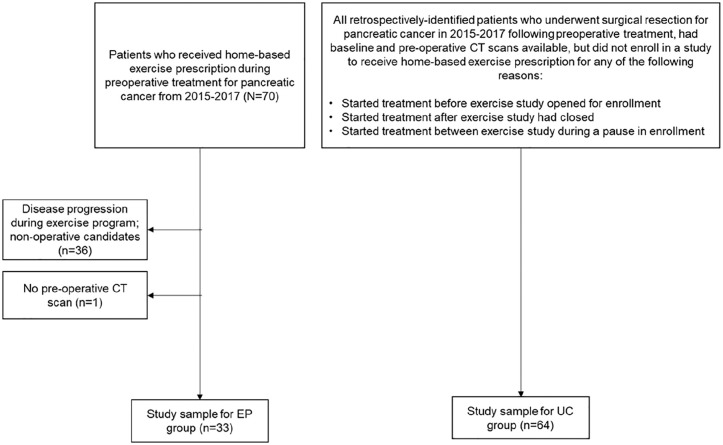
Figure 1 depicts the enrollment and identification of the study groups and the timepoints for skeletal muscle data collection relative to preoperative therapy. Data for the exercise program (EP) arm of the analysis were collected from a prospective study examining adherence to a home-based exercise prescription during preoperative chemotherapy and/or radiation therapy performed from 2015 to 2017 at the University of Texas MD Anderson Cancer Center (ClinicalTrials.gov Identifier NCT02295956). Of 73 patients who were approached and offered enrollment in this previous study, 70 (96%) consented and enrolled. The study inclusion and exclusion criteria have been described previously.4,27,28 In brief, participants had biopsy-proven pancreatic ductal adenocarcinoma, plans for at least 6 weeks of preoperative treatment prior to the planned pancreatectomy, and no medical conditions precluding independent exercise. All participants received exercise clearance based on their responses to the Physical Activity Readiness Questionnaire (PAR-Q)32 and consultations with physicians in MD Anderson Cancer Center’s Departments of Surgical Oncology, Internal Medicine, and Physical Medicine and Rehabilitation, as necessary. We previously reported findings from this study.27,28 Briefly, 65/70 (93%) of patients completed follow up data collection but with varying adherence to exercise recommendations. Among the 57 participants with evaluable exercise logs, 36/57 (63%) adhered to the weekly aerobic exercise recommendation, and 15/57 (26%) adhered to the weekly resistance training recommendation, based on average weekly self-reported exercise minutes.27,28 Of the 70 participants enrolled in the previous study, 33 were eligible for the present analysis27,28; of the 37 participants who were ineligible, 36 (97%) did not undergo surgery and 1 (3%) did not have a T1 CT scan (Figure 2).
Figure 1.
Study schema.
Abbreviations: CT, computerized tomography; EP, exercise program; T0, baseline/treatment planning; T1, follow-up/preoperative visit; UC, usual care.
aFollowed prospectively as exercise program participants.
bIdentified retrospectively (no formal exercise programming).
Figure 2.
Flowchart of study participants.
Abbreviations: CT, computerized tomography; EP, exercise program; UC, usual care.
Patients included in the usual care (UC) cohort of this analysis included all eligible patients who had undergone pancreatectomy following preoperative therapy but who had presented outside the enrollment period for the prospective exercise study described above. Specifically, these patients presented (1) in 2015 prior to the opening of enrollment in the exercise study, (2) in 2015 during a several-month pause in exercise study enrollment, or (3) following the completion of exercise study enrollment in early 2017. The UC patients received no formal exercise programming from MD Anderson. Thus, the UC cohort was a convenience sample of all patients who underwent surgical resection for pancreatectomy in 2015 to 2017 following preoperative treatment (the same general timeframe as EP participants), but they were not offered enrollment in the exercise feasibility study.
All research activities were approved by MD Anderson’s Institutional Review Board via protocols 2014-0702 and RCR01-112. All patients in the EP group provided informed consent.
Exercise Prescription
As described previously,27,28 EP participants were encouraged to perform home-based aerobic and strengthening exercise from the time of their initial treatment planning visits (T0) until their preoperative planning appointments (T1). Exercise prescriptions included at least 60 minutes per week of moderate-intensity aerobic exercise (such as brisk walking) and at least 60 minutes per week of strengthening exercises using resistance tubes. The aerobic exercise prescription was attenuated from ACSM Exercise Guidelines for Cancer Survivors in order to account for potential for fatigue and other side effects during systemic therapy for pancreatic cancer. However, patients were encouraged to exceed these recommendations as they felt able. A total of 60 minutes of resistance training per week was the approximate time requirement for performing 2 complete, full-body sessions, but participants were encouraged to perform resistance training in shorter bouts throughout the week if preferred. Participants were encouraged to perform aerobic exercise in bouts of at least 10 minutes at a time. Participants received portable, graded resistance tube sets to perform strengthening exercises targeting all major muscle groups. Following resistance training guidelines,33,34 patients were encouraged to perform 3 sets of 10 to 12 repetitions for each of 8 strengthening exercises in a given session and to aim for moderate exercise intensity based on perceived exertion. Participants thus started the program with different resistance levels based on their starting strength, and they were encouraged to increase resistance when a given resistance became easy. Exercises were organized into 3 categories targeting the upper body, lower body, and abdominal muscle groups, and participants were encouraged to select 2 to 3 exercises for each muscle group in a given strengthening session. Resistance tube exercises included chest press, row, shoulder raises, biceps curls, triceps extensions, seated leg press, front and reverse hip lifts, abdominal rotations, and seated crunches. Research staff with training in kinesiology and ACSM Cancer Exercise Training certification provided instructions for performing all strengthening exercises safely and with proper form. Participants completed daily exercise logs to record their minutes of aerobic and strengthening exercise and wore accelerometers to measure physical activity objectively. Self-reported exercise and accelerometer physical activity data were available for only the EP participants because UC participants were not enrolled in a program through which these measurements were collected. Research staff contacted participants once every 2 weeks to monitor exercise performance and safety and encourage exercise adherence.
Skeletal Muscle Characteristics
Skeletal muscle quantity (skeletal muscle index [SMI]) and quality (skeletal muscle density [SMD]) were assessed using CT scans performed at both T0 and T1. CT “slices” from the midpoint of the third lumbar vertebra were selected for analysis, and skeletal muscle cross-sectional areas (CSAs, in cm2) were quantified using SliceOmatic software (version 5.0, rev-4a2, TomoVision) using established cut points for pixel density (in Hounsfield units [HUs]).4,35 CT scans were analyzed by 2 trained researchers with overlap on 20% of the sample for quality control. CT scan assessments were not blinded by participant group because EP participants’ scans were evaluated as they became available throughout the feasibility study and UC participants’ scans were collected retrospectively. However, CT scan assessments were blinded regarding the time point at which they were obtained (T0 vs T1). The skeletal muscle CSA in each scan was standardized to the patient’s stature (square of height in meters) to ascertain the SMI in cm2/m2. The average pixel radiodensity (in HUs) from all tissue coded for SMI measurement was recorded to assess the SMD. The time between scans was calculated in weeks, and to account for differences in the times between scans and to improve interpretability, rates of change in the SMI and SMD between each scan were calculated (cm2/m2/week and HUs/week, respectively). Published, sex-specific cut points defining sarcopenia were applied to the SMI to identify participants with sarcopenia at baseline for group comparisons.36
Statistical Analyses
Statistical analyses were performed using SPSS version 24 (IBM, 2016). SMI measurement inter-rater reliability for the overlapping 20% of CT scans assessed by both coders was calculated using the intraclass correlation coefficient. Descriptive statistics were computed for all study variables and stratified by EP versus UC groups. Independent t-tests and chi-square tests were used to assess differences between groups. Paired t-tests were used to assess intragroup differences in skeletal muscle variables between T0 and T1. Independent t-tests were used to compare mean changes in skeletal muscle variables from T0 to T1 in the EP and UC groups.
Multivariable linear regression models were used to examine associations between the EP and UC groups and the rates of change in skeletal muscle variables between T0 and T1. Sex and age were included as covariates in these linear regression models based on characteristics known to affect body composition and skeletal muscle loss. All other clinicodemographic variables were tested for bivariate associations with the skeletal muscle variables or evidence of differences between the EP and UC groups and included as covariates in linear regression models accordingly. The a priori criterion for covariate inclusion was evidence of an association or group difference at P < .1. This criterion was used to include potential covariates that may contribute to the relationships between group and outcome given the nonrandomized design of the study, its sample size limitations, and the relationships between clinical and treatment variables.37 Model fit and assumptions for linear regression were verified for all models. Additional sensitivity analyses involving the treatment sequence variable were conducted to check the robustness of the regression findings (ie, substituting a dichotomous variable measuring receipt of chemotherapy for the 4-category treatment sequence variable). P-values < .05 were considered statistically significant for all analyses.
Results
Participants
Table 1 reports demographic and clinical characteristics for the 2 study cohorts. The final study population included 33 EP participants and 64 UC patients. There were no significant differences between the cohorts in regard to age, sex, baseline performance status, baseline radiographic tumor stage, the percentage who had sarcopenia at baseline, the time between the T0 and T1 CT scans, or the type of operation (all P > .05). However, the cohorts differed significantly (P = .04) regarding the treatments they received between T0 and T1.
Table 1.
Clinicodemographic Characteristics for the EP and UC Groups.a
| EP group (n = 33) | UC group (n = 64) | P | |
|---|---|---|---|
| Age, y ± SD | 67.7 ± 6.8 | 65.0 ± 8.9 | .12 |
| Sex, n (%) | .57 | ||
| Female | 14 (42.4) | 31 (48.4) | |
| Male | 19 (57.6) | 33 (51.6) | |
| Baseline performance status (ECOG),b n (%) | .80 | ||
| 0 | 11 (33.3) | 19 (29.7) | |
| 0/1 | 1 (3.0) | 1 (1.6) | |
| 1 | 19 (57.6) | 42 (65.6) | |
| 2 | 2 (6.1) | 2 (3.1) | |
| Baseline radiographic tumor stage,c n (%) | .11 | ||
| I | 25 (75.8) | 33 (51.6) | |
| II | 8 (24.2) | 27 (42.2) | |
| III | 0 (0.0) | 3 (4.7) | |
| IV | 0 (0.0) | 1 (1.6) | |
| Sarcopeniad at baseline, n (%) | 21 (64) | 40 (63) | .91 |
| Treatment sequence relative to CT scans, n (%) | .04 | ||
| T0 CT, chemotherapy, chemoradiation, T1 CT | 12 (36.4) | 33 (51.6) | |
| Previous chemotherapy, T0 CT, chemoradiation, T1 CT | 4 (12.1) | 6 (9.4) | |
| T0 CT, chemoradiation, T1 CT | 12 (36.4) | 8 (12.5) | |
| T0 CT, chemotherapy, T1 CT | 5 (15.1) | 17 (26.5) | |
| Weeks between T0 and T1 CT scans, mean ± SD | 19.2 ± 10.3 | 22.3 ± 9.5 | .15 |
| Surgery type, n (%) | .46 | ||
| Pancreaticoduodenectomy (Whipple) or total pancreatectomy | 30 (90.9) | 52 (81.3) | |
| Distal pancreatectomy | 3 (9.1) | 12 (18.8) | |
| Preoperative physical activity and exercise during exercise program enrollment, mean weekly min ± SD | — | ||
| Self-reported moderate-to-vigorous aerobic exercise | 118.7 ± 65.3 | Unknown | |
| Self-reported resistance exercise | 42.3 ± 38.2 | Unknown | |
| Accelerometer moderate-to-vigorous physical activity | 170.0 ± 103.0 | Unknown |
Bold indicates significance at P < .05.
Abbreviations: CT, computed tomography; ECOG, eastern cooperative oncology group; EP, exercise program; SD, standard deviation; T0, baseline/treatment planning; T1, follow-up/preoperative visit; UC, usual care.
All clinicodemographic characteristics were abstracted from electronic medical records and correspond to baseline (T0). Exercise data were self-reported or collected using accelerometers.
Performance status from Zubrod et al.38
Staging from Katz et al.39
Cut points from Prado et al.36
The EP cohort reported a mean of 118.7 ± 65.3 minutes of moderate-to-vigorous intensity aerobic exercise per week and 42.3 ± 38.2 minutes of resistance exercise per week between T0 and T1. Objective (accelerometer) physical activity monitoring registered 170.0 ± 103.0 minutes of moderate-to-vigorous physical activity per week between T0 and T1. Among this cohort, 28/33 (85%) and 9/33 (27%) adhered to weekly aerobic and resistance exercise recommendations, respectively, based on average weekly self-reported exercise volumes.
Anthropometric and Body Composition Changes
The reliability of skeletal muscle CSA coding between the 2 researchers who analyzed CT scans was excellent (intraclass correlation coefficient = 0.99). Table 2 reports changes in body mass index (BMI), SMI, and SMD by group. The mean BMI of both cohorts decreased between T0 and T1 (P < .05), and the extent to which the BMI declined was similar between cohorts (P = .3). The mean SMI of the UC cohort, but not that of the EP cohort, declined between T0 and T1 (P = .005 and P = .7, respectively) and the extent to which the SMI decreased differed significantly between groups (P = 0.03). The mean SMD of both groups did not change significantly between T0 and T1.
Table 2.
Anthropometric and Body Composition Changes for the EP and UC Groups.
| EP group |
UC group |
P for between-group difference in change from T0 to T1** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | Within- group change from T0 to T1 | P * | T0 | T1 | Within- group change from T0 to T1 | P * | ||
| BMI (kg/m2), mean ± SD | 27.1 ± 5.4 | 26.6 ± 4.9 | −0.5 ± 1.4 | .04 | 27.3 ± 4.8 | 26.4 ± 4.0 | −0.9 ± 2.1 | .01 | .3 |
| SMI (cm2/m2), mean ± SD | 46.7 ± 10.2 | 46.9 ± 9.1 | 0.2 ± 3.2 | .7 | 45.8 ± 5.4 | 44.4 ± 9.0 | −1.4 ± 3.8 | .005 | .03 |
| SMD (HU), mean ± SD | 37.4 ± 9.9 | 38.8 ± 9.8 | 1.4 ± 6.1 | .2 | 37.6 ± 8.7 | 37.3 ± 9.0 | −0.3 ± 6.9 | .7 | .2 |
Bold indicates significance at P < .05.
Abbreviations: BMI, body mass index; EP, exercise program; HU, Hounsfield unit; SD, standard deviation; SMD, skeletal muscle density; SMI, skeletal muscle index; T0, baseline/treatment planning; T1, follow-up/preoperative visit; UC, usual care.
Paired t-test (within-group change from T0 to T1).
Independent t-test (comparing changes from T0 to T1 between groups).
Linear Regression Models
Table 3 reports the results of the linear regression models for rates of change in muscular variables. Models were adjusted for age, sex, and treatment sequence. EP participation was associated with a mean gain of .11 cm2/m2 in SMI per week from T0 to T1 (P = .02). There was no significant difference in the rate of SMD change between the EP and UC groups (P = .2).
Table 3.
Linear Regression Outputs Modeling Rate of Change in Muscular Variables (Unstandardized Coefficients).
| β for difference between groups | t | P | |
|---|---|---|---|
| Rate of change in the SMI (cm2/m2/week) | .11 | 2.45 | .02 |
| Rate of change in SMD (HU/week) | .10 | 1.19 | .2 |
The UC group was the reference in both models. The models were adjusted for age, sex, and treatment sequence.
Bold indicates significance at P < .05.
Abbreviations: HU, Hounsfield unit; SMD, skeletal muscle density; SMI, skeletal muscle index; UC, usual care.
Sensitivity Analysis
In the sensitivity analysis for the combined study groups, there were no significant differences in the skeletal muscle changes between patients who received chemotherapy and those who did not (all P > .05). Substituting receipt of chemotherapy for treatment sequence in multivariable regression models did not affect the model’s significance and had no significant effects on regression coefficients (data not shown). Therefore, the final regression models presented included the treatment sequence variable.
Discussion
In this study, we compared changes in skeletal muscle in a cohort of patients who participated in a prospective study of exercise while undergoing preoperative therapy for pancreatic cancer with those of a contemporary cohort who received preoperative therapy but no formal exercise recommendations. Our examination of skeletal muscle changes included measures of both muscle quantity (SMI) and quality (SMD). We found that UC patients experienced a significant reduction in the SMI during preoperative therapy, while EP participants did not. Further, we found that EP participation was associated with better SMI maintenance in a multivariable regression model.
SMI and SMD were the endpoints of this study; together, they reflect skeletal muscle health. SMI provides a measure of an individual’s muscularity relative to stature. However, muscle tissue can have a wide range of lipid infiltration and, thus, density, ranging from low density (high lipid content) to high density (low lipid content). The higher the lipid content of skeletal muscle, the poorer its “quality,” or potential strength and function. SMD provides a proxy measure for muscle quality by indicating the average radiodensity of all muscle tissue included in the SMI measurement. Our findings suggest that participating in a multimodal, prehabilitation exercise program helps patients maintain skeletal muscle mass during preoperative pancreatic cancer treatment but does not improve muscle density.
Our findings regarding SMI change in the UC group in this study were similar to those of Cooper et al4 in their 2015 study of patients who underwent pancreatic tumor resection (mean SMI loss of 1.3 cm2/m2 in the 2015 study compared to mean loss of 1.4 cm2/m2 in the present study). These similar findings in similar cohorts provide further evidence highlighting the need for programs to help patients maintain muscle mass during therapy for pancreatic cancer. Previous studies among patients with more prevalent cancers have demonstrated the potential for exercise to improve skeletal muscle health,11 and it is important to highlight that this improvement can occur even among patients with pancreatic cancer—a disease characterized by muscle loss and cachexia. A recent large, retrospective cohort study of colorectal cancer patients found a higher risk of extended postoperative hospitalizations, postoperative complications, and mortality among those with low skeletal muscle mass or density.40 Though our study was not powered to compare postoperative outcomes between cohorts, future studies should determine how exercise prehabilitation—perhaps via mitigation of adverse changes in skeletal muscle—helps improve these outcomes.
The favorable and statistically significant SMI changes in the EP group compared to the UC group contrast with the lack of significant findings regarding SMD. SMD is an important physiological indicator of healthy skeletal muscle, and recent evidence suggests that SMD may be more important than SMI as a predictor of physical functioning among cancer survivors.41 Reductions in intramuscular adipose tissue and myosteatosis were reported after a resistance training intervention involving older adults in various states of chronic disease, including cancer survivors.42 It is important to note that the resistance training program in this prior study was supervised and rigorous, with progression and intensity monitored in person by physical therapists or exercise physiologists. EP participants in our study were more successful in achieving the aerobic exercise recommendation than the resistance training recommendation; though we are unable to describe the physical activity levels of patients in the UC cohort, it is possible that a difference in overall physical activity between the two groups may have led to differences in SMI maintenance.
Potential explanations for the lack of a significant difference in SMD changes include the small sample size, suboptimal adherence to resistance training recommendations, inadequate resistance loads due the unsupervised (home-based) nature of the intervention, or the difficulty of improving SMD during the complex metabolic sequelae associated with pancreatic cancer diagnosis and treatment. Nutritional interventions to complement exercise and focus on fat loss may also be important to reduce the fatty infiltration of skeletal muscle and increase SMD. Participants in both the EP and UC groups consulted with clinical dietitians to manage nutritional concerns prior to pancreatic tumor resection, as per usual care, but there was no standardized nutritional intervention. It may be important for future iterations of the home-based exercise program to incorporate remote monitoring or supervision strategies that improve resistance training adherence and encourage progress or to add a standardized nutrition aspect (ie, protein supplementation) to the program. In general, findings from our study highlight the potential of a relatively simple, home-based exercise program to help patients mitigate skeletal muscle loss during treatments leading up to pancreatic cancer resection. Demonstration of significantly different changes in SMD with exercise may require larger study samples, more rigorous resistance training and nutritional intervention, or a combination of these factors.
This study has important strengths and limitations. The exceptionally high recruitment rate in the study that produced the EP group may be attributable to the intervention design and focus on feasibility. Once enrolled, participants received specific, home-based exercise recommendations and encouragement to gradually increase their exercise to reach them. During recruitment, potential participants received clear communication that difficulty achieving exercise recommendations would not preclude study completion. We used validated measures of the SMI and SMD collected from participants’ abdominal CT scans to provide accurate measures of skeletal muscle variables at relevant clinical time points. By measuring both the SMI and SMD, we included measures of skeletal muscle quantity and quality, both of which have been identified as important prognostic factors and independent predictors of physical function among cancer survivors.25,26 It is important to emphasize that this study was not randomized, but instead compared participants from a single-arm feasibility study to a retrospectively-identified group of patients who were not enrolled in the study but underwent preoperative treatment in the same general time frame. CT scans from the EP group were analyzed before the UC comparison group was identified. However, skeletal muscle data collection was blinded by time point, with assessors unaware of whether CT scans represented anthropometrics at T0 or T1.
We tested for clinicodemographic differences between groups and identified a difference in preoperative treatment patterns relative to CT scans. UC participants more frequently received chemotherapy during the study period defining CT scan-based skeletal muscle measures. This may be a particularly important clinical difference between the groups, as chemotherapy is associated with cachexia and muscle loss that could help explain the difference in the preoperative SMI changes observed between them. Our sensitivity analysis, which showed that there were no statistically significant differences in the SMI or SMD changes based on receiving or not receiving chemotherapy, may help to alleviate concerns regarding potential confounding due to treatment differences. While we adjusted for the differences in treatment patterns in multivariable models comparing rates of skeletal muscle change between groups, this strategy does not account for bias introduced by the non-randomized study design. Though our observational results are promising, future, randomized trials in which individuals undergoing the same treatment regimen are randomized to formal exercise training against usual care will be critical to demonstrate the specific benefits of exercise for skeletal muscle maintenance during pancreatic cancer treatment.
We have no data regarding UC patients’ exercise behavior during the preoperative treatment period. These patients were not referred to a formal exercise program by MD Anderson physicians, and it is unknown whether they received encouragement to exercise or pursued any exercise training independently. However, most Americans are insufficiently active and cancer survivors generally perform less than the recommended levels of activity.43,44 While our findings regarding the mitigation of skeletal muscle loss are promising given the simple, home-based exercise program in which EP participants engaged and their favorable results compared to those of a similar group lacking the same program, we must conduct a true randomized, controlled trial before making any causal inferences about exercise benefits.
Our findings have important implications for research and practice. Beyond implementing randomized designs, future studies should include comparisons of fitness, physical functioning, and quality of life outcomes between study groups and extend examination of these outcomes to the postoperative period for comparisons of functional and physiological recovery from pancreatectomy. There are important opportunities to increase the rigor of exercise programming in order to improve exercise adherence and examine the resultant improvements in skeletal muscle health. We have previously described the importance of home-based exercise programming in this cancer treatment context given patients’ tendency to undergo chemotherapy close to home and to then return to MD Anderson for surgery.45 Even within this context, however, tele-supervision via videoconferencing may increase resistance training accountability, improve exercise self-efficacy, and ensure that participants are progressing (ie, continuing to safely overload their skeletal muscles’ capabilities) over time. We aim to implement and test this strategy in a future rendition of this home-based exercise program during preoperative treatment for pancreatic cancer.
From a practice perspective, our findings suggest that simple exercise prescription, instruction, and monitoring may help patients with resectable pancreatic cancer mitigate preoperative skeletal muscle loss and potentially improve their treatment outcomes. The American College of Sports Medicine recently published guidelines and strategies encouraging cancer clinicians to prescribe exercise for patients and survivors.33,46 In the absence of capabilities or resources to provide formal exercise programming, these strategies may be implemented in busy oncology clinics with relative ease and may provide sufficient stimulus for many patients to improve their treatment outcomes with exercise.
Conclusions
In this study, we found that participating in a home-based exercise program during preoperative pancreatic cancer treatment was associated with maintenance of the SMI. Our findings are insufficient to conclude that participating in an exercise program is associated with benefits regarding the maintenance of SMD, which may be an important predictor of physical function and the risk of impairments. Relatively simple exercise prescriptions and programming may help patients with resectable pancreatic cancer mitigate preoperative muscle loss. More research is needed to understand the potential benefits of the skeletal muscle outcomes from preoperative exercise for patients with pancreatic cancer.
Acknowledgments
We acknowledge Laura L. Russell of Editing Services, Research Medical Library at The University of Texas MD Anderson Cancer Center for her contribution to editing this work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the MD Anderson Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment; a faculty fellowship from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment; the Knox Family Foundation; the Cancer Prevention and Research Institute of Texas Training Grant/MD Anderson Cancer Prevention Research Training Program (RP170259; Dr. Shine Chang, PI), the Transdisciplinary Research on Energetics and Cancer (TREC) Training Workshop R25CA203650 (Dr. Melinda Irwin, PI), and the NIH/NCI under awards number P30CA016672 and 5R21CA218732-02.
ORCID iDs: Nathan H. Parker  https://orcid.org/0000-0002-8947-7942
https://orcid.org/0000-0002-8947-7942
Jessica Gorzelitz  https://orcid.org/0000-0001-9230-0593
https://orcid.org/0000-0001-9230-0593
An Ngo-Huang  https://orcid.org/0000-0003-4797-4147
https://orcid.org/0000-0003-4797-4147
References
- 1. Cloyd JM, Katz MH, Prakash L, et al. Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg. 2017;21:164-174. [DOI] [PubMed] [Google Scholar]
- 2. Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395-2406. [DOI] [PubMed] [Google Scholar]
- 4. Cooper AB, Slack R, Fogelman D, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:2416-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749-5756. [DOI] [PubMed] [Google Scholar]
- 6. Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levolger S, Van Vugt J, De Bruin R, IJzermans J. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448-1458. [DOI] [PubMed] [Google Scholar]
- 9. Mei KL, Batsis JA, Mills JB, Holubar SD. Sarcopenia and sarcopenic obesity: do they predict inferior oncologic outcomes after gastrointestinal cancer surgery? Perioper Med. 2016;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45:2080-2090. [DOI] [PubMed] [Google Scholar]
- 12. Singh F, Newton RU, Galvão DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. Surg Oncol. 2013;22:92-104. [DOI] [PubMed] [Google Scholar]
- 13. Silver JK. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin Oncol Nurs. 2015;13:13-30. [DOI] [PubMed] [Google Scholar]
- 14. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715-727. [DOI] [PubMed] [Google Scholar]
- 15. Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295-317. [DOI] [PubMed] [Google Scholar]
- 16. Madzima TA, Ormsbee MJ, Schleicher EA, Moffatt RJ, Panton LB. Effects of resistance training and protein supplementation in breast cancer survivors. Med Sci Sports Exerc. 2017;49:1283-1292. [DOI] [PubMed] [Google Scholar]
- 17. Nilsen TS, Raastad T, Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54:1805-1813. [DOI] [PubMed] [Google Scholar]
- 18. Visovsky C. Muscle strength, body composition, and physical activity in women receiving chemotherapy for breast cancer. Integr Cancer Ther. 2006;5:183-191. [DOI] [PubMed] [Google Scholar]
- 19. Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38:2045-2052. [DOI] [PubMed] [Google Scholar]
- 20. Galvão DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65:856-864. [DOI] [PubMed] [Google Scholar]
- 21. Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937-947. [DOI] [PubMed] [Google Scholar]
- 22. Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072-1082. [DOI] [PubMed] [Google Scholar]
- 23. Carli F, Gillis C, Scheede-Bergdahl C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncol. 2017;56:128-133. [DOI] [PubMed] [Google Scholar]
- 24. Montalvo RN, Hardee JP, VanderVeen BN, Carson JA. Resistance exercise’s ability to reverse cancer-induced anabolic resistance. Exerc Sport Sci Rev. 2018;46:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, Lin JB, Wu MH, et al. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J Cachexia Sarcopenia Muscle. 2019;10:814-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinberg MS, Shachar SS, Muss HB, et al. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018;24:278-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ngo-Huang A, Parker NH, Wang X, et al. Home-based exercise during preoperative therapy for pancreatic cancer. Langenbecks Arch Surg. 2017;402:1175-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parker NH, Ngo-Huang A, Lee RE, et al. Physical activity and exercise during preoperative pancreatic cancer treatment. Support Care Cancer. 2019;27:2275-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perri G, Prakash L, Qiao W, et al. Response and survival associated With first-line FOLFIRINOX vs gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 2020;155:832-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151:e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487-3495. [DOI] [PubMed] [Google Scholar]
- 32. Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci. 1992;17:338-345. [PubMed] [Google Scholar]
- 33. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 35. Cloyd JM, Nogueras-González GM, Prakash LR, et al. Anthropometric changes in patients with pancreatic cancer undergoing preoperative therapy and pancreatoduodenectomy. J Gastrointest Surg. 2018;22:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [DOI] [PubMed] [Google Scholar]
- 37. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zubrod C, Schneiderman M, Frei E, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960:11:7-33. [Google Scholar]
- 39. Katz MHG, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58: 111-125. [DOI] [PubMed] [Google Scholar]
- 40. Xiao J, Caan BJ, Cespedes Feliciano EM, et al. Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surg. 2020;155:942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williams GR, Deal AM, Muss HB, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget. 2017;8:33658-33665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marcus R, Addison O, Kidde J, Dibble L, Lastayo P. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS One. 2017; 12:e0182554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. US Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Published February 2018. Accessed July, 2020. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
- 45. Parker NH, Lee RE, O’Connor DP, et al. Supports and barriers to home-based physical activity during preoperative treatment of pancreatic cancer: a mixed-methods study. J Phys Act Health. 2019;16:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468-484. [DOI] [PMC free article] [PubMed] [Google Scholar]