Abstract
Background:
Growing evidence suggests psilocybin, a naturally occurring psychedelic, is a safe and promising pharmacotherapy for treatment of mood and substance use disorders when administered as part of a structured intervention. In most trials to date, psilocybin dose has been administered on a weight-adjusted basis rather than the more convenient procedure of administering a fixed dose.
Aims:
The present post hoc analyses sought to determine whether the subjective effects of psilocybin are affected by body weight when psilocybin is administered on a weight-adjusted basis and when psilocybin is administered as a fixed dose.
Methods:
We analyzed acute subjective drug effects (mystical, challenging, and intensity) associated with therapeutic outcomes from ten previous studies (total N = 288) in which psilocybin was administered in the range 20 to 30 mg/70 kg (inclusive). Separate multivariate regression analyses examined the relationships between demographic variables including body weight and subjective effects in participants receiving 20 mg/70 kg (n = 120), participants receiving 30 mg/70 kg (n = 182), and participants whose weight-adjusted dose was about 25 mg (to approximate the fixed dose that is currently being evaluated in registration trials for major depressive disorder) (n = 103).
Results:
In the 20 mg/70 kg and 30 mg/70 kg weight-adjusted groups, and in the fixed dose group, no significant associations were found between subjective effects and demographic variables including body weight or sex. Across a wide range of body weights (49 to 113 kg) the present results showed no evidence that body weight affected subjective effects of psilocybin.
Conclusions:
These results suggest that the convenience and lower cost of administering psilocybin as a fixed dose outweigh any potential advantage of weight-adjusted dosing.
Keywords: Hallucinogen, psilocybin, psychedelic, weight-adjusted dosing, fixed dosing, serotonin, mystical experience
Introduction
The past decade has seen growing interest in the therapeutic potential of classic psychedelic drugs, defined here as hallucinogenic substances with primary activity at the serotonin 2A receptor (5-HT2AR). Psilocybin is a naturally occurring psychedelic that is a prodrug of psilocin, to which it is rapidly converted in the body, and whose psychoactive effects are primarily mediated via 5-HT2AR agonist activity (Nichols, 2016). Recent open-label pilot studies have found psilocybin to be a potentially effective aid for treatment of depression (Carhart-Harris et al., 2016, 2017, 2018; Davis et al., 2020), alcohol dependence (Bogenschutz et al., 2015), and tobacco dependence (Johnson et al., 2014, 2017). Psilocybin has also shown efficacy in controlled trials for alleviating symptoms of anxiety and depression in patients with life-threatening cancer diagnoses (Griffiths et al., 2016; Grob et al., 2011; Ross et al., 2016). Effect size of psilocybin treatment in conjunction with supportive counseling has generally been moderate to large (i.e., placebo-controlled Hedge’s gs from 0.82 to 0.83; Goldberg et al., 2020), and therapeutic effects have been observed to last at least 6 months after one to three doses of psilocybin (Bogenschutz et al., 2015; Carhart-Harris et al., 2018; Griffiths et al., 2016; Johnson et al., 2017; Ross et al., 2016). Most of these therapeutic studies (Bogenschutz et al., 2015; Griffiths et al., 2016; Grob et al., 2011; Johnson et al., 2014; Ross et al., 2016) used body weight-adjusted doses (e.g., 30 mg/70 kg), in which the psilocybin dose administered is adjusted proportionately with the participant’s body weight. Notably, one open-label trial of psilocybin for treatment-resistant depression showed significant reductions in depressive symptoms when psilocybin was administered as a fixed dose (Carhart-Harris et al., 2016). Although fixed dosing regimens are more convenient and less costly than weight-adjusted dosing, weight-adjusted dosing is recommended for some drugs to account for weight-based differences in bioavailability and pharmacodynamic response, thereby increasing safety and efficacy (Pan et al., 2016). With the developing interest in making psilocybin available for medical treatment it will be important to determine whether weight-adjusted dosing is necessary for optimizing psilocybin’s therapeutic efficacy, or whether a fixed dose regimen would be comparably effective.
To date, various acute subjective drug effects, typically rated at the conclusion of psilocybin sessions, have emerged as among the most robust predictors of psilocybin therapeutic efficacy and other enduring aftereffects. For instance, acute mystical-type effects, which include a sense of unity, sacredness, and deeply felt positive mood (Barrett et al., 2015; MacLean et al., 2012), have shown significant positive associations with therapeutic outcomes in depressed/anxious cancer patients (Griffiths et al., 2016; Ross et al., 2016), cigarette smokers (Garcia-Romeu et al., 2014; Johnson et al., 2017), alcohol-dependent individuals (Bogenschutz et al., 2015), and depressed patients (Roseman et al., 2018). Other persisting benefits associated with psilocybin-occasioned mystical experience have been reported in healthy participants, such as an enduring sense of personal meaning and increased well-being (Griffiths et al., 2008, 2018). Self-reported intensity of psilocybin effects has also shown significant association with therapeutic efficacy for alcohol dependence (Bogenschutz et al., 2015). Challenging experiences during psilocybin administration, colloquially known as “bad trips,” comprise another notable class of subjective effects that may influence psilocybin’s therapeutic efficacy. Challenging experiences can include strong and often difficult emotions such as paranoia, grief, or fear. Survey data on challenging experiences from recreational psilocybin users suggest even highly challenging experiences can be associated with increased well-being and perceived benefits in retrospect (Barrett et al., 2017; Carbonaro et al., 2016), though these relationships have not been systematically assessed in clinical studies to date.
The present report provides post hoc analyses of acute subjective effect data in 288 participants from ten previous studies in which participants were administered psilocybin doses in the therapeutic range 20 to 30 mg/70 kg to examine the following research questions: (1) Does the effect of psilocybin at a moderate weight-adjusted dose of 20 mg/70 kg vary as a function of body weight? (2) Does the effect of psilocybin at a high weight-adjusted dose of 30 mg/70 kg vary as a function of body weight? (3) Does the effect of psilocybin at a fixed dose (about 25 mg, to approximate the fixed dose that is currently being evaluated in registration trials for major depressive disorder) vary as a function of body weight? For the first two research questions, if heavier individuals showed greater (i.e., more pronounced) subjective effects than lighter individuals, then this would suggest that weight-adjusted dosing led to unintended differences between individuals in acute effects, and therefore weight-adjustment may be undesirable. For the third research question, if lighter individuals who received about 25 mg showed greater subjective effects than heavier individuals who received about 25 mg, then this would suggest that weight-adjusted dosing may be effective in correcting for weight-based differences in drug effects, and therefore may be more appropriate than fixed dosing.
Material and methods
Data were analyzed from ten studies conducted at Johns Hopkins University School of Medicine between 2001 and 2018 (total N = 288), in which adults were administered body weight-adjusted doses of psilocybin of 20 to 30 mg/70 kg (inclusive). These studies were approved by the Johns Hopkins Medicine Institutional Review Board and all participants provided informed consent. We have focused specifically on psilocybin doses between 20 and 30 mg/70 kg as this range has been identified as safe and most likely to yield therapeutic outcomes in clinical populations (Bogenschutz et al., 2015; Carhart-Harris et al., 2016; Davis et al., 2020; Griffiths et al., 2016; Johnson et al., 2014; Ross et al., 2016), and positive persisting effects in healthy volunteers (Barrett et al., 2020; Griffiths et al., 2006, 2008, 2011, 2018).
Measures
Participant demographics
Data on participant age, race, sex, weight, and absolute psilocybin dose administered were compiled for this secondary post hoc analysis from ten studies conducted at the Johns Hopkins Bayview Medical Center between February 2001 and August 2018. Study samples included healthy volunteers both with and without prior hallucinogen use (Barrett et al., 2018, 2020; Carbonaro et al., 2018; Griffiths et al., 2006, 2008, 2011), cigarette smokers seeking to quit smoking (Johnson et al., 2014, 2017), patients with a life-threatening cancer diagnosis and symptoms of anxiety or depression (Griffiths et al., 2016), novice meditators (Griffiths et al., 2018), meditation practitioners with a long-term daily practice (clinicaltrials.gov: NCT02145091), religious professionals (clinicaltrials.gov: NCT02243813), and people with major depressive disorder (Davis et al., 2020).
Mystical Experience Questionnaire
The Mystical Experience Questionnaire (MEQ30) is a psychometrically validated 30-item scale designed to assess the occurrence and intensity of mystical-type experiences occasioned by psilocybin across four factors: mystical (including items assessing unity, noetic quality, and sacredness), positive mood, transcendence of time and space, and ineffability, with each factor comprising an MEQ30 subscale (Barrett et al., 2015; MacLean et al., 2012). Volunteers used a 6-point response scale (0: None/not at all, 1: So slight cannot decide, 2: Slight, 3: Moderate, 4: Strong/equivalent in degree to any other strong experience; 5: Extreme/more than any other time in my life and stronger than 4) to rate the degree to which they experienced each of a series of subjective effects during their psilocybin session. Total MEQ30 score is expressed as the percentage of the total possible ratings on the scale. A “complete mystical experience” was defined as a score ⩾60% of the maximum possible score on each of the four subscales of the MEQ30 (Barrett et al., 2015). Psilocybin-occasioned mystical experiences have shown significant associations with increased personality openness (MacLean et al., 2011), improved outcomes in psilocybin-facilitated alcohol and tobacco dependence interventions (Bogenschutz et al., 2015; Garcia-Romeu et al., 2014; Johnson et al., 2017), and decreased anxiety and depressive symptoms in cancer patients (Griffiths et al., 2016; Ross et al., 2016). Participants in each study completed the MEQ30 at the end of each psilocybin session day approximately 7 h after drug administration.
Challenging Experience Questionnaire
The Challenging Experience Questionnaire (CEQ) is a 26-item measure assessing seven dimensions of psychedelic-occasioned challenging experiences: grief, fear, death, insanity, isolation, physical distress, and paranoia, with each dimension comprising a CEQ subscale (Barrett et al., 2016). Volunteers used a 6-point response scale [0: None/not at all, 1: So slight cannot decide, 2: Slight, 3: Moderate, 4: Strong; 5: Extreme (more than ever before in my life)] to indicate the degree to which they experienced each of a series of subjective effects during their psilocybin session. Total CEQ score is expressed as the percentage of the total possible ratings on the scale. This questionnaire was administered in a subset of studies (Barrett et al., 2020; Carbonaro et al., 2018; Davis et al., 2020; Griffiths et al., 2016; NCT02145091 and NCT02243813). Participants in each study responded to CEQ items upon conclusion of the psilocybin sessions, approximately 7 h after capsule administration.
Intensity
At the end of each psilocybin session, participants were asked to rate the peak intensity of drug effects since administration on a scale from 0 (Not at all) to 4 (Extreme).
Data analysis
Multivariate regression analyses were used to test for associations between subjective drug effects (i.e., mystical effects, challenging effects, and intensity) as endogenous variables and demographic variables (i.e., age, race, sex, and weight) as exogenous variables. Each endogenous variable was fixed to unit variance, whereas covariances between endogenous variables as well as variances and covariances of exogenous variables were estimated. Analyses were conducted separately in three subsets of pooled data: drug administration sessions in which participants received 20 mg/70 kg of psilocybin (n = 120), drug administration sessions in which participants received 30 mg/70 kg of psilocybin (n = 182), and drug administration sessions in which participants received an absolute dose between 23 and 27 mg (inclusive) of psilocybin (consisting of weight-adjusted doses between 20 and 30 mg/70 kg; n = 103). For the latter, these parameters (i.e., 23 to 27 mg) were chosen to provide as much clinically relevant data as possible within a constrained dose range approximating the 25 mg fixed dose being used in current clinical trials of psilocybin for depression. Some participants contributed data to more than one analysis. In the case that the same volunteer was administered a given dose of psilocybin on more than one occasion, only the responses and ratings from the first exposure to that given dose were used in these analyses.
Analysis of the observations from 20 mg/70 kg dosing sessions and 30 mg/70 kg dosing sessions included participant weight as an exogenous variable, but did not include the absolute dose of psilocybin that was administered as an exogenous variable, because participant weight and absolute dose were perfectly collinear. Given the high prevalence of white volunteers, and the very low prevalence of volunteers endorsing any other race, race was coded as a dummy variable indicating white or not. Bonferroni correction for multiple comparisons provided an alpha level of p < 0.004 (i.e., .05/12 comparisons) for statistical significance. For the analysis of observations in which participants received an absolute dose of about 25 mg, separate models were fit including body mass index (BMI) instead of weight (Supplemental Materials). Additional models including dummy codes for study membership were estimated, and can be found in Supplemental Materials. Data were analyzed using the lavaan toolbox (Rosseel, 2012) in R Statistical Software (v 3.5.0; R Core Team, 2018), and plotted using GraphPad Prism 8.4.1 for Mac (GraphPad Software, Inc., La Jolla, CA).
Results
Subjective effects in weight-adjusted dosing (20 mg/70 kg)
Participant demographics are presented in Table 1. Overall, participants who received a weight-adjusted moderate dose of 20 mg/70 kg psilocybin (n = 120) had a mean (SD) age of 42.2 (12.6) years, and 53% were female (n = 64). Participants’ body weight varied across a 2.3-fold range from 49 to 111 kg, with absolute doses ranging from 14.0 to 31.6 mg (inclusive). On average, subjective effects were characterized by moderately high MEQ30 scores and intensity ratings, and low to moderate CEQ scores overall. No statistically significant relationships were found between psilocybin subjective effects (endogenous variables) and demographic variables (exogenous variables) (Table 2). Data on MEQ30 total score, CEQ total score, and intensity rating by body weight are presented in Figure 1. Findings from models including dummy codes for study membership did not substantively differ from models that did not include these dummy coding variables with the exception of Carbonaro et al. (2018), where challenging effects were attenuated relative to other trials (Supplemental Materials).
Table 1.
Participant characteristics by psilocybin dose.
| Demographics | 20 mg/70 kg dose, n = 120 | 30 mg/70 kg dose, n = 182 | Absolute doses of 23 to 27 mg, n = 103 | |||
|---|---|---|---|---|---|---|
| Male, n (%) | 56 (46.7) | 80 (44.0) | 52 (50.5) | |||
| White, n (%) | 107 (89.2) | 158 (86.8) | 89 (86.4) | |||
| Age (years) at study intake, mean (SD)—range | 42.2 (12.6) | 22–65 | 42.7 (12.2) | 22–69 | 46.9 (13.1) | 22–71 |
| Weight (kg), mean (SD)—range | 72.4 (13.6) | 49–111 | 73.0 (14.3) | 49–113 | 71.0 (11.9) | 54–94 |
| Body mass index (BMI), mean (SD)—range | 24.3 (3.5) | 17.5–32.5 | 24.6 (3.8) | 17.5–35.9 | 23.9 (3.4) | 18–32.5 |
| Absolute psilocybin dose administered (mg), mean (SD)—range | 20.7 (3.9) | 14.0–31.6 | 31.3 (6.1) | 21.0–48.6 | 25.1 (1.2) | 23.0–26.9 |
| Mystical Experience Questionnaire (MEQ30) total score*, mean (SD)—range | 65.4 (24.2) | 6.0–99.3 | 70.7 (23.6) | 7.3–100.0 | 70.1 (22.6) | 2.7–100.0 |
| Challenging Experience Questionnaire (CEQ) total score*, mean (SD)—range | 30.1 (17.4) | 3.3–80.0 | 32.6 (17.1) | 0.4–93.1 | 31.3 (17.9) | 0–76.7 |
| Drug effect intensity rating, mean (SD)—range | 3.1 (0.9) | 0–4 | 3.4 (0.9) | 0–4 | 3.2 (1.0) | 0–4 |
| “Complete” mystical experience, n (%) | 50 (41.7) | 98 (53.8) | 53 (51.5) | |||
| Healthy volunteers, n (%) | 97 (80.8) | 148 (81.3) | 70 (70) | |||
| Treatment-seeking smokers, n (%) | 15 (12.5) | 27 (14.8) | 19 (18.4) | |||
| Depressed/anxious cancer patients, n (%) | 0 (0) | 1 (0.5) | 11 (10.7) | |||
| Major depressive disorder, n (%) | 8 (6.7) | 6 (3.3) | 3 (2.9) | |||
MEQ30 and CEQ total scores are expressed as the percentage of the total possible score on each scale, with a minimum possible score of 0 and a maximum possible score of 100.
Table 2.
Multivariate regression results for participants who received 20 mg/70 kg (n = 120) demonstrating no statistically significant relationships between psilocybin subjective effects and demographic variables.
| Estimate | SE | z-value | p-value | |
|---|---|---|---|---|
| MEQ30 total | ||||
| Age | 0 | 0.002 | 0.270 | 0.787 |
| Race | 0.009 | 0.071 | 0.131 | 0.896 |
| Sex | −0.043 | 0.053 | −0.813 | 0.416 |
| Weight (kg) | 0.003 | 0.002 | 1.307 | 0.191 |
| CEQ total | ||||
| Age | 0 | 0.001 | −0.118 | 0.906 |
| Race | 0.015 | 0.051 | 0.287 | 0.774 |
| Sex | −0.076 | 0.038 | −2.000 | 0.045 |
| Weight (kg) | 0.002 | 0.001 | 1.307 | 0.191 |
| Intensity | ||||
| Age | −0.004 | 0.007 | −0.661 | 0.509 |
| Race | 0.353 | 0.262 | 1.351 | 0.117 |
| Sex | −0.189 | 0.195 | −0.970 | 0.332 |
| Weight (kg) | 0.007 | 0.007 | 0.962 | 0.336 |
SE: standard error; CEQ: Challenging Experience Questionnaire; MEQ30: Mystical Experience Questionnaire.
Figure 1.
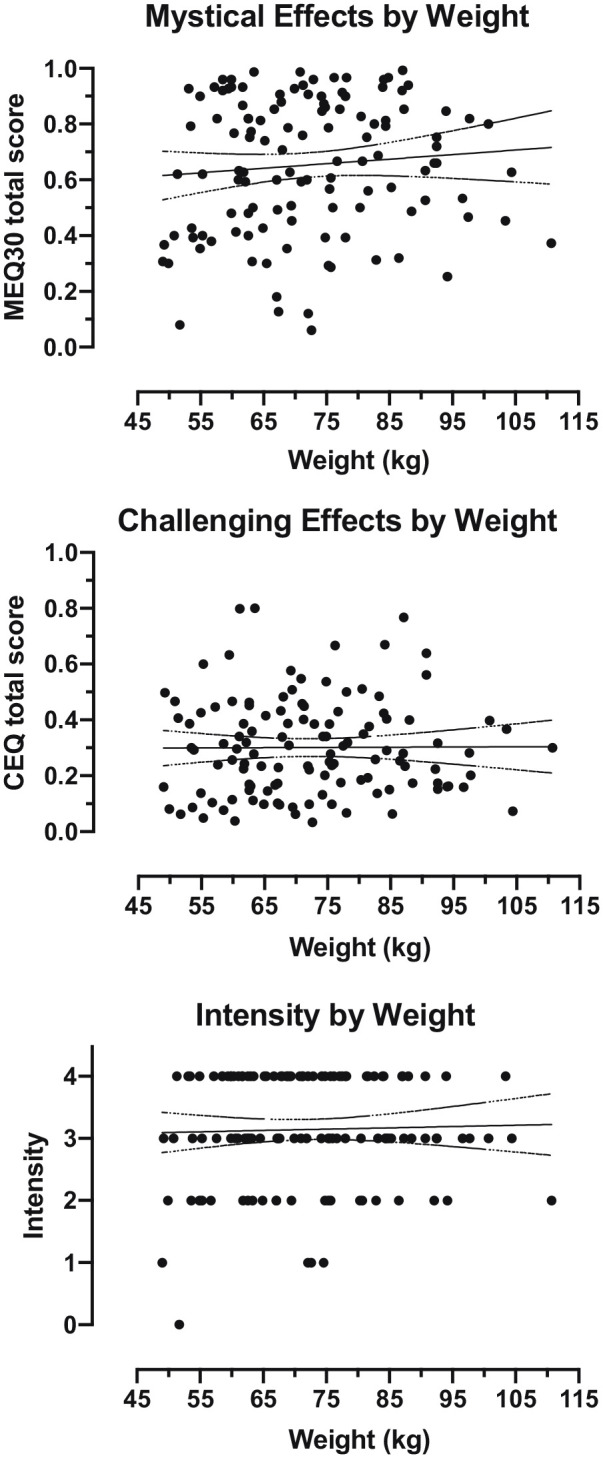
Subjective effects by body weight in participants who received 20 mg/70 kg psilocybin (n = 120). Each data point represents an individual participant. Solid line shows best fit linear regression. Dotted lines show 95% confidence intervals for the reported slope.
Subjective effects in weight-adjusted dosing (30 mg/70 kg)
Participants who received a weight-adjusted high dose of 30 mg/70 kg psilocybin (n = 182) had a mean (SD) age of 42.7 (12.2) years, and 56% were female (n = 102). Participants’ body weight varied across a 2.3-fold range from 49 to 113 kg, with absolute doses ranging from 21.0 to 48.6 mg (inclusive). On average, subjective effects were characterized by high MEQ30 scores and intensity ratings, and low to moderate CEQ scores overall. No statistically significant relationships were found between psilocybin subjective effects and demographic variables (Table 3). Data on MEQ30 total score, CEQ total score, and intensity rating by body weight are presented in Figure 2. Findings from models including dummy codes for study membership did not substantively differ from models that did not include these dummy coding variables with the exception of Carbonaro et al. (2018), in which intensity ratings were attenuated relative to other trials (Supplemental Materials).
Table 3.
Multivariate regression results for participants who received 30 mg/70 kg (n = 182) demonstrating no statistically significant relationships between psilocybin subjective effects and demographic variables.
| Estimate | SE | z-value | p-value | |
|---|---|---|---|---|
| MEQ30 total | ||||
| Age | −0.002 | 0.001 | −1.265 | 0.206 |
| Race | −0.057 | 0.038 | −1.504 | 0.133 |
| Sex | −0.009 | 0.039 | −0.234 | 0.815 |
| Weight (kg) | 0.001 | 0.001 | 0.606 | 0.544 |
| CEQ total | ||||
| Age | −0.001 | 0.001 | −0.818 | 0.413 |
| Race | −0.039 | 0.027 | −1.431 | 0.152 |
| Sex | −0.017 | 0.028 | −0.615 | 0.539 |
| Weight (kg) | 0.001 | 0.001 | 0.930 | 0.353 |
| Intensity | ||||
| Age | −0.005 | 0.005 | −1.000 | 0.317 |
| Race | −0.090 | 0.139 | −0.647 | 0.517 |
| Sex | 0.008 | 0.143 | 0.057 | 0.955 |
| Weight (kg) | 0.006 | 0.005 | 1.292 | 0.196 |
SE: standard error; CEQ: Challenging Experience Questionnaire; MEQ30: Mystical Experience Questionnaire.
Figure 2.
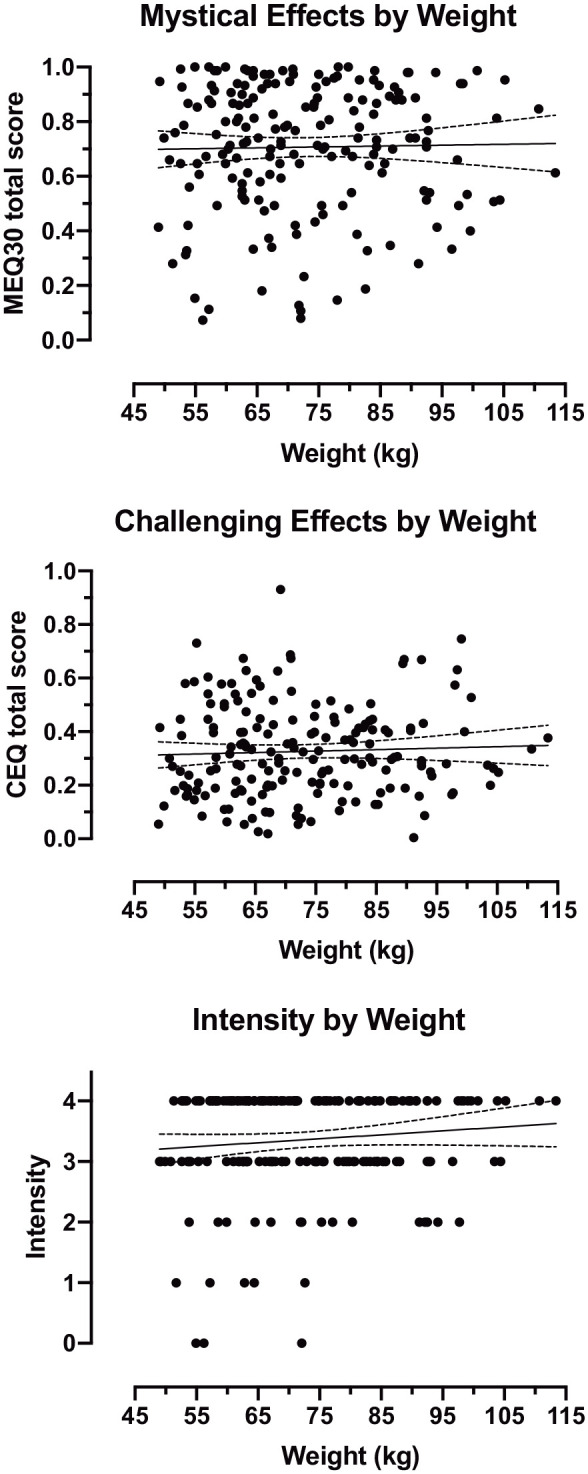
Subjective effects by body weight in participants who received 30 mg/70 kg psilocybin (n = 182). Each data point represents an individual participant. Solid line shows best fit linear regression. Dotted lines show 95% confidence intervals for the reported slope.
Subjective effects in absolute doses of 23 mg to 27 mg
To approximate the effects of using a fixed dose regimen, a subsample of 103 individuals across a 1.7-fold range of body weights (54 to 94 kg) who received similar absolute doses between 23 and 27 mg (inclusive) of psilocybin were also examined. In this subset of individuals, no significant associations were found between psilocybin subjective effects and demographic variables (Table 4). Data on MEQ30 total score, CEQ total score, and intensity rating by body weight are presented in Figure 3. Separate models including BMI or dummy codes for study membership did not substantively differ in outcomes (Supplemental Materials).
Table 4.
Multivariate regression analysis results for participants who received 23 to 27 mg (inclusive) psilocybin (n = 103) demonstrating no statistically significant relationships between psilocybin subjective effects and demographic variables.
| Estimate | SE | z-value | p-value | |
|---|---|---|---|---|
| MEQ30 total | ||||
| Age | −0.001 | 0.002 | −0.777 | 0.437 |
| Race | −0.064 | 0.068 | −0.944 | 0.345 |
| Sex | −0.032 | 0.052 | −0.625 | 0.532 |
| Weight (kg) | 0.001 | 0.002 | 0.287 | 0.774 |
| CEQ total | ||||
| Age | −0.001 | 0.001 | −0.803 | 0.422 |
| Race | 0.032 | 0.053 | 0.613 | 0.540 |
| Sex | −0.093 | 0.040 | −2.312 | 0.021 |
| Weight (kg) | 0.002 | 0.002 | 1.073 | 0.283 |
| Intensity | ||||
| Age | −0.006 | 0.008 | −0.838 | 0.402 |
| Race | 0.055 | 0.296 | 0.184 | 0.854 |
| Sex | −0.262 | 0.225 | −1.163 | 0.744 |
| Weight (kg) | 0.005 | 0.010 | 0.570 | 0.568 |
SE: standard error; CEQ: Challenging Experience Questionnaire; MEQ30: Mystical Experience Questionnaire.
Figure 3.
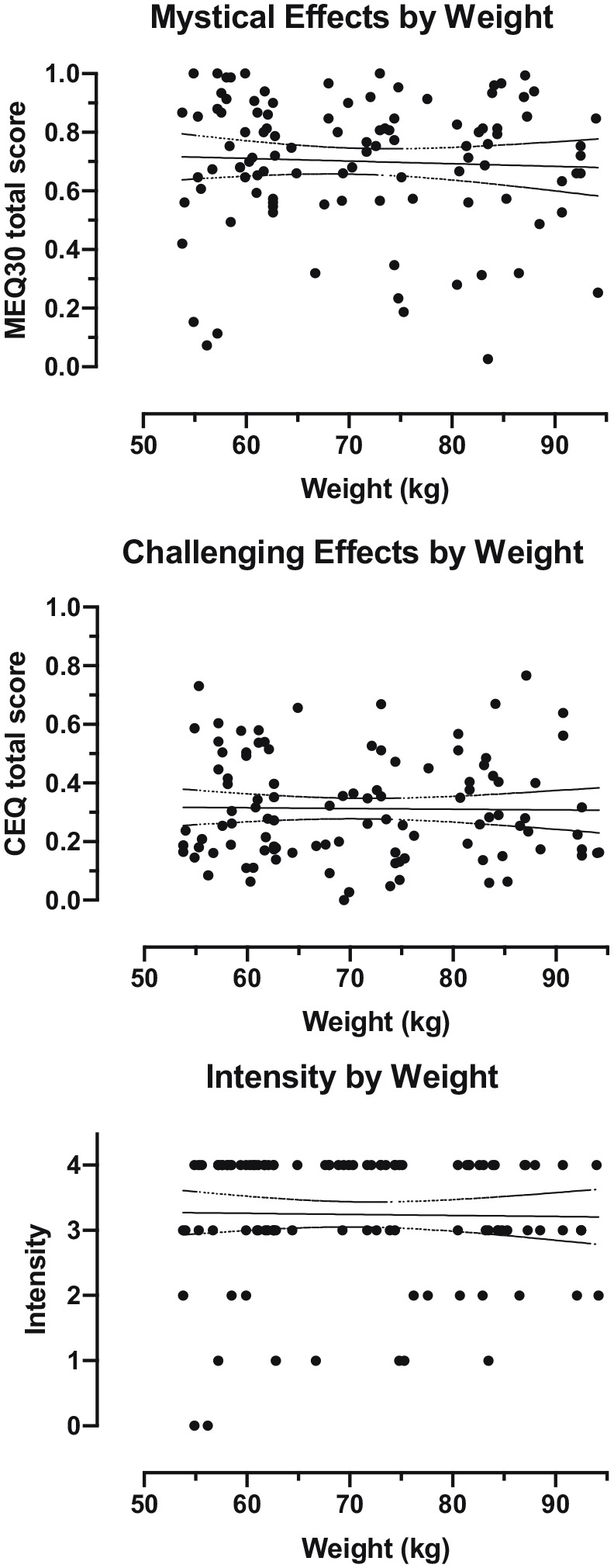
Subjective effects by body weight in participants who received 23 to 27 mg (inclusive) psilocybin (n = 103). This dose range was chosen to approximate the 25 mg fixed dose being used in current clinical trials of psilocybin for depression. Each data point represents an individual participant. Solid line shows best fit linear regression. Dotted lines show 95% confidence intervals for the reported slope.
Discussion
Post hoc analyses of pooled data from several previous studies that administered body weight-adjusted doses of psilocybin of 20 mg/70 kg and 30 mg/70 kg to participants with a 2.3-fold range of body weights (49 to 113 kg) found no significant associations between age, race, sex, or weight of the participant and psilocybin subjective effects. In a smaller subsample of individuals (n = 103) receiving 23 to 27 mg (inclusive) of psilocybin across a 1.7-fold range of body weights (54 to 94 kg), no significant associations were found between demographic variables (including body weight) and subjective drug effects. In neither the body weight-adjusted nor fixed dosing analyses did participant body weight have a significant impact on subjective drug effects of clinical relevance. Therefore, the results were somewhat mixed in the sense that the overall lack of significant effects did not provide definitive evidence supporting either weight-adjusted or fixed dosing. Contrary to the assumption implicit in most contemporary research with psilocybin, these findings do not indicate an advantage of weight-adjusted dosing over the simpler method of fixed dosing. In analyzing weight-adjusted doses in the 20 mg/70 kg and 30 mg/70 kg groups, we did not find significant evidence that weight-adjusted dosing produced unintended stronger effects in heavier individuals, which would be expected if weight-adjusted dosing was unnecessary to produce comparable effects across individuals of different body weights. However, in analyzing fixed doses approximating 25 mg, we also did not find significant evidence that lighter individuals showed stronger effects, which would be expected if weight-adjusted dosing was necessary to produce comparable effects across individuals of different body weights. Taken together, the present results suggest that with respect to subjective effects, and ostensibly clinical efficacy of psilocybin, the more convenient and less costly fixed dosing regimen may be as effective as weight-adjusted dosing.
Prospective research comparing weight-adjusted to fixed doses with regard to clinical efficacy in particular mental health conditions would be necessary to definitively demonstrate clinical superiority of either dosing strategy, which was not directly addressed in the present analysis that included mostly healthy volunteers and focused primarily on subjective drug effects. However, because research is currently underway examining clinical populations with fixed doses of psilocybin (e.g., NCT03866174 and NCT03775200), those studies have the potential to demonstrate efficacy of fixed psilocybin dosing, which would make rigorous comparison of weight-adjusted vs. fixed dosing unnecessary for future clinical practice.
The present results are consistent with a recent pharmacokinetic study of psilocybin, which found in a simulated model that use of a fixed oral psilocybin dose of 25 mg may result in psilocin AUC and Cmax exposures similar to those from a weight-adjusted (0.3 mg/kg) oral dose (Brown et al., 2017). Our findings are also consistent with results showing that fixed oral doses of 25 mg psilocybin were associated with decreased treatment-resistant depression symptoms, although participant body weight or BMI data for this trial were not reported (Carhart-Harris et al., 2016, 2018).
Psilocybin-occasioned increases in mindfulness have recently been found to correlate negatively with changes in 5-HT2AR binding from pre- to post-psilocybin administration, suggesting potential biological and psychological therapeutic mechanisms of psilocybin (Madsen et al., 2020). BMI, a measure closely related to body weight, has shown positive associations with 5-HT2AR binding in the cortex, but no relationship to tobacco or alcohol use (Erritzoe et al., 2009), highlighting the complex interaction of serotonergic function, body weight, and effects of different drugs. These findings raise the question of whether observed variability in subjective effects of psilocybin may be driven by psychological, neurological, or pharmacogenetic factors distinct from body weight and absolute dose administered, and if so, to what extent. Similarly, it is unclear what may have driven observed differences in challenging effects and intensity ratings among participants from the Carbonaro et al. (2018) study (Supplemental Tables SC and SD). It is possible that the participants’ greater prior hallucinogen use and younger age on average relative to participants from other studies might have influenced these outcomes (mean classic hallucinogen uses = 60.9; mean age = 28.5 years).
There are some notable limitations regarding this study that constrain generalizability of the current findings. Analyses were conducted post hoc, and only data from weight-adjusted psilocybin sessions were available, thereby limiting the ability to provide decisive conclusions with regard to fixed dosing. The majority of the participants were healthy volunteers, raising further questions about applicability of findings to clinical populations. Prospective research is needed to compare weight-adjusted and fixed psilocybin dosing, preferably among a well-defined clinical population. Furthermore, the smaller sample size of individuals who received doses around 25 mg may have limited statistical power relative to other analyses presented, possibly hindering the ability to detect effects.
Racial homogeneity of the majority white study samples examined here is another important limitation, and remains an ongoing challenge for clinical hallucinogen research that must be addressed as this work expands more broadly (Michaels et al., 2018). Factors such as lack of sufficient funding for research on therapeutic effects of hallucinogens have made it difficult to recruit populations from lower socioeconomic status backgrounds, who are often unable to take time away from work or childcare responsibilities to participate in time-intensive research studies without financial compensation. In addition, underrepresentation of people of color in scientific and clinical research settings (Henningfield et al., 2020), and distrust of biomedical research institutions due to historical mistreatment of minority populations are also likely factors influencing the low prevalence of non-white participants in research studies like those presented here (Nisbet and Fahy, 2013; Skloot, 2017). These issues require long-term and systemic work to make substantive progress in equity of access, representation, and inclusion of people of color within research and medical settings, areas in which we are actively striving to make improvements.
The present analyses found no evidence for sex differences in subjective effects of psilocybin. Trend level findings in the 20 mg/70 kg and restricted 25 mg psilocybin datasets suggested women may score marginally higher on self-reported challenging effects (CEQ) than men. It is possible that women were more likely to report feelings such as grief and physical distress than their male counterparts or felt more comfortable disclosing these due to cultural norms or expectations (e.g.,Kring and Gordon, 1998). Liechti et al. (2001) found similar sex differences in the psychoactive effects of MDMA across studies using weight-adjusted doses (N = 74), with female participants scoring higher on perceptual changes, thought disturbances, fear of loss of body control, and experiencing more adverse effects, while males exhibited greater acute increases in blood pressure. However, previous results from pooled analyses of psilocybin effects in 261 healthy volunteers found no significant sex differences in drug response (Studerus et al., 2012). Thus, data indicate the possibility of sex differences in psilocybin effects that may have clinical relevance, a topic that should be explored further in future research.
Previous studies in healthy volunteers that examined weight-based psilocybin doses over either a 2.7-fold range (Studerus et al., 2012) or a 6-fold range (Griffiths et al., 2011) showed that higher psilocybin doses were associated with stronger effects. The present study examining a 1.5-fold range of weight-based doses (from 20 to 30 mg/70 kg) showed only a dose-related trend (Table 1), which was consistent with the findings between these two doses in the Griffiths et al. (2011) study. Studerus et al. (2012) additionally found that lower age was associated with greater psilocybin-related cognitive impairment and loss of self-control, though we found no significant relationship between age and subjective effects. This discrepancy may be related to age differences between study samples, with Studerus et al.’s (2012) participants having a mean (SD) age of 27.8 (6.0) years, compared with the current sample with mean (SD) ages from 42.2 (12.6) to 45.6 (12.4) years. Furthermore, Studerus et al. (2012) found no significant associations between BMI and psilocybin response in healthy volunteers, congruent with our findings.
Across a wide range of body weights (49 to 113 kg) the present results showed no evidence that body weight affected the subjective effects of psilocybin. From a logistic standpoint for medications development, fixed dosing of psilocybin is preferable because it allows for standardized manufacture of a single dose form, which is less costly and more convenient than body weight-adjusted dosing. However, our results also show substantial variability in response to psilocybin across individuals, independent of body weight, suggesting some patients may necessitate higher dosage in order to receive clinical benefit based on factors yet to be determined. Personality factors (i.e., absorption), mental state before drug sessions (e.g., emotional excitability), and environment in which the drug is administered (e.g., PET scanner) have been associated with subjective responses to psilocybin (Studerus et al., 2012), consistent with long-standing assertions that psychedelic drug effects may be heavily influenced by extra-pharmacological factors often referred to as “set and setting” (Hartogsohn, 2016). Although we did not analyze such variables here, they clearly warrant careful additional consideration, along with other genetic, psychological, and diagnostic factors that may have an impact on the safety and efficacy of psilocybin-assisted interventions.
The present results strongly suggest that body weight is not a major factor in determining the subjective effects of psilocybin across a range of populations. These data indicate that administering a fixed dose of psilocybin may provide comparable subjective effects, and presumably clinical efficacy, as weight-adjusted dosing for future clinical work with psilocybin. Future trials should assess the clinical efficacy of fixed dosing with psilocybin in more diverse participant populations and should incorporate pharmacogenetic and neurological testing in addition to prospective research on optimal set and setting to inform best practices in the therapeutic administration of psilocybin.
Supplemental Material
Supplemental material, sj-pdf-1-jop-10.1177_0269881121991822 for Optimal dosing for psilocybin pharmacotherapy: Considering weight-adjusted and fixed dosing approaches by Albert Garcia-Romeu, Frederick S Barrett, Theresa M Carbonaro, Matthew W Johnson and Roland R Griffiths in Journal of Psychopharmacology
Acknowledgments
Mary Cosimano, Margaret Klinedinst, Patrick Johnson, Matthew Bradstreet, Rosemary Scavullo-Flickinger, Fred Reinholdt, Samantha Gebhart, Grant Glatfelter, Toni White, Laura Doyle, Ethan Hurwitz, Nathan Sepeda, Jefferson Mattingly, Darrick May, Alan Davis, Nora Belblidia, and John Clifton assisted in data collection. Hillary Jackson assisted with manuscript proofreading and formatting. Annie Umbricht, Darrick May, and Leticia Nanda provided medical screening and coverage. William A Richards provided valuable clinical consultation. Gayane Yenokyan provided statistical consulting and support.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Griffiths is on the board of directors of the Heffter Research Institute. Dr Johnson has an advisory relationship with the following organizations regarding the medical development of psychedelics and related compounds: AWAKN Life Sciences Inc., Beckley Psychedelic Ltd., Entheogen Biomedical Corp., Field Trip Psychedelics Inc., Mind Medicine Inc., Otsuka Pharmaceutical Development & Commercialization Inc., Silo Pharma, Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was provided by the Heffter Research Institute, and by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Support for Dr Griffiths was provided in part by NIDA Grant R01DA003889. We would like to acknowledge support for the statistical analysis from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health (grant no. 1UL1TR001079).
ORCID iD: Albert Garcia-Romeu  https://orcid.org/0000-0003-2182-1644
https://orcid.org/0000-0003-2182-1644
Supplemental material: Supplemental material for this article is available online.
References
- Barrett FS, Bradstreet MP, Leoutsakos JMS, et al. (2016) The Challenging Experience Questionnaire: Characterization of challenging experiences with psilocybin mushrooms. Journal of Psychopharmacology 30: 1279–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Carbonaro TM, Hurwitz E, et al. (2018) Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: Effects on cognition. Psychopharmacology (Berlin) 235: 2915–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Doss MK, Sepeda ND, et al. (2020) Emotions and brain function are altered up to one month after a single high dose of psilocybin. Scientific Reports 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2015) Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. Journal of Psychopharmacology 29: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2017) Neuroticism is associated with challenging experiences with psilocybin mushrooms. Personality and Individual Differences 117: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology 29: 289–299. [DOI] [PubMed] [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, et al. (2017) Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clinical Pharmacokinetics 56: 1543–1554. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Bradstreet MP, Barrett FS, et al. (2016) Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. Journal of Psychopharmacology 30: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Johnson MW, Hurwitz E, et al. (2018) Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: Similarities and differences in subjective experiences. Psychopharmacology (Berlin) 235: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, et al. (2018) Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology (Berlin) 235: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. (2016) Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 3: 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, et al. (2017) Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Scientific Reports 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, et al. (2020) Effects of psilocybin-assisted therapy for major depressive disorder: A randomized clinical trial. JAMA Psychiatry. Epub ahead of print 4 November 2020. DOI: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, et al. (2009) Brain serotonin 2A receptor binding: Relations to body mass index, tobacco and alcohol use. Neuroimage 46: 23–30. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Griffiths R, Johnson W. (2014) Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Current Drug Abuse Reviews 7: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Pace BT, Nicholas CR, et al. (2020) The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry Research 284: 1–4. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of Psychopharmacology (Oxford) 30: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011) Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berlin) 218: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2018) Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. Journal of Psychopharmacology (Oxford) 32: 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, et al. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. Journal of Psychopharmacology (Oxford) 22: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, et al. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berlin) 187: 268–283. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, et al. (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of General Psychiatry 68: 71–78. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2016) Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. Journal of Psychopharmacology (Oxford) 30: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fields S, Anthony JC, et al. (2020) Advancing equity, diversity, and inclusion in the American College of Neuropsychopharmacology (ACNP): Advances, challenges, and opportunities to accelerate progress. Neuropsychopharmacology. Epub ahead of print 3 August 2020. DOI: 10.1038/s41386-020-0784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, et al. (2014) Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology (Oxford) 28: 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR. (2017) Long-term follow-up of psilocybin-facilitated smoking cessation. The American Journal of Drug and Alcohol Abuse 43: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. (1998) Sex differences in emotion: Expression, experience, and physiology. Journal of Personality and Social Psychology 74: 686–703. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology (Berlin) 154: 161–168. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology (Oxford) 25: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Leoutsakos JMS, Johnson MW, et al. (2012) Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. Journal for the Scientific Study of Religion 51: 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MK, Fisher PM, Stenbæk DS, et al. (2020) A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. European Neuropsychopharmacology 33: 71–80. [DOI] [PubMed] [Google Scholar]
- Michaels TI, Purdon J, Collins A, et al. (2018) Inclusion of people of color in psychedelic-assisted psychotherapy: A review of the literature. BMC Psychiatry 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. (2016) Psychedelics. Pharmacological Reviews 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet MC, Fahy D. (2013) Bioethics in popular science: Evaluating the media impact of The Immortal Life of Henrietta Lacks on the biobank debate. BMC Medical Ethics 14: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Zhu L, Chen M, et al. (2016) Weight-based dosing in medication use: What should we know? Patient Preference and Adherence 10: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/ (accessed 10 June 10 2020) [Google Scholar]
- Roseman L, Nutt DJ, Carhart-Harris RL. (2018) Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Frontiers in Pharmacology 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. Journal of Psychopharmacology (Oxford) 30: 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. (2012) Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). Journal of Statistical Software 48: 1–36. [Google Scholar]
- Skloot R. (2017) The immortal life of Henrietta Lacks. Broadway Paperbacks. [Google Scholar]
- Studerus E, Gamma A, Kometer M, et al. (2012) Prediction of psilocybin response in healthy volunteers. PLoS One 7: e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jop-10.1177_0269881121991822 for Optimal dosing for psilocybin pharmacotherapy: Considering weight-adjusted and fixed dosing approaches by Albert Garcia-Romeu, Frederick S Barrett, Theresa M Carbonaro, Matthew W Johnson and Roland R Griffiths in Journal of Psychopharmacology


