Abstract
Aims
Keyhole limpet haemocyanin (KLH) immunization is a clinical model for the evaluation of human antibody responses. The current study evaluated the anti‐KLH antibody response after KLH immunization and the delayed‐type hypersensitivity response following intradermal KLH administration, using objective imaging techniques.
Methods
Healthy male subjects aged 24.5 ± 5.4 years were randomized to intramuscular immunization with 100 μg KLH (n = 12) or placebo (n = 3). Anti‐KLH antibody (Ig) M and IgG titres were determined before and every 7 days after KLH immunization for a total of 28 days. Twenty‐one days after the immunization, all subjects received 1 μg KLH intradermally. Prior to and 2 days after intradermal KLH administration, skin blood perfusion, erythema and oedema were quantified using noninvasive imaging tools. Repeated measures ANCOVAs were used to analyse data.
Results
Anti‐KLH IgM and IgG titres increased after KLH immunization compared to placebo (estimated difference [ED]: 37%, 95% confidence interval [CI]: 19–51% and ED: 68%, 95% CI: 56–76% respectively). Upon intradermal KLH administration an increase in skin blood perfusion (ED: 10.9 arbitrary units (AU), 95% CI: 1.4–20.4 AU) and erythema (ED: 0.3 AU, 95% CI: 0.1–0.5 AU) was observed in KLH‐immunized subjects compared to placebo.
Conclusion
KLH immunization followed by intradermal KLH administration resulted in increased anti‐KLH IgM and IgG titres and a delayed‐type hypersensitivity response quantified by an increase in skin blood perfusion and erythema. Using noninvasive imaging tools the KLH model has the potential to serve as an objective tool to study the pharmacodynamics of T‐cell‐directed immunomodulatory drugs.
Keywords: antigens, biomarkers, drug development, efficacy, healthy subjects, imaging, immunosuppressants, inflammation, pharmacodynamics, vaccine
What is already known about this subject
In vivo challenges for evaluation of the adaptive immune response in humans are currently not well‐characterized.
Keyhole limpet haemocyanin (KLH) is a neo‐antigen driving an adaptive immune response.
Delayed‐type hypersensitivity (DTH) response to KLH challenge is often scored subjectively and objective, noninvasive quantification using a continuous numerical scale is preferred.
What this study adds
The DTH response in KLH‐immunized subjects was objectively quantified by imaging, but remained undetected by visual inspection.
There was no clear correlation between the anti‐KLH antibody response and the DTH response.
This KLH model can serve as an objective tool to study pharmacodynamic effects of B‐ or T‐cell‐directed immunomodulatory drugs.
1. INTRODUCTION
Autoreactive T cells play an essential role in immune‐mediated diseases including type 1 diabetes mellitus, 1 autoimmune arthritis, 2 multiple sclerosis 3 , 4 and psoriasis. 5 Novel immunomodulatory drugs targeting the adaptive immune system and specifically T cells are often investigated in healthy subjects as part of the development program. However, evaluation of the pharmacological activity of such immunomodulatory drugs is challenging since a target engagement biomarker is not constitutively expressed in a healthy population. An in vivo immune challenge in which T cells are activated could serve as an alternative approach. By inducing an antigen‐specific T‐cell response in healthy subjects, the effect of investigational drugs targeting the adaptive immune system could be quantified. However, in vivo challenges for evaluation of the adaptive immune response in humans are currently not well‐characterized.
Keyhole limpet haemocyanin (KLH) is a metalloprotein considered to be a suitable immunization antigen for studying cell‐mediated immune responses. 6 It has been used in many clinical trials and found to be safe. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 KLH is available in 2 different formulations. High molecular weight KLH consists of native KLH, predominantly a didecamer of roughly 4–8 MDa. Subunit KLH is dissociated native KLH known as immunocyanin, each subunit is approximately 400 kDa. As the immunogenicity of subunit KLH is lower compared to native KLH, 17 subunit KLH has been combined with an adjuvant such as aluminium hydroxide to provide a more potent immune response. 18 , 19 KLH was found to elicit a T‐cell‐dependent immune response following 1–3 KLH immunizations. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Immunization doses of 8 μg up to 5000 μg KLH have been reported, with 100 μg KLH being most frequently used. 20 , 21 Commonly, the KLH‐specific immune response is measured by quantifying the anti‐KLH antibody response by enzyme‐linked immunosorbent assay. 8 , 9 , 10 , 11 , 13 , 14 , 15 , 16 , 19 , 21 , 22 , 23 , 24 However, the mechanism of the KLH‐induced immune response is not fully understood. KLH drives an innate immune response through activation of nuclear factor κ‐B, partially mediated via spleen tyrosine kinase and extracellular‐signal‐regulated kinase pathways, both associated with inflammatory responses, apoptosis and phagocytosis. 25 In parallel, KLH is recognized as a neo‐antigen driving an adaptive immune response. This cellular immune response can be evoked and studied in vivo by intradermal administration of a second KLH dose, 2–3 weeks after the initial intramuscular KLH immunization. The intradermal KLH administration induces a delayed‐type IV hypersensitivity (DTH) response at the intradermal injection site. 8 , 9 , 11 , 13 , 14 , 15 , 16 , 26 This KLH‐induced DTH response may serve as a model for the clinical evaluation of future drugs that modulate the adaptive immunity. In previous studies the KLH‐mediated DTH response was only measured subjectively by visual inspection of the skin to assess the presence of induration and erythema 7 , 8 , 9 , 10 , 11 , 13 , 14 , 15 , 16 , 26 , 27 and none of the studies has objectively quantified the erythema and induration response using noninvasive instruments. A positive skin reaction is often defined as an induration of ≥5 mm. This method of reporting DTH skin response is subject to inter‐rater variability leading up to 12% reclassification of the skin response 28 and is often scored categorically. Furthermore, measurement of small distances with a ruler can easily provide imprecise results and observer bias. Objective, noninvasive quantification of skin blood perfusion, induration and erythema using a continuous numerical scale would be preferred.
Therefore, a clinical trial was designed to objectively quantify KLH‐specific DTH responses, in relation to KLH‐specific circulating antibody responses. As such, this study aimed to evaluate KLH immunization with a subsequent intradermal KLH administration as a challenge model for characterization of the adaptive immune responses in man implementing objective measures. This model could potentially be used in future clinical pharmacology studies with drugs targeting the immune system in healthy subjects.
2. METHODS
This was a randomized, double blind, placebo‐controlled study in 15 healthy subjects. The study was conducted at the Centre for Human Drug Research, Leiden, The Netherlands. The Declaration of Helsinki was the principle for trial execution. The study protocol was approved by the Medical Ethics Committee Medisch Ethische Toetsingscommissie van de Stichting Beoordeling Ethiek Biomedisch Onderzoek (Assen, the Netherlands). All subjects provided written informed consent prior to any study activity.
2.1. Subjects
Healthy male subjects aged 18–45 years with a body mass index between 18 and 35 kg/m2 were included in the trial. The health status was verified by a detailed medical history, a complete physical examination, vital signs, 12‐lead electrocardiogram and laboratory test (including hepatic and renal panels, complete blood count, virology and urinalysis). Subjects were not eligible if they had any disease associated with immune system impairment, or received immunomodulatory medication within 30 days of enrolment. Subjects with known previous exposure to KLH were excluded.
2.2. Study design and treatments
A timeline overview of the study design is shown in Figure 1. Subjects were randomized to intramuscular KLH immunization (n = 12) or placebo (n = 3). On the first study day, 100 μg of subunit KLH (Immucothel, Biosyn Corporation, Carlsbad CA, USA), adsorbed to 900 μg aluminium hydroxide (Alhydrogel, Brenntag AG, Essen, Germany), was used for immunization in the deltoid muscle of the left arm. The KLH‐specific immune response was monitored for 28 days by quantification of blood serum titres of anti‐KLH antibodies. In addition, all participants received an intradermal KLH administration (1 μg Immucothel, no adjuvant), 21 days after intramuscular KLH immunization in the left ventral forearm for induction of DTH. Prior to and 2 days after the intradermal KLH administration, the skin DTH response was quantified as described in more detail below. Matching areas on the right ventral forearm were used as untreated control.
FIGURE 1.
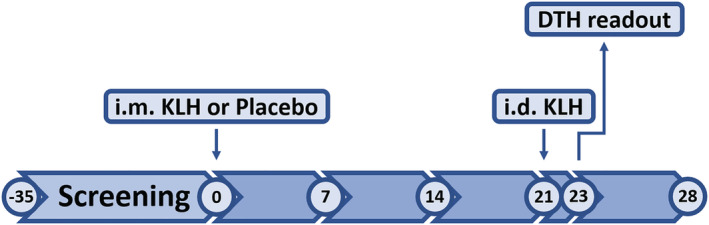
Study timeline. Numbers represent visit days; i.m. = intramuscular; i.d. = intradermal; KLH = keyhole limpet haemocyanin; DTH = delayed type hypersensitivity
2.3. Antibody responses
Anti‐KLH IgM and IgG titres in serum were quantified by enzyme‐linked immunosorbent assay (Ardena Bioanalytical Laboratory, Assen, the Netherlands). Microwell plates were precoated with KLH (BCI‐ImmuneActivator, Intracel Resources LLC. Rockville, Maryland, USA). For human antibodies specifically raised against KLH, no species‐specific (polyclonal) reference material was available. Therefore, a pool of positive serum samples was used as positive control and a pool of human serum samples naive for KLH was used as negative control as well as a negative reagent control. Bound human anti‐KLH IgM antibodies were detected by adding an anti‐human IgM‐horseradish peroxidase conjugate. Ratios relative to baseline were calculated based on mean optical density for all samples collected after KLH immunization. The lower and upper limits of quantification for anti‐KLH IgG and IgM were baseline‐corrected optical densities of 0.060 and 3.900, respectively.
2.4. Skin blood perfusion
Skin blood perfusion measurements were performed with laser speckle contrast imaging (LSCI; PeriCam PSI System, Perimed AB, Järfälla, Sweden) in a temperature‐controlled room with a temperature around 22°C, after subjects were accommodated to the temperature for at least 15 minutes. The camera to forearm distance was standardized to 12.5 cm. An area of 7 × 7 cm was measured with a frame rate of 21 images/s. Dedicated software (PimSoft, Perimed AB, Järfälla, Sweden) was used to capture LSCI recordings of at least 30 seconds. The recording with the strongest response was used to define a circular region of interest at the intradermal injection site. Area size‐matched regions of interest of the intradermal injection sites and untreated control sites were identified in all other recordings and skin blood perfusion (indicated as basal flow) was quantitatively measured and expressed in arbitrary units (AUs). The homogeneity of skin blood perfusion in the region of interest (indicated as flare) was expressed as values that are +1 standard deviation from the mean basal flow within the region. The flare was also quantitatively assessed and expressed in AUs.
2.5. Erythema and oedema
Erythema was quantified with several modalities: multispectral imaging (Antera 3D, Miravex, Dublin, Ireland), colorimetry (DSM II ColorMeter, Cortex Technology, Hadslund, Denmark) and automated 2D photography (FotoFinder Bodystudio ATBM, FotoFinder Systems GmbH, Bad Birnbach, Germany). Quantification of target site oedema was performed with multispectral imaging. Induration, erythema, tenderness and pain were also assessed using a validated toxicity grading scale (TGS), 29 which was performed by an experienced physician (M.S.).
The multispectral imaging camera captures images of 5 × 5 cm without exposure to ambient light. The image with the strongest response in average redness was used to define a circular region of interest at the intradermal injection site. Area size‐matched regions of interest of the intradermal injection sites and untreated control sites were identified in all other recordings. Erythema (indicated as CIELab colour space a* value and average redness) was quantitatively assessed and expressed in AUs. Oedema height, area and volume were also quantitatively assessed and expressed in mm, mm2 and mm3 respectively using Antera 3D software.
Colorimetry was performed using the DSM II ColorMeter, measuring erythema 3 consecutive times and reported as the CIELab a* value. The average of 3 measurements was used for statistical analysis.
Automated 2D photographs of both forearms were obtained using the FotoFinder bodystudio ATBM. Intradermal injection sites on both forearms were identified in the captured images and erythema index was calculated using colour correction software (QPcolorsoft 501, QPcard AB, Everöd, Sweden) and image processing software (ImageJ, National Institutes of Health, Bethesda, MD, USA).
2.6. Statistics
All statistical programming was conducted with SAS 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). The randomization code was generated using SAS by a study‐independent statistician. Subjects were randomized to intramuscular KLH immunization or placebo in a 4:1 ratio in a consecutive order starting with the lowest number. The randomization code was only made available for data analysis after study completion. Demographic and baseline variables were summarized by allocation to intramuscular KLH or placebo immunization. Anti‐KLH antibody and cell‐mediated immunity endpoints were analysed with a mixed model analysis of covariance (ANCOVA) with treatment, time and treatment by time as fixed factors and subject, subject by treatment and subject by time as random factors and the (average) baseline measurement as covariate. The Kenward–Roger approximation was used to estimate denominator degrees of freedom and model parameters were estimated using the restricted maximum likelihood method. Anti‐KLH IgM and IgG titres, erythema index quantified from ATBM captured photographs and oedema area and volume quantified by multispectral imaging required log transformation. The general treatment effect and specific contrasts were reported with the estimated difference (ED) and the 95% confidence interval (CI), the least square mean (LSM) estimates and the P‐value. For anti‐KLH IgM and IgG titres additional contrasts were calculated per time point. Graphs of the LSM estimates over time by treatment were presented with 95% CI as error bars, as well as change from baseline LSM estimates. To correlate the antibody with the DTH responses the following ratios were calculated: anti‐KLH IgM and IgG antibody titres at week 3 vs baseline, LSCI (basal flow and flare) and multispectral imaging (average redness and CIELab a*) levels 2 days post‐intradermal KLH administration vs baseline. Spearman rank correlations between anti‐KLH antibodies and DTH responses were performed. Based on the generated data, power calculations were performed supporting future trials investigating the effect of immunomodulatory drugs based on this KLH model. Sample sizes were calculated for anti‐KLH antibodies, skin blood perfusion by LSCI and erythema by multispectral imaging assuming similar variability, based on an anticipated drug‐dependent inhibition of the KLH‐induced response of 75%.
3. RESULTS
3.1. Baseline characteristics
The study was conducted between February and May 2017. Fifteen healthy, male subjects were enrolled in the study, 12 receiving an immunization with KLH and 3 receiving placebo. All subjects received an intradermal administration of KLH and all subjects completed the study and were included in the analysis population. Baseline characteristics were comparable between the treatment groups (Table 1). No serious adverse events or deaths occurred during the study. One subject reported mild discomfort upon touch after intradermal KLH administration at the injection site which resolved within 2 days. No other adverse events occurred that were considered related to KLH immunization or intradermal KLH administration.
TABLE 1.
Baseline characteristics. Parameters are shown as mean (standard deviation). BMI = body mass index
| All subjects | KLH | Placebo | |
|---|---|---|---|
| n = 15 | n = 12 | n = 3 | |
| Age (y) | 24.5 (5.4) | 24.7 (6.1) | 23.7 (0.6) |
| Weight (kg) | 80.8 (8.5) | 82.2 (8.7) | 75.6 (5.8) |
| Height (cm) | 181.7 (9.0) | 183.1 (8.9) | 175.9 (8.2) |
| BMI (kg/m2) | 24.6 (2.9) | 24.6 (2.8) | 24.6 (4.1) |
| Haemoglobin (mmol/L) | 9.37 (0.46) | 9.44 (0.45) | 9.07 (0.47) |
| Leucocytes (*109/L) | 6.48 (1.71) | 6.35 (1.90) | 6.99 (0.53) |
| Eosinophils (*109/L) | 0.26 (0.60) | 0.30 (0.67) | 0.13 (0.03) |
| Basophils (*109/L) | 0.05 (0.04) | 0.05 (0.04) | 0.03 (0.01) |
| Neutrophils (*109/L) | 3.71 (1.14) | 3.60 (1.25) | 4.15 (0.43) |
| Lymphocytes (*109/L) | 1.94 (0.41) | 1.90 (0.42) | 2.09 (0.40) |
| Monocytes (*109/L) | 0.52 (0.18) | 0.51 (0.18) | 0.58 (0.15) |
3.2. Antibody responses
Intramuscular KLH immunization resulted in an increase in circulating anti‐KLH IgM and IgG titres (Figure 2A and B). Titres started to rise after day 7 and reached a plateau at day 14–21. The increase in antibody titres was significant compared to placebo (ED: 37%, 95% CI: 19–51%, P = .002 and ED: 68%, 95% CI: 56–76%, P < .0001 for IgM and IgG, respectively; Table 2). Also at individual time points, treatment group contrasts reached a statistically significant difference at 2, 3 and 4 weeks after vaccination (Figure 2A and 1B).
FIGURE 2.
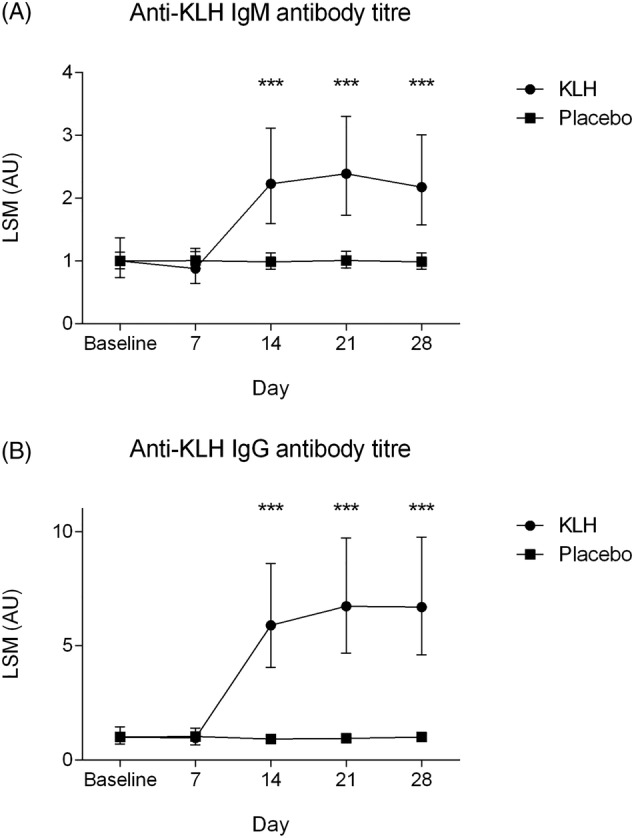
(A) Anti‐keyhole limpet haemocyanin (KLH) IgM antibody titres and (B) anti‐KLH IgG antibody titres over time by treatment group. Data are shown as least square means with 95% confidence interval. Asterisks indicate significance between groups, ***P < .001
TABLE 2.
Summary statistics for pharmacodynamic endpoints. LSM = least square means; KLH, keyhole limpet haemocyanin; ED = estimated difference; i.m. = intramuscular; i.d. = intradermal; AU = arbitrary unit. a P < .05, b P < .01, c P < .001, d P < .0001
| LSM | ED (95% CI) | |||||
|---|---|---|---|---|---|---|
| Pharmacodynamic parameter | KLH | Placebo | KLH vs placebo | |||
| Anti‐KLH antibodies | ||||||
| Anti‐KLH IgM (% change) | 1.59 | 1.00 | 37.3% (19.4–51.2%)b | |||
| Anti‐KLH IgG (% change) | 3.03 | 0.98 | 67.7% (56.3–76.1%)d | |||
| LSM | ED (95% CI) | |||||
|---|---|---|---|---|---|---|
| i.m. KLH i.d. KLH (n = 12) | i.m. placebo i.d. KLH (n = 3) | i.m. KLH or placebo i.d. untreated (n = 15) | i.m. KLH i.d. KLH vs | i.m. KLH i.d. KLH vs | i.m. placebo i.d. KLH vs | |
| i.m. placebo i.d. KLH | i.m. KLH or placebo i.d. untreated | i.m. KLH or placebo i.d. untreated | ||||
| Skin blood perfusion | ||||||
| LSCI | ||||||
| Basal flow (AU) | 42.89 | 31.97 | 31.14 | 10.92 (1.41–20.44)a | 11.75 (6.59–16.91)c | −0.83 (−9.75–8.10) |
| Flare (AU) | 79.57 | 74.72 | 74.84 | 4.86 (1.24–8.48)a | 4.74 (2.59–6.88)c | 0.12 (−3.33–3.58) |
| Erythema | ||||||
| Multispectral imaging | ||||||
| Average redness (AU) | 1.17 | 0.91 | 0.97 | 0.26 (0.05–0.47)a | 0.21 (0.09–0.32)b | 0.05 (−0.14–0.25) |
| CIELab a* (AU) | 14.88 | 11.79 | 12.88 | 3.09 (0.84–5.34)b | 2.00 (0.79–3.22)b | 1.09 (−1.04–3.22) |
| Colorimetry | ||||||
| CIELab a* (AU) | 13.05 | 11.37 | 11.45 | 1.69 (−0.29–3.66) | 1.61 (0.57–2.65)b | 0.08 (−1.72–1.88) |
| Erythema index | ||||||
| Erythema index (% change) | 62.58 | 61.39 | 57.45 | 1.9% (−13.6 to 15.3%) | 8.2% (1.5 to 14.4%)a | −6.8% (−21.8 to 6.3%) |
| Oedema | ||||||
| Multispectral imaging | ||||||
| Oedema height (mm) | 0.17 | 0.06 | 0.04 | 0.11 (−0.01–0.22) | 0.12 (0.06–0.19)b | −0.02 (−0.13–0.09) |
| Oedema area (% change) | 8.28 | 4.15 | 4.74 | 49.9% (−1.85 × 106 to 100%) | 42.8%(−7.00 × 104 to 100%) | 12.4%(−5.32 × 107 to 100%) |
| Oedema volume (% change) | 1.45 | 0.41 | 0.88 | 71.4%(−1.46 × 107 to 100%) | 39.2%(−1.68 × 105 to 100%) | 53.0%(−4.47 × 108 to 100%) |
3.3. DTH response
A statistically significant increase in skin blood perfusion as determined by LSCI basal flow (ED: 10.9 AU, 95% CI: 1.4–20.4 AU, P = .026) and flare (ED: 4.9 AU, 95% CI: 1.2–8.5 AU, P = .011) was observed at the KLH intradermal injection site on the left ventral forearm in the KLH immunized group compared to placebo (Table 2 and Figure 3A and B). When compared to the untreated control area (on the right ventral forearm), there was also a significant increase in basal flow and flare at the KLH intradermal injection site (on the left ventral forearm) in the KLH immunized group (ED: basal flow 11.8 AU, 95% CI: 6.6–16.9 AU, P < .001, ED: flare 4.7 AU, 95% CI: 2.6–6.9 AU, P < .001) whilst no statistically significant difference was observed in the placebo treated group (ED: −0.8 AU, 95% CI: −9.8 to 8.1 AU, P = .85 and ED: 0.1 AU, 95% CI: −3.3 to 3.6 AU, P = .94). LSCI basal flow illustrations 2 days after intradermal KLH administration of a single subject per group are shown in Figure 4.
FIGURE 3.
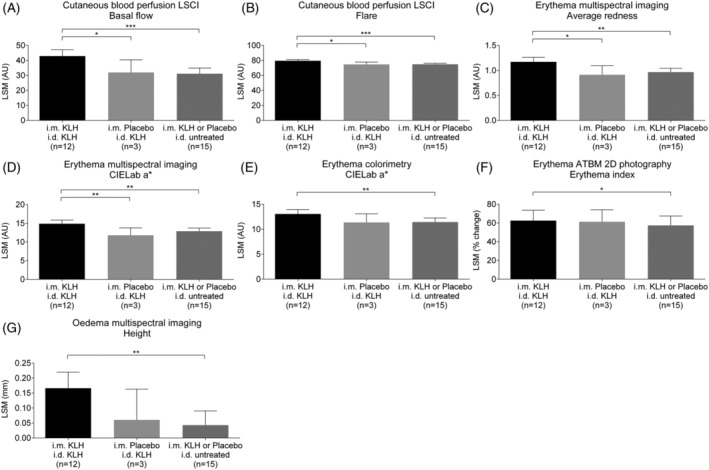
Skin blood perfusion assessed as (A) laser speckle contrast imaging (LSCI) basal flow and (B) LSCI flare, erythema assessed as (C) average redness, (D) CIELab a* with multispectral imaging, (E) CIELab a* with colorimetry and (F) erythema index with ATBM 2D photography and (G) oedema height with multispectral imaging by treatment group. Treatment groups are defined as subjects receiving intramuscular (i.m.) keyhole limpet haemocyanin (KLH) immunization and intradermal (i.d.) KLH administration (n = 12), intramuscular placebo immunization and intradermal KLH administration (n = 3) and both immunization groups combined (KLH or placebo) and no intradermal administration (untreated arm; n = 15). Data are shown as change from baseline least square means (LSM) with 95% CI. * P < .05, ** P < .01 and *** P < .001
FIGURE 4.
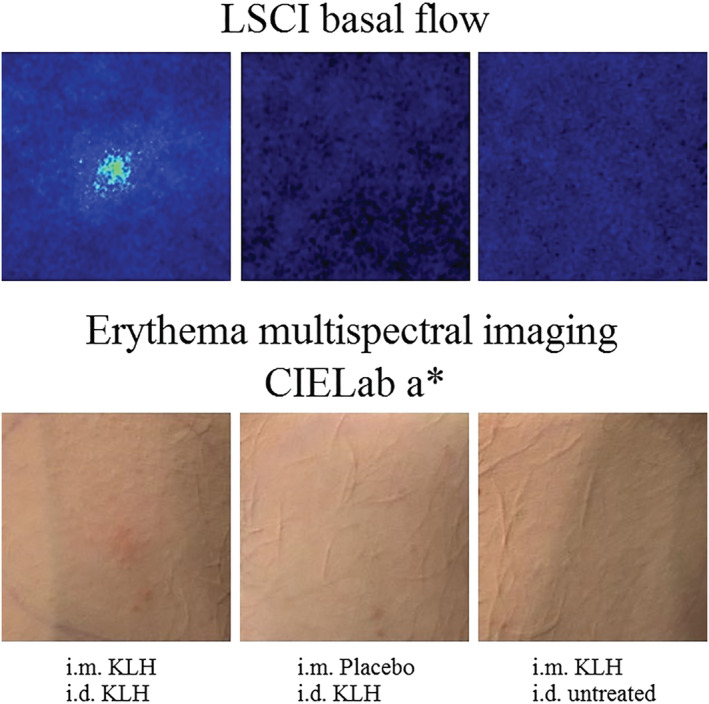
Illustrations of laser speckle contrast imaging (LSCI) basal flow and erythema assessed as CIELab a* with multispectral imaging 2 days after intradermal keyhole limpet haemocyanin (KLH) administration of a subject treated with intramuscular KLH immunization and intradermal KLH administration (left images), intramuscular placebo immunization and intradermal KLH administration (middle images) and intramuscular KLH immunization and untreated control arm (right images)
Erythema quantified with multispectral imaging was significantly increased at the KLH intradermal injection site on the left ventral forearm in the KLH immunized group compared to placebo, both for average redness (ED: 0.3 AU, 95% CI: 0.1–0.5 AU, P = .017) as well as CIELab colour space a* value (ED: 3.1 AU, 95% CI: 0.8–5.3 AU, P = .009; Table 2 and Figure 3C and D). A significant increase in erythema, expressed as average redness and as CIELab a* value was also observed when comparing the KLH intradermal injection site to the untreated control area (ED: 0.2 AU, 95% CI: 0.1–0.3 AU, P = .002 and ED: 2.0 AU, 95% CI: 0.8–3.2 AU, P = .003, respectively). No differences were observed in average redness and CIELab a* value between the KLH intradermal injection site and the untreated control area in the placebo treated group (ED: 0.1 AU, 95% CI: −0.1 to 0.3 AU, P = .58 and ED: 1.1 AU, 95% CI: −1.0 to 3.2 AU, P = .30, respectively). Illustrations of erythema quantified as CIELab a* with multispectral imaging 2 days after intradermal KLH administration of a single subject per group are shown in Figure 4.
Erythema quantified by colorimetry (CIELab a* value) and by colour‐corrected automated photography (erythema index) showed statistically significant differences between the KLH intradermal injection site and the untreated control area in the KLH immunized group (ED: 1.6 AU, 95% CI: 0.6–2.7 AU, P = .005 and ED: 8%, 95% CI: 2–14%, P = .021, respectively; Table 2 and Figure 3E and F). However, differences between the KLH intradermal injection site in the KLH immunized group and the placebo group were not statistically significant (ED: 1.7 AU, 95% CI: −0.3 to 3.7 AU, P = .09 and ED: 2%, 95% CI: −14 to 15%, P = .78).
Similarly, oedema height quantified by multispectral imaging was significantly increased at the KLH intradermal injection site compared to the untreated control area in the KLH immunized group (ED: 0.1 mm, 95% CI: 0.1–0.2 mm, P = .002; Table 2 and Figure 3G). However, no statistically significant difference was observed at the KLH intradermal injection site between the KLH immunized group and placebo (ED: 0.1 mm, 95% CI: −0.01 to 0.2 mm, P = .07). No differences were observed in oedema area and volume between the treatment groups (Table 2).
Based on the TGS, only 1 subject reported tenderness directly after intradermal KLH administration categorized as mild discomfort upon touching, which had disappeared within 2 days. There were no visual changes in erythema and tactile examination showed no induration based on the TGS at the intradermal injection sites during DTH readout.
3.4. Correlations and power calculation
Spearman nonparametric rank correlation between anti‐KLH IgM and LSCI flare showed a statistically significant positive correlation r = 0.67 (P = .033). No other statistically significant correlations between anti‐KLH antibodies and DTH responses (LSCI and multispectral imaging) were observed.
Based on the observed KLH responses and observed variability, a sample size of at least 12 per group would be required to detect a 75% inhibition of the KLH‐induced anti‐KLH IgM and IgG antibody response, the DTH skin blood perfusion response quantified by LSCI (basal flow and flare) and the DTH erythema response quantified by multispectral imaging (average redness and CIELab a*) using a parallel study design, with an α of .05 and a power of 80%. To detect a 75% inhibition of only the anti‐KLH IgM and IgG antibody response following KLH immunization a sample size of at least 4 per group would be required. The most sensitive readout based on the KLH responses is the anti‐KLH IgG antibody response requiring a sample size of at least 2 per group and the least sensitive readout is the LSCI basal flow requiring a sample size of at least 12 per group to detect a 75% inhibition of the responses using a parallel study design, with an α of .05 and a power of 80%.
4. DISCUSSION
In this study we evaluated the response of healthy subjects to KLH immunization, by the quantification of anti‐KLH IgM and IgG and the DTH response of the skin upon intradermal KLH administration. Our study confirms that KLH immunization and intradermal KLH administration are well‐tolerated and result in a primary antibody response against KLH. Intradermal KLH administration resulted in a DTH response in KLH‐immunized subjects that was quantified as increased skin blood perfusion and erythema by imaging, but that remained undetected by visual inspection. Based on our findings, KLH immunization followed by an intradermal KLH administration may serve as a model for quantification of adaptive immune responses in healthy subjects, potentially for future use in clinical pharmacology studies with drugs targeting the adaptive immune system.
Multiple studies have used a KLH challenge model including a DTH response to evaluate the pharmacodynamic effects of immunomodulatory drugs such as cyclosporine treatment in bone marrow transplant patients, 30 methotrexate and rituximab treatment in patients with rheumatoid arthritis 10 and multiple immunomodulatory drugs in renal transplant patients. 14 Recent studies investigating novel targets of immunomodulatory drugs in healthy subjects used a KLH challenge to show a significant decrease in anti‐KLH antibody response of >90% compared to placebo 31 , 32 ; however, the cell‐mediated immune response using either in vivo (DTH response) or ex vivo (lymphocyte proliferation assays) testing was not evaluated in these studies.
The DTH response has been reported to be primarily induced by a type IV hypersensitivity reaction in the skin involving antigen presenting cells that display antigens using major histocompatibility complex class II molecules to dermal cluster of differentiation 4 (CD4+) T‐cells. 33 This causes activation of and an increase in dermal CD4 + T‐cells, which usually takes up to 48–72 hours to reach a maximum response. 8 , 9 , 11 , 13 , 14 , 15 , 16 , 26 Subsequent cytokine secretion, such as interleukin (IL) 2 and interferon‐γ, by activated CD4 + T‐cells primarily causes proliferation of CD8 + T‐cells and attraction of macrophages that migrate and infiltrate the affected area. 33 Tumour necrosis factor α secretion by activated T‐cells induces prostacyclin release from endothelial cells that promote vasodilation and increased permeability, resulting in increased blood perfusion, erythema and oedema. 34 Previous studies have measured the DTH response to intradermal KLH administration as induration 7 , 8 , 9 , 10 , 11 , 13 , 14 , 16 , 26 , 27 and erythema 13 , 27 expressed as the largest diameter of the skin reaction or the average of orthogonal diameters measured with either a ruler or the ball point pen technique. 35 In line with our results, previous studies were also unable to detect a DTH response subjectively by visual inspection 9 , 15 or the response was only positive in a portion of the treated population. 13 , 26 This might be attributed to a low immunization dose and/or a low subsequent intradermal administration dose. 6 The devices used in the present study were able to detect small changes in the DTH response compared to the traditional categorical scale for erythema and induration suggesting a higher sensitivity of the imaging techniques. Importantly, the DTH response in the current study was quantified objectively on continuous numerical scales, which makes the impact of inter‐rater variability minimal, which is inherent to subjective DTH scoring approaches. LSCI and multispectral imaging have, to our knowledge, not yet been used before in the investigation of DTH skin reactions. Based on the results observed in this study, these techniques may acquire a prominent role in objectively evaluating DTH skin reactions in future clinical trials.
The systemic cell‐mediated immune response to KLH can be quantified ex vivo by lymphocyte proliferation assays, although the intra‐assay and interindividual variability is high. 8 , 11 , 12 , 13 , 14 , 19 , 22 , 24 Also, other techniques, such as ELISPOT, ex vivo cytokine production assays or cell activation based on L‐selectin expression suffer from the same limitations. 19 , 22 , 36 Although we performed cell‐based assays in the present study to quantify the systemic cellular response to KLH, we were unable to detect systemic and significant antigen‐specific circulating T‐cell responses in KLH immunized subjects. This may be explained by the aforementioned bioanalytical variability and the number of antigen‐specific T‐cells in the circulation, which was assumed to be very low at the KLH dose that we selected (single vaccination, 100 μg of subunit KLH). However, the KLH‐driven skin responses after intradermal re‐challenge of KLH‐immunized volunteers proves a KLH‐specific T‐cell response upon KLH immunization. The skin response after intradermal KLH administration contains both a type IVa DTH component as a result of increased interferon‐γ secretion by T‐helper 1 (Th1) cells, as well as a type IVb DTH component characterized by increased IL‐5, IL‐4 and IL‐13 production in Th2 cells involved in KLH immunization. 37
The fact that we did not observe a clear correlation between the anti‐KLH antibody response and the DTH response suggests that the DTH response to intradermal KLH administration is unlikely to be driven by the antibody response to the initial KLH immunization. This underlines the value of the antibody response and the DTH response as 2 KLH‐driven but mechanistically independent phenomena, 1 reflecting B‐cell‐mediated responses and the other reflecting T‐cell‐mediated responses.
As stated earlier, we administered a relatively low dose of KLH (100 μg), vaccinated only once and used a KLH subunit, which is less immunogenic than high‐molecular weight KLH. Therefore, the elicited immune responses were relatively mild (no apparent systemic KLH‐specific T‐cells, no positive DTH response by visual inspection). Future research including multiple primary and booster KLH formulations could shed light on the KLH exposure versus effect relationship. Furthermore, such studies could include specific pharmacological interventions modulating T‐ and B‐cell responses (e.g. corticosteroids, compounds resulting in suppression of nuclear factor of activated T cells, modulators of co‐stimulatory molecules) to establish a benchmark for testing pharmacodynamic effects of novel immunomodulatory drugs in healthy subjects.
We were unable to detect an ex vivo systemic cell‐mediated immune response to KLH and an in vivo positive DTH response by visual inspection in the current study, probably due to the selection of a KLH monomer with aluminium adjuvant, immunization dose and the dosing regimen. Future research including multiple primary and booster KLH formulations and various intervals is needed to characterize and optimize the outcome measures with noninvasive instruments in the current trial. Furthermore, a pharmacological intervention modulating the adaptive immune response should be included in order to establish a benchmark for testing pharmacodynamic effects of novel immunomodulatory drugs in healthy subjects.
5. CONCLUSION
In this study, KLH immunization resulted in the release of anti‐KLH antibodies and intradermal KLH administration following initial KLH immunization produced an objectively measured and quantifiable increase in skin blood perfusion and erythema as DTH response. Importantly, these effects remained undetected upon visual inspection, underlining the importance of sensitive and objective imaging techniques for evaluation of dermal responses. Our KLH model has the potential to serve as an objective measurement tool to study the pharmacodynamic effects of B‐ or T‐cell‐directed immunomodulatory drugs.
ACKNOWLEDGEMENTS
This study was, in part, supported by an educational grant from Kymab Ltd, Cambridge, UK.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
M.M. devised the project, the main conceptual ideas and manuscript outline together with P.G. and M.S. P.G. and M.S. worked out technical details and study design. J.B., R.R. and M.v.D. provided expertise on the project setup. M.S. coordinated the clinical trial under supervision of M.M. and P.G. D.Z. designed and carried out statistics. J.P. and N.B. provided partial funding for the clinical trial. M.S. wrote the manuscript. All authors provided critical feedback and helped shape the manuscript.
Saghari M, Gal P, Ziagkos D, et al. A randomized controlled trial with a delayed‐type hypersensitivity model using keyhole limpet haemocyanin to evaluate adaptive immune responses in man. Br J Clin Pharmacol. 2021;87:1953–1962. 10.1111/bcp.14588
The authors confirm that the Principal Investigator for this paper is Matthijs Moerland and the Medical Responsibility is Pim Gal. Together they had direct clinical responsibility for subjects.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. 2017;127(8):2881‐2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothe K, Raulien N, Kohler G, Pierer M, Quandt D, Wagner U. Autoimmune arthritis induces paired immunoglobulin‐like receptor B expression on CD4(+) T cells from SKG mice. Eur J Immunol. 2017;47(9):1457‐1467. [DOI] [PubMed] [Google Scholar]
- 3. Malik S, Want MY, Awasthi A. The emerging roles of gamma–delta T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front Immunol. 2016;7:14. 10.3389/fimmu.2016.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salou M, Nicol B, Garcia A, Laplaud D‐A. Involvement of CD8+ T cells in multiple sclerosis. Front Immunol. 2015;6:604. 10.3389/fimmu.2015.00604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagchi S, He Y, Zhang H, et al. CD1b‐autoreactive T cells contribute to hyperlipidemia‐induced skin inflammation in mice. J Clin Invest. 2017;127(6):2339‐2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swaminathan A, Lucas RM, Dear K, McMichael AJ. Keyhole limpet haemocyanin ‐ a model antigen for human immunotoxicological studies. Br J Clin Pharmacol. 2014;78(5):1135‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palestine AG, Roberge F, Charous BL, Lane HC, Fauci AS, Nussenblatt RB. The effect of cyclosporine on immunization with tetanus and keyhole limpet hemocyanin (KLH) in humans. J Clin Immunol. 1985;5(2):115‐121. [DOI] [PubMed] [Google Scholar]
- 8. Smith A, Vollmer‐Conna U, Bennett B, Wakefield D, Hickie I, Lloyd A. The relationship between distress and the development of a primary immune response to a novel antigen. Brain Behav Immun. 2004;18:65‐75. [DOI] [PubMed] [Google Scholar]
- 9. Boulton C, Meiser K, David OJ, Schmouder R. Pharmacodynamic effects of steady‐state fingolimod on antibody response in healthy volunteers: a 4‐week, randomized, placebo‐controlled, parallel‐group, multiple‐dose study. J Clin Pharmacol. 2012;52(12):1879‐1890. [DOI] [PubMed] [Google Scholar]
- 10. Bingham CO 3rd, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64‐74. [DOI] [PubMed] [Google Scholar]
- 11. Valdez H, Smith KY, Landay A, et al. Response to immunization with recall and neoantigens after prolonged administration of an HIV‐1 protease inhibitor‐containing regimen. ACTG 375 team. AIDS Clinical Trials Group. Aids. 2000;14(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 12. Ferbas J, Belouski SS, Horner M, et al. A novel assay to measure B cell responses to keyhole limpet haemocyanin vaccination in healthy volunteers and subjects with systemic lupus erythematosus. Br J Clin Pharmacol. 2013;76(2):188‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boelens PG, Fonk JC, Houdijk AP, et al. Primary immune response to keyhole limpet haemocyanin following trauma in relation to low plasma glutamine. Clin Exp Immunol. 2004;136(2):356‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rentenaar RJ, van Diepen F, Meijer RT, et al. Immune responsiveness in renal transplant recipients: mycophenolic acid severely depresses humoral immunity in vivo. Kidney Int. 2002;62(1):319‐328. [DOI] [PubMed] [Google Scholar]
- 15. Grant RW, Mariani RA, Vieira VJ, et al. Cardiovascular exercise intervention improves the primary antibody response to keyhole limpet hemocyanin (KLH) in previously sedentary older adults. Brain Behav Immun. 2008;22:923‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell‐mediated responses in men. J Appl Physiol. 2004;97(2):491‐498. 10.1152/japplphysiol.01404.2003 [DOI] [PubMed] [Google Scholar]
- 17. Lebrec H, Hock MB, Sundsmo JS, et al. T‐cell‐dependent antibody responses in the rat: forms and sources of keyhole limpet hemocyanin matter. J Immunotoxicol. 2014;11(3):213‐221. [DOI] [PubMed] [Google Scholar]
- 18. Wilson‐Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009;98(4):1278‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller JS, Curtsinger J, Berthold M, et al. Diminished neo‐antigen response to keyhole limpet hemocyanin (KLH) vaccines in patients after treatment with chemotherapy or hematopoietic cell transplantation. Clin Immunol. 2005;117(2):144‐151. [DOI] [PubMed] [Google Scholar]
- 20. Jurincic‐Winkler CD, Metz KA, Beuth J, Klippel KF. Keyhole limpet hemocyanin for carcinoma in situ of the bladder: a long‐term follow‐up study. Eur Urol. 2000;37(Suppl 3):45‐49. [DOI] [PubMed] [Google Scholar]
- 21. Gallegos AM, Hoerger M, Talbot NL, et al. Toward identifying the effects of the specific components of mindfulness‐based stress reduction on biologic and emotional outcomes among older adults. J Altern Complement Med. 2013;19(10):787‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spazierer D, Skvara H, Dawid M, et al. T helper 2 biased de novo immune response to keyhole limpet Hemocyanin in humans. Clin Exp Allergy. 2009;39(7):999‐1008. [DOI] [PubMed] [Google Scholar]
- 23. van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100(6):2257‐2259. [PubMed] [Google Scholar]
- 24. Kondratenko I, Amlot PL, Webster AD, Farrant J. Lack of specific antibody response in common variable immunodeficiency (CVID) associated with failure in production of antigen‐specific memory T cells. MRC immunodeficiency group. Clin Exp Immunol. 1997;108(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yasuda K, Ushio H. Keyhole limpet hemocyanin induces innate immunity via Syk and Erk phosphorylation. EXCLI J. 2016;15:474‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith AJ, Vollmer‐Conna U, Bennett B, Hickie IB, Lloyd AR. Influences of distress and alcohol consumption on the development of a delayed‐type hypersensitivity skin test response. Psychosom Med. 2004;66(4):614‐619. [DOI] [PubMed] [Google Scholar]
- 27. Belson A, Schmidt T, Fernando D, et al. Characterisation of the clinical and activated T cell response to repeat delayed‐type hypersensitivity skin challenges in human subjects, with KLH and PPD, as a potential model to test T cell‐targeted therapies. Inflamm Res. 2016;65(5):389‐404. [DOI] [PubMed] [Google Scholar]
- 28. Pouchot J, Grasland A, Collet C, Coste J, Esdaile JM, Vinceneux P. Reliability of tuberculin skin test measurement. Ann Intern Med. 1997;126(3):210‐214. [DOI] [PubMed] [Google Scholar]
- 29. Food and Drug Administration . Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. https://www.fda.gov/media/73679/download [accessed October 2019].
- 30. Amlot PL, Hayes AE, Gray D, Gordon‐Smith EC, Humphrey JH. Human immune responses in vivo to protein (KLH) and polysaccharide (DNP‐Ficoll) neoantigens: normal subjects compared with bone marrow transplant patients on cyclosporine. Clin Exp Immunol. 1986;64(1):125‐135. [PMC free article] [PubMed] [Google Scholar]
- 31. Shi R, Honczarenko M, Zhang S, et al. Pharmacokinetic, Pharmacodynamic, and safety profile of a novel anti‐CD28 domain antibody antagonist in healthy subjects. J Clin Pharmacol. 2017;57(2):161‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poirier N, Blancho G, Hiance M, et al. First‐in‐human study in healthy subjects with FR104, a Pegylated monoclonal antibody fragment antagonist of CD28. J Immunol. 2016;197:4593‐4602. [DOI] [PubMed] [Google Scholar]
- 33. King TC. 2 ‐ Inflammation, Inflammatory Mediators, and Immune‐Mediated Disease. In: King TC, ed. Elsevier's Integrated Pathology. Philadelphia: Mosby; 2007:21‐57. [Google Scholar]
- 34. Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5(1):7. [PubMed] [Google Scholar]
- 35. Sokal JE. Editorial: measurement of delayed skin‐test responses. N Engl J Med. 1975;293(10):501‐502. [DOI] [PubMed] [Google Scholar]
- 36. Kantele A, Häkkinen MP, Zivny J, Elson CO, Mestecky J, Kantele JM. Humoral immune response to keyhole limpet haemocyanin, the protein carrier in cancer vaccines. Clin. Dev Immunol. 2011;2011:1‐6. 10.1155/2011/614383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uzzaman A, Cho SH. Chapter 28: classification of hypersensitivity reactions. Allergy Asthma Proc. 2012;33(Suppl 1):96‐99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


