Abstract
Aims
Tacrolimus is a critical dose drug and to avoid under‐ and overexposure, therapeutic drug monitoring is standard practice. However, rejection and drug‐related toxicity occur despite whole‐blood tacrolimus pre‐dose concentrations ([Tac]blood) being on target. Monitoring tacrolimus concentrations at the target site (within peripheral blood mononuclear cells; [Tac]cells) may better correlate with drug‐efficacy. The aim of this study was to (1) investigate the relationship between [Tac]blood and [Tac]cells, (2) identify factors affecting the tacrolimus distribution in cells and whole‐blood, and (3) study the relationship between [Tac]cells and clinical outcomes after kidney transplantation.
Methods
A total of 175 renal transplant recipients were prospectively followed. [Tac]blood and [Tac]cells were determined at Months 3, 6 and 12 post‐transplantation. Patients were genotyped for ABCB1 1199G>A and 3435C>T, CYP3A4 15389C>T, and CYP3A5 6986G>A. Data on rejection and tacrolimus‐related nephrotoxicity and post‐transplant diabetes mellitus were collected.
Results
Correlations between [Tac]blood and [Tac]cells were moderate to poor (Spearman's r = 0.31; r = 0.41; r = 0.61 at Months 3, 6 and 12, respectively). The [Tac]cells/[Tac]blood ratio was stable over time in most patients (median intra‐patient variability 39.0%; range 3.5%–173.2%). Age, albumin and haematocrit correlated with the [Tac]cells/[Tac]blood ratio. CYP3A5 and CYP3A4 genotype combined affected both dose‐corrected [Tac]blood and [Tac]cells. ABCB1 was not significantly related to tacrolimus distribution. Neither [Tac]blood nor [Tac]cells correlated with clinical outcomes.
Conclusions
The correlation between [Tac]blood and [Tac]cells is poor. Age, albumin and haematocrit correlate with the [Tac]cells/[Tac]blood ratio, whereas genetic variation in ABCB1, CYP3A4 and CYP3A5 do not. Neither [Tac]blood nor [Tac]cells correlated with clinical outcomes.
Keywords: kidney transplantation, peripheral blood mononuclear cell, pharmacogenetics, tacrolimus, therapeutic drug monitoring
What is already known about this subject
Lymphocytes are the target cells of the immunosuppressant tacrolimus.
The whole‐blood concentration is the standard matrix for therapeutic drug monitoring of tacrolimus.
Tacrolimus whole‐blood concentrations do not optimally predict rejection and toxicity.
The intra‐lymphocytic tacrolimus concentration may correlate better with clinical outcomes than whole‐blood concentrations.
What this study adds
The correlation between peripheral blood mononuclear cells and whole‐blood tacrolimus concentrations is poor.
Age, albumin and haematocrit are correlated with the PBMC/whole‐blood tacrolimus concentration ratio.
Single‐nucleotide polymorphisms in ABCB1, CYP3A4 or CYP3A5 do not explain inter‐patient variability in tacrolimus distribution.
The PBMC tacrolimus concentration is not associated with rejection nor tacrolimus‐related toxicity in the first 3 months after kidney transplantation.
1. INTRODUCTION
Although transplant recipients undoubtedly benefit from therapeutic drug monitoring (TDM), a considerable number of patients experience acute rejection, despite tacrolimus whole‐blood pre‐dose concentrations ([Tac]blood) being within the therapeutic range. 1 , 2 , 3 Long‐term allograft failure is also an important problem, with 3–5% of kidney allografts being lost annually after the first transplant year, mainly as a result of rejection and tacrolimus‐related nephrotoxicity. 4 , 5 , 6
The immunosuppressive effect of tacrolimus is mediated through the inhibition of calcineurin within lymphocytes. In clinical practice, however, whole‐blood is the matrix used for TDM. In whole‐blood, tacrolimus is distributed extensively into erythrocytes, which do not contribute to alloreactivity. This may explain why multiple studies could not find a correlation between [Tac]blood and rejection in solid organ transplant recipients. 3 , 7 , 8 , 9 A better correlation with drug efficacy can be expected from direct quantification of the tacrolimus concentration at the target site. Tacrolimus measurement in peripheral‐blood mononuclear blood cells (PBMCs; [Tac]cells), which represent a blood compartment enriched with lymphocytes, has been proposed as a superior method to monitor tacrolimus treatment. 10 , 11 , 12 In liver transplant recipients, [Tac]cells significantly correlated with both the development and the severity of rejection. 8 Moreover, previous studies reported poor to moderate correlations between [Tac]cells and [Tac]blood, indicating that [Tac]blood does not always reflect the concentration at the target site. 8 , 11 , 13 , 14 , 15 , 16 , 17
This poor correlation may reflect the activity of the efflux transporter protein ABCB1 in mononuclear cell membranes. Differences in ABCB1 activity may result in differences in intra‐lymphocytic tacrolimus accumulation and tacrolimus pharmacodynamics. 18 , 19 , 20 In case of a high ABCB1 activity, both [Tac]cells and the [Tac]cells/[Tac]blood ratio are expected to be low. In a study by Capron et al., different ABCB1 single‐nucleotide polymorphisms (SNPs) were associated with reduced ABCB1 activity and higher [Tac]cells, while having no effect on [Tac]blood in 96 renal transplant recipients. 18
Tacrolimus metabolism is mediated by the cytochrome P450 enzymes CYP3A4 and CYP3A5. 21 The CYP3A5*3 variant allele has been associated consistently with low CYP3A5 enzymatic activity and a low tacrolimus dose requirement as compared to the CYP3A5*1 allele. 22 , 23 , 24 SNPs in the CYP3A4 gene have also been associated with tacrolimus pharmacokinetics. 24 , 25 However, little is known about the influence of CYP3A4 and CYP3A5 genotype on [Tac]cells. Capron et al. observed significantly lower dose‐adjusted [Tac]cells and [Tac]blood in CYP3A5 expressers 18 and Tron et al. did not find any association between CYP3A4 and CYP3A5 genotype and either [Tac]cells or [Tac]blood. 17
In this study, the relationship between [Tac]cells and [Tac]blood in kidney transplant recipients was investigated, as well as the influence of genetic variability in the ABCB1, CYP3A4 and CYP3A5 genes on [Tac]cells. A total of 175 renal transplant recipients were followed prospectively and blood and PBMCs were collected at 3, 6 and 12 months post‐transplantation in order to study which matrix correlated best with the occurrence of allograft rejection or tacrolimus‐related toxicity.
2. METHODS
2.1. Patients and sample collection
This study is a post hoc analysis and includes renal allograft recipients who participated in a randomized, controlled clinical trial that compared the efficacy of standard, bodyweight‐based tacrolimus dosing with CYP3A5 genotype‐based tacrolimus dosing. 26 In this trial, patients were followed until the third postoperative month. For the present study, sample collection (but not the collection of clinical data) was extended up until Month 12.
Only patients with an available [Tac]cells and [Tac]blood 3 months post‐transplantation were included in this analysis. Patients with a [Tac]cells with a red level >2 (see paragraph on [Tac]cells measurement below), were excluded from the analysis. The study was approved by the institutional review board of the Erasmus MC, University Medical Center, Rotterdam (Medical Ethical Review Board number 2010‐080). All patients provided written informed consent for the study.
Patients received basiliximab induction therapy followed by triple immunosuppression consisting of tacrolimus, mycophenolate mofetil (MMF) and prednisolone, as described by Shuker et al. 26 Patients were randomized to receive a tacrolimus starting dose (Prograft®; Astellas Pharma, Leiden, The Netherlands) twice daily based on either bodyweight alone or a combination of CYP3A5 genotype plus bodyweight. After the measurement of the first tacrolimus pre‐dose concentration on the third postoperative day (the first steady state concentration), routine TDM was performed aiming for a target [Tac]blood of 10.0–15.0 ng/mL (week 1–2), 8.0–12.0 ng/mL (week 3–4), and 5.0–10.0 ng/mL (after week 4) post‐transplantation. For further details regarding the immunosuppressive therapy, the reader is referred to Shuker et al. 26
2.2. End points
The correlation between [Tac]blood and [Tac]cells was studied at 3, 6 and 12 months post‐transplantation. The [Tac]cells/[Tac]blood ratio was evaluated over time and the intra‐ and inter‐patient variability of this ratio are described. Covariate factors affecting this ratio were investigated. Age, gender, haematocrit, serum albumin, serum creatinine, ABCB1 genotype (wildtype vs. one allele variant vs. >one allele variant), and CYP3A genotype (extensive vs. intermediate vs. poor metabolizers; see below for more information on categorization) genotype were evaluated as potential predictors. Also, the influence of pharmacogenetic variability in ABCB1 1199G>A, ABCB1 3435C>T, CYP3A4 and CYP3A5 genotype on both [Tac]cells and [Tac]blood was investigated. Finally, the relationship between the tacrolimus distribution and rejection and tacrolimus‐related nephrotoxicity was evaluated.
2.3. Rejection and tacrolimus‐related toxicity
Biopsy‐proven acute rejection (BPAR) was registered up until the third postoperative month. Renal biopsies were performed for cause only (no protocol biopsies) and were reviewed in a blinded fashion by two independent pathologists and were graded according to the 2013 Banff classification of renal allograft rejection. 27 Data on the presence of post‐transplant diabetes mellitus (PTDM) and tacrolimus‐induced nephrotoxicity were collected, as these events may be associated with tacrolimus treatment. PTDM was defined as the use of glucose‐lowering medical therapy up until Month 3 after transplantation in a patient not needing such treatment before transplantation. Tacrolimus‐related nephrotoxicity was defined as any ≥15% increase of serum creatinine with a return to baseline after tacrolimus dose reduction and after exclusion of other causes of renal transplant function deterioration. 26 The estimated glomerular filtration rate (eGFR) was calculated using the abbreviated MDRD study equation. 28
2.4. Whole‐blood tacrolimus concentration measurement
[Tac]blood was determined by two immunoassays: the antibody‐conjugated magnetic immunoassay (ACMIA) on a Dimension platform (Siemens Healthcare, N.V., The Hague, The Netherlands) and the enzyme multiplied immunoassay technique (EMIT; Siemens Healthcare N.V.). In the first two years of the trial, [Tac]blood was measured exclusively by the ACMIA, after which measurements were performed exclusively by the EMIT. 26 ACMIA and EMIT immunoassays demonstrated a high correlation (r = 0.97). 26
2.5. PBMC tacrolimus concentration measurement
PBMCs were isolated from whole‐blood pre‐dose samples at Months 3, 6 and 12 post‐transplantation, using a Ficoll separation technique. The whole procedure was performed at room temperature. Cells were counted (Sysmex XOP‐300 cell counter), resuspended in PBS, snap frozen in liquid nitrogen and stored until analysis at −80°C in aliquots of 1×106 cells per vial. To determine the presence of erythrocytes, which could affect the measured tacrolimus concentration, the redness of the cell pellet after PBMC isolation was rated on a scale from 0 to 8 by visual inspection (Figure S1 in the Supporting Information). Samples with a red level above 2, were excluded from this study. [Tac]cells were measured using a LC–MS/MS method, as described previously. 29 , 30
2.6. Genotyping
DNA for the genotyping of CYP3A5*3 6986G>A (rs776746) was extracted from peripheral‐blood leukocytes by use of the Blood DNA kit (Qiagen, Courtaboeuf, France). Genotyping was performed using TaqMan Assay reagents for allelic discrimination (Applied Biosystems, Courtaboeuf, France) with a 7900 Applied Biosystems thermal cycler as previously described. 22 , 26 , 31 Genotyping of the ABCB1 1199G>A (rs2229109), ABCB1 3435C>T (rs1045642) and CYP3A4*22 15389C>T (rs35599367) alleles was performed using TaqMan Assay reagents for allelic discrimination (San Diego, USA) with a 7900 Applied Biosystems thermal cycler. 22 , 31 All genotypings were performed according to standard laboratory procedures in an ISO15189 certified laboratory.
2.7. Statistical analysis
All analyses were performed using R (Version 3.5.3). 32 Categorical variables are described as number of cases with proportions. Continuous variables with a non‐parametric distribution are described as median with interquartile range (IQR). The Mann–Whitney U and the Kruskal‐Wallis test (for multiple groups) were used to compare non‐parametric variables. Spearman's correlation coefficient was used to determine correlations between non‐parametric variables. After a significant result from the Kruskal‐Wallis test, post hoc tests were performed using a pairwise Mann–Whitney U‐test with Bonferroni correction. The Friedman test was used to compare non‐parametric paired data of multiple groups. Intra‐patient variability was calculated for each patient with complete data on the [Tac]cells/[Tac]blood ratio by the following formula: coefficient of variation (CV) %CV = Xsd/Xmean×100. Here, Xsd represents the standard deviation, and Xmean the mean of the [Tac]cells/[Tac]blood ratios of Months 3, 6 and 12. The inter‐patient variability was calculated at Months 3, 6 and 12 with the following formula: inter‐patient variability % = Xtsd/Xtmean×100, where Xtsd and Xtmean represent the standard deviation and the mean of the [Tac]cells/[Tac]blood ratios at a certain time point. For the combined CYP3A4 and CYP3A5 analysis, patients were categorized as poor, intermediate and extensive metabolizers as proposed by Elens et al. 33 For the combined analysis of the ABCB1 genotype, patients were categorized as ‘wildtype’ if they had no variant alleles (ABCB1 3435CC and ABCB1 1199GG), as ‘one variant allele’ if they had one variant allele (either ABCB1 3435 T or ABCB1 1199A), and as ‘>one variant allele’ if they had more than one variant allele at the ABCB1 3435 and 1199 positions together. After the removal of an outlier (Cooks distance >10), a multiple linear regression analysis was performed to assess the contribution of covariates on the [Tac]cells/[Tac]blood ratio. A logistic regression analysis was performed to assess the relationship between the [Tac]cells/[Tac]blood ratio and clinical outcomes, which included BPAR, drug‐related nephrotoxicity and PTDM.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 34 , 35
3. RESULTS
3.1. Baseline characteristics
A total of 175 renal transplant recipients were included in this analysis. Of the 237 patients included in the original trial by Shuker et al., 26 61 patients were excluded, based on: no available [Tac]cells (n = 36) or [Tac]blood (n = 16) at Month 3 post‐transplantation, or a red level above 2 (see paragraph on [Tac]cells measurement; n = 9; Figure S2 in the Supporting Information). One patient was excluded from the analysis because he was considered an outlier (Cooks distance > 10). Table 1 summarizes the baseline characteristics.
TABLE 1.
Baseline characteristics
| n = 175 | |
|---|---|
| Age (years | 56.0 (IQR 46.0–64.0) |
| Gender | |
| Male | 113 (64.6%) |
| Female | 62 (35.4%) |
| Ethnicity | |
| Caucasian | 146 (83.4%) |
| Asian | 16 (9.1%) |
| Black | 10 (5.7%) |
| Other | 3 (1.7%) |
| Body weight (kg) | 80.9 (IQR 69.4–92.3) |
| Height (cm) | 174.5 (IQR 166.3–182.0) |
| BMI (kg/m2) | 25.9 (IQR 23.7–29.6) |
| Primary kidney disease | |
| Diabetic nephropathy | 31 (17.7%) |
| Polycystic kidney disease | 34 (19.4%) |
| Glomerulonephritis | 8 (4.6%) |
| Hypertensive nephropathy | 30 (17.1%) |
| Reflux disease/chronic pyelonephritis | 12 (6.9%) |
| Focal segmental glomerulosclerosis | 5 (2.9%) |
| IgA nephropathy | 8 (4.6%) |
| Obstructive nephropathy | 7 (4.0%) |
| Other | 23 (13.1%) |
| Unknown | 17 (9.7%) |
| Number of kidney transplantation | |
| 1st | 162 (92.6%) |
| 2nd | 11 (6.3%) |
| 3rd | 2 (1.1%) |
| PRA% | |
| <15% | 165 (94.3%) |
| ≥15%) | 10 (5.7%) |
| Peak PRA% | |
| <15% | 147 (84.0%) |
| ≥15% | 28 (16.0%) |
| Serum creatinine (Month 3; μmol/L) | 125.0 (104.2–150.8) |
| Haematocrit (Month 3; L/L) | 0.37 (0.34–0.40) |
| Serum albumin (Month 3; g/L) | 46.0 (44.0–48.0) |
| BMI, body mass index; PRA, panel reactive antibodies. | |
3.2. Relationship between whole‐blood and PBMC tacrolimus concentrations
Tacrolimus concentrations were measured at Month 3 (n = 175), 6 (n = 130) and 12 (n = 54) post‐transplantation. The median [Tac]blood decreased from 7.5 ng/mL (IQR 6.1–9.2) at Month 3, to 6.6 ng/mL (IQR 5.6–8.2) and 5.9 ng/mL (IQR 4.3–7.7) at Months 6 and 12, respectively (Table S1 in the Supporting Information). The median [Tac]cells also decreased from 26.5 pg/106 cells (IQR 18.8–37.0) at Month 3, to 23.8 pg/106 cells (IQR 16.5–33.4) and 20.8 pg/106 cells (IQR 12.5–31.4) at Months 6 and 12, respectively.
The correlations between [Tac]cells and [Tac]blood at Months 3, 6 and 12 post‐transplantation were poor to moderate with Spearman's correlation coefficients of r = 0.31, r = 0.41 and r = 0.61, respectively (Figures 1A–C).
FIGURE 1.
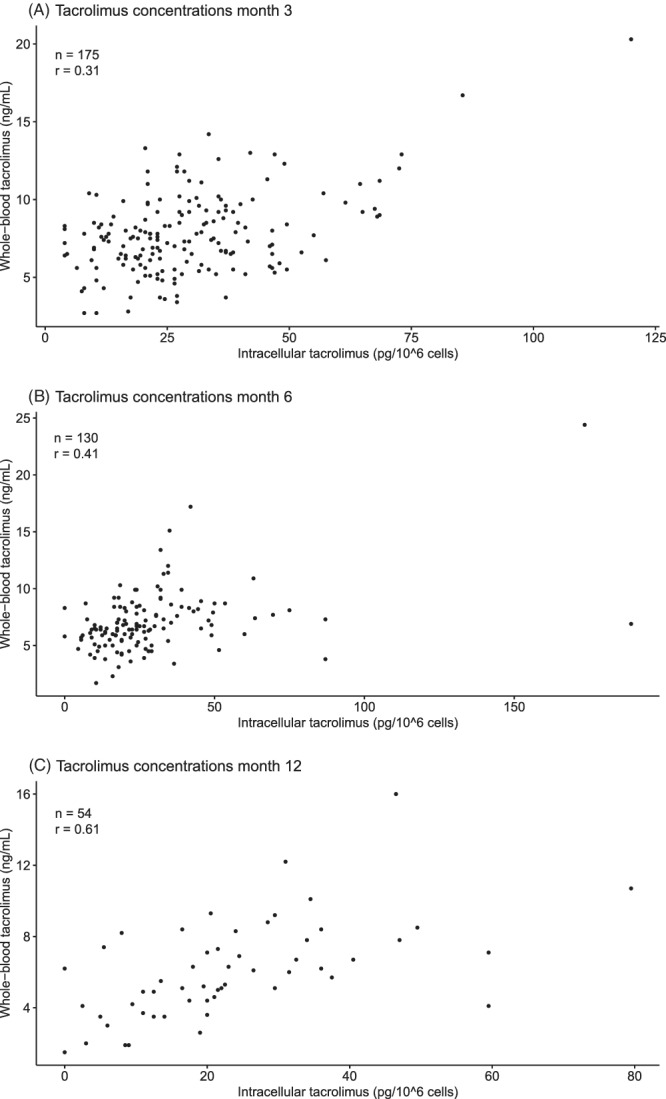
Scatter plots illustrating the distribution of whole‐blood and PBMC tacrolimus concentrations in the 3rd (A), 6th (B) and 12th (C) month after transplantation with Spearman's correlation coefficient
3.3. The PBMC to whole‐blood tacrolimus concentration ratio over time
Next, the evolution of the [Tac]cells/[Tac]blood ratio over time was investigated (Figure 2A and B). The median [Tac]cells/[Tac]blood ratio was 3.5 (IQR 2.4–5.4) at Month 3, 3.5 (IQR 2.4–5.1) at Month 6 and 3.6 (2.5–4.7) at Month 12 post‐transplantation (Table S1 in Supporting Information). For patients with an available [Tac]cells at all time points (n = 45), the [Tac]cells/[Tac]blood ratio did not change significantly over time (P = 0.71). The median intra‐patient variability of the [Tac]cells/[Tac]blood ratio in these patients was 39.0% and ranged from 3.5% to 173.2%. The inter‐patient variability was higher, with 51.8%, 82.0% and 60.5% at Months 3, 6 and 12, respectively. The variability in the [Tac]cells/[Tac]blood ratio of the 15 patients with the highest intra‐patient variability could not be explained by a change in haematocrit or albumin (data not shown). In two patients the high intra‐patient variability in the [Tac]cells/[Tac]blood ratio could be explained by a [Tac]cells under the limit of quantification, causing a high ratio.
FIGURE 2.
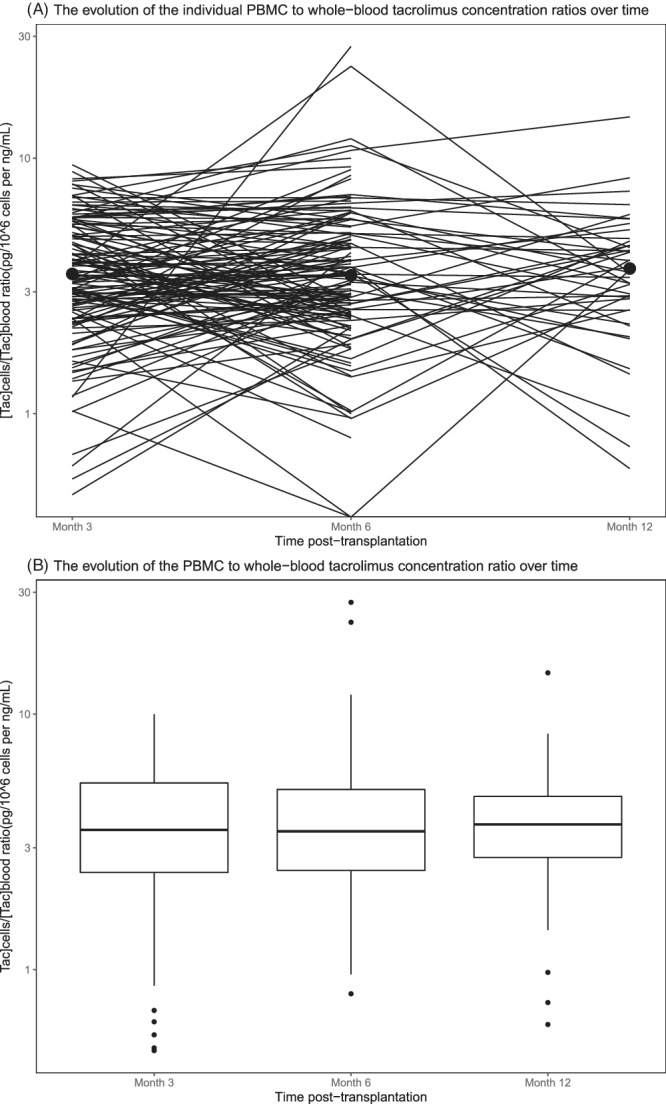
Spaghetti plot (on a logarithmic scale; A) and a boxplot (B) illustrating the evolution of the tacrolimus PBMC to whole‐blood ratio together with its median 3, 6 and 12 months after transplantation
3.4. Relationship between tacrolimus concentrations and genotypes
Allelic frequencies of ABCB1, CYP3A5 and CYP3A4 are depicted in Table S2 in the Supporting Information. The ABCB1 3435C>T, CYP3A5 and CYP3A4 SNPs were in Hardy–Weinberg equilibrium (all P > 0.05), but ABCB1 1199G>A genotype was not (P = 0.005491). However, only two patients were homozygous for the variant allele (1199AA), and with expected counts below five, the chi‐square approximation may be incorrect.
3.4.1. ABCB1
At all time points, no significant difference in the [Tac]cells/[Tac]blood ratio was observed between patients with different ABCB1 genotypes (for both ABCB1 1199G>A and 3435C>T). Also, no differences between the groups were observed in tacrolimus doses, unadjusted‐ and dose‐adjusted tacrolimus concentrations 3 months post‐transplantation (P > 0.05; Table S3 and S4 in the Supporting Information). The [Tac]cells/[Tac]blood ratio was numerically, but not significantly, higher in the 3435T allele carriers compared to non‐carriers at Month 3 (median 3.9 (IQR 2.5–5.5) vs. 3.1 (IQR 2.2–4.0); P = 0.06). At Months 6 and 12 post‐transplantation, no differences in [Tac]cells/[Tac]blood ratios were present between the different genotypes. Moreover, [Tac]cells/dose was not significantly different in 3435T carriers compared to non‐carriers (median 4.6 (IQR 2.9–8.4) vs. 5.3 (IQR 3.7–6.4); P = 0.96).
Also when ABCB1 1199G>A and 3435C>T genotypes were pooled, no significant differences were observed in either the (dose‐corrected) [Tac]cells, [Tac]blood, nor the [Tac]cells/[Tac]blood ratio between patients without allelic variants, those carrying one variant and those harbouring more than one variant in the ABCB1 genes (Table 2).
TABLE 2.
The association between pooled ABCB1 1199G>A and 3435C>T genotype and tacrolimus concentrations
| ABCB1 genotype | Wildtype | One variant allele | >One variant allele | P‐value |
|---|---|---|---|---|
| Month 3 | (n = 37) | (n = 79) | (n = 51) | |
| Dose (mg) | 5.0 (4.0–7.0) | 6.0 (4.0–8.0) | 6.0 (4.0–9.0) | 0.48 |
| [Tac]blood (ng/mL) | 7.8 (6.4–9.0) | 7.5 (6.0–9.6) | 7.3 (5.9–8.6) | 0.65 |
| [Tac]blood/dose | 1.7 (0.9–2.1) | 1.5 (0.8–2.1) | 1.3 (0.8–1.9) | 0.33 |
| [Tac]cells (pg/106 cells) | 24.5 (16.0–33.0) | 27.0 (19.5–40.5) | 28.5 (19.8–35.8) | 0.29 |
| [Tac] cells/dose | 5.3 (3.7–6.4) | 4.6 (3.0–8.7) | 4.5 (2.8–7.3) | 0.68 |
| [Tac]cells/[Tac]blood | 3.1 (1.8–4.0) | 3.9 (2.8–5.4) | 3.6 (2.4–5.6) | 0.12 |
| Month 6 | (n = 29) | (n = 61) | (n = 35) | |
| [Tac]cells/[Tac]blood | 3.5 (2.4–4.3) | 3.4 (2.4–5.4) | 3.7 (2.5–5.1) | 0.72 |
| Month 12 | (n = 15) | (n = 17) | (n = 20) | |
| [Tac]cells/[Tac]blood | 3.8 (2.7–4.4) | 3.6 (2.9–4.3) | 3.3 (2.4–5.6) | 0.99 |
[Tac]cells indicates the unadjusted tacrolimus pre‐dose concentration in peripheral blood mononuclear cell concentration. [Tac]blood indicates the unadjusted tacrolimus pre‐dose whole blood concentration. Data is shown as median (IQR). P denotes the comparison between groups by Kruskal‐Wallis test.
3.4.2. CYP3A metabolic phenotype
CYP3A4 and CYP3A5 genotypes were combined to classify patients as poor, intermediate and extensive CYP3A metabolizers. The CYP3A metabolic phenotype was associated with tacrolimus concentrations (Table 3). Three months post‐transplantation, the tacrolimus dose was significantly different between poor, intermediate and extensive metabolizers (median 3.0 mg (IQR 2.8–5.5) vs. 5.0 mg (IQR 4.0–6.0) vs. 10.0 mg (IQR 9.0–12.0) respectively; P < 0.001). In addition, the dose‐adjusted [Tac]blood was significantly different between these groups (median 2.3 (IQR 1.7–3.1) vs. 1.6 (IQR 1.1–2.1) vs. 0.7 (IQR 0.5–0.9) respectively, P < 0.001). Also, the dose‐adjusted [Tac]cells was significantly higher in poor and intermediate metabolizers, compared to extensive metabolizers (median 7.3 (IQR 4.9–8.3) vs. 5.3 (IQR 3.6–8.8) vs. 2.4 (IQR 1.3–3.6), respectively, P < 0.001). The unadjusted [Tac]cells and [Tac]blood were not significantly associated with CYP3A metabolizing status. The [Tac]cells/[Tac]blood ratio was significantly different between the groups at Month 6 post‐transplantation (median 2.3 (IQR 1.9–2.9) vs. 3.6 (IQR 2.5–5.1) vs. 3.9 (IQR 2.6–5.8); P = 0.02). Pairwise testing revealed a significant difference only between poor and intermediate metabolizers.
TABLE 3.
Poor, intermediate and extensive metabolizers and the association with tacrolimus concentrations
| Metabolizers | Poor | Intermediate | Extensive | P‐value |
|---|---|---|---|---|
| Month 3 | (n = 16) | (n = 117) | (n = 33) | |
| Dose (mg) | 3.0 (2.8–5.5) b | 5.0 (4.0–6.0) c | 10.0 (9.0–12.0) b , c | <0.001 |
| [Tac]blood (ng/mL) | 8.2 (7.0–9.8) | 7.5 (6.1–9.0) | 7.5 (6.1–9.2) | 0.59 |
| [Tac]blood/dose | 2.3 (1.7–3.1) a , b | 1.6 (1.1–2.1) a , c | 0.7 (0.5–0.9) b , c | <0.001 |
| [Tac]cells (pg/106 cells) | 25.3 (18.8–29.6) | 27.5 (19.0–37.0) | 24.0 (17.5–34.5) | 0.47 |
| [Tac]cells/dose | 7.3 (4.9–8.3) b | 5.3 (3.6–8.8) c | 2.4 (1.3–3.6) b , c | <0.001 |
| [Tac]cells/[Tac]blood | 3.0 (2.5–4.3) | 3.8 (2.6–5.6) | 3.5 (2.1–4.2) | 0.25 |
| Month 6 | (n = 14) | (n = 83) | (n = 25) | |
| [Tac]cells/[Tac]blood | 2.3 (1.9–2.9) a | 3.6 (2.5–5.1) a | 3.9 (2.6–5.8) | 0.02 |
| Month 12 | (n = 6) | (n = 36) | (n = 9) | |
| [Tac]cells/[Tac]blood | 3.6 (2.9–4.3) | 3.6 (2.4–4.6) | 3.6 (2.9–5.8) | 0.91 |
[Tac]cells indicates the unadjusted tacrolimus pre‐dose concentration in peripheral blood mononuclear cell concentration. [Tac]blood indicates the unadjusted tacrolimus pre‐dose whole blood concentration. Data is shown as median (IQR). P denotes the comparison between groups by Kruskal‐Wallis test.
P < 0.05 comparing poor metabolizers to intermediate metabolizers with Bonferroni correction.
P < 0.05 comparing poor metabolizers to extensive metabolizers with Bonferroni correction.
P < 0.05 comparing intermediate metabolizers to extensive metabolizers with Bonferroni correction.
3.5. Covariate factors determining the PBMC to whole‐blood tacrolimus concentration ratio
In a multiple linear regression analysis, age and albumin were positive predictors for the [Tac]cells/[Tac]blood ratio (β = 0.0229, P = 0.048; β = 0.1275, P = 0.007, respectively), whereas haematocrit was a negative predictor for the [Tac]cells/[Tac]blood ratio (β = −16.138, P < 0.001; Table 4).
TABLE 4.
Multivariable linear regression PBMC to whole‐blood tacrolimus concentration ratio
| Dependent variable | β | SE | P‐value |
|---|---|---|---|
| (Intercept) | 2.000 | 2.578 | |
| Age | 0.0229 | 0.0115 | 0.048 |
| Gender (female) | −0.4003 | 0.4011 | 0.320 |
| ABCB1 (one variant) | 0.7236 | 0.4105 | 0.080 |
| ABCB1 (>one variant) | 0.7988 | 0.4482 | 0.077 |
| CYP3A (poor) | 0.0101 | 0.6363 | 0.987 |
| CYP3A4 (intermediate) | 0.6405 | 0.3798 | 0.094 |
| Serum albumin | 0.1275 | 0.0463 | 0.007 |
| Serum creatinine | −0.0011 | 0.0046 | 0.813 |
| Haematocrit | −16.138 | 4.070 | 0.0001 |
The effect of changes in these predictors on the median [Tac]cells/[Tac]blood ratio was estimated to evaluate clinical relevance. A 10‐year increase in age would result in a 0.23 higher [Tac]cells/[Tac]blood ratio. Considering the median of 3.5 pg/106 cells per ng/mL as baseline [Tac]cells/[Tac]blood ratio, this would correspond with an increase in the ratio of 6.6%. A 5.0 g/L increase in albumin (i.e. from 45.0 to 50.0 g/L) would increase the [Tac]cells/[Tac]blood ratio by 0.64, corresponding to an increase of 18.2% of the median ratio. A 0.1 L/L increase in haematocrit (i.e. from 0.35 to 0.45 L/L) would result in a decrease in the [Tac]cells/[Tac]blood ratio of 1.61, which corresponds to a decrease of 46.1% in the ratio.
3.6. Tacrolimus concentrations and clinical outcomes
3.6.1. Rejection
Fourteen out of 175 patients (8.0%) developed BPAR within the first three postoperative months. Five patients (2.9%) were diagnosed with a borderline rejection and in three patients (1.7%) a rejection episode was presumed (i.e. treated but not histologically confirmed). The other 153 patients remained rejection free. No statistically significant differences were observed between patients with and without BPAR in either [Tac]cells (median 24.8 pg/106 cells (IQR 18.4–34.8) vs. 27.0 pg/106 cells (IQR 19.0–37.0); P = 0.54), [Tac]blood (median 7.0 ng/mL (IQR 4.9–8.3) vs. 7.7 ng/mL (IQR 6.4–9.2); P = 0.18) or the [Tac]cells/[Tac]blood ratio (median 3.8 (IQR 2.4–5.6) vs. 3.5 (IQR 2.4–4.9); P = 0.67; Table 5). In a regression analysis, the [Tac]cells/[Tac]blood ratio was not a significant predictor for rejection occurring within the first 3 months after transplantation, when corrected for age, current and highest PRA, and total HLA mismatches (Table S5 in the Supporting Information).
TABLE 5.
Tacrolimus concentrations and clinical outcomes at Month 3 post‐transplantation
| Yes | No | P‐value | |
|---|---|---|---|
| BPAR | (n = 14) | (n = 153) | |
| [Tac]blood (ng/ml) | 7.0 (4.9–8.3) | 7.7 (6.4–9.2) | 0.18 |
| [Tac]cells (pg/106 cells) | 24.8 (18.4–34.8) | 27.0 (19.0–37.0) | 0.54 |
| [Tac]cells/[Tac]blood | 3.8 (2.4–5.6) | 3.5 (2.4–4.9) | 0.67 |
| Nephrotoxicity | (n = 52) | (n = 123) | |
| [Tac]blood (ng/ml) | 7.3 (5.8–8.4) | 7.8 (6.2–9.2) | 0.38 |
| [Tac]cells (pg/106 cells) | 26.5 (20.3–33.6) | 27.0 (18.3–38.3) | 0.91 |
| [Tac]cells/[Tac]blood | 3.6 (2.7–5.4) | 3.5 (2.4–5.1) | 0.60 |
| PTDM | (n = 36) | (n = 139) | |
| [Tac]blood (ng/ml) | 7.0 (6.0–8.9) | 7.6 (6.2–9.2) | 0.26 |
| [Tac]cells (pg/106 cells) | 28.0 (20.1–38.8) | 26.5 (18.5–36.5) | 0.58 |
| [Tac]cells/[Tac]blood | 4.0 (2.7–5.9) | 3.5 (2.4–5.0) | 0.26 |
[Tac]cells indicates the unadjusted tacrolimus pre‐dose concentration in peripheral blood mononuclear cell concentration. [Tac]blood indicates the unadjusted tacrolimus pre‐dose whole blood concentration. Data is shown as median (IQR). P denotes the comparison by the Mann–Whitney U‐test. BPAR, biopsy‐proven acute rejection; PTDM, post‐transplant diabetes mellitus.
3.6.2. Tacrolimus‐related toxicity
Fifty‐two (29.7%) patients suffered from tacrolimus‐related nephrotoxicity within the first 3 months post‐transplantation. No significant differences existed in [Tac]blood (median 7.3 ng/mL (IQR 5.8–8.4) vs. 7.8 ng/mL (IQR 6.2–9.2); P = 0.38) or [Tac]cells (median 26.5 pg/106 cells (IQR 20.3–33.6) vs. 27.0 pg/106 cells (IQR 18.3–38.3); P = 0.91) between recipients with or without nephrotoxicity. No significant difference was found in the [Tac]cells/[Tac]blood ratio at the third postoperative month (median 3.6 (IQR 2.7–5.4) vs. 3.5 (IQR 2.4–5.1); P = 0.60; Table 5).
Thirty‐six (20.6%) patients were diagnosed with PTDM within the first 3 months after kidney transplantation. No significant differences were observed between patients with and without PTDM in [Tac]blood (median 7.0 ng/mL (IQR 6.0–8.9) vs. 7.6 ng/mL (IQR 6.2–9.2); P = 0.26), [Tac]cells (median 28.0 pg/106 cells (IQR 20.1–38.8) vs. 26.5 pg/106 cells (IQR 18.5–36.5); P = 0.58) or the [Tac]cells/[Tac]blood ratio (median 4.0 (IQR 2.7–5.9) vs. 3.5 (IQR 2.4–5.0); P = 0.26; Table 5). In regression analysis, the [Tac]cells/[Tac]blood ratio was not a significant predictor for either drug‐related nephrotoxicity (univariate) or PTDM (corrected for age, BMI and creatinine) 3 months post‐transplantation (Tables S6 and S7 in the Supporting Information).
4. DISCUSSION
In this study, 175 patients were prospectively followed to evaluate tacrolimus PBMC concentrations after kidney transplantation. The relationship between [Tac]cells and [Tac]blood was poor to moderate. Age, albumin and haematocrit were associated with the [Tac]cells/[Tac]blood ratio, whereas pharmacogenetic variability was not. Three months post‐transplantation, this ratio was not associated with clinical outcomes.
The poor to moderate correlation between [Tac]cells and [Tac]blood after solid organ transplantation has been reported by other research groups. 8 , 11 , 13 , 14 , 15 , 16 , 17 In kidney transplant recipients with a stable kidney function, a moderate correlation between [Tac]cells and [Tac]blood was observed (r = 0.67), which corresponds to the observation in the present study. 11 The poor correlation might be explained by factors affecting the tacrolimus distribution and indicates that [Tac]blood does not properly reflect the [Tac]cells. Under the assumption that the tacrolimus concentration at the target site correlates better with its immunosuppressive effect (which remains, however, to be demonstrated), a poor correlation can explain why [Tac]blood predicts clinical outcomes poorly. Monitoring the [Tac]cells has therefore been proposed as a more meaningful matrix. 12 Interestingly, the relationship between [Tac]cells and [Tac]blood seemed to improve with time after transplantation in the present study. However, this may have been caused by the decreasing number of available tacrolimus measurements over time.
The [Tac]cells/[Tac]blood ratio appeared to be stable in most patients (median CV 39.0%). This observation is in line with the observations of Klaasen et al., who found a median CV of 45% in the [Tac]cells/[Tac]blood ratios measured at 1 week, 6 weeks and 1 year after renal transplantation. 13 Inter‐patient variability was higher than the intra‐patient variability, suggesting that an individual's ratio might be useful in the prediction of future tacrolimus ratios and dose requirements.
Inter‐ and intra‐patient variability in the tacrolimus distribution might be explained by covariate factors. Covariate factors known to affect the [Tac]cells/[Tac]blood ratio allow the identification of patients at risk for extremely high or low ratios. These patients might have a higher risk for adverse clinical outcomes. In the present study, age and albumin were positive predictors and haematocrit was a negative predictor for the [Tac]cells/[Tac]blood ratio. In whole‐blood, tacrolimus is highly bound to erythrocytes. A higher haematocrit may therefore reflect a higher tacrolimus binding capacity, which limits the shift of tacrolimus to the intracellular compartment and decreases the [Tac]cells/[Tac]blood ratio. Age has been associated with lower tacrolimus metabolism and dose requirement. 36 The present results suggest that age is also involved in the distribution of tacrolimus, although the effect of a one‐year increase in age on the [Tac]cells/[Tac]blood ratio was limited (0.02 per year). The positive correlation between albumin and the [Tac]cells/[Tac]blood ratio is not easily explained. Theoretically, a higher albumin concentration might be expected to increase tacrolimus binding, resulting in a decrease in the [Tac]cells/[Tac]blood ratio. As reduced serum albumin concentrations have been associated with inflammation (which occurs frequently in transplant recipients), an explanation for the positive relationship might be underlying inflammatory disease. 37 , 38 Inflammation has been associated with lower albumin concentrations and reduced CYP3A activity. 39 , 40 Lower CYP3A activity might in turn lower the [Tac]cells/[Tac]blood ratio. Factors that were previously associated with the tacrolimus distribution are ABCB1 genotype, total plasma protein concentration, sex, haematocrit and time after transplantation. 11 , 18
SNPs in the ABCB1 gene have been associated with reduced activity of this efflux transporter, 41 , 42 which might affect the distribution of tacrolimus. 20 In the present study, both ABCB1 1199G>A and 3435C>T genotypes were not associated with [Tac]cells or the [Tac]cells/[Tac]blood ratio. The [Tac]cells/[Tac]blood ratio was, at Month 3, numerically, but not significantly higher in 3435T allele carriers compared to non‐carriers. Also ABCB1 1199G>A and 3435C>T genotype combined was not predictive for the [Tac]cells/[Tac]blood ratio. Previous research on the importance of different ABCB1 SNPs for the tacrolimus distribution in solid organ transplant recipients was inconclusive. A study in 214 kidney transplant recipients with a stable graft function also found that ABCB1 genotype (including 3435C>T) was not a significant predictor for the [Tac]cells/[Tac]blood ratio. 11 In contrast, in a study including 96 renal transplant recipients, ABCB1 genotype was a positive predictor for the [Tac]cells/[Tac]blood ratio 7 days post‐transplantation and [Tac]cells was strongly associated with ABCB1 genotype (including both 3435C>T and 1199G>A). 18 In 32 liver transplant recipients, Tron et al. found an association between ABCB1 1199G>A and [Tac]cells, but not between ABCB1 3435C>T genotypes and [Tac]cells. 17 In a study in 150 liver transplant recipients, Elens et al. found that ABCB1 (1199G>A and 2677G>T/A) genotype significantly influenced hepatic tissue tacrolimus concentrations, whereas the impact of ABCB1 on [Tac]blood was negligible. 43
Tacrolimus is extensively metabolized by CYP3A enzymes. 21 , 24 , 44 , 45 In this study, we confirm the importance of CYP3A polymorphisms for tacrolimus dose requirement. CYP3A4 and CYP3A5 genotype combined (as poor, intermediate and extensive metabolizers) was significantly associated with tacrolimus dose requirement as well as [Tac]cells and [Tac]blood. The [Tac]cells/[Tac]blood ratio was not significantly different between these groups. Our results indicate that CYP3A‐related tacrolimus metabolism affects both dose‐corrected [Tac]cells and [Tac]blood, but not the distribution of tacrolimus. Capron et al. observed lower dose‐adjusted [Tac]cells in CYP3A5 expressers compared to non‐expressers, which is in line with the present results. 18 In 32 liver transplant recipients, no difference in either [Tac]cells or [Tac]blood (not dose‐corrected) between different CYP3A4 and CYP3A5 genotypes was found. 17
Although the use of tacrolimus and TDM has greatly improved transplant outcomes, the relationship between [Tac]blood and clinical outcomes is still unclear. Multiple studies, including a meta‐analysis including 1304 patients, showed no correlation between tacrolimus exposure and clinical outcomes. 3 , 7 , 8 In everyday clinical practice, rejection and drug‐related toxicity still occur despite [Tac]blood being within the tacrolimus target pre‐dose concentration. Also, in a time‐to‐event analysis, longitudinal exposure to tacrolimus was not associated with a composite clinical endpoint (including rejection, cytomegalovirus infection and death). 9 Therefore, it was hoped that monitoring tacrolimus concentrations at the target site would better correlate with drug efficacy and clinical outcomes. 10 , 12 In the present study, no associations were observed between [Tac]cells, [Tac]blood or the [Tac]cells/[Tac]blood ratio and either the risk of BPAR, tacrolimus‐related nephrotoxicity or PTDM at the third postoperative month. This might be (in part) explained by the fact that intracellular tacrolimus concentrations were measured at a fixed time point, which was often different from the moment of diagnosis. To overcome this shortcoming, in an ongoing prospective trial performed by our research group, (intracellular) tacrolimus concentrations are measured on the morning of a for‐cause renal biopsy. Another explanation for the lack of associations between tacrolimus concentrations and clinical outcomes is the variability in the tacrolimus concentration measurement, due to the use of immunoassays instead of the more sensitive LC–MS/MS method or red blood cell contamination. Also the use of TDM might in part explain the lack of correlation, as alternative tacrolimus concentrations are necessary to be able to show an association with clinical outcomes. Finally, it is possible that both whole‐blood and intracellular tacrolimus concentrations are not the right matrix for monitoring tacrolimus exposure. Future studies may also focus on other matrices to monitor tacrolimus treatment, which might have a stronger correlation with clinical outcomes, such as for example, the T lymphocyte subset of PBMCs or the unbound (or free) tacrolimus concentrations. 2 , 46 , 47 , 48
Previous studies evaluated the association between [Tac]cells and drug efficacy but results are contradictory. In 213 kidney transplant recipients, [Tac]cells was associated with T cell activation, 11 whereas in 10 liver transplant recipients no association between [Tac]cells and calcineurin activity was found. 49 Interestingly, in the latter study, the one patient that experienced rejection (despite adequate [Tac]blood), had area‐under the PBMC concentration–time curves (AUCs) that were four times lower than the mean AUCs of the other patients. The differences in the association between [Tac]cells and T cell function might be explained by the difference in sample size, as the last study may have been underpowered. In 90 liver transplant recipients on tacrolimus mono‐therapy, [Tac]cells significantly correlated with both the development and the severity of rejection. 8 Lower tacrolimus [Tac]cells was associated with higher histological grades of rejection, while rejection grade was not associated with [Tac]blood. However, these results have never been replicated. Klaasen et al. did not find any significant differences in [Tac]cells in kidney transplant recipients with and without (sub)clinical rejection. 13 These conflicting results might be explained by differences in transplanted organs (liver vs. kidney), co‐medication (tacrolimus mono‐therapy vs. a combination of basiliximab induction therapy, glucocorticoids and MMF), and time post‐transplantation (1–7 days vs. 1 week–1 year post‐transplantation), as these factors associated with tacrolimus pharmacokinetics and/or the risk of rejection. In the present study, the ABCB1 genotype of the donor was not evaluated, which may be more relevant to the development of CNI‐related nephrotoxicity than the recipient genotype. 50 , 51 , 52
This prospective study included a large number of patients in whom both an intracellular tacrolimus concentration was measured as well as genotyping for CYP3A4, CYP3A5 and ABCB1 was performed. Moreover, the data quality from the present analysis is high, as these data were collected as part of a randomized, controlled clinical trial. A limitation of the present analysis is that [Tac]blood was measured with immunoassays. This relates to the fact that this was a post hoc analysis of a randomized, controlled clinical trial and that at that time tacrolimus was not routinely measured with more sensitive techniques such as LC–MS/MS. Second, [Tac]cells measurement can also be affected by the presence of erythrocytes. In the present analysis, samples were excluded from the analysis based on visual red level of the cell pellets, as at the time of PBMC isolation it was not yet known that red blood cells can affect the measurement of [Tac]cells. However, in future studies it is recommended to add a red‐blood cell lysis step. 30 Also, a high number of missing values was observed, especially at 12 months post‐transplantation, which can be explained by the fewer hospital appointments with increasing time after transplantation. Since most endpoints concerned data from the first three postoperative months, the effect on the conclusions are expected to be limited. Another limitation of the study design that might explain why no association between tacrolimus concentrations and clinical outcomes was found, is the fact that samples were collected at a fixed time‐point (Month 3), whereas rejection or drug‐related toxicity could be diagnosed at any time‐point during the first three post‐transplant months (biopsies were performed for cause only). Both [Tac]cells and [Tac]blood may have changed after diagnosis due to pharmacokinetic changes or dose adaptations by the attending physicians. Future studies should therefore investigate [Tac]cells at the time of a for‐cause biopsy, when protocol biopsies are taken or at the time of clinical diagnosis of an adverse event.
5. CONCLUSION
In conclusion, in this prospective study including 175 renal transplant recipients, a poor correlation between [Tac]cells and [Tac]blood was observed, although this correlation seemed to improve with time after transplantation. The tacrolimus distribution over PBMC and whole‐blood appears to be relatively stable over time in individual patients. Predictors for the [Tac]cells/[Tac]blood ratio were age, albumin and haematocrit, whereas ABCB1 genotype was not significantly related to tacrolimus distribution. CYP3A genotype affects both dose‐corrected [Tac]cells and [Tac]blood, but not the tacrolimus distribution. [Tac]cells did not correlate with clinical outcomes 3 months post‐transplantation. Based on the outcomes of this study, it is not recommended to implement monitoring of [Tac]cells as they were not more predictive of clinical outcome than whole‐blood concentrations.
COMPETING INTERESTS
This was an investigator‐initiated study. D.A. Hesselink has received grant support (paid to his institution) from Astellas Pharma, Chiesi Farmaceutici SpA and Bristol Myers‐Squibb, as well as lecture and consulting fees from Astellas Pharma, Chiesi Farmaceutici SpA, Novartis Pharma and Vifor Pharma. In the last 3 years T. van Gelder has received lecture fees and study grants from Chiesi and Astellas, in addition to consulting fees from Roche Diagnostics, Vitaeris, Astellas and Aurinia Pharma. In all cases money has been transferred to hospital accounts, and none has been paid to his personal bank accounts. T. van Gelder does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. All authors had full access to all the data and had final responsibility for the contents of this publication and the decision to submit for publication.
CONTRIBUTORS
M.I. Francke was involved in the data collection, analysis of the data and the writing of the manuscript. D.A. Hesselink was involved in the design of the trial, its performance and the writing of the manuscript. Y. Li, B.C.P Koch, L.E.A. de Wit, R.H.N. van Schaik, L. Yang, and C.C. Baan were involved in the performance of the trial and approved the final version of this manuscript. T. van Gelder was involved in the design of this trial and approved the final version of this manuscript. B.C.M. de Winter was involved in the design of the trial, its performance and the writing of the manuscript.
Supporting information
Figure S1. Red level scale
Figure S2. Flowchart of patient inclusion and exclusion
Table S1. Tacrolimus concentrations in whole‐blood and in PBMCs
Table S2. Genotype and allele frequencies
Table S3. The association between ABCB1 3435C>T and tacrolimus concentrations
Table S4. The association between ABCB1 1199G>A and tacrolimus concentrations
Table S5. Logistic regression biopsy‐proven acute rejection
Table S6. Logistic regression tacrolimus‐related nephrotoxicity
Table S7. Logistic regression post‐transplant diabetes mellitus
ACKNOWLEDGEMENTS
The authors would like to acknowledge the help of Ms S. Bahmany with the development and operation of the PBMC tacrolimus assay and Mrs N. Shuker and Mrs R. Bouamar with the performance of the randomized controlled trial. The authors are grateful to the research nurses Mrs M.J. Boer Verschragen, Ms M. Cadogan and Mrs N.J. de Leeuw‐van Weenen for their valuable contribution to this clinical study. The authors also wish to acknowledge the important contributions of Prof. Dr S.P. Berger, Ms S. El Bouazaoui, Ms I. Buijt, Drs I. Noorlander, Ir. M. van Vliet, and Prof. Dr J. J. Weening. The authors want to thank Mr E. de Jonge for the genotyping analysis.
Francke MI, Hesselink DA, Li Y, et al. Monitoring the tacrolimus concentration in peripheral blood mononuclear cells of kidney transplant recipients. Br J Clin Pharmacol. 2021;87:1918–1929. 10.1111/bcp.14585
The authors confirm that the PI for this paper is Dennis A. Hesselink and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Taylor PJ, Franklin ME, Tai CH, Pillans PI. Therapeutic drug monitoring of tacrolimus by liquid chromatography‐tandem mass spectrometry: is it truly a routine test? J Chromatogr B Analyt Technol Biomed Life Sci. 2012;883‐884:108‐112. [DOI] [PubMed] [Google Scholar]
- 2. Brunet M, van Gelder T, Asberg A, et al. Therapeutic drug monitoring of tacrolimus‐personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261‐307. [DOI] [PubMed] [Google Scholar]
- 3. Bouamar R, Shuker N, Hesselink DA, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized‐controlled clinical trials. Am J Transplant. 2013;13(5):1253‐1261. [DOI] [PubMed] [Google Scholar]
- 4. de Jonge H, Metalidis C, Naesens M, Lambrechts D, Kuypers DR. The P450 oxidoreductase *28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5‐expressing renal recipients. Pharmacogenomics. 2011;12(9):1281‐1291. [DOI] [PubMed] [Google Scholar]
- 5. Nankivell BJ, P'Ng CH, O'Connell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100(8):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 6. Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody‐mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388‐399. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez‐Peralvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta‐analysis. Am J Transplant. 2012;12(10):2797‐2814. [DOI] [PubMed] [Google Scholar]
- 8. Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P. Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study. Transpl Int. 2012;25(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 9. Daher Abdi Z, Prémaud A, Essig M, et al. Exposure to mycophenolic acid better predicts immunosuppressive efficacy than exposure to calcineurin inhibitors in renal transplant patients. Clin Pharmacol Ther. 2014;96(4):508‐515. [DOI] [PubMed] [Google Scholar]
- 10. Capron A, Haufroid V, Wallemacq P. Intra‐cellular immunosuppressive drugs monitoring: a step forward towards better therapeutic efficacy after organ transplantation? Pharmacol Res. 2016;111:610‐618. [DOI] [PubMed] [Google Scholar]
- 11. Han SS, Yang SH, Kim MC, et al. Monitoring the intracellular tacrolimus concentration in kidney transplant recipients with stable graft function. PLoS ONE. 2016;11(4):e0153491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Gelder T, Klupp J, Sawamoto T, Christians U, Morris RE. ATP‐binding cassette transporters and calcineurin inhibitors: potential clinical implications. Transplant Proc. 2001;33(3):2420‐2421. [DOI] [PubMed] [Google Scholar]
- 13. Klaasen RA, Bergan S, Bremer S, et al. Longitudinal study of tacrolimus in lymphocytes during the first year after kidney transplantation. Ther Drug Monit. 2018;40(5):558‐566. [DOI] [PubMed] [Google Scholar]
- 14. Pensi D, De Nicolo A, Pinon M, et al. First UHPLC‐MS/MS method coupled with automated online SPE for quantification both of tacrolimus and everolimus in peripheral blood mononuclear cells and its application on samples from co‐treated pediatric patients. J Mass Spectrom. 2017;52(3):187‐195. [DOI] [PubMed] [Google Scholar]
- 15. Pensi D, De Nicolo A, Pinon M, et al. An UPLC‐MS/MS method coupled with automated on‐line SPE for quantification of tacrolimus in peripheral blood mononuclear cells. J Pharm Biomed Anal. 2015;107:512‐517. [DOI] [PubMed] [Google Scholar]
- 16. Lemaitre F, Antignac M, Fernandez C. Monitoring of tacrolimus concentrations in peripheral blood mononuclear cells: application to cardiac transplant recipients. Clin Biochem. 2013;46(15):1538‐1541. [DOI] [PubMed] [Google Scholar]
- 17. Tron C, Woillard JB, Houssel‐Debry P, et al. Pharmacogenetic‐whole blood and intracellular pharmacokinetic‐pharmacodynamic (PG‐PK2‐PD) relationship of tacrolimus in liver transplant recipients. PLoS ONE. 2020;15(3):e0230195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capron A, Mourad M, De Meyer M, et al. CYP3A5 and ABCB1 polymorphisms influence tacrolimus concentrations in peripheral blood mononuclear cells after renal transplantation. Pharmacogenomics. 2010;11(5):703‐714. [DOI] [PubMed] [Google Scholar]
- 19. Dessilly G, Elens L, Panin N, et al. ABCB1 1199G>A genetic polymorphism (Rs2229109) influences the intracellular accumulation of tacrolimus in HEK293 and K562 recombinant cell lines. PLoS ONE. 2014;9(3):e91555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vafadari R, Bouamar R, Hesselink DA, et al. Genetic polymorphisms in ABCB1 influence the pharmacodynamics of tacrolimus. Ther Drug Monit. 2013;35(4):459‐465. [DOI] [PubMed] [Google Scholar]
- 21. Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53(2):123‐139. [DOI] [PubMed] [Google Scholar]
- 22. Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR‐1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74(3):245‐254. [DOI] [PubMed] [Google Scholar]
- 23. Press RR, Ploeger BA, den Hartigh J, et al. Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther Drug Monit. 2009;31(2):187‐197. [DOI] [PubMed] [Google Scholar]
- 24. Lloberas N, Elens L, Llaudo I, et al. The combination of CYP3A4*22 and CYP3A5*3 single‐nucleotide polymorphisms determines tacrolimus dose requirement after kidney transplantation. Pharmacogenet Genomics. 2017;27(9):313‐322. [DOI] [PubMed] [Google Scholar]
- 25. Lunde I, Bremer S, Midtvedt K, et al. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur J Clin Pharmacol. 2014;70(6):685‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shuker N, Bouamar R, van Schaik RH, et al. A randomized controlled trial comparing the efficacy of Cyp3a5 genotype‐based with body‐weight‐based tacrolimus dosing after living donor kidney transplantation. Am J Transplant. 2016;16(7):2085‐2096. [DOI] [PubMed] [Google Scholar]
- 27. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d‐negative antibody‐mediated rejection and antibody‐associated arterial lesions. Am J Transplant. 2014;14(2):272‐283. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247‐254. [DOI] [PubMed] [Google Scholar]
- 29. Bahmany S, de Wit LEA, Hesselink DA, et al. Highly sensitive and rapid determination of tacrolimus in peripheral blood mononuclear cells by liquid chromatography‐tandem mass spectrometry. Biomed Chromatogr. 2019;33(1):e4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemaitre F, Vethe NT, D'Avolio A, et al. Measuring intracellular concentrations of calcineurin inhibitors: expert consensus from the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) expert panel. Ther Drug Monit. 2020;42(5):665‐670. [DOI] [PubMed] [Google Scholar]
- 31. van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48(10):1668‐1671. [PubMed] [Google Scholar]
- 32. R‐CoreTeam R . A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019; https://www.R-project.org/ [Google Scholar]
- 33. Elens L, Bouamar R, Hesselink DA, et al. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin Chem. 2011;57(11):1574‐1583. [DOI] [PubMed] [Google Scholar]
- 34. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. Br J Pharmacol. 2019;176(S1):S297‐S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander SPH, Kelly E, Mathie A, et al. The Concise Guide to PHARMACOLOGY 2019/20: Transporters. Br J Pharmacol. 2019;176(S1):S397‐S493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrews LM, Hesselink DA, van Schaik RHN, et al. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Franken LG, Masman AD, de Winter BCM, et al. Hypoalbuminaemia and decreased midazolam clearance in terminally ill adult patients, an inflammatory effect? Br J Clin Pharmacol. 2017;83(8):1701‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J Parenter Enteral Nutr. 2019;43(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute‐phase response. Br J Cancer. 2002;87(3):277‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kugler N, Klein K, Zanger UM. MiR‐155 and other microRNAs downregulate drug metabolizing cytochromes P450 in inflammation. Biochem Pharmacol. 2020;171:113725. [DOI] [PubMed] [Google Scholar]
- 41. Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15(10):693‐704. [PubMed] [Google Scholar]
- 42. Kimchi‐Sarfaty C, Oh JM, Kim IW, et al. A ‘silent’ polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525‐528. [DOI] [PubMed] [Google Scholar]
- 43. Elens L, Capron A, Kerckhove VV, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17(10):873‐883. [DOI] [PubMed] [Google Scholar]
- 44. Macphee IA, Fredericks S, Tai T, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P‐glycoprotein correlate with dose requirement. Transplantation. 2002;74(11):1486‐1489. [DOI] [PubMed] [Google Scholar]
- 45. Mourad M, Wallemacq P, De Meyer M, et al. Biotransformation enzymes and drug transporters pharmacogenetics in relation to immunosuppressive drugs: impact on pharmacokinetics and clinical outcome. Transplantation. 2008;85(7 Suppl):S19‐S24. [DOI] [PubMed] [Google Scholar]
- 46. In 't Veld AE, Grievink HW, Saghari M, et al. Immunomonitoring of tacrolimus in healthy volunteers: the first step from PK‐ to PD‐based therapeutic drug monitoring? Int J Mol Sci. 2019;20(19):4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stienstra NA, Sikma MA, van Dapperen AL, de Lange DW, van Maarseveen EM. Development of a simple and rapid method to measure the free fraction of tacrolimus in plasma using ultrafiltration and LC‐MS/MS. Ther Drug Monit. 2016;38(6):722‐727. [DOI] [PubMed] [Google Scholar]
- 48. Bittersohl H, Schniedewind B, Christians U, Luppa PB. A simple and highly sensitive on‐line column extraction liquid chromatography‐tandem mass spectrometry method for the determination of protein‐unbound tacrolimus in human plasma samples. J Chromatogr A. 2018;1547:45‐52. [DOI] [PubMed] [Google Scholar]
- 49. Lemaitre F, Blanchet B, Latournerie M, et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clin Biochem. 2015;48(6):406‐411. [DOI] [PubMed] [Google Scholar]
- 50. Picard N, Bergan S, Marquet P, et al. Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther Drug Monit. 2016;38(Suppl 1):S57‐S69. [DOI] [PubMed] [Google Scholar]
- 51. Woillard JB, Rerolle JP, Picard N, et al. Donor P‐gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long‐term follow‐up. Clin Pharmacol Ther. 2010;88(1):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hauser IA, Schaeffeler E, Gauer S, et al. ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine‐related nephrotoxicity after renal transplantation. J Am Soc Nephrol. 2005;16(5):1501‐1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Red level scale
Figure S2. Flowchart of patient inclusion and exclusion
Table S1. Tacrolimus concentrations in whole‐blood and in PBMCs
Table S2. Genotype and allele frequencies
Table S3. The association between ABCB1 3435C>T and tacrolimus concentrations
Table S4. The association between ABCB1 1199G>A and tacrolimus concentrations
Table S5. Logistic regression biopsy‐proven acute rejection
Table S6. Logistic regression tacrolimus‐related nephrotoxicity
Table S7. Logistic regression post‐transplant diabetes mellitus
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


