Abstract
This study explores the relationships among anxiety, sensitivity to sensory stimuli, and picky eating (PE). An earlier study in 95 children ages 5–10 found that sensory sensitivity fully mediated the relationship between anxiety and picky eating. We replicated this finding in a sample of 158 children, ages 8–17, and in 813 young adult college students. As in the previous child sample, the relationship between anxiety and picky eating appears to be mediated by sensory sensitivity. This relationship extends into adolescence and young adulthood and holds even in a sample of children with obsessive-compulsive-spectrum and anxiety disorders. However, there may be developmental differences in the relationship between sensory sensitivity and PE; the magnitude of this relationship was significantly greater for children than young adults. Although there was a trend towards a stronger relationship in a subsample of young adults with high anxiety, the effect was still smaller than that observed in children, suggesting that this difference is developmental and not completely driven by higher anxiety in the child sample. Sensory sensitivity is a candidate mechanism of picky eating, although the cross-sectional nature of this study means that we cannot address whether it is an etiological or maintaining mechanism, or both. Implications for behavioral treatment of picky eating in clinically anxious and non-clinical samples are discussed.
1. Introduction
Picky eating is characterized by refusal of most new and familiar foods based on aversions to their sensory properties (American Psychiatric Association, 2013; Taylor, Wernimont, Northstone, & Emmett, 2015). Once thought to be a normative childhood phase, picky eating that is more pronounced than is age-typical from young childhood throughout adulthood is increasingly understood to be problematic from both nutritional and psychosocial perspectives. Some degree of picky eating behavior is relatively common across the lifespan, with most prevalence estimates ranging from 20 to 30% in middle childhood and adulthood (e.g., Cole, An, Lee, & Donovan, 2017; Kauer, Pelchat, Rozin, & Zickgraf, 2015; Taylor et al., 2015; Zickgraf, Franklin, & Rozin, 2016). Picky eating is related to growth faltering and constipation in early childhood, limited fruit/vegetable intake and dietary variety in childhood and adulthood, stress, anxiety, and family conflict for parents of childhood picky eaters, negative self-concept, eating-related anxiety, impaired eating-related quality of life in children and adults, and risk for malnutrition in the elderly (e.g., Brown, Vander Schaaf, Cohen, Irby, & Skelton, 2016; Tharner et al., 2015; Dubois, Farmer, Girard, & Peterson, 2007; Galloway, Lee, & Birch, 2003; Jaeger, Rasmussen, & Prescott, 2017; Maitre et al., 2014; Maiz & Balluerka, 2018; Ramos-Paúl et al., 2014; Wildes, Zucker, & Marcus, 2012; Zickgraf et al., 2016; Zickgraf & Schepps, 2016). When severe, picky eating can lead to symptoms of avoidant/restrictive food intake disorder (ARFID), a diagnosis for individuals with restrictive eating, not driven by fear of fatness or desire to lose weight, that results in weight loss/growth faltering, nutritional deficiency, and/or psychosocial interference (American Psychiatric Association, 2013).
Picky eating across the lifespan has been linked to symptoms of depression, obsessive compulsive disorder, and anxiety (Kauer et al., 2015; Mascola, Bryson, & Agras, 2010; Micali et al., 2011; Wildes et al., 2012; Zickgraf et al., 2016), and to externalizing symptoms in children (Jacobi, Schmitz, & Agras, 2008). To our knowledge, however, only one study has explored the prospective relationship between picky eating and psychopathology. Zucker et al. (2015) found concurrent associations between picky eating and anxiety, depression, and attention deficit/hyperactivity symptoms in a large, representative sample of 2–5 year old children; longitudinally, baseline picky eating, controlling for baseline anxiety, predicted more symptoms of generalized and overall anxiety symptoms an average of two years later (Zucker et al., 2015). Further study is needed to begin to disentangle the pattern of relationships between picky eating and psychopathology, and to elucidate transdiagnostic mechanisms, including temperamental risk and maintaining factors, that can help to explain comorbidity. Understanding the nature of the relationship between picky eating and psychopathology has implications for interventions aimed at broadening dietary variety and promoting healthier eating in picky eaters.
One proposed link between psychopathology and selective eating is temperamental sensory sensitivity. Sensory sensitivity refers to the tendency to have lower sensory thresholds and faster perception of sensory inputs; sensory over-responsivity is the closely-related tendency to be bothered or distressed by sensory experiences at levels of intensity that others would not find aversive (e.g., Steinsbekk, Bonneville-Roussy, Fildes, Llewellyn, & Wichstrom, 2017). In one longitudinal childhood study, sensory sensitivity, but not other temperamental factors including negative affectivity and surgency, predicted the persistence of PE from age 4 to 6 (Steinsbekk, Bonneville-Roussy, Fildes, Llewellyn, & Wichstrøm, 2017). Picky eating is concurrently associated with sensory over-responsivity in both adults and children (e.g., Kauer et al., 2015; Smith, Roux, Naidoo, & Venter, 2005). Sensory sensitivity might be a heritable route to picky eating; in one study of parent/child dyads, parent tactile sensitivity was strongly associated with child food neophobia (Coulthard & Sahota, 2016). Elevated sensory sensitivity has been observed in clinical samples with anxiety and obsessive compulsive disorders (e.g., Conelea, Carter, & Freeman, 2014), and children with sensory sensitivity have elevated symptoms of anxiety and depression compared to peers (e.g., Ben-Sasson, Carter, & Briggs-Gowan, 2009). Sensory sensitivity is highly heritable, and overlapping heritability has been found to account for comorbidity of sensory over-responsivity and anxiety in childhood (Van Hulle, Schmidt, & Goldsmith, 2012).
Green and Ben-Sasson proposed three potential causal models to explain the anxiety/sensory over-responsivity overlap (2010). Elevated anxiety might drive sensory over-responsivity via heightened awareness and monitoring of the environment and/or through cognitive appraisals of benign environmental inputs as salient and potentially dangerous. Conversely, sensory over-responsivity might contribute to the development of anxiety via repeated pairings of benign environmental stimuli with aversive sensations (e.g., specific phobia) or by creating an uncomfortable and unpredictable environment conducive to anxiety. It is also possible that a third mechanism (e.g., amygdala over-activity) drives the development of both anxiety and sensory over-responsivity (Green & Ben-Sasson, 2010). Regardless of the etiological mechanism, once anxiety and sensory over-responsivity begin to be expressed there is likely a bidirectional maintaining relationship between the two; for example, anxiety might increase attention to, and negative attributions of, sensory information, and sensory discomfort might increase experienced distress or anticipatory anxiety. Avoidance of distressing sensory situations maintains anxiety related to these sensations by preventing both extinction learning and sensory habituation; picky eating behavior, defined as rejection of foods due to aversions to their sensory properties, could be one example of a sensory avoidance behavior through which anxiety and sensory sensitivity are each expressed (e.g., Green & Ben-Sasson, 2010).
Given the findings linking picky eating across the lifespan to both sensory over-responsivity and anxiety, an understanding of how these constructs are related might help to guide the development of interventions aimed at reducing picky eating behavior. For example, if anxiety is independently linked to picky eating, exposure-based and cognitive interventions aimed at reducing avoidance and habituating to anxiety provoked by food would be indicated. If, as one previous study in a sample of 5–10 year old children has suggested, the picky eating/anxiety relationship is fully mediated by sensory over-responsivity, interventions would need to take into account, for example, emotional reactions other than anxiety (e.g., aversion) and the contributions of multiple sensory modalities (Farrow & Coulthard, 2012).
The current study adds to the literature on the relationships between anxiety and sensory sensitivity in predicting childhood picky eating by replicating Farrow and Coulthard’s mediation finding in a clinical sample of older children and adolescents, ages 8–17, and a sample of young adults, ages 18–22. We also explore potential developmental and/or clinical differences in the magnitude of the relationships among sensory sensitivity, anxiety, and picky eating by comparing effect sizes for anxious children vs. young adults, and anxious vs. non-anxious young adults. Finally, we used a measure of sensory sensitivity with minimal content overlap with picky eating. The original authors measured sensory sensitivity using the Sensory Profile, which has separate scales assessing sensitivity to taste, smell, texture, sounds, and sight (Dunn, 1999). The items used to measure sensitivity to taste and smell appear to capture picky eating behavior, (e.g., “avoids foods/food smells that are part of typical children’s diet”), conflating the experience of gustatory sensitivity (having low threshold for awareness of gustatory sensations) with behaviors consistent with picky eating (e.g., avoidance of familiar foods). In this study, we used a measure of sensory sensitivity that assesses aversion to sensory experiences, but not avoidance or other behavioral responses to sensation, and removed all items referring to specific foods or types of food (see below, Methods).
The aims of this study are to understand whether sensory sensitivity mediates the anxiety/picky eating relationship in clinically anxious children and adolescents and in young adult college students, and to compare the magnitude of the zero-order relationships between children/adolescents and young adults, for the first time using measures of sensory sensitivity and picky eating with minimal content overlap. Although the young adult sample was not recruited for anxiety, we compared the subsample scoring in the potentially clinical range on our anxiety measure to the sample scoring in the minimal anxiety range to explore the degree to which differences in the zero-order relationship between sensory sensitivity and picky eating between our child and young adult samples could be explained by developmental changes vs. anxiety level.
2. Methods
2.1. Participants
2.1.1. Child sample
We recruited 182 children ages 5–17 in treatment for an anxiety or obsessive compulsive spectrum disorder at a university-based specialty anxiety/OCD clinic. Children with a diagnosis of avoidant/restrictive food intake disorder or a primary diagnosis of a mood disorder or oppositional defiant disorder were not eligible for the study. Children who attended a single intake visit and did not return to the clinic for treatment were not eligible. Of 224 families approached, 203 (90.6%) agreed to participate. Of these, 21 did not return their completed forms, resulting in a completion rate of 89.7%. Because the MASC is validated for ages 8–17, 22 five, six, and seven-year-old participants were excluded from this analysis (10.8%). Three additional participants were excluded because their data were incomplete. The final sample included 158 participants. Most were early in treatment: 55% had had 1–5 sessions, 16.3% had had 6–10 session, and 28.7% had had 11 or more treatment sessions (mean number of sessions = 10.2, SD = 15.73). A majority of survey respondents (77%) were mothers; 13% were fathers, one set of parents responded together (0.6%), and one custodial grandmother responded (0.6%). Five respondents specified that a child under 11 filled out the questionnaire with the help of a parent (3.1%) and nine teenagers (5.6%) responded independently.
2.1.2. College student sample
Undergraduates enrolled in psychology courses at the University of Pennsylvania were recruited to participate in survey research for course credit. Five hundred and thirty-four participants were recruited during the fall 2016 semester for a study about parent/child resemblance in eating behavior, and 311 participants were recruited in spring 2017 through a study description that made no mention of eating behavior. Complete data were available from 813 students (96%).
2.2. Measures
2.2.1. Demographics
Parents of the child participants reported their own race/ethnicity, age, family income, and their child’s race/ethnicity and current year in school on the Conners-March Developmental Questionnaire (Conners, 1996). Undergraduate participants reported their race/ethnicity, age, and parents’ income. In both samples, income was reported on a 10-point scale ranging from “under $10,000 per year” to “$100,000 or more per year.”
2.2.2. Anxiety
Parents of the child participants responded to the Multidimensional Anxiety Scale for Children-2 (MASC-2), a measure of physiological, cognitive, and somatic anxiety symptoms in children ages 8–17 (March, Sullivan, Stallings, & Conners, 1997). The MASC-2 items had excellent internal consistency (α = 0.91). Undergraduate participants responded to the 7-item Anxiety scale from the Depression, Anxiety, and Stress Scale, short form (DASS-21; Henry & Crawford, 2005). The DASS-21 had excellent internal consistency (α = 0.91). Raw DASS-A scores were multiplied by 2 for comparison with norms and clinical severity ranges based on the original 42-item DASS: no anxiety: 0–7, mild anxiety: 8–9, moderate anxiety: 10–14, severe anxiety: 15–19, very severe anxiety: ≥20 (Lovibond & Lovibond, 1995).
2.2.3. Sensory sensitivity
In both samples, sensitivity to non-gustatory sensations was assessed using 68 items of the 77-item sensory over-responsivity inventory (sensOR; Schoen, Miller, & Green, 2008). The sensOR lists experiences and sensations across seven sensory domains including taste, touch, smell, audition, vision, kinesthetic/body positioning, and movement. For each item, participants are asked to indicate whether or not it bothers or irritates them (or their child). Eleven items that refer to the sensory properties of foods or food neophobia (e.g., “spicy foods,” “chewy foods,” “new foods”) were removed to reduce content overlap with picky eating/food neophobia. Two items that referred to eating experiences (e.g., “getting crumbs on mouth while eating,” “getting hands messy while eating”) were retained because they did not seem to relate to choice or rejection of specific foods, but rather to choice of eating utensils or mealtime behaviors. The modified sensOR assessed sensitivity across 6 modalities, excluding taste. The sensOR was designed for use in individuals of all ages and developed and validated in samples ranging from 3 to 55 years in age (Schoen et al., 2008). The modified sensOR had good internal consistency in both samples: child α = 0.80, undergraduate α = 0.91.
2.2.4. Picky eating
Picky eating was assessed using the three-item picky eating (PE) subscale of the nine-item ARFID screen (NIAS; Zickgraf & Ellis, 2018). The NIAS is a validated self-report measure of picky eating in adults; items from the picky eating scale were modified for parent report on the child sample in the present study (e.g., “I dislike many foods that other people eat” changed to “My child dislikes many foods that other kids his/her age eat”). Undergraduates responded to the adult self-report version. The NIAS-PE scale had good internal consistency in both samples: child α = 0.93, undergraduate α = 0.84.
2.2.5. Food neophobia
Food neophobia was measured only in the undergraduate sample, using the food neophobia scale (FNS), a widely used 10-item measure of avoidance of novel foods (Pliner & Hobden, 1992). The FNS had excellent internal consistency: α = 0.93.
2.3. Procedures
2.3.1. Child sample
Parents and children were approached in the clinic waiting room before a scheduled appointment and invited to complete a paper-and-pencil questionnaire packet about the child’s personality and eating habits. Although children were encouraged to participate in completing the questionnaires, all measures were formatted for parent report about children. Parents provided informed consent for their child to participate in the study, and children provided assent. Participants were offered a $5 incentive for participating. All measures and procedures were approved by the Institutional Review Board at the University of Pennsylvania.
During the clinic intake process parents were asked to provide demographic information, including parent age, child race and ethnicity, family income, and education attainment of parents/caregivers on the Conners-March Developmental Questionnaire, which was sent to families through REDCap (Harris, Taylor, Thielke, Payne, Gonzolez, & Conde, 2009) at the time they made their intake appointment. Families were instructed to complete the demographic survey prior to their intake. Demographic information was unavailable from 45 families (28%).
2.3.2. College student sample
Participants completed all questionnaires in a single online session. Students participated in this research in exchange for course credit and provided informed consent prior to beginning the study. Procedures and measures were approved by the Institutional Review Board.
2.4. Data analysis
We employed the same analytic methods as Farrow and Coulthard (2012). In both samples, two-tailed zero-order Pearson’s correlations among picky eating/food neophobia, anxiety, sensory sensitivity, and child/student demographic variables were examined. We used independent sample t-tests and Fisher’s r-to-z transformation to compare means and correlations between the child and undergraduate samples. Mediation analyses estimating direct and indirect effects were used to explore whether sensory sensitivity accounted for the relationship between anxiety and picky eating/FN, using the PROCESS macro (v 3.0) for SPSS (Preacher & Hayes, 2008). Sample means were compared for the NIAS and sensOR using t-tests, and r-to-z transformation was used to compare the magnitude of correlations between the samples.
3. Results
3.1. Sample characteristics
3.1.1. Child sample
The children were 41.8% female and 57.6% male. Mean child age at the time surveys were administered was 12.61 years (2.93). Including both primary and secondary diagnoses, 35.4% of participants had an OCD diagnosis, 30.4% had an OC-spectrum diagnosis (e.g., tics, trichotillomania, skin-picking), and 70.3% had an anxiety disorder diagnosis. Fifteen percent had a secondary ADHD diagnosis and 13.9% had a secondary mood diagnosis. Five participants (3.2%) had a historical diagnosis of autism spectrum disorder, two (1.2%) had another developmental disorder, and two had a learning disability (1.3%). The clinic intake did not include formal assessments of learning or developmental disabilities, so these diagnoses were not ruled out in other participants.
The families who provided demographic information (116, 72%) reported high income and parental education attainment. 72% reported family income of $100,000 or greater, and only 6.0% reported family income less than $50,000 per year. 70% of both mothers and fathers had at least a college education; 27.6% of fathers and 26.7% of mothers held a master’s-level degree and 9.5% of fathers and 10.3% of mothers held a doctoral degree. Children in the subsample with demographic data were largely White (84.6%); 4.3% were African American/Black, 6% were Asian, 0.9% were First Nations, 0.9% were multiracial, and 1.7% chose not to disclose race. Most participants indicated non-Hispanic ethnicity (89.6%), with 4.3% identifying their child as Hispanic and 5.1% declining to disclose ethnicity.
3.1.2. Undergraduate sample
The student sample was 70% female and 30% male. Almost all students (97.3%) were between ages 18–22 (range 18–57, M = 19.88, SD = 2.49); data on age were missing for one student. The student sample was 52.3% White, 24.7% Asian, 8.5% multiracial, 7.5% Hispanic/Latinx, and 4.8% African American; 2.3% did not provide data on race/ethnicity.
3.2. Descriptive statistics
The child sample’s mean score on the MASC was above the score range observed in normative samples (e.g., 30–38; Ivarsson, 2006; Thor Olason, Blondahl Sighvatsson, & Smari, 2004). Children with no primary anxiety disorder or OCD diagnosis (e.g., tics or trichotillomania/excoriation disorder only, 9.9%) reported significantly lower scores than the rest of the sample: 60.42 ± 16.01 vs. 39.63 ± 14.91; t (158) = 4.96, d = 1.34, p < .001. The undergraduate sample mean score on the DASS-A falls within the measure’s non-anxious range (Lovibond & Lovibond, 1995). See Table 1 for sample means.
Table 1.
Sample means.
| Sample |
t(971) | d | ||
|---|---|---|---|---|
| Child | Undergraduate | |||
| NIAS picky eating (0–15) | 5.81 (4.85) | 4.28 (3.60) | 4.68** | 0.43 |
| Food neophobia (0–50) | NA | 17.77 (10.23) | - | |
| SensOR (0–70) | 6.80 (5.49) | 11.96 (9.08) | 6.90** | 0.63 |
| Multidimensional anxiety scale for | 58.33 (17.02) | NA | - | |
| children (MASC) (0–177) |
||||
| DASS-21 Anxiety (0–42) | NA | 6.31 (6.99) | - | |
p < .001.
Undergraduate participants scored significantly higher than children on the sensOR, whereas children scored significantly higher on the NIAS-PE. The undergraduate sample was split according to anxiety severity, with participants scoring 10 or greater on the DASS-A characterized as having moderate-severe anxiety (n = 210) and those with a score of 9 or less as having absent or mild anxiety (n = 603; Lovibond & Lovibond, 1995). When anxious and non-anxious undergraduates were compared on these measures, students scoring in the moderate-severe range of anxiety reported higher scores on the sensOR (10.15 ± 7.70 vs. 17.16 ± 10.65, t(288.68) = 8.77, d = 0.75, p < .001), NIAS-PE (4.10 ± 3.60 vs. 4.81 ± 3.60, t(811) = 2.50, d = 0.20, p = .01), and FNS (17.14 ± 10.10 vs. 19.58 ± 10.66, t(811) = 3.0, d = 0.24, p = .003).
3.3. Correlational analyses
Correlations among the variables in the child and undergraduate samples are presented in Table 2. Fisher’s r-to-z transformation was used to compare the magnitude of the correlations among anxiety, sensory sensitivity, and picky eating in the child vs. undergraduate sample. The relationship between picky eating and sensory sensitivity was of greater magnitude in the child vs. undergraduate sample (z = 3.0, p = .003). The magnitude of the relationship between sensory sensitivity and picky eating (child sample) and sensory sensitivity and food neophobia (undergraduate sample) also differed at a trend level (z = 1.98, p = .05). The magnitudes of the relationships between sensory sensitivity and anxiety (z = 0.5, p = .60) and picky eating and anxiety (z = 0.9, p = .35) did not differ.
Table 2.
Zero-order correlations.
| Child sample | Undergraduate sample | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | ||
| 1 | Picky eating | 1 | 1 | |||||
| 2 | Sensory sensitivity | .40** | 1 | .16** | 1 | |||
| 3 | Anxiety | .20* | .33** | 1 | .12** | .37** | 1 | |
| 4. | Food neophobia | – | – | – | .74** | .25** | .11* | 1 |
p < .001
p < .05.
The magnitude of the correlation between sensory sensitivity and picky eating between the anxious and non-anxious undergraduates differed at a trend level, with a stronger relationship observed in the high-anxiety sample, although both effect sizes were significantly smaller than the effect size from the child sample: (ranxious = .22, rnonanxious = 0.11, z = 1.35, p = .08). Correlations between anxiety and picky eating/neophobia and sensory sensitivity were not calculated due to restricted range in the non-anxious sample.
3.4. Mediation analyses
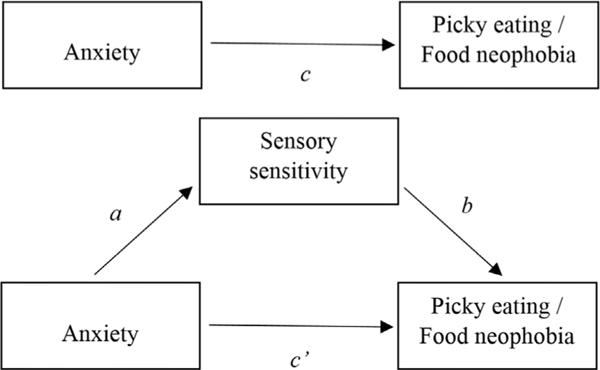
The hypothesized mediation model is displayed in Fig. 1. Mediation analysis involves two steps, and the final model involves four coefficients/paths. The first step is the regression of the mediator variable (M) on the independent variable (X), in this case, sensory sensitivity (M) on anxiety (X). The coefficient of the X variable on the M variable is represented by path a in Fig. 1. The second step is the regression of the X and M variables on the dependent variable (selective eating, e.g., pickiness or food neophobia). The second mediation model yields an overall R2, and coefficients for the effect of the M variable (path b), the indirect effect of the X variable via the M variable (the product of paths a*b), the direct effect of the X variable controlling for the M variable (path c’), and the total effect of the X variable (path c, the sum of the direct and indirect effects).
Fig. 1.
Mediation model (conceptual).
In both samples, sensory sensitivity mediated the relationship between anxiety and picky eating. In the undergraduate sample, the same was true for the relationship between anxiety and food neophobia. See Table 3 for path coefficients and regression results for each step. For the child sample, Step 1 of the mediation model (path a) was significant: R2 = 0.12, F(1,156) = 21.92, p < .001, as was step 2 (paths b, c, c’): R2 = 0.17, F(2,155) = 15.31, p < .001. In the undergraduate sample, Step 1 was significant (R2 = 0.01, F(2, 812) = 12.09, p < .001), as was Step 2 for each selective eating dependent variable: for picky eating, R2 = 0.04, F(2, 812) = 12.09, p < .001, and for food neophobia, R2 = 0.06, F(2,810) = 27.44, p < .001.
Table 3.
Mediation path coefficients.
| B(SE) | CI | t | p | |
|---|---|---|---|---|
| Child sample (Picky eating, 0–15) | ||||
| Path a: Sensory sensitivity ~ Anxiety | 0.11 (0.02) | [0.07, 0.16] | 4.68 | < .001 |
| Path b: Sensory sensitivity | 0.33 (.07) | [0.19, 0.47] | 4.68 | < .001 |
| Path c: Anxiety total | 0.062 (0.02) | [0.02, 0.11] | 2.77 | .006 |
| Path c’: Anxiety direct | 0.025 (0.02) | [−0.02, 0.07] | 1.12 | .27 |
| Anxiety indirect | 0.037 (0.01) | [0.02, 0.06] | 3.70 | < .001 |
| Undergraduate sample (Picky eating, 0–15) | ||||
| Path a: Sensory sensitivity ~ Anxiety | 0.48 (0.04) | [0.40, 0.57] | 22.40 | < .001 |
| Path b: Sensory sensitivity | 0.05 (.02) | [0.03, 0.08] | 3.69 | < .001 |
| Path c: Anxiety total | 0.062 (0.02) | [0.03, 0.10] | 3.48 | .001 |
| Path c’: Anxiety direct | 0.036 (0.02) | [−0.002, 0.07] | 1.88 | .06 |
| Anxiety indirect | 0.026 (0.01) | [0.01, 0.04] | 2.60 | .009 |
| Undergraduate sample (Food neophobia, 0–50) | ||||
| Path a: Sensory sensitivity ~ Anxiety | 0.49 (0.04) | [0.40, 0.57] | 11.50 | < .001 |
| Path b: Sensory sensitivity | 0.28 (.04) | [0.19, 0.36] | 6.66 | < .001 |
| Path c: Anxiety total | 0.16 (0.06) | [0.06, 0.26] | 3.16 | .002 |
| Path c’: Anxiety direct | 0.028 (0.05) | [−0.08, 0.13] | 0.52 | .61 |
| Anxiety indirect | 0.13 (0.03) | [0.08, 0.19] | 4.33 | < .001 |
4. Discussion
Researchers are beginning to explore the correlates of picky eating and food neophobia, but there is a need for more theory-driven research aimed at understanding the etiology and maintenance of picky eating behavior. In this study, we found that children and young adults who are anxious are more sensitive to sensory inputs, and that this elevated sensitivity in turn predicts picky eating behavior and, for young adults, food neophobia. The relationship between sensory sensitivity and picky eating (and, in undergraduates, sensory sensitivity and food neophobia) was independent of anxiety. This suggests that individuals who are more sensitive to sensory inputs, regardless of whether they are also anxious, are more likely to be picky eaters.
Although sensory sensitivity emerged as a mediator in these analyses, there is evidence to suggest that elevated responsiveness to typical sensations might be a shared, heritable risk factor for both feeding problems and affective disorders, including, but not limited to anxiety (e.g., Green, Ben-Sasson, Soto, & Carter, 2012; Van Hulle et al., 2012; 2017). There is also evidence to suggest that changes in anxiety and cortisol levels drive changes in sensory over-sensitivity (Corbett, Muscatello, & Blain, 2016). More work is needed to clarify the relationship between sensory sensitivity and anxiety, but it appears that sensory sensitivity, like anxiety, may be both state- and trait-like (e.g., Green & Ben-Sasson, 2010). Future mechanistic research on picky eating/food neophobia should explore sensory sensitivity and its temporal interactions with anxiety as both an etiological and maintaining factor. For example, infants who are high in sensory sensitivity have been found to express more distress and resistance to novel foods, which may limit their exposure to dietary variety very early in life and set them on a course to develop picky eating (e.g., Steinsbekk et al., 2017). Later in life, individuals high in food neophobia and picky eating experience arousal and anxiety, and demonstrate anxiety-related attentional biases, when presented with images of new or disliked foods (e.g., Maratos & Staples, 2015; Raudenbush & Capiola, 2012). Anxiety triggered by exposure to disliked foods might lead to increases in state sensory sensitivity, thereby confirming expectancies that the food will be aversive and unpalatable and reinforcing future avoidance of new foods.
The mediation relationship between sensory sensitivity and picky eating was moderately strong in the child sample, but smaller in the adult sample for both picky eating and food neophobia. The adult and child samples also differed in the magnitude of zero-order relationships between sensory sensitivity and picky eating, but notably, not in the magnitude of the relationship between anxiety and sensory sensitivity. This suggests that for older individuals, influences on picky eating/food neophobia other than sensory sensitivity may be more significant than for children. There is evidence from longitudinal and experimental studies that exposure to dietary variety or novel foods is associated with increased acceptance of fruits and vegetables and lower levels of picky eating and food neophobia (e.g., Maier, Chabanet, Schaal, Issanchou, & Leathwood, 2007; Vilela, Hetherington, Olivera, & Lopes, 2018). As children grow older, they have more opportunities—and more individual differences in opportunity—for exposure to food and habituation to its sensory properties, potentially helping to account for developmental differences in the strength of the relationship between sensory sensitivity and picky eating. However, this difference in magnitudes might be partially explained by other differences between the child and adult samples: the child participants were drawn from a treatment-seeking clinical population, and most had anxiety disorder or OCD diagnoses. When undergraduate participants with minimal or no anxiety were compared to those with moderate to severe anxiety, there was a trend towards a stronger relationship in the anxious sample, although the magnitude was still smaller than that observed in the anxious children. Participants who are sensitive to sensation, but not anxious, might be less likely than more anxious individuals to avoid sensations they find aversive, possibly accounting for the weaker relationship between picky eating and sensory sensitivity in this subsample.
In each sample, sensory sensitivity accounted for less than 15% of the variance in picky eating/food neophobia. Other trait-level candidate predictors of childhood picky eating include reward-related and homeostatic appetitive traits including satiety responsiveness and enjoyment of food (Tharner et al., 2014) and cognitive rigidity. Cognitive rigidity is a candidate endophenotype for anorexia nervosa and autism spectrum disorders, both of which are characterized by rigid and restrictive eating behavior (e.g., Dell’Osso et al., 2018). Demographic factors that influence the availability of a variety of foods during childhood, including parental education and economic resources, also influence the early development of food preferences (e.g., Mustonen, Oerlemans, & Tuorlia, 2012).
Although sensory sensitivity accounted for the relationship between anxiety and picky eating in both samples, the cross-sectional nature of this study limits conclusions about the treatment implications of this finding. Anxiety is linked to both trait and state sensory sensitivity. However, there have not been studies of the impact of anxiety treatment (e.g., exposure and response prevention, cognitive behavioral therapy) on sensory processing. Future studies in treatment-seeking clinical samples should explore whether changes in anxiety severity are associated with, and precede, changes in sensory sensitivity, and whether changes in either anxiety or sensory sensitivity precede changes in picky eating.
Picky eating usually emerges before age 6 and can be observed in the first year of life (Mascola et al., 2010). Anxiety disorders have a median onset in late school-age (e.g., 11 years, Kessler et al., 2005; Ramsawh, Weisberg, Dyck, Stout, & Keller, 2011), although subclinical symptoms may begin earlier. If anxiety emerges later than picky eating, it is unlikely that anxiety-driven increases in sensory sensitivity cause picky eating. In this study, the relationship between sensory sensitivity and picky eating was independent of anxiety, supporting the hypothesis that sensory sensitivity is a shared temperamental risk factor for both anxiety and picky eating. If this is the case, interventions for picky eating should target sensory sensitivity, and changes in picky eating behavior should not be expected during interventions for traditional anxiety disorders.
This study replicates, and extends into a new age group and larger sample, Farrow & Coulthard, 2012 finding that sensory sensitivity mediates the relationship between picky eating and anxiety in childhood. In addition, by using the sensOR to measure sensory sensitivity, we were able to address a methodological limitation of the original study: the use of items from the short sensory profile that assess oral sensory sensitivity by measuring behaviors consistent with picky eating (e.g., “avoids foods/food smells that are part of typical children’s diet”). Items from the sensOR that refer to responses to food textures and tastes were removed in the present study, yielding a sensory sensitivity variable that captures sensitivity in modalities other than taste and oral texture. This study provides stronger evidence that the relationship between sensory sensitivity and picky eating is 1) not specific to oral/gustatory sensory modalities, and 2) not accounted for by overlapping item content.
This study shares many of the limitations Farrow and Coulthard (2012) noted in their original study. Data were cross-sectional, and for the child sample also relied on parent report about children. We replicated our findings with self-reported data from adults, which supports the validity of conclusions drawn from parent report about children. Although we observed a difference in the magnitude of the selective eating/sensory sensitivity relationship between children and adults, conclusions about the developmental trajectory of this relationship are limited by both the cross-sectional design and potential differences between the two samples other than participant age that could not be fully accounted for by our analyses comparing undergraduates who scored above and below a severity cut-off on the self-reported anxiety measure.
Participants in the child sample were treatment-seeking, suggesting that their anxiety symptoms were noticeable and impairing their functioning, whereas we did not collect data on whether or not undergraduate participants were currently in, or had a history of, treatment for anxiety, mood, eating, or OC-spectrum disorders. The anxious undergraduate sample is therefore not directly comparable to the treatment-seeking child clinical sample in terms of anxiety symptom severity, and levels of comorbid symptomatology in undergraduates was not measured or controlled. Data from the child sample were parent-reported. Because parent report may rely more on observable behaviors than internal states, to which self-reporting participants have more ready access, the relationship between sensory sensitivity and picky eating might have been inflated in this sample because both were based on observable behaviors. Data from our child sample may therefore have only reflected sensory sensitivities that children act on (e.g., by avoiding or expressing distress), whereas the undergraduate data may have included report of sensory sensitivities that participants experience but, because they are more minor, because of intentional suppression of responses, or because of habituation to uncomfortable sensations during development, do not express behaviorally. Because picky eating may be conceptualized as a form of sensory avoidance, it may be more strongly related to the tendency to avoid uncomfortable sensations than to the tendency to experience them.
As noted in the discussion, future research should address the longitudinal relationship between early sensory sensitivity and picky eating in middle childhood and beyond. Mechanistic research on picky eating should explore the simultaneous and combined effects of multiple temperamental and environmental predictors, including sensory sensitivity, cognitive rigidity, and parental feeding practices/early dietary exposure to develop a fuller understanding of the factors that cause and maintain picky eating across development. Because data on food neophobia were not available for the child sample, future research should also address the potential mediation relationship between anxiety and sensory sensitivity for food neophobia in general and clinical child samples. Given the strong correlations usually reported between food neophobia and pickiness in children (and the large correlation observed in the present adult sample), we would expect the mediation relationship to be similar, as it was in our undergraduate participants.
There is increasing recognition of the need for effective interventions to reduce picky eating behavior and promote dietary flexibility and variety. The ARFID diagnosis has brought attention to the serious implications of severe picky eating for growth, nutrition, and social functioning (American Psychiatric Association, 2013), but subclinical picky eating is also associated with poor diet, distress, and interference (e.g., Brown et al., 2016; Ramos-Paúl et al., 2014; Wildes et al., 2012; Zickgraf et al., 2016; Zickgraf & Schepps, 2016). An understanding of the physiological, cognitive, and affective mechanisms that maintain maladaptive picky eating will aid the development of efficient, targeted treatments. Future study is now needed on whether exposure-based treatments are effective for reducing sensory aversion/avoidance and hypersensitivity, and what modifications need to be made to traditional exposure paradigms.
Acknowledgements
This research was funded by the Teece Fellowship, awarded to the first author in 2016–17. The authors would like to thank Martin Franklin for his assistance with data collection and Ralph Zickgraf for editing the final manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.appet.2018.06.023.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Ben-Sasson A, Carter AS, & Briggs-Gowan MJ (2009). Sensory over-responsivity in elementary school: Prevalence and social-emotional correlates. Journal of Abnormal Child Psychology, 37(5), 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Vander Schaaf EB, Cohen GM, Irby MB, & Skelton JA (2016). Association of picky eating and food neophobia with weight: A systematic review. Childhood Obesity, 12(4), 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NC, An R, Lee SY, & Donovan SM (2017). Correlates of picky eating and food neophobia in young children: A systematic review and meta-analysis. Nutrition Reviews, 75(7), 516–532. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Carter AC, & Freeman JB (2014). Sensory over-responsivity in a sample of children seeking treatment for anxiety. Journal of Developmental and Behavioral Pediatrics: JDBP, 35(8), 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. (1996). The conners/march developmental questionnaire. Toronto, ON: MultiHealth Systems Inc. [Google Scholar]
- Corbett BA, Muscatello RA, & Blain SD (2016). Impact of sensory sensitivity on physiological stress response and novel peer interaction in children with and without autism spectrum disorder. Frontiers in Neuroscience, 10, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard H, & Sahota S. (2016). Food neophobia and enjoyment of tactile play: Associations between preschool children and their parents. Appetite, 97, 155–159. [DOI] [PubMed] [Google Scholar]
- Dell’Osso L, Carpita B, Gesi C, Cremone IM, Corsi M, Massimetti E, … Ricca V. (2018). Subthreshold autism spectrum disorder in patients with eating disorders. Comprehensive Psychiatry, 81, 66–72. [DOI] [PubMed] [Google Scholar]
- Dubois L, Farmer A, Girard M, Peterson K, & Tatone-Tokuda F. (2007). Problem eating behaviors related to social factors and body weight in preschool children: A longitudinal study. International Journal of Behavioral Nutrition and Physical Activity, 4(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. (1999). Sensory profile: User’s manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Farrow CV, & Coulthard H. (2012). Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite, 58(3), 842–846. [DOI] [PubMed] [Google Scholar]
- Galloway AT, Lee Y, & Birch LL (2003). Predictors and consequences of food neophobia and pickiness in young girls. Journal of the American Dietetic Association, 103(6), 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, & Ben-Sasson A. (2010). Anxiety disorders and sensory over-responsivity in children with autism spectrum disorder: Is there a causal relationship? Journal of Autism and Developmental Disorders, 40(12), 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Ben-Sasson A, Soto TW, & Carter AS (2012). Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. Journal of Autism and Developmental Disorders, 42(6), 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44(2), 227–239. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Lemery-Chalfant K, & Goldsmith HH (2017). Parent-offspring transmission of internalizing and sensory over-responsivity symptoms in adolescence. Journal of Abnormal Child Psychology, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, Schmidt NL, & Goldsmith HH (2012). Is sensory over-responsivity distinguishable from childhood behavior problems? A phenotypic and genetic analysis. Journal of Child Psychology and Psychiatry, 53(1), 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson T. (2006). Normative data for the multidimensional anxiety scale for children (MASC) in Swedish adolescents. Nordic Journal of Psychiatry, 60(2), 107–113. [DOI] [PubMed] [Google Scholar]
- Jacobi C, Schmitz G, & Agras WS (2008). Is picky eating an eating disorder? International Journal of Eating Disorders, 41(7), 626–634. [DOI] [PubMed] [Google Scholar]
- Jaeger SR, Rasmussen MA, & Prescott J. (2017). Relationships between food neophobia and food intake and preferences: Findings from a sample of New Zealand adults. Appetite, 116, 410–422. [DOI] [PubMed] [Google Scholar]
- Kauer J, Pelchat ML, Rozin P, & Zickgraf HF (2015). Adult picky eating. Phenomenology, taste sensitivity, and psychological correlates. Appetite, 90, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behaviour Research and Therapy, 33(3), 335–343. [DOI] [PubMed] [Google Scholar]
- Maier A, Chabanet C, Schaal B, Issanchou S, & Leathwood P. (2007). Effects of repeated exposure on acceptance of initially disliked vegetables in 7-month old infants. Food Quality and Preference, 18(8), 1023–1032. [Google Scholar]
- Maitre I, Van Wymelbeke V, Amand M, Vigneau E, Issanchou S, & Sulmont-Rossé C. (2014). Food pickiness in the elderly: Relationship with dependency and malnutrition. Food Quality and Preference, 32, 145–151. [Google Scholar]
- Maiz E, & Balluerka N. (2018). Trait anxiety and self-concept among children and adolescents with food neophobia. Food Research International, 105, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Maratos FA, & Staples P. (2015). Attentional biases towards familiar and unfamiliar foods in children. The role of food neophobia. Appetite, 91, 220–225. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, & Conners CK (1997). The multidimensional anxiety scale for children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry, 36(4), 554–565. [DOI] [PubMed] [Google Scholar]
- Mascola AJ, Bryson SW, & Agras WS (2010). Picky eating during childhood: A longitudinal study to age 11 years. Eating Behaviors, 11(4), 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micali N, Simonoff E, Elberling H, Rask CU, Olsen EM, & Skovgaard AM (2011). Eating patterns in a population-based sample of children aged 5 to 7 years: Association with psychopathology and parentally perceived impairment. Journal of Developmental and Behavioral Pediatrics, 32(8), 572–580. [DOI] [PubMed] [Google Scholar]
- Mustonen S, Oerlemans P, & Tuorila H. (2012). Familiarity with and affective responses to foods in 8–11-year-old children. The role of food neophobia and parental education. Appetite, 58(3), 777–780. [DOI] [PubMed] [Google Scholar]
- Pliner P, & Hobden K. (1992). Development of a scale to measure the trait of food neophobia in humans. Appetite, 19(2), 105–120. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Ramos-Paúl R, J Marriage B, Ruiz Debeza R, Oliveros Leal L, Ros Mar L, et al. (2014). Impact of picky eating on level of family stress in healthy children between the ages of 3 and 6 years. The Open Nutrition Journal, 8(1). [Google Scholar]
- Ramsawh HJ, Weisberg RB, Dyck I, Stout R, & Keller MB (2011). Age of onset, clinical characteristics, and 15-year course of anxiety disorders in a prospective, longitudinal, observational study. Journal of Affective Disorders, 132(1), 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush B, & Capiola A. (2012). Physiological responses of food neophobics and food neophilics to food and non-food stimuli. Appetite, 58(3), 1106–1108. [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, & Green KE (2008). Pilot study of the sensory over-responsivity scales: Assessment and inventory. American Journal of Occupational Therapy, 62(4), 393–406. [DOI] [PubMed] [Google Scholar]
- Smith AM, Roux S, Naidoo NR, & Venter DJ (2005). Food choices of tactile defensive children. Nutrition, 21(1), 14–19. [DOI] [PubMed] [Google Scholar]
- Steinsbekk S, Bonneville-Roussy A, Fildes A, Llewellyn CH, & Wichstrøm L. (2017). Child and parent predictors of picky eating from preschool to school age. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Wernimont SM, Northstone K, & Emmett PM (2015). Picky/fussy eating in children: Review of definitions, assessment, prevalence and dietary intakes. Appetite, 95, 349–359. [DOI] [PubMed] [Google Scholar]
- Tharner A, Jansen PW, Kiefte-de Jong JC, Moll HA, Hofman A, et al. (2015). Bidirectional associations between fussy eating and functional constipation in preschool children. The Journal of Pediatrics, 166(1), 91–96. [DOI] [PubMed] [Google Scholar]
- Tharner A, Jansen PW, Kiefte-de Jong JC, Moll HA, van der Ende J, Jaddoe VW, … Franco OH (2014). Toward an operative diagnosis of fussy/picky eating: A latent profile approach in a population-based cohort. International Journal of Behavioral Nutrition and Physical Activity, 11(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor Olason D, BLÖNDAHL Blondahl Sighvatsson M, & Smari J. (2004). Psychometric properties of the multidimensional anxiety scale for children (MASC) among Icelandic schoolchildren. Scandinavian Journal of Psychology, 45(5), 429–436. [DOI] [PubMed] [Google Scholar]
- Vilela S, Hetherington MM, Oliveira A, & Lopes C. (2018). Tracking diet variety in childhood and its association with eating behaviours related to appetite: The generation XXI birth cohort. Appetite, 123, 241–248. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Zucker NL, & Marcus MD (2012). Picky eating in adults: Results of a web-based survey. International Journal of Eating Disorders, 45(4), 575–582. [DOI] [PubMed] [Google Scholar]
- Zickgraf HF, & Ellis JM (2018). Initial validation of the nine item avoidant/restrictive food intake disorder screen (NIAS): A measure of three restrictive eating patterns. Appetite, 128, 32–42. [DOI] [PubMed] [Google Scholar]
- Zickgraf HF, Franklin ME, & Rozin P. (2016). Adult picky eaters with symptoms of Avoidant/Restrictive Food Intake Disorder: Comparable distress and comorbidity but different eating behaviors compared to those with disordered eating symptoms. Journal of eating disorders, 4(1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickgraf HF, & Schepps K. (2016). Fruit and vegetable intake and dietary variety in adult picky eaters. Food Quality and Preference, 54, 39–50. [Google Scholar]
- Zucker N, Copeland W, Franz L, Carpenter K, Keeling L, Angold A, et al. (2015). Psychological and psychosocial impairment in preschoolers with selective eating. Pediatrics, 136(3), e582–e590. [DOI] [PMC free article] [PubMed] [Google Scholar]