Abstract
Patient: Male, 20-year-old
Final Diagnosis: e-Cigarette or vaping product use-associated lung injury • pulmonary embolism • renal infarction • superficial venous thrombosis
Symptoms: Cough • fever • hemoptysis • nausea • shortness of breath • vomiting • weight loss
Medication: —
Clinical Procedure: —
Specialty: Critical Care Medicine • Pulmonology
Objective:
Rare co-existance of disease or pathology
Background:
In 2019, the US Centers for Disease Control and Prevention (CDC) described the criteria for the diagnosis of e-cigarette or vaping product use-associated lung injury (EVALI), which may be caused by contamination of delta-9-tetrahydrocannabinoid (THC) e-liquids with vitamin E acetate. This report describes a case of a 20-year-old man with a history of recreational drug use that included vaping, who presented with EVALI and a coagulopathy associated with thrombotic events.
Case Report:
We present a 20-year-old patient who worked at a cannabidiol (CBD) manufacturing facility with a history of e-cigarette use and polysubstance abuse in remission who presented with respiratory and gastrointestinal symptoms accompanied by 50-pound weight loss over 6 months. The patient had been vaping with nicotine and THC-containing e-cigarettes multiple times per day for 1.5 years. He met the CDC surveillance criteria for EVALI, consisting of respiratory symptoms and infiltrates on imaging within 90 days of vaping, and was found to have eosinophilic pneumonia secondary to THC-containing e-cigarette use. Additionally, thrombi were detected in the pulmonary arteries, right saphenous vein, and right ventricle. A segmental infarct was noted in the inferior pole of the left kidney.
Conclusions:
We present the second case report potentially linking e-cigarette use with clinically significant thrombogenesis, the first with both arterial and venous thromboses. This report demonstrates the importance of taking a history of e-cigarette use in patients presenting with lung injury. Although EVALI and the diagnostic criteria have only recently been described, systemic effects, including coagulopathy, are now being reported.
Keywords: Acute Lung Injury, Cannabis, Tobacco Use Cessation Products, Venous Thromboembolism, Volatile Organic Compounds
Background
Since entering the US market in 2007, e-cigarettes used for vaping have gained widespread popularity, especially among adolescents and young adults [1]. By late 2018, over 3.6 million adolescents had been affected by the vaping epidemic, as e-cigarette use increased in that year alone by 77.8% and 46.2% among high school students and young adults, respectively [1,2]. Marketed as a safe alternative to traditional cigarettes, e-cigarettes largely appeal to younger demographics through their convenient cartridge-based delivery systems and formerly diverse array of flavors [3].
In 2019, however, increased e-cigarette use gave rise to an outbreak of respiratory illness, which the Centers for Disease Control and Prevention (CDC) coined e-cigarette, or vaping, product use-associated lung injury (EVALI) [4]. By February 2020, over 2800 cases of EVALI had been reported, with 68 confirmed deaths throughout the United States [5]. Although the exact toxicology of e-cigarettes remains unclear, products containing delta-9-tetrahydrocannabinol (THC) contaminated by vitamin E acetate have been primarily implicated in the EVALI outbreak [4,6].
EVALI involves a combination of gastrointestinal, constitutional, and pulmonary symptoms that can rapidly progress to severe hypoxemia and respiratory failure [5,7,8]. It remains a diagnosis of exclusion, based on symptoms, history of recent e-cigarette use, vital signs, and imaging studies. While not intended for diagnostic purposes, the CDC’s surveillance criteria for confirmed EVALI cases include the following: (1) E-cigarette use within 90 days of symptom onset, (2) presence of pulmonary infiltrates on imaging, and (3) negative workup for pulmonary infections or other disease processes [7–10]. Acute eosinophilic pneumonia, diagnosed by >25% eosinophils on bronchoalveolar lavage (BAL), in addition to characteristic symptoms (febrile illness and hypoxemic respiratory failure), with a detailed history and clinical evaluation, is yet another complication of e-cigarette use, albeit rarely reported. It is not clear whether this rarity is due to lack of documentation of eosinophilia on BAL in recent studies or clinical rarity [11,12].
Additionally, e-cigarettes have been found to enhance platelet activation and thrombotic potential in animal studies, thereby theoretically increasing the risk of cardiovascular disease and acute clot formation [13,14]. Only one case report of pulmonary embolism associated with EVALI has been documented [15]. Cannabis use has been associated with multiple case reports of clinically significant arterial and venous thromboses [16–18]. Although animal models have demonstrated no association between cannabidiol (CBD) and increased thrombogenesis or platelet activation, there is no literature analyzing the effect of THC on thrombohemostasis or platelet activity [19].
Although increased arterial and venous thrombosis with cigarette use in humans has been extensively demonstrated, there is a lack of literature on e-cigarette use increasing thrombo-genesis in humans [20,21]. Carbon monoxide, produced by the combustion of tobacco, has been implicated as one of the many harmful or potentially harmful chemical constituents that increases thrombogenesis [20]. However, e-cigarettes have been shown to produce little to no carbon monoxide [21]. Nicotine, the main active ingredient with addictive properties in cigarettes and e-cigarette products, has been implicated in increased thrombogenesis. Studies have demonstrated that nicotine modifies hemodynamics, resulting in increased shear force on arterial endothelial cells, and additionally has an effect on thrombohemostatic factors; however, the latter likely only plays a minor role in increasing atherothrombotic events [22]. This report is of a case of a 20-year-old man with a history of recreational drug use that included vaping, who presented with EVALI and a coagulopathy associated with thrombotic events.
Case Report
A 20-year-old man with current e-cigarette use and a past medical history of polysubstance abuse, in remission, presented to the emergency department (ED) with worsening shortness of breath, hemoptysis, subjective fevers, rigors, nausea, and severe vomiting for 9 days. He was previously evaluated 7 days prior and was prescribed albuterol and ondansetron, which provided inadequate relief of his symptoms. His symptoms progressively worsened over the next 3 days and he was re-evaluated and started on ceftriaxone and doxycycline with a presumptive diagnosis of pneumonia. After multiple urgent care and family medicine clinic visits due to the patient’s poor clinical progression, minimal improvement of his dyspnea with home supplemental oxygen, and newly developed hemoptysis, the patient presented to the ED.
In the ED, the patient reported e-cigarette use with nicotine products multiple times a day for approximately 1.5 years, averaging about one 2-mL cartridge of “vape juice” daily. He also vaped marijuana and THC-containing products occasionally 6 months prior to his presentation and had a history of polysubstance abuse, including heroin and methamphetamine, that was in remission for 3 years. He denied any history of intravenous drug use. In the past 6 months, he reported a 50-pound weight loss associated with decreased appetite and early satiety which he contributed to his new job at a CBD manufacturing facility where he was exposed to multiple chemicals in vaporized, aerosolized, and pulverized forms. He did not use any occupational or industrial personal protective equipment at this job. The patient denied any recent travel outside of the country, but had been to Arizona in the past year. He denied other exposures to mold or large animals.
On physical exam, he was observed to be a cachectic male patient in apparent respiratory distress. Vital signs showed a temperature of 38.0°C, a blood pressure of 136/94, a heart rate of 136 beats/min, a respiratory rate of 40/min, an oxygen saturation of 80% on room air, and a body mass index of 17.62. The patient had slightly dry mucous membranes. He was tachypneic with normal lung sounds bilaterally. His cardiovascular exam revealed tachycardia. There were no other significant physical exam findings. Due to his acute hypoxic respiratory failure, the patient was placed on supplemental oxygen and admitted to the Intensive Care Unit.
Initial laboratory studies were significant for an elevated white blood cell count of 24.3 K/µL with an elevated absolute eosinophil count of 1.2 K/µL, a hemoglobin of 14.9 g/dL, a platelet count of 247 K/µL, a creatinine level of 0.7 mg/dL, an international normalized ratio of 1.4, a mild transaminitis, an elevated lactate dehydrogenase level of 609, and an elevated lactate level of 3.3. Antiphospholipid syndrome (APS) and systemic lupus erythematosus workup were unremarkable. Extensive infection workup was unremarkable, including a negative workup for human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and tuberculosis. Urine toxicology was positive for THC, but was otherwise unremarkable. Blood cultures were negative for bacterial or fungal growth.
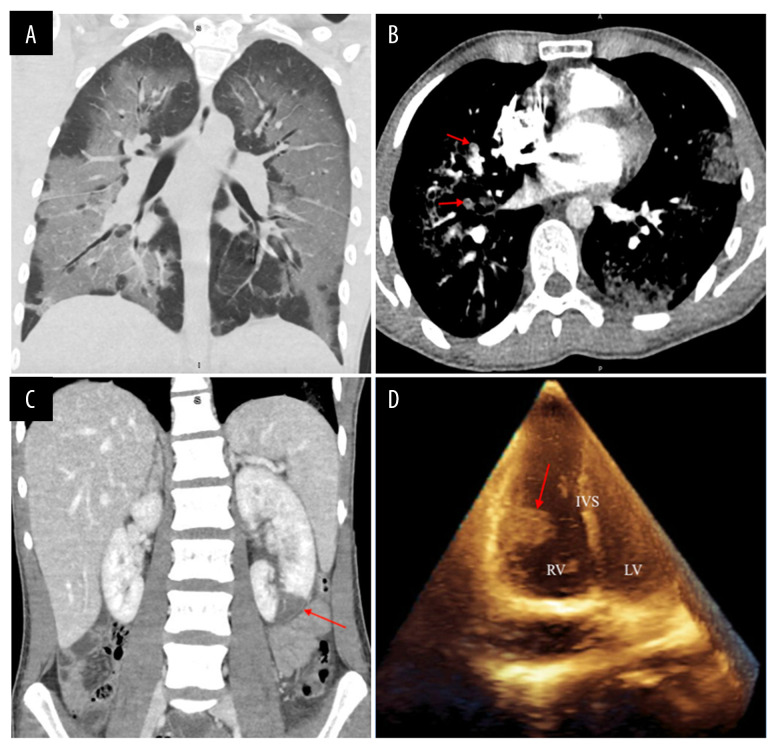
Chest X-ray showed diffuse bilateral lung opacities, which were greatest at the lung bases and most prominent on the right. A computed tomography (CT) of the chest showed confluent bilateral ground-glass pulmonary opacities with septal thickening and relative subpleural sparing in a lower lobe-predominant distribution (Figure 1A). A CT angiography showed multiple bilateral pulmonary emboli (Figure 1B). CT scan of the abdomen and pelvis also showed a wedge-shaped hypoattenuation of the inferior pole of the left kidney, which was concerning as it might indicate a segmental infarct (Figure 1C). A transthoracic echocardiogram was remarkable for a 2.0×1.3 cm mobile mass on the free wall of the right ventricle and a 0.8×0.5 cm mobile mass attached to the chordae. These were believed to be thrombi, but no patent foramen ovale or ventricular septal defect was identified (Figure 1D). Lower-extremity Doppler ultrasound demonstrated a superficial venous thrombus in the right saphenous vein, but no deep venous thromboses.
Figure 1.
(A) Computed tomography of the chest demonstrating confluent bilateral ground-glass pulmonary opacities with septal thickening and relative subpleural sparing in a lower lobe-predominant distribution; (B) Computed tomography angiography of the chest demonstrating multiple bilateral pulmonary emboli (red arrows); (C) Computed tomography of the abdomen and pelvis demonstrating a large left renal infarct (red arrows); (D) Transthoracic echocardiogram demonstrating a large mobile mass on the free wall of the right ventricle (RV – right ventricle; LV – left ventricle; IVS – interventricular septum).
The patient was started on broad-spectrum antibiotics and solumedrol for possible pneumonia. Bronchoscopy with BAL showed 39% eosinophils and 50–60% lipid-laden macrophages when stained with oil red O dye. No lung biopsy was performed, and we were unable to obtain a histological slide showing BAL. Lavage culture was negative for infection. He was also started on a heparin drip for his multiple arterial and venous thromboses.
Based on his presentation of acute hypoxemic respiratory failure in the setting of failed outpatient antibiotic therapy for pneumonia and a history of heavy e-cigarette use, we suspected that the patient’s symptoms were due to e-cigarette-related acute lung injury. This patient effectively met the CDC’s surveillance criteria for EVALI, as he had a history of vaping within 90 days of symptom onset, pulmonary infiltrates on both chest X-ray and CT scan, and a negative workup for both infection and autoimmune disease [14,15]. In addition, we suspected that the most likely etiology of his arterial and venous emboli was activation of the coagulation system caused by a profound inflammatory state secondary to e-cigarette-related products.
Subsequently, the patient’s antibiotics were discontinued, and he was transitioned to a 10-day course of prednisone. Heparin was transitioned to therapeutic enoxaparin and later switched to apixaban prior to discharge. The patient was discharged, with follow-up for thrombosis, pulmonology, and hematology/oncology, to primary care clinics with plans for anticoagulation for a minimum of 3 months. Genetic workup for additional thrombophilias and hypercoagulability workup in the out-patient setting were unremarkable. Hematology and oncology consultants suggested that hypereosinophilic syndrome and malignancy were inconsistent with his presentation. His follow-up echocardiogram 5 months later showed complete resolution of the right ventricular thrombi. As a result, the patient’s apixaban was discontinued and he was encouraged to continue with his cessation of e-cigarette use. The patient’s written informed consent for publication of case details and images was obtained.
Discussion
Our case not only illustrates the adverse pulmonary effects of THC-containing e-cigarettes due to vitamin E acetate, but also warns of the thrombogenic potential associated with EVALI [6]. E-cigarette use has become a significant public health concern in the adolescent and young adult populations, especially since the identification and surge of EVALI cases in 2019 [11]. Though considered generally safe for oral intake, vegetable glycerin and propylene glycol, 2 chemicals commonly found in “e-liquids”, can form toxic aldehydes when heated; these, in turn, can impair the function of alveolar macrophages, disrupt surfactant homeostasis, increase oxidative stress, and promote aberrant platelet activation [1,23]. Additionally, many flavoring agents found in “e-liquids”, such as diacetyl, appear to exert cytotoxic effects, forming toxic carbonyls when aerosolized [23]. By tinkering with pulmonary gene expression, inhaled diacetyl appears to alter the cytoskeletal and ciliary structure of bronchial epithelial cells, inducing airway injury and promoting a fibroproliferative response that can lead to bronchiolitis obliterans or “popcorn lung” [23,24]. Because our patient presented prior to the FDA ban on flavorings in “e-liquids”, his “vape juice” most likely contained an assortment of flavoring agents and additives, in conjunction with propylene glycol and vegetable glycerin [23]. Frequent inhalation of these substances likely predisposed our patient to acute lung injury.
The adverse effects of the patient’s e-cigarette use were possibly confounded by his occupational exposure to aerosolized CBD or vaporized solvents such as ethanol or toluene. Cannabis manufacturing facilities can additionally expose workers to organic dusts including endotoxins, fungi, and bacteria, as well as volatile organic compounds, such as diacetyl, ethanol, and toluene [25–27]. In mouse models, chronic exposure to ethanol vapors has been shown to increase pulmonary inflammatory cell infiltration (not associated with significant lung injury, however), but there was no mention of augmenting thrombogenesis [28]. There has been one case report of inhalation of toluene causing acute lung injury in a factory worker; however, this study did not document BAL results, and larger studies of toluene toxicity fail to demonstrate other pulmonary or thrombogenic adverse effects of toluene [29,30]. While our patient had no evidence of infection, his occupational hazards might have promoted respiratory inflammation and irritation.
One explanation for the patient’s weight loss in the context of gastrointestinal symptoms and concurrent e-cigarette use containing THC could be cannabinoid hyperemesis syndrome. One of many proposed mechanisms of cannabinoid hyper-emesis syndrome hypothesizes that THC accumulates in cerebral fat, which may lead to toxicity and emesis in sensitive patients [31]. Gastrointestinal adverse effects of nicotine include increased risk of gastric ulceration and occasionally diarrhea and nausea [32]. Although he did not have signs or symptoms suggestive of gastric ulceration, nicotine may have played a minor role in his nausea and vomiting. Although a viral syndrome could not be definitively ruled out as the cause of the patient’s respiratory and gastrointestinal symptoms, his 6-month history of weight loss was not consistent with that of a viral syndrome. Chronic eosinophilic pneumonia is additionally a possibility given the patient’s chronic exposure to e-cigarettes and chemicals in his workplace and profound weight loss over 6 months. Chronic eosinophilic pneumonia has an insidious onset with weight loss, fever, cough, and dyspnea. A BAL of greater than 40% eosinophils is demonstrated in over 80% of these patients and peripheral eosinophilia is demonstrated in 88–95% of these patients [33–35]. Although his BAL eosinophil count was 39%, it is very likely that the constellation of his symptoms could be due to chronic eosinophilic syndrome elicited by e-cigarettes, as he had complete resolution of symptoms without the need for steroids on outpatient follow-up. Lastly, dehydration from gastrointestinal losses could have played a role in thrombogenesis in our patient, as multiple studies have linked dehydration and hypernatremia with increased thrombogenesis [36–39]. Hypernatremia has been shown to stimulate production of multiple proinflammatory signaling molecules and thrombogenic molecules by endothelial cells, most notably von Willebrand factor, a key initiator of the clotting cascade [38,39]
The addition of THC to e-cigarettes has proven particularly detrimental to users’ pulmonary function. About 83% of EVALI cases thus far have been linked to THC or CBD-containing products, and vitamin E acetate has been identified as a potential culprit in THC-related toxicity [4,6]. Vitamin E acetate, often used as a thickening agent in e-liquids containing THC, has been detected in a preponderance of BAL samples from EVALI patients, implicating its role in the development of respiratory distress. Tocopherols, such as vitamin E acetate, stimulate the transition of phosphatidylcholines from a gel to a liquid crystalline phase. By altering the properties of surfactants’ components, vitamin E acetate can destabilize the pulmonary surface tension that is required for respiration [40,41]. Additionally, heating vitamin E acetate can generate ketene, an extremely reactive gas that can cause severe pulmonary toxicity at high concentrations [42]. This combination of ketene formation and surfactant dysfunction likely contributed to our patient’s acute hypoxemic respiratory failure, as he reported regular use of THC-containing e-cigarette products.
EVALI is characterized by a constellation of respiratory, constitutional, and gastrointestinal symptoms, which can ultimately progress to respiratory failure, necessitating mechanical ventilation. Lab findings include increased inflammatory markers, mild transaminitis, and leukocytosis. The histopathology of EVALI is diverse, ranging from acute eosinophilic and organizing pneumonia to diffuse alveolar damage and acute fibrinous pneumonitis [1]. Foamy macrophages and vacuolated pneumocytes, although nonspecific, have been consistently identified in EVALI patients, regardless of histopathologic pattern. Although several reports have attributed this to exogenous lipoid pneumonia, the histological and radiological evidence for this has been lacking, pointing instead towards a chemical pneumonitis [4]. Our patient’s bronchoscopy was consistent with this finding, as his BAL showed 50–60% lipid-laden macrophages. Meanwhile, the patient’s eosinophilia, both peripherally and on BAL (39% eosinophils), is consistent with a histopathological pattern of acute eosinophilic pneumonia [38–40]. Finally, the CT scan of his chest revealed confluent ground-glass opacities, a common imaging finding in EVALI and acute eosinophilic pneumonia.
This patient’s presentation was particularly unique due to the arteriovenous thromboses complicating his disease course from the outset. Inpatient and outpatient coagulopathy workups were unremarkable for our patient. To our recollection, there is only one other case report documenting pulmonary thromboembolism associated with e-cigarette use, which was detected upon re-admission after an episode of EVALI with appropriate inpatient venous thromboembolism prophylaxis [15]. However, our case is unique because both arterial and venous thromboses were identified early in the patient’s hospital admission, making prolonged supine positioning less likely a risk factor in developing venous thromboembolism. Although data remain limited, e-cigarettes have been linked to platelet activation and thrombus formation in animal models. Even brief exposure to nicotine-containing e-cigarettes has been shown to increase arterial stiffness and carotid artery occlusion in animal studies, suggesting a prothrombotic effect [13]. Upregulation of platelet aggregation and adhesion has also been observed in animal studies following e-cigarette use, resulting in a hypercoagulable state overall [13,46]. Additionally, inflammation itself is a known stimulus of thrombogenesis, as it promotes the activation of endothelial cells and platelets, resulting in upregulation of the coagulation cascade [47]. As evidenced by his leukocytosis and meeting 4 out of 4 systemic inflammatory response syndrome criteria, the patient was likely in a heightened proinflammatory state secondary to his acute eosinophilic pneumonia; this, in conjunction with theoretical vape-induced platelet aggregation and coagulation, likely gave way to our patient’s systemic thromboses [48].
Complications notwithstanding, the management of EVALI and acute eosinophilic pneumonia involves both pharmacological therapy and supportive care. Because EVALI is a diagnosis of exclusion, many patients receive empiric antibiotic therapy until infectious agents can be more definitively excluded. Steroids represent the mainstay of care, for both EVALI and acute eosinophilic pneumonia, and their dosing and duration depend primarily on the severity of patients’ symptoms. Additionally, supplemental oxygen and mechanical ventilation may be necessary for patients undergoing respiratory decompensation [49]. Our patient’s respiratory symptoms resolved on a 10-day course of prednisone, coupled with supportive care during his hospital stay. A 5-month course of anticoagulation was also administered to protect against further thrombogenesis.
Conclusions
We report a case of EVALI with a pattern of acute eosinophilic pneumonia, complicated by multiple arteriovenous thromboses presumed secondary to widespread inflammation. To the best of our knowledge, only one case of post-EVALI thrombo-genesis has been recorded and our case is the first to demonstrate both arterial and venous thromboses. Given the increasing prevalence and limited regulation of e-cigarettes, this report further demonstrates the importance of taking a history of e-cigarette use in patients presenting with lung injury. Although EVALI and the diagnostic criteria have only recently been described, systemic effects that include coagulopathy are now being reported.
Footnotes
Conflict of Interest
None.
References:
- 1.Chalmers S, Von Buchwald CL, Gajic O. VpALI – vaping-related acute lung injury: A new killer around the block. Mayo Clin Proc. 2019;94(12):2534–45. doi: 10.1016/j.mayocp.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014–2018. JAMA. 2019;322(18):1824–27. doi: 10.1001/jama.2019.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madison MC, Landers CT, Gu B, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine, 2020. J Clin Invest. 2019;129(10):4290–304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christiani D. Vaping-induced acute lung injury. N Engl J Med. 2020;382(10):960–62. doi: 10.1056/NEJMe1912032. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Outbreak of Lung Injury Associated with E-Cigarette Use, or Vaping. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#epi-chart.
- 6.Duffy B, Li L, Lu S, et al. Analysis of cannabinoid-containing fluids in illicit vaping cartridges recovered from pulmonary injury patients: Identification of vitamin E acetate as a major diluent. Toxics. 2020;8(1):8. doi: 10.3390/toxics8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatham-Stephens K, Roguski K, Jang Y, et al. Characteristics of hospitalized and nonhospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury – United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1076–80. doi: 10.15585/mmwr.mm6846e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic-cigarette-product use – interim guidance. MMWR Morb Mortal Wkly Rep. 2019;68(36):787–90. doi: 10.15585/mmwr.mm6836e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin – Final report. N Engl J Med. 2020;382:903–16. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 10.Aldy K, Cao DJ, Weaver MM, et al. E-cigarette or vaping product use-associated lung injury (EVALI) features and recognition in the emergency department. J Am Coll Emerg Physicians Open. 2020;1(5):1090–96. doi: 10.1002/emp2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philit F, Etienne-Mastroianni B, Parrot A, et al. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med. 2002;166(9):1235–39. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 12.Arter ZL, Wiggins A, Hudspath C, et al. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. doi: 10.1016/j.rmcr.2019.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qasim H, Karim ZA, Silva-Espinoza JC, et al. Short-term e-cigarette exposure increases the risk of thrombogenesis and enhances platelet function in mice. J Am Heart Assoc. 2018;7(15):e009264. doi: 10.1161/JAHA.118.009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: What do we know so far? Vasc Health Risk Manag. 2019;15:159–74. doi: 10.2147/VHRM.S175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Light PM, McBride CL, Fajilan A, Kwon M. Unprovoked pulmonary embolism despite prophylaxis as a sequela of e-cigarette or vape-associated lung injury (EVALI). D39. CASE REPORTS IN VAPING INDUCED LUNG DISEASE/ Thematic Poster Session. American Thoracic Society. 2020:A6681–A6681. [Google Scholar]
- 16.Marchetti D, Spagnolo A, De Matteis V, et al. Coronary thrombosis and marijuana smoking: A case report and narrative review of the literature. Drug Test Anal. 2016;8(1):56–62. doi: 10.1002/dta.1898. [DOI] [PubMed] [Google Scholar]
- 17.Salhan D, Abdulfattah O, Roy S, et al. Cannabis-induced VTE: Is it a safe recreational drug? Chest. 2016;150(4):909A. [Google Scholar]
- 18.Dahdouh Z, Roule V, Lognoné T, et al. Cannabis and coronary thrombosis: What is the role of platelets? Platelets. 2012;23(3):243–45. doi: 10.3109/09537104.2011.601824. [DOI] [PubMed] [Google Scholar]
- 19.Grambow E, Strüder D, Klar E, et al. Differential effects of endogenous, phyto and synthetic cannabinoids on thrombogenesis and platelet activity. BioFactors. 2016;42(6):581–90. doi: 10.1002/biof.1294. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen VG, Hafner DT, Steinbrenner EB. Tobacco smoke-induced hyper-coagulation in human plasma: role of carbon monoxide. Blood Coagul Fibrinolysis. 2013;24(4):405–10. doi: 10.1097/MBC.0b013e32835d5458. [DOI] [PubMed] [Google Scholar]
- 21.Caponnetto P, Maglia M, Prosperini G, et al. Carbon monoxide levels after inhalation from new generation heated tobacco products. Respir Res. 2018;19(1):164. doi: 10.1186/s12931-018-0867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol. 2013;33(7):1460–67. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 23.Chun LF, Moazed F, Calfee CS, et al. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313:L193–206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landman ST, Dhaliwal I, Mackenzie CA, et al. Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. CMAJ. 2019;191(48):E1321–31. doi: 10.1503/cmaj.191402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson M, Reed S, Oosthuizen J, et al. Occupational health and safety in cannabis production: An Australian perspective. Int J Occup Environ Health. 2018;24(3-4):75–85. doi: 10.1080/10773525.2018.1517234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couch JR, Grimes GR, Green BJ, et al. Review of NIOSH Cannabis-related health hazard evaluations and research. Ann Work Expo Health. 2020;64(7):693–704. doi: 10.1093/annweh/wxaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster GRB, Sarna LP. Cannabinoid extraction method. U.S. Patent No. 6,403,126. 11 Jun. 2002.
- 28.Mouton AJ, Maxi JK, Souza-Smith F, et al. Alcohol vapor inhalation as a model of alcohol-induced organ disease. Alcohol Clin Exp Res. 2016;40:1671–78. doi: 10.1111/acer.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singhai A. Toluene-induced acute lung injury. Med J Dr. DY Patil Univ. 2013;6(1):82. [Google Scholar]
- 30.Camara-Lemarroy CR, Rodríguez-Gutiérrez R, Monreal-Robles R, et al. Acute toluene intoxication–clinical presentation, management and prognosis: A prospective observational study. BMC Emerg Med. 2015;15:19. doi: 10.1186/s12873-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Zimmermann AE. Cannabinoid hyperemesis syndrome. Hosp Pharm. 2013;48(8):650–55. doi: 10.1310/hpj4808-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu WK, Cho CH. The pharmacological actions of nicotine on the gastrointestinal tract. J Pharmacol Sci. 2004;94(4):348–58. doi: 10.1254/jphs.94.348. [DOI] [PubMed] [Google Scholar]
- 33.Alam M, Burki NK. Chronic eosinophilic pneumonia: A review. South Med J. 2007;100(1):49–53. doi: 10.1097/01.smj.0000242863.17778.1d. [DOI] [PubMed] [Google Scholar]
- 34.Marchand E, Reynaud-Gaubert M, Lauque D, et al. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P) Medicine (Baltimore) 1998;77(5):299–312. doi: 10.1097/00005792-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Jederlinic PJ, Sicilian L, Gaensler EA. Chronic eosinophilic pneumonia. A report of 19 cases and a review of the literature. Medicine (Baltimore) 1988;67(3):154–62. doi: 10.1097/00005792-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Kelly J, Hunt BJ, Lewis RR, et al. Dehydration and venous thromboembolism after acute stroke. QJM. 2004;97(5):293–96. doi: 10.1093/qjmed/hch050. [DOI] [PubMed] [Google Scholar]
- 37.Elias S, Hoffman R, Saharov G, et al. Dehydration as a possible cause of monthly variation in the incidence of venous thromboembolism. Clin Appl Thromb Hemost. 2016;22(6):569–74. doi: 10.1177/1076029616649435. [DOI] [PubMed] [Google Scholar]
- 38.Dmitrieva NI, Burg MB. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci USA. 2014;111(17):6485–90. doi: 10.1073/pnas.1404809111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dmitrieva NI, Burg MB. Elevated sodium and dehydration stimulate inflammatory signaling in endothelial cells and promote atherosclerosis. PLoS One. 2015;10(6):e0128870. doi: 10.1371/journal.pone.0128870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boudi FB, Patel S, Boudi A, Chan C. Vitamin E acetate as a plausible cause of acute vaping-related illness. Cureus. 2019;11(12):e6350. doi: 10.7759/cureus.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D, O’Shea DF. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. PNAS. 2020;117(12):6349–55. doi: 10.1073/pnas.1920925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47(1):15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 44.Arter ZL, Wiggins A, Hudspath C, et al. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. doi: 10.1016/j.rmcr.2019.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamada T, Yamashita Y, Tomioka H. Acute eosinophilic pneumonia following heat-not-burn cigarette smoking. Respirol Case Rep. 2016;4(6):e00190. doi: 10.1002/rcr2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hom S, Chen L, Wang T, Ghebrehiwet B, et al. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016;27:694–702. doi: 10.3109/09537104.2016.1158403. [DOI] [PubMed] [Google Scholar]
- 47.Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–38. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 49.Blagev DP, Harris D, Dunn AC. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: A prospective observational cohort study. Lancet. 2019;394(10214):2073–83. doi: 10.1016/S0140-6736(19)32679-0. [DOI] [PubMed] [Google Scholar]