Abstract
Skin cancer remains the most common groups of cancers globally and the incidence continues to rise. Although localized skin cancers tend to have excellent outcomes following surgical excisions, the less common cases that become surgically unresectable or metastatic have been associated with poor prognosis and suboptimal treatment responses to cytotoxic chemotherapy. Development of monoclonal antibodies to programmed cell death-1 receptor and its ligand (PD-1/PD-L1) have transformed the management of metastatic melanoma, squamous cell carcinoma, and Merkel cell carcinoma. These agents, as monotherapies, are associated with approximately 40–60% response rates, many of which persist durably. Further efficacy is observed with combination immunotherapy in advanced melanoma. Early reports suggest similar activity in locally advanced or metastatic basal cell carcinoma. In this review, we describe common molecular features of skin cancers that may render them particularly susceptible to anti-PD-1/PD-L1 and detail results from key clinical trials of these agents across skin cancers. Overall, the superior response rates of skin cancer to anti-PD-1/PD-L1 compared to other solid tumor types is likely due, at least in part, to a high mutational burden and, in Merkel cell carcinoma, viral etiology. Although melanoma has been rigorously studied in the setting of anti-PD-1/PD-L1 treatment, more research is needed for the other skin cancer types to establish toxicity profiles, responses, and quality of life outcomes.
1. Introduction:
Treatment with antibodies to programmed death-1 receptor and its ligand (anti-PD-1/PD-L1) has revolutionized the management of advanced and metastatic cancer. These treatments block the interaction of PD-1 and PD-L1, reversing cancer-mediated immune exhaustion. This pharmacologic blockade augments the anticancer T-cell activity, and produces clinical responses in a variety of cancers.1,2 These agents are now approved in 17 different cancer types, with many more indications expected.3,4 Additionally, when compared to conventional cytotoxic chemotherapy used in advanced stage cancer, anti-PD-1/PD-L1 agents generally produce more durable responses with fewer side effects.5
Skin cancer remains the most common cancer globally and the incidence continues to rise. In the United States alone, there are an estimated 5 million cases of skin cancer annually with an associated economic impact of $8.1 billion.6 While many cutaneous malignancies can be treated with surgical resection alone, there are limited treatment options for metastatic disease. More recently, anti-PD-1/PD-L1 agents have demonstrated remarkable activity across the skin cancer spectrum and have emerged as a cornerstone in the management of unresectable or metastatic disease. (Figure 1)7,8 In fact, the response rates for patients with advanced skin cancer largely surpass those of all other solid tumors, and trail only those patients with Hodgkin Lymphoma across the full range of cancers.
Figure 1:
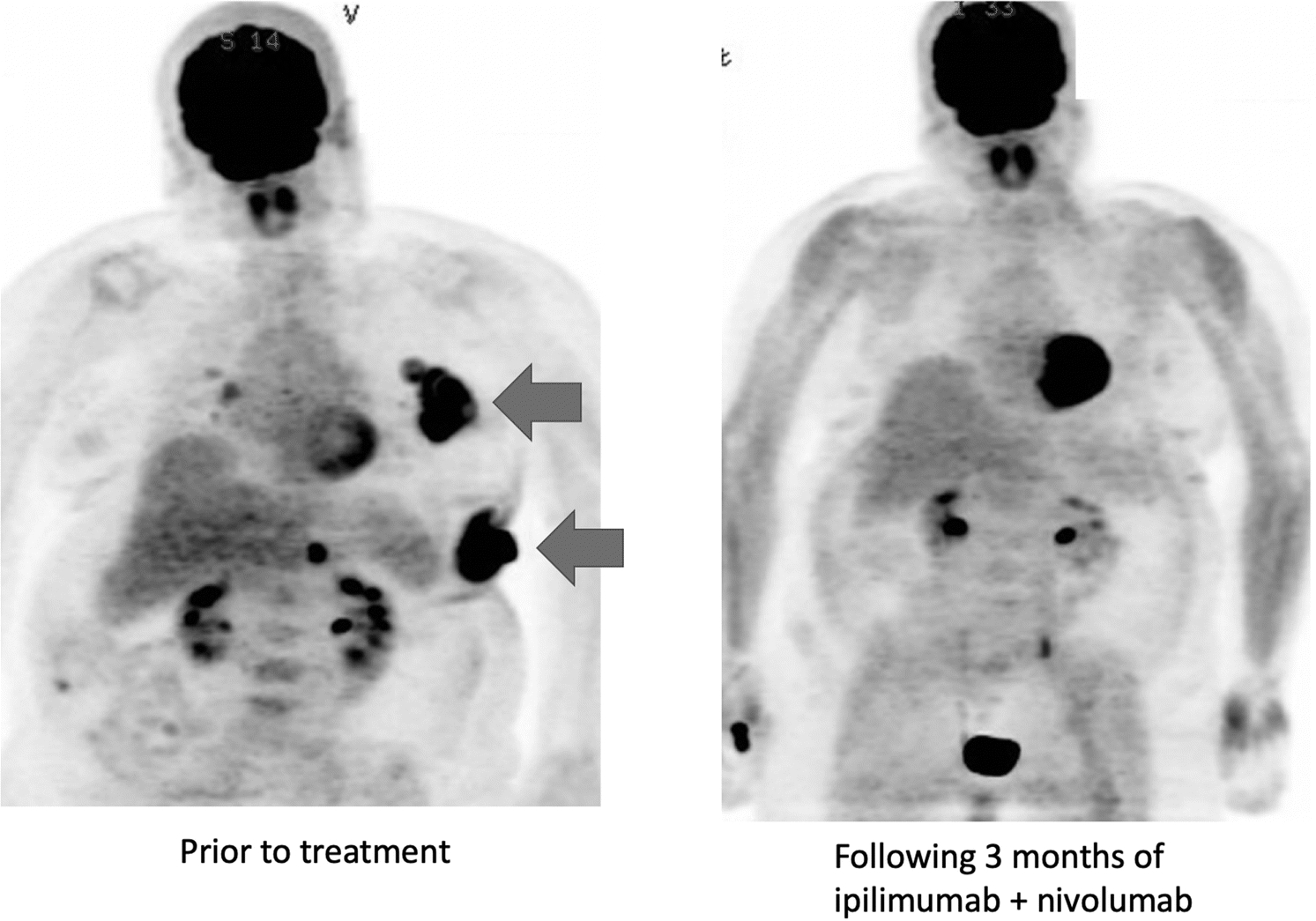
Patient with widespread metastatic melanoma including bulky lymph node and subcutaneous metastases (arrows) prior to treatment (left) and following 3 months of ipilimumab and nivolumab, showing resolution of metastatic disease (right). This image is from the author’s personal files.
This review seeks to synthesize the current data on the role of anti-PD-1/PD-L1 in the treatment of melanoma and non-melanoma skin cancer with an emphasis on efficacy, long-term durability, and toxicity profile.
2. Search Strategy
Studies between January 1st, 2010 and January 1st, 2020 were identified. Studies were excluded if they were not in English. PubMed was used to retrieve articles and keywords included “PD-1”, “PD-L1”, “immune checkpoint inhibitor”, “pembrolizumab”, “nivolumab”, “atezolizumab”, “cemiplimab”, “avelumab”, “toxicities”, “efficacy”, “melanoma”, “merkel cell carcinoma”, “basal cell carcinoma”, and “squamous cell carcinoma”. Articles that contained primary data from large clinical trials were emphasized.
3. Discussion
3.1. Overview of findings
Among the reviewed skin cancer types, melanoma9–16 has been the most studied, with multiple rigorous, multi-center randomized controlled trials to establish efficacy and toxicity profiles with anti-PD-1/PD-L1 therapy. MCC7,17,18 and SCC8 have also been studied fairly extensively, although most of the published work comprises multicenter, non-randomized phase II studies without a control group due to their relative infrequency and poorly active controls. Management with anti-PD-1/PD-L1 has become the standard pharmacologic approach to treating these cancers in the advanced setting. Although BCC is the least aggressive skin cancer, case reports and case series have detailed success with anti-PD-1/PD-L1 in the advanced stages (locally advanced/unresectable and metastatic). However, large randomized controlled trials are currently lacking for the use of anti-PD-1/PD-L1 in BCC.19
Of particular interest, compared to other advanced stage malignancies treated with anti-PD-1/PD-L1, skin cancers display among the highest response rates (40–60%) and the most durable responses. One feature common across skin cancers that potentially contributes to this phenomenon is the high degree of somatic mutations.20 Many studies have suggested that high numbers of mutations (tumor mutational burden; TMB) is correlated with response to anti-PD-1/PD-L1, both within and individual cancer, and between cancers.21–25 One potential explanation for this phenomenon is that as mutations accumulate, the likelihood of generating immunogenic neoepitopes (e.g. proteins that appear foreign to the immune system) increases accordingly.26 However, the increase in response rate does not appear proportional (e.g. tumors with 10 mutations/MB do not have 5 times the likelihood of response as those with 2 mutations/MB), and a threshold effect appears to occur (although the specific threshold may differ across cancers). Despite these unresolved complexities, the relationship between TMB and response appears quite relevant for skin cancers. The one exception to this usual correlation are MCC associated with polyomavirus infections. These tumors appear to respond well to anti-PD-1/PD-L1, despite having an extremely low mutational load, which may relate to the immunogenicity of the viral infection. While anti-PD-1/PD-L1 has produced promising outcomes in the treatment of advanced skin cancer, further research is needed to characterize the long-term outcomes in these patients; particularly those with non-melanoma skin cancer. (Table 1)
Table 1:
Summary of response to anti-PD-1/PD-L1 in each skin cancer
| Response Rate | Median PFS (months) | Median OS (months) | Long-term OS (5-years) | Long-term PFS (5-years) | |
|---|---|---|---|---|---|
| Melanoma | |||||
| Anti-PD-1 monotherapy (pembrolizumab30,31,100, nivolumab9,14) | 34%30,31, 33%30,31, 43%14 | 5.530,31, 4.130,31, 6.914 | Not reached14,30,31 | 44%9, 38.7%100 | 29%9, 45.7% (48-month rate)100 |
| Anti-PD1 +CTLA-4 inhibitor (nivolumab + ipilimumab14) | 59%14 | 11.514 | Not reached14 | 52%9 | 36%9 |
| Squamous Cell Carcinoma | |||||
| Anti-PD-1 monotherapy (cemiplimab8, pembrolizumab53) | 47%8, 50%8, 42%53 | Not reached8, 7.053 | Not reached8,53 | - | - |
| Merkel Cell Carcinoma | |||||
| Pembrolizumab69 (previously untreated) | 56%69 | 9.069 | - | - | - |
| Avelumab18 (previously treated) | 32%18 | 2.718 | 11.318 | - | - |
3.2. Malignant Melanoma
Although melanoma has a low incidence compared to BCC and SCC, it is more aggressive with a higher mortality, and results in 7000–10,000 deaths annually in the United States.27 Therefore, the most abundant clinical trial data using immune checkpoint inhibitor therapy has been in melanoma. Prior to the introduction of anti-PD-1/PD-L1 in melanoma treatment, ipilimumab, a CTLA-4 inhibitor, was the first checkpoint inhibitor approved by the FDA for this patient population. This agent was the first to improve overall survival in advanced melanoma, and was approved in 2011.28 Although only associated with a 10–15% response rate, many patients experienced durable benefit, with a persistent ~20% long-term survival rate even beyond 10 years.29 Although this study provided an important proof of principle for the durable activity of immune checkpoint inhibitors, most patients still failed to respond to treatment, and high-grade immune related adverse events (irAEs) occurred in up to 1/3 of patients.
Currently, the only FDA approved anti-PD-1/PD-L1 agents in melanoma are pembrolizumab (FDA approval 2014) and nivolumab +/− ipilimumab (FDA approval 2015). Several studies have directly compared anti-PD-1 agents with ipilimumab. The KEYNOTE-006 trial was a large phase III study comparing pembrolizumab with ipilimumab.30,31 The study included 834 patients with advanced melanoma and compared two regimens of pembrolizumab (given every 2 weeks and every 3 weeks) with ipilimumab (every 3 weeks). Both pembrolizumab arms were associated with superior progression free survival (PFS) (median 5.5 months vs median 4.1 months vs 2.8 months, hazard ratio: 0.61) and overall survival (OS) (median not reached for both pembrolizumab arms vs 16 months, hazard ratio: 0.68). The rates of response were also superior in the pembrolizumab groups compared to ipilimumab (33.7% vs 32.9% vs 11.9%), although median time to response was similar (86 vs 85 vs 87 days). Similar to other published data, severe immune-related adverse events (grade 3+) were higher in patients treated with ipilimumab compared to pembrolizumab at 2 and 3 weeks (19.9% vs 13.3% vs 10.1%). Additionally, the rate of discontinuation due to treatment-related adverse events was lower in the pembrolizumab groups. In both groups, the most common treatment-related adverse events were fatigue, diarrhea, rash, and pruritus. Adverse events are due to removal of self-tolerance mechanisms by treatment. Thyroiditis (hypo and hyperthyroidism) was most frequently observed with pembrolizumab while colitis and hypophysitis were more commonly seen with ipilimumab. A full discussion of these toxicities can be found in the following reviews.32,33
Additionally, the phase III CheckMate 067 trial compared nivolumab, ipilimumab, and the combination of these two agents.14 A number of pre-clinical and early clinical studies suggested that this strategy would be synergistic in activating anti-tumor immune responses in a non-redundant fashion.34 This study revealed improved response rates with the combination compared to nivolumab or ipilimumab monotherapy (59% vs 43% vs 19%, respectively) as well as PFS (median 11.5 vs 6.9 vs 2.9 months). Similarly, the 5-year outcomes of this trial indicated a superior overall survival with combination therapy (5-year OS rate 52%) versus monotherapy with nivolumab (5-year OS rate 44%) or ipilimumab (5-year OS rate 26%), although this did not quite meet statistical significance between the combination and nivolumab group.9 Importantly, no deterioration in quality of life outcomes were observed in any of the treatment groups at 5-year follow-up. However, despite a better tumor response, combination therapy significantly increased the risk of acute grade 3/4 immune-related adverse events compared to nivolumab and ipilimumab monotherapy (59% vs 23% vs 28%).9,12,14 Although toxicities were increased with the combination, treatment related deaths were fairly rare overall; subsequent studies have demonstrated a 0.36–1.2% death rate from toxicities.35 Also, notably, the responses to anti-PD-1/PD-L1 are often durable, even after treatment discontinuation. Specifically, after planned discontinuation, responses are durable in 80–90% of patients, likely accounting for long-term survival in patients that initially respond.36–38
Anti-PD-1/PD-L1 has been increasingly used in the adjuvant setting after surgical resection. Similar to treatment in the metastatic setting, adjuvant treatment was first approved with ipilimumab monotherapy, as improved OS was noted compared with placebo in patients with resected stage III melanoma (accompanied by substantial toxicities).39 However, recent trials have demonstrated superior outcomes with anti-PD-1/PD-L1 in the adjuvant setting compared to CTLA-4 inhibitor monotherapy and placebo.3,10,11 Specifically, the recurrence-free survival rate was superior with nivolumab compared to ipilimumab (70.5% vs 60.8%, hazard ratio: 0.65) and pembrolizumab compared to placebo (75.4% vs 61.0%, hazard ratio: 0.57). Additionally, using adjuvant anti-PD-1/PD-L1 monotherapy results in significantly fewer immune-related adverse events than CTLA-4 inhibitors.10 At the time of this writing, no mature OS data has been published from these studies.
Of all tumor types, melanoma has the highest rate of brain metastasis and central nervous system involvement requires unique considerations.40,41 Immune checkpoint inhibitors recently displayed promising results in the treatment of melanoma that is metastatic to the brain. In 2018, the CheckMate 204 trial demonstrated that nivolumab combined with ipilimumab displayed favorable response rates (56% complete or partial response) in patients with brain metastases, with largely concordant intra- vs. extracranial responses.15 This study was limited to patients without symptoms or concurrent corticosteroid use, and largest tumor diameter of 3.0 cm. Other studies have suggested anti-PD-1/PD-L1 monotherapy is associated with much lower intracranial response rates (20–25%).16,42 In 2019, findings from the phase II ABC trial demonstrated superior intracranial/extracranial responses in patients receiving ipilimumab vs nivolumab (51%/57%) compared to nivolumab alone (20%/29%) and nivolumab in patients with symptoms and prior failed treatment (6%/25%).43 In addition, patients with symptomatic brain metastases and those requiring steroids rarely respond to treatment, thus suggesting that local therapy is needed (radiation and/or surgery) prior to starting treatment.
Of note, different histologic subtypes of melanoma also have different response rates, a finding which is likely linked to TMB. Desmoplastic melanomas, which most often occur in older men with chronically sun-exposed skin, have by far the highest TMB of all melanomas, have 70% response rates to anti-PD-1 monotherapy. On the other hand, non-cutaneous melanomas (including those of mucosal and uveal origin) have dramatically lower TMB, and accordingly have much lower response rates to treatment (~5% for uveal, ~20% for mucosal).24,44,45 Of note, patients appear to have similar response rates whether or not they have BRAF mutations. Combination of anti-PD-1/PD-L1 with BRAF +/− MEK inhibitors are in development.46
3.3. Squamous Cell Carcinoma
SCC is one of the most common skin cancer types, representing 20–50% of all skin cancers.47,48 Compared to other cancer types, SCC does not have a high propensity to evolve into metastatic or advanced disease. In fact, a recent large retrospective study analyzing the long-term outcomes of patients with cutaneous SCC found only a 1.3% rate of metastasis and a 4.3% rate of local recurrence.49 With this, standard therapy for most SCC is local resection. However, in the presence of inoperable, recurrent, locally advanced, or metastatic disease, primary surgical management is often not sufficient. Unfortunately, treatment with traditional systemic therapies, such as platinum-based chemotherapy, produces a suboptimal response profile.50 EGFR inhibitors such as cetuximab may have superior activity, but is still associated with disease progression in <1 year on therapy.51
However, recent data has suggested that treatment with anti-PD-1/PD-L1, specifically cemiplimab, the only FDA approved (2018) anti-PD-1/PD-L1 agent for cutaneous SCC, has demonstrated favorable outcomes. Specifically, in 2018 Migden et al reported results of a trial using cemiplimab in patients with advanced or metastatic cutaneous SCC.8 This study reported outcomes on both a phase I cohort of 26 patients with advanced disease and a phase II group of 59 patients with metastatic disease. In the advanced disease group, the response rate was 50.0% with a median time to response of 2.3 months. Additionally, the duration of response surpassed 6 months in 54% of the patients that responded. Notably there was a 19.2% rate of grade 3/4 adverse events, three deaths due to disease progression, and 1 death due to a severe immune-related adverse event. In the metastatic cohort, the response rate was 47% (4 complete, 24 partial) with a median time to response of 1.9 months. The median duration of response was not reached but surpassed 6 months in 57% of the patients that responded. At 12 months, the estimated rates for PFS and OS were 53% and 81%, respectively. Immune-related adverse events caused treatment cessation in four patients (7%) and was the cause of death in one patient. There was a 42.4% rate of grade 3/4 adverse events, although treatment was largely well-tolerated. Cemiplimab has since been FDA approved as the only therapy for advanced cutaneous SCC and is now a standard first-line treatment option.52
Aside from cemiplimab, other anti-PD-1 therapies, primarily pembrolizumab and nivolumab have displayed promising results. The most comprehensive data was presented at the 2018 American Society of Clinical Oncology annual meeting reporting the results of the phase 2 CARSKIN trial.53 In this study, 19 patients with unresectable cutaneous SCC who were chemotherapy-naive received pembrolizumab monotherapy. The response rate was 42% (7 partial and 1 complete) with a 58% disease control rate and median progression free survival of 7 months. Of note, immune-related adverse events occurred in 63% of the study population.
A number of other case reports have also demonstrated that nivolumab and pembrolizumab are effective treatment options in both chemotherapy pre-treated and naive patients.54–60
3.4. Merkel Cell Carcinoma
MCC has the lowest incidence of the reviewed skin cancers, with approximately 1600 cases in the United States annually.61 Relative to SCC and BCC, MCC is very aggressive with a 5-year mortality rate close to 50%.62 The etiology of MCC is multifactorial. Similar to other skin cancers, ultraviolet light exposure and immunosuppression are major risk factors. MCC development is also associated with merkel cell polyomavirus, likely explaining its higher incidence in the immunosuppressed population.
Localized MCC is commonly treated with surgical resection and/or radiotherapy. In metastatic MCC, chemotherapy is often given in regimens used to treat high-grade neuroendocrine tumors such as cisplatin and etoposide. Although associated with high initial rates of response, benefit lacks durability and is associated with a high rate of severe treatment-related toxicities in this frequently elderly population with substantial comorbidities.63–65 Interestingly, histopathologic analysis of MCC revealed PD-1 or PD-L1 expression in approximately 50% of specimens.66 Persistent viral infections has been shown to yield high levels of PD-1 receptors.67 Notably, however, virus negative MCC, particularly those with an ultraviolet light signature, display higher TMB.68 Overall, these findings led to further investigation into the role of anti-PD-1/PD-L1 in the treatment of MCC.
Currently, avelumab (FDA approval 2017) and pembrolizumab (FDA approval 2018) are the only approved anti-PD-1/PD-L1 for MCC. To date, the largest study of checkpoint inhibitors in MCC is a phase II trial in 88 patients who had previously progressed on chemotherapy treated with avelumab The response rate was 32% (8 complete, 20 partial) with an incidence of 5% for grade 3/4 adverse events.18 Notably, avelumab for MCC has a comparable response rate to pembrolizumab in treatment naïve patients, and both should be considered as front-line therapy.17,69 Pembrolizumab has been studied in a phase II trial with 26 patients.69 This study assessed patients without prior treatment and showed a 56% response rate (4 complete, 10 partial), progression free survival at 6 months of 67%, and a 15% rate of severe immune-related toxicities requiring treatment cessation. 65% of patients in this study had MCC tumors positive for Merkel cell polyomavirus. Importantly, treatment with anti-PD1 as first-line therapy has displayed long-term durable responses in this population. In a phase II trial, 50 treatment-naïve patients treated with pembrolizumab displayed 24-month OS and PFS rates of 69%, and 48%, respectively.70 Nivolumab is also being trialed in a phase I/II study with 25 patients.71 The overall response rate was 68% with 67% of the tumors testing positive for merkel cell polyomavirus and an incidence of grade 3/4 toxicities of 20%. Overall, outcomes for virus-positive vs. negative patients appears to be very similar across studies despite their distinct biology. Given the generally more aggressive course of virus-negative patients, one could suggest that the degree of benefit may be particularly pronounced in this population.72
3.5. Basal Cell Carcinoma
BCC is the most common skin cancer with an estimated incidence of 550,000 annually in the United States.73 BCC is typically not aggressive and reaches the metastatic stages in <0.01% of cases.74 In most patients, treatment with surgical resection or topical agents are effective. However, in the rare cases of metastatic disease or locally-advanced unresectable presentation, treatment with radiotherapy and/or systemic therapy may be warranted. Sonic hedgehog inhibitors vismodegib (FDA approval 2012) and sonidegib (FDA approval 2015) were historically used for advanced and metastatic BCC.75 Although these agents produce frequent tumor responses, with a response rate of 30–43%, adverse events are significant and frequently cause treatment discontinuation.76,77 Further, many patients ultimately develop acquired resistance and require other therapies.
Recently, with the success of anti-PD-1/PD-L1 in other skin cancer types, there has been interest in testing the efficacy and toxicity profile of these agents in advanced or metastatic BCC. Interestingly, histopathologic analysis has demonstrated high levels PD-1 or PD-L1 expression in the majority of BCC specimens, suggesting anti-PD-1/PD-L1 could be effective in treating these tumors.78 Currently, there are no FDA approved anti-PD-1/PD-L1 agents for BCC, but the largest endeavor is a phase II trial studying cemiplimab in patients with advanced BCC after prior failure of hedgehog inhibitors.79,80 However, the trial is currently in progress with no finalized results.
To date, the published data on the safety and efficacy of anti-PD-1/PD-L1 in advanced BCC is limited to isolated case reports with largely positive results. The largest collection of published data involves 16 patients with advanced BCC treated with pembrolizumab monotherapy or pembrolizumab given in combination with vismodegib.81 The overall response rate for the total cohort was 38%, but patients treated with pembrolizumab monotherapy displayed a superior response rate compared to the combined therapy group (44% vs 29%). In 2018, Fischer et al also reported a case of near-complete response to pembrolizumab after failure with hedgehog inhibitors.82 The isolated findings suggest rigorous examination through a large multi-center randomized controlled trial is needed to fully define the efficacy of anti-PD-1/PD-L1 treatment in advanced or metastatic BCC. Notably, there are currently two ongoing clinical trials studying pembrolizumab and ipilimumab with nivolumab in patients with advanced BCC.83,84
4. Treatment-related toxicities
Immune-related adverse events are common with anti-PD-1/PD-L1 treatment and can virtually affect any organ system.33,85 Most adverse events occur within the first 12 weeks of treatment and fortunately commonly resolve with low-dose glucocorticoid therapy.86 However, more severe toxicities are not uncommon and can require high-dose glucocorticoids, systemic immunosuppression (i.e. infliximab), or treatment discontinuation. In some cases, toxicities can result in chronic impairment or death.87 Interestingly, the risk of developing toxicities appears to not be dose-dependent and the development of toxicities might be associated with an improved response to therapy.88–90 Specifically, when using nivolumab with ipilimumab, there is a significantly higher rate of treatment-related adverse events with a 10 mg/kg preparation of ipilimumab, but no dose response relationship between nivolumab and ipilimumab.91,92 Therefore, to minimize toxicities, lower doses of ipilimumab (1 mg/kg) should be used.
The most common acute immune-related adverse events impact the skin, GI tract (colitis), liver (hepatitis), musculoskeletal system, pulmonary system (pneumonitis), and endocrine system (hypophysitis, thyroiditis). In rare cases, toxicities impacting the cardiac (myocarditis) and neurologic system (encephalitis) can also occur.93–95 Although these toxicities occur more frequently with the inclusion of CTLA-4 inhibitors, the majority of treated patients exhibit at least some mild symptoms.85,86 In addition to acute toxicities, recent studies have described persistent, chronic toxicities primarily affecting the endocrine, nervous, pulmonary, and musculoskeletal systems.96–99 While prior research has focused on the characterization of acute toxicities, with a growing number of long-term survivors treated with anti-PD-1/PD-L1 therapy, more research is needed to describe chronic and rare adverse events.
5. Conclusion
Similar to many other cancer types, treatment with anti-PD-1/PD-L1 in skin cancer is revolutionizing management and outcomes. Overall, the available data suggests that the superior response rates of skin cancer to anti-PD-1/PD-L1 compared to other solid tumor types is likely due to a high mutational burden and viral etiology associated with MCC. Although melanoma has been rigorously studied in the setting of anti-PD-1/PD-L1 treatment, more research is needed for the other skin cancer types to establish toxicity profiles and responses. Future studies should attempt to characterize the long-term clinical responses, toxicities, and patient-reported outcomes in this population.
Key Points:
The use of antibodies to programmed cell death-1 receptor and its ligand (PD-1/PD-L1) has revolutionized treatment in patients with advanced skin cancer and produced long-term, durable responses in this population.
The response of skin cancer to anti-PD-1/PD-L1 largely surpasses all other solid tumors, likely due unique molecular features of these cancers.
While anti anti-PD-1/PD-L1 agents have been rigorously studied in advanced melanoma, more long-term, multi-center controlled trials are needed with these agents in non-melanoma skin cancer.
Funding
This work was funded by NCCN Young Investigators Award (DBJ), American Cancer Society Institutional Research Grant (DBJ), National Institutes of Health, grant numbers: K23 CA204726 (DBJ), NIH R01CA227481 (DBJ).
Footnotes
Conflict of Interest
Douglas B. Johnson serves on advisory boards for Array Biopharma, BMS, Incyte, Jansen, Merck, and Novartis, and receives research funding from BMS and Incyte, and travel support from Genentech. J. Randall Patrinely Jr and Anna K. Dewan declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
References
- 1.Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J. 2018;24(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokota K, Uchi H, Uhara H, et al. Adjuvant therapy with nivolumab versus ipilimumab after complete resection of stage III/IV melanoma: Japanese subgroup analysis from the phase 3 CheckMate 238 study. J Dermatol. 2019;46(12):1197–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 6.Chen JT, Kempton SJ, Rao VK. The Economics of Skin Cancer: An Analysis of Medicare Payment Data. Plastic and Reconstructive Surgery – Global Open. 2016;4(9):e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379(4):341–351. [DOI] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 10.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. [DOI] [PubMed] [Google Scholar]
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. [DOI] [PubMed] [Google Scholar]
- 15.Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 17.D’Angelo SP, Russell J, Lebbe C, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018;4(9):e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: An investigator-initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80(2):564–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulson KG, Lahman MC, Chapuis AG, Brownell I. Immunotherapy for skin cancer. Int Immunol. 2019;31(7):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandal R, Samstein RM, Lee KW, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science. 2019;364(6439):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature. 2018;553(7688):347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DB, Frampton GM, Rioth MJ, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res. 2016;4(11):959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 27.Guy GP Jr., Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–596. [PMC free article] [PubMed] [Google Scholar]
- 28.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015;33(17):1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–1862. [DOI] [PubMed] [Google Scholar]
- 31.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 32.Johnson DB, Chandra S, Sosman JA. Adverse Events Associated With Immune Checkpoint Inhibitors-Reply. Jama. 2019;321(12):1219–1220. [DOI] [PubMed] [Google Scholar]
- 33.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30(7):1154–1161. [DOI] [PubMed] [Google Scholar]
- 37.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauci ML, Lanoy E, Champiat S, et al. Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome upon Treatment Discontinuation. Clin Cancer Res. 2019;25(3):946–956. [DOI] [PubMed] [Google Scholar]
- 39.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 40.Glitza Oliva IC, Schvartsman G, Tawbi H. Advances in the systemic treatment of melanoma brain metastases. Ann Oncol. 2018;29(7):1509–1520. [DOI] [PubMed] [Google Scholar]
- 41.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 42.Kluger HM, Chiang V, Mahajan A, et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J Clin Oncol. 2019;37(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long GV, Atkinson VG, Lo S, et al. Long-term outcomes from the randomized phase II study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets): Anti-PD1 brain collaboration (ABC). Annals of Oncology. 2019;30:v534. [Google Scholar]
- 44.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122(21):3354–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan RJ, Hamid O, Gonzalez R, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. 2019;25(6):929–935. [DOI] [PubMed] [Google Scholar]
- 47.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247. [DOI] [PubMed] [Google Scholar]
- 48.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatology. 2015;151(10):1081–1086. [DOI] [PubMed] [Google Scholar]
- 49.Khan K, Mykula R, Kerstein R, et al. A 5-year follow-up study of 633 cutaneous SCC excisions: Rates of local recurrence and lymph node metastasis. J Plast Reconstr Aesthet Surg. 2018;71(8):1153–1158. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Okuyama R, Saida T, Uhara H. Platinum and anthracycline therapy for advanced cutaneous squamous cell carcinoma. Int J Clin Oncol. 2013;18(3):506–509. [DOI] [PubMed] [Google Scholar]
- 51.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29(25):3419–3426. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed SR, Petersen E, Patel R, Migden MR. Cemiplimab-rwlc as first and only treatment for advanced cutaneous squamous cell carcinoma. Expert Rev Clin Pharmacol. 2019;12(10):947–951. [DOI] [PubMed] [Google Scholar]
- 53.Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first line therapy in patients with unresectable squamous cell carcinoma of the skin: Interim results of the phase 2 CARSKIN trial. Journal of Clinical Oncology. 2018;36(15_suppl):9534–9534. [Google Scholar]
- 54.Borradori L, Sutton B, Shayesteh P, Daniels GA. Rescue therapy with anti-programmed cell death protein 1 inhibitors of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: preliminary experience in five cases. British Journal of Dermatology. 2016;175(6):1382–1386. [DOI] [PubMed] [Google Scholar]
- 55.Tran DC, Colevas AD, Chang ALS. Follow-up on Programmed Cell Death 1 Inhibitor for Cutaneous Squamous Cell Carcinoma. JAMA Dermatology. 2017;153(1):92–94. [DOI] [PubMed] [Google Scholar]
- 56.Blum V, Müller B, Hofer S, et al. Nivolumab for recurrent cutaneous squamous cell carcinoma: three cases. European Journal of Dermatology. 2018;28(1):78–81. [DOI] [PubMed] [Google Scholar]
- 57.Delaitre L, Martins-Hericher J, Truchot E, et al. [Regression of cutaneous basal cell and squamous cell carcinoma under pembrolizumab]. Ann Dermatol Venereol. 2019. [DOI] [PubMed] [Google Scholar]
- 58.van Baar MLM, Guminski AD, Ferguson PM, Martin LK. Pembrolizumab for cutaneous squamous cell carcinoma: Report of a case of inoperable squamous cell carcinoma with complete response to pembrolizumab complicated by granulomatous inflammation. JAAD Case Rep. 2019;5(6):491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assam JH, Powell S, Spanos WC. Unresectable cutaneous squamous cell carcinoma of the forehead with MLH1 mutation showing dramatic response to Programmed Cell Death Protein 1 Inhibitor Therapy. Clin Skin Cancer. 2016;1(1):26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevenson ML, Wang CQ, Abikhair M, et al. Expression of Programmed Cell Death Ligand in Cutaneous Squamous Cell Carcinoma and Treatment of Locally Advanced Disease With Pembrolizumab. JAMA Dermatol. 2017;153(4):299–303. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am Surg. 2015;81(8):802–806. [DOI] [PubMed] [Google Scholar]
- 62.Hughes MP, Hardee ME, Cornelius LA, Hutchins LF, Becker JC, Gao L. Merkel Cell Carcinoma: Epidemiology, Target, and Therapy. Curr Dermatol Rep. 2014;3:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5(9):2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatia S, Storer BE, Iyer JG, et al. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases From the National Cancer Data Base. J Natl Cancer Inst. 2016;108(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villani A, Fabbrocini G, Costa C, Carmela Annunziata M, Scalvenzi M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol Ther (Heidelb). 2019;9(2):209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afanasiev OK, Yelistratova L, Miller N, et al. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19(19):5351–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knepper TC, Montesion M, Russell JS, et al. The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy. Clin Cancer Res. 2019;25(19):5961–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. New England Journal of Medicine. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nghiem P, Bhatia S, Lipson EJ, et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. Journal of Clinical Oncology. 2019;37(9):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Topalian SL, Bhatia S, Hollebecque A, et al. Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC). Cancer Research. 2017;77(13 Supplement):CT074–CT074. [Google Scholar]
- 72.Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J Invest Dermatol. 2017;137(4):819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: A retrospective cohort study. J Am Acad Dermatol. 2016;75(5):957–966.e952. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen-Nielsen M, Wang L, Pedersen L, et al. The incidence of metastatic basal cell carcinoma (mBCC) in Denmark, 1997–2010. Eur J Dermatol. 2015;25(5):463–468. [DOI] [PubMed] [Google Scholar]
- 75.Axelson M, Liu K, Jiang X, et al. U.S. Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19(9):2289–2293. [DOI] [PubMed] [Google Scholar]
- 76.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Apalla Z, Papageorgiou C, Lallas A, et al. Spotlight on vismodegib in the treatment of basal cell carcinoma: an evidence-based review of its place in therapy. Clin Cosmet Investig Dermatol. 2017;10:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lipson EJ, Lilo MT, Ogurtsova A, et al. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer. 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewis KD, Fury MG, Stankevich E, et al. 1240TiPPhase II study of cemiplimab, a human monoclonal anti-PD-1, in patients with advanced basal cell carcinoma (BCC) who experienced progression of disease on, or were intolerant of prior hedgehog pathway inhibitor (HHI) therapy. Annals of Oncology. 2018;29(suppl_8). [Google Scholar]
- 80.Stein JE, Brothers P, Applebaum K, et al. A phase 2 study of nivolumab (NIVO) alone or plus ipilimumab (IPI) for patients with locally advanced unresectable (laBCC) or metastatic basal cell carcinoma (mBCC). Journal of Clinical Oncology. 2019;37(15_suppl):TPS9595–TPS9595. [Google Scholar]
- 81.Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: An investigator-initiated, proof-of-concept study. Journal of the American Academy of Dermatology. 2019;80(2):564–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fischer S, Hasan Ali O, Jochum W, Kluckert T, Flatz L, Siano M. Anti-PD-1 Therapy Leads to Near-Complete Remission in a Patient with Metastatic Basal Cell Carcinoma. Oncol Res Treat. 2018;41(6):391–394. [DOI] [PubMed] [Google Scholar]
- 83.ClinicalTrials.gov. Nivolumab and Ipilimumab in Treating Patients With Rare Tumors. NCT02834013. National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT02834013. Accessed.
- 84.ClinicalTrials.gov. TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People With Advanced Stage Cancer (TAPUR). NCT02693535. https://clinicaltrials.gov/ct2/show/NCT02693535. Accessed.
- 85.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018;4(1):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. Jama. 2018;320(16):1702–1703. [DOI] [PubMed] [Google Scholar]
- 87.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Attia P, Phan GQ, Maker AV, et al. Autoimmunity Correlates With Tumor Regression in Patients With Metastatic Melanoma Treated With Anti–Cytotoxic T-Lymphocyte Antigen-4. Journal of Clinical Oncology. 2005;23(25):6043–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Downey SG, Klapper JA, Smith FO, et al. Prognostic Factors Related to Clinical Response in Patients with Metastatic Melanoma Treated by CTL-Associated Antigen-4 Blockade. Clinical Cancer Research. 2007;13(22):6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quach HT, Dewan AK, Davis EJ, et al. Association of Anti-Programmed Cell Death 1 Cutaneous Toxic Effects With Outcomes in Patients With Advanced Melanoma. JAMA Oncol. 2019;5(6):906–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622. [DOI] [PubMed] [Google Scholar]
- 92.Lebbe C, Meyer N, Mortier L, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. J Clin Oncol. 2019;37(11):867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181(4):2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schneider S, Potthast S, Komminoth P, Schwegler G, Bohm S. PD-1 Checkpoint Inhibitor Associated Autoimmune Encephalitis. Case Rep Oncol. 2017;10(2):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cappelli LC, Brahmer JR, Forde PM, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. 2018;48(3):553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes. 2018;67(8):1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson DB, Taylor KB, Cohen JV, et al. Anti-PD-1-induced pneumonitis is associated with persistent imaging abnormalities in melanoma patients. Cancer Immunology Research. 2019:canimm.0717.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. [DOI] [PubMed] [Google Scholar]


