Dear Editor,
In this Journal, Tang and colleagues recently discussed the emergence of a new Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) variant in the UK and the necessity to determine its impact on society and healthcare capacity.1 Tenerife (Canary Islands, Spain) is one of the top destinations for UK travelers all year long and was an air corridor until late 20202 as the coronavirus disease 2019 (COVID-19) had <50 cases of accumulated incidence per 100,000 inhabitants in the last 14 days (AI14) until late September, retaining one of the lowest case levels among all Spanish communities since the decrease of the first wave during April 2020. Starting in December 2020, Tenerife faced an unexplained rapid increase of COVID-19 cases compared to the rest of the Archipelago as AI14 jumped from <130 cases between August and late November 2020 to 248 cases by the 23rd of December 2020. As a consequence, border restrictions and mobility limitations between municipalities from the 19th of December 2020 until the 2nd of January 2021 were imposed.
The emergence of novel SARS-CoV-2 variants is generating widespread concern, as they harbor a constellation of mutations that might lead to increased transmissibility3 and/or immune evasion from previous infection or vaccination.4 The lineage B.1.1.7 (variant of concern [VOC]−202012/01; also known as clade 20I/501Y.V1), firstly identified in the UK in December 2020,1 , 5 has rapidly spread worldwide (https://cov-lineages.org/global_report_B.1.1.7.html). By mid-January 2021, this VOC had an accumulated prevalence over 76% among all UK sequences despite restrictions in many of the affected areas.5 Among the mutations characterizing this lineage, the deletion predicting the loss of the amino acids 69 and 70 (Δ69/70) in the S protein has been associated with a “Spike gene target failure” (SGTF) for two commercial RT-qPCR kits for COVID-19 diagnosis.6 Here we aimed to monitor the SARS-CoV-2 B.1.1.7 lineage cases in Tenerife by leveraging the SGTF during the RT-qPCR followed by viral genome sequencing of the prioritized samples.
The study was conducted at the University Hospital Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain) and its review board approved the study (CHUNSC_2020_24). We assessed nasopharyngeal swabs from COVID-19/SARS-CoV-2 patients corresponding to independent outbreaks declared from 18th of December 2020 to 25th of February 2021. Routine COVID-19 diagnosis was conducted as described elsewhere.7
We first used the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific) to monitor the SGTF in the COVID-19 positive patients as it has been suggested to occur by the presence of the Δ69/70 deletion6 that is known to have arisen in multiple lineages.8 We used this assay to assess all RNA extracts (Zymo Quick DNA/RNA kit, Zymo) from COVID-19 positives in the period. Thermocycling was conducted on a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific) following manufacturers recommendations.
Then, samples were selected for sequencing when categorized as SGTF and if they showed a cycle threshold (Ct)<30 for any of the targets assessed. The libraries were obtained using the COVIDSeq Test (Illumina, Inc.), covering the whole SARS-CoV-2 genome and including internal controls consisting of 11 human mRNA targets. The procedure followed the manufacturers recommendations and quality control steps. Sequencing was conducted on a NextSeq 550 (Illumina) instrument on High Output mode with 36 bp single end reads. Per sample consensus sequences and variants against the reference (NC_045512.2) were obtained based on the DRAGEN COVIDSeq Test v1.2.2 pipeline (Illumina, Inc.). Positive and negative amplification controls were included in each run. To be able to recover the FASTA sequences that were not automatically provided by DRAGEN COVIDSeq Test, the fastq files were reprocessed with DRAGEN Lineage v3.5.0 with the following specifications: coverage threshold of 20, virus detection threshold of 5, virus negative threshold of 5, and a human control threshold of 3. The PANGOLIN software suite v2.1.7 (https://github.com/cov-lineages/pangolin) was then used for lineage and clade classification following the nomenclature of Rambaut et al.9 Nextclade v.0.12.0 was used for variant calling and functional predictions. The AI14 data was retrieved from the COVID-19 data hub from Gobierno de Canarias (https://www.gobiernodecanarias.org/principal/coronavirus/acceso_datos.html; accessed March 6th, 2021).
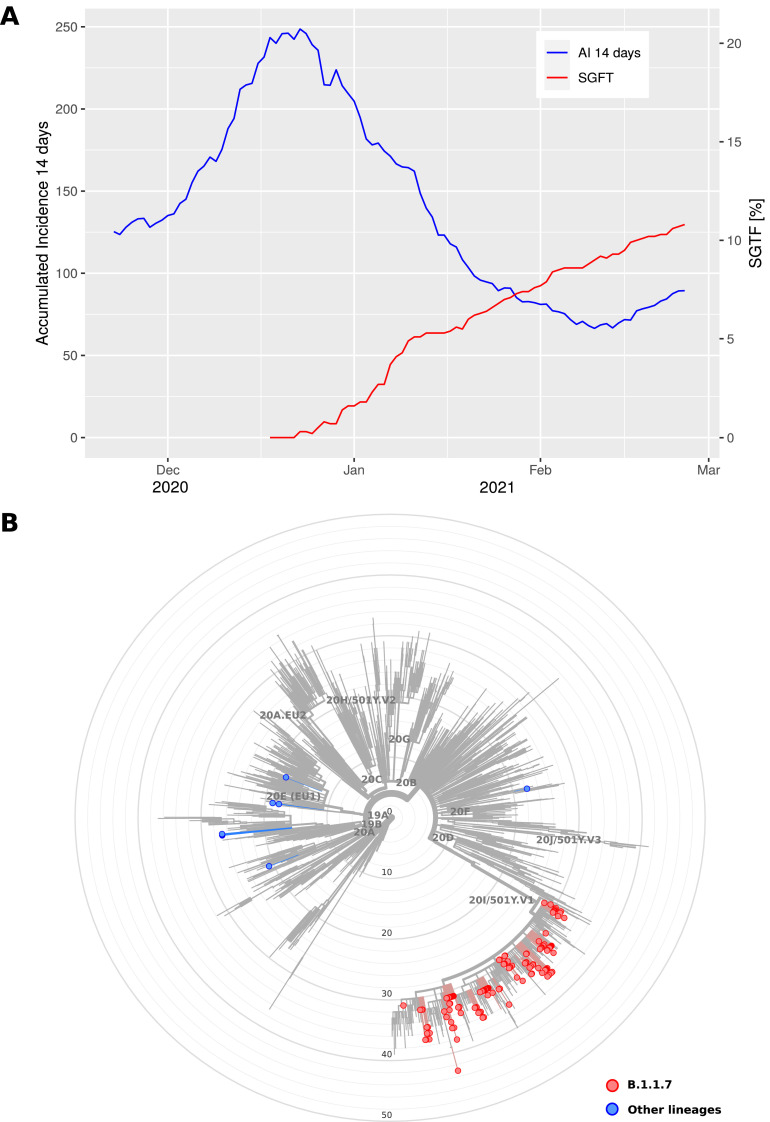
In the analysis period, a total of 2091 COVID-19 positive samples were assessed, resulting in 226 SGTF samples identified and reaching a maximum of 10.7% at the end of the period under study. Remarkably, we detected a rapid increase in the prevalence of SGTF over this period, rising sharply during the Christmas season and steadily after December 28th, 2020 (Fig. 1 A). We were able to sequence 147 (mean depth of coverage ± SD: 48X ± 9) out of the 226 SGTF samples in the period. The sequenced genomes of SARS-CoV-2 with SGTF were assigned to five different lineages where B.1.1.7 (VOC-202012/01) was the predominant (93.2%). Eleven of these had lower sequence quality (based on the aggregation of four metrics: missing data, mixed sites, private mutations, mutation clusters) and were not included in downstream analysis. Among those classified as B.1.1.7 lineage, the sequences carried between nine and 17 of the lineage-defining mutations,8 with 93.4% of the sequences including ≥15 (Fig. 1B). Other lineages that were linked to the SGTF in this study were classified as B.1.258 (5), B.1.177 (3), B.1.221 (1), and B.1.1.222 (1).
Fig. 1.
Monitoring the SARS-CoV-2 B.1.1.7 lineage in Tenerife (Spain) between 18th of December 2020 and 25th of February 2021. (A) Accumulated incidence per 100,000 inhabitants in the last 14 days (AI 14 days) and spike gene target failure (SGTF) cases per day in the study period. (B) Maximum likelihood phylogenetic tree of SARS-CoV-2 sequences characterized by the spike gene target failure (SGTF) in the period (B.1.1.7 lineages in red; other lineages in blue). The tree also includes relationships with other 1902 genome sequences representative of major clades, labelled based on Nextstrain definitions, from Auspice v2.23.0 (as of 2021-03-07) and sampled between December 2019 and March 2021 for reference (light gray) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Based on RT-qPCR SGTF and viral genome sequencing, this study supports the local transmission of SARS-CoV-2 B.1.1.7 lineage in Tenerife since late December 2020. Despite vaccination campaign started in Spain on the 27th of December 2020 and given that it has not reached a large fraction of the population yet, the possibility that the B.1.1.7 lineage is also responsible for increased transmission in the rest of the Archipelago is high. Although neglecting a protective effect from vaccination, the models for some major cities in Europe indicate that the B.1.1.7 lineage will be the dominant by mid-March 2021 even if accounting for non-pharmaceutical interventions.10 We anticipate that the data reported here should be taken as an underestimate as there will be genomes of the B.1.1.7 lineage that escape detection by RT-qPCR SGTF. We also assume that there is potential that some of the samples may derive from non-resident travelers that could have introduced an upward bias in the estimated AI14.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We deeply acknowledge the University Hospital Nuestra Señora de Candelaria and the Instituto Tecnológico y de Energías Renovables board of directors for their strong support and assistance in accessing diverse resources used in the study.
Funding
This work was supported by Cabildo Insular de Tenerife [grants CGIEU0000219140 and “Apuestas científicas del ITER para colaborar en la lucha contra la COVID-19″]; the agreement with Instituto Tecnológico y de Energías Renovables (ITER) to strengthen scientific and technological education, training research, development and innovation in Genomics, Personalized Medicine and Biotechnology [grant number OA17/008]; Ministerio de Ciencia e Innovación [grant numbers RTI2018-093747-B-100 and RTC-2017-6471-1], co-funded by the European Regional Development Fund (ERDF); Lab P2+ facility [grant number UNLL10 - 3E-783], co-funded by the ERDF and “Fundación CajaCanarias”; and the Spanish HIV/AIDS Research Network [grant number RIS-RETIC, RD16/0025/0011], co-funded by Instituto de Salud Carlos III and by the ERDF; and RIS-3 Canarias Strategy - “María del Carmen Betancourt y Molina” Program, “Consejería de Economía, Conocimiento y Empleo, Gobierno de Canarias” [grant number ProID2020010093]. The funders had no role in the study design, collection, analysis and interpretation of data, in the writing of the manuscript or in the decision to submit the manuscript for publication.
References
- 1.Tang J.W., Tambyah P.A., Hui D.S. Emergence of a new SARS-CoV-2 variant in the UK. J Infect. 2021;82:e27–e28. doi: 10.1016/j.jinf.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department for Transport and Foreign, Commonwealth & Development Office, Government of UK. Canary Islands, Denmark, Maldives and Mykonos added to travel corridor exempt list; 2020 Oct 22. Available from: https://www.gov.uk/government/news/canary-islands-denmark-maldives-and-mykonos-added-to-travel-corridor-exempt-list.
- 3.Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9:E20–E21. doi: 10.1016/S2213-2600(21)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine — preliminary report. N Engl J Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Toole A.O., Hill V., Pybus O.G., Watts A., Bogoch I.I., Khan K., et al. 2021. Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2.https://virological.org/t/tracking-the-international-spread-of-sars-cov-2-lineages-b-1-1-7-and-b-1-351-501y-v2/592 Feb 4 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA COVID-19 Update . 2021. Genetic variants of SARS-CoV-2 may lead to false negative results with molecular tests for detection of SARS-CoV-2 - letter to clinical laboratory staff and healthcare providers.https://www.fda.gov/medical-devices/letters-health-care-providers/genetic-variants-sars-cov-2-may-lead-false-negative-results-molecular-tests-detection-sars-cov-2 Jan 8 Available from: [Google Scholar]
- 7.Alcoba-Florez J., Gil-Campesino H., García-Martínez de Artola D., González-Montelongo R., Valenzuela-Fernández A., Ciuffreda L., et al. Sensitivity of different RT-qPCR solutions for SARS-CoV-2 detection. Int J Infect Dis. 2020;99:190–192. doi: 10.1016/j.ijid.2020.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rambaut A., Holmes E.C., O'Toole Á., Hill V., McCrone J.T., Ruis C., et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gozzi N., Chinazzi M., Davis J.T., Mu K., Pastore y Piontti A., Ajelli M., et al. medRxiv; 2021. Estimating the spreading and dominance of SARS-CoV-2 VOC 202012/01 (lineage B.1.1.7) across Europe. Forthcoming [February 23] [DOI] [Google Scholar]