Abstract
Background
Lopinavir-ritonavir is a repurposed drug for coronavirus disease-2019 (COVID-19). In this study, a pooled effect of lopinavir-ritonavir on mortality, virological cure, radiological improvement and safety profile in COVID-19 patients has been evaluated.
Methods
The databases were searched for comparative randomized controlled studies evaluating the efficacy and/or safety of lopinavir-ritonavir in COVID-19 patients. The mortality outcome was pooled as a risk difference (RD) with 95% CI. The virological cure, radiological improvement and adverse events were pooled as risk ratio (RR) with 95% CI. All outcomes were pooled using the Mantle-Hanzle method random effect model. The heterogeneity was assessed using the I2 test.
Results
Out of 82 full text assessed, seven studies were included in the analysis. The included studies had five different control interventions: supportive care (n = 4), umifenovir (arbidol) (n = 2), navaferon (recombinant anti-tumour and anti-virus protein) (n = 1), lopinavir-ritonavir + novaferon (n = 1) and lopinavir-ritonavir + interferon beta 1b + ribavirin (n = 1). Lopinavir-ritonavir group did not show significant difference in mortality [RD: 0.00 (95% CI: −0.01, 0.02), I2 = 0], virological cure [RR: 1.06 (95% CI: 0.85, 1.31), I2 = 0%], radiological improvement [RR: 0.81 (95% CI: 0.62, 1.05)] and adverse events [RR: 2.59 (95% CI: 0.17, 38.90), I2 = 75%] than supportive care. Similarly, no difference was observed for any efficacy outcomes between lopinavir-ritonavir and other control interventions. We observed significantly high risk of adverse events with lopinavir-ritonavir as compared to umifenovir [RR: 2.96 (95% CI: 1.42–6.18); I2 = 0%].
Conclusion
There is no benefit of the addition of lopinavir-ritonavir to the standard care in COVID-19 patients.
Keywords: Lopinavir, Ritonavir, COVID-19, Virological cure, Safety, Meta-analysis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) belongs to the beta Coronaviridae family, like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [1] and is responsible for the current pandemic of Corona Virus Disease 2019 (COVID-19). After infection, patients present with mild to severe symptoms, including fever, cough, sore throat, rhinorrhea, severe pneumonia, and septic shock. Few patients infected with SARS-CoV-2 develop acute respiratory distress syndrome, leading to a high rate of admission to intensive care units and ultimately death in severe cases [2], [3]. The clinical presentation of COVID-19 greatly resembles SARS-CoV infection [3].
Currently, no antiviral treatment is proven to be effective in SARS-CoV-2 infection. Optimized supportive care remains the mainstay of therapy for it. Therefore, identifying drug treatment options as soon as possible is critical for the response to the COVID-19 outbreak.
The Lopinavir-ritonavir combination is one of the repurposed drugs currently used in the treatment of COVID-19. They are protease inhibitors used for treating HIV infection. Both these drugs are suggested to inhibit SARS-CoV 3C-like protease enzyme [1]. The protease is considered a key enzyme in coronavirus polyprotein processing [4]. In vitro study of Choy et al. suggested that lopinavir could achieve the therapeutic working concentration of 26.63 μM against SARS-CoV-2 in Vero E6 cells [5]. However, ritonavir alone did not show the in vitro activity against SARS-CoV-2. Another in vitro study by Kang et al. observed significant antiviral activity of a combination of lopinavir-ritonavir against SARS-CoV-2 [6]. A recent animal study by Park et al. did not show in vivo efficacy of lopinavir-ritonavir in SARS-CoV-2 infection in the ferret model [7]. The current use of lopinavir-ritonavir is mainly based on the experience of the use of lopinavir-ritonavir in SARS and MERS. A systematic review of lopinavir-ritonavir therapy in patients of SARS and MERS suggested better clinical outcomes and a lower risk of ARDS or death. However, the evidence was mainly based on case reports and retrospective case-series [8].
The literature for the use of lopinavir-ritonavir in the treatment of COVID-19 is now available in the form of randomized controlled trials. So, this systematic review aimed to find out the efficacy and safety outcomes in COVID-19 patients treated with lopinavir-ritonavir combination through the meta-analytic summary.
Material and methods
Study identification
Two investigators independently systematically searched the databases: PubMed, medrxiv.org, biorxib.org, mediterranee-infection.com/pre-prints-ihu, Google Scholar and CNKI. The search terms were: (Lopinavir AND Ritonavir) AND (COVID-19 OR coronavirus OR SARS-CoV-2). The last search was run on 27th December 2020. There were no language and time restriction to include the published articles. Titles, abstracts and full articles (if required) were assessed for deciding the eligibility of retrieved articles. Any disagreements were resolved by discussion and consensus among the authors.
Selection criteria
Inclusion criteria
-
•
Randomized controlled clinical trials (open labelled or blinded studies)
-
•
Comparative trials of lopinavir-ritonavir with any other interventions (placebo, supportive care or any other treatment modalities)
-
•
studies evaluating the efficacy and/or safety of Lopinavir-ritonavir in confirmed COVID-19 patients (any age, either sex)
Exclusion criteria:
-
•
Studies with less than 10 participants in the treatment arms
-
•
Non-SARS-CoV-2 studies,
-
•
Non-comparative clinical studies, in vitro or cell culture, animal studies,
-
•
Non-research articles (e.g. review articles, meta-analysis) and duplicate publications.
Types of interventions
Use of lopinavir-ritonavir irrespective of dose and duration of therapy was considered. All treatment modalities including supportive care or placebo were considered as the control arm. The interventions as an add on to the lopinavir-ritonavir treatment arm were analyzed separately.
Risk of bias assessment of included studies
Two investigators assessed the methodological quality of the included randomized studies as per revised Cochrane “risk of bias assessment tool for the randomized controlled clinical trials (ROB-II)” [9]. Each study was assessed for the possibility of risk of bias in the following five domains: a process of randomization, deviations from the intended interventions, missing outcome data, outcome measurement and selection of the reported results. Each domain was categorized into low, high or having some concerns in the risk of bias assessment [9]. Any disagreements were resolved by discussion and consensus among the authors.
Data extraction
The following data were extracted in a Microsoft Excel sheet, 2016: first author, publication year, study design, study site, baseline data of study population in treatment arms (age, gender, the severity of the disease, days of illness to start of treatment), lopinavir-ritonavir and comparator interventions (dosage, duration and route of administration), supportive care and efficacy (mortality, virological cure, radiological improvement in treatment arms), safety (adverse events) data and intention to treat analysis. The data were cross-checked to ensure the accuracy of extraction.
Efficacy outcomes
Mortality, virological cure and radiological improvement between patients who received lopinavir-ritonavir and control interventions at the end of the study period were analyzed based on the type of control interventions. At the end of study period data were used in case of multiple time-point estimations of outcomes in the included studies. The intention to treat the study population was used to estimate the efficacy outcomes.
Safety outcome
The safety outcomes were the number of adverse events between lopinavir-ritonavir and control interventions at the end of the study period. Adverse events were analyzed based on the type of control interventions using the safety population. All patients who received lopinavir-ritonavir and control interventions irrespective of per-protocol dose and duration were considered as a safety population.
Data synthesis
All outcomes were the dichotomous variables. The mortality outcome was summarized as a risk difference (RD) with 95% CI. The virological cure, radiological improvement and adverse events were summarized as risk ratio (RR) with 95% CI. All outcomes were pooled using the Mantle-Hanzle method. The fixed effect or random model was used to estimate the meta-analytic summary. In case of substantial heterogeneity, the random effect model was preferred over the fixed effect model. The heterogeneity was assessed using the I 2 test. The heterogeneity was considered as 25% – low, 50% – moderate and 75% – high. The publication bias was assessed visually through a ‘funnel plot’. It was plotted using [log (OR)] and standard error of efficacy and safety outcomes.
The sensitivity analyses of efficacy and safety outcomes were performed based on the risk of bias assessment as per ROB-II tool. The meta-analytic summary was estimated by excluding studies showing ‘some concern’ or ‘high’ risk of bias.
The meta-analysis was performed through ‘Review manager software version 5.3′.
Results
Study characteristics
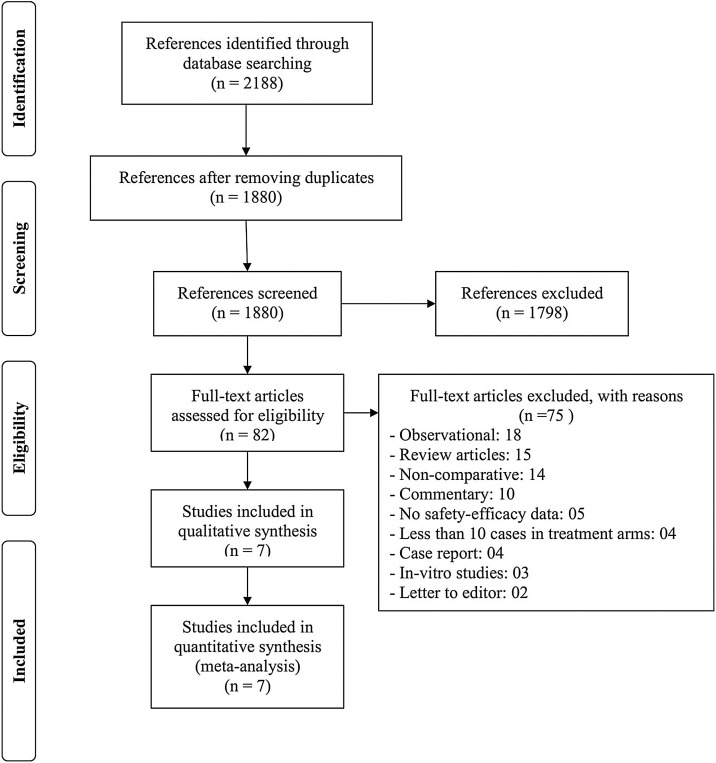
From the literature search, we retrieved 2188 references and assessed 82 full-text articles. A total of 7 randomized controlled studies fulfilling the selection criteria were included in the analysis (Fig. 1 ). Among 7 included studies, there were total 9 control interventions with five different modalities: supportive care (n = 4), umifenovir (n = 2), novaferon (n = 1), lopinavir-ritonavir + novaferon (n = 1) and lopinavir-ritonavir + interferon beta 1b + ribavirin (n = 1). Umifenovir is a generic name of arbidol. It is used as an antiviral drug for influenza in Russia and China. Novaferon is a recombinant anti-tumour and anti-virus protein, which is developed using cDNA sequences of 12 human interferon subtypes. Its nucleotide sequences are 89% homologous to human interferon α-2b. The general characteristics of all included studies are presented in Table 1 [10], [11], [12], [13], [14], [15], [16].
Fig. 1.
Study selection–PRISMA flow diagram.
Table 1.
General and baseline characteristics of included studies.
| Study | Design | Study period (Months-Year) and Study site | Study Population | Total number of participants randomized/completed | Age | Male Gender (%) | Co-existing conditions (%) | Severity status (%) | Drug, dose and duration of interventions |
Follow up duration (Days) | Outcome evaluated | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD years/Median (IQR)* | Median (IQR)* | LR | Comparator | |||||||||
| Cao et al., 2020 [10] | Randomized, Open labelled trial | Jan 2020 to Feb 2020 (China) | All Hospitalized COVID-19 patients with evidence of lower respiratory tract involvement | Total: 199/196LR: 99/96 SC: 100/100 |
LR: 58.0 (50.0–68.0)*SC: 58.0 (48.0–68.0)* | LR: 61.6SC: 59.0 | LR: DM (10.1) CrVD (5.1) SC: DM (13.0) CrVD (8.0) |
NEWS2 score at Day 1 LR: 5.0 (4.0–6.0)* SR: 5.0 (4.0–7.0)* |
LR (400–100 mg) orally every 12 h for 14 days | SC | 28 | Mortality, Virological clearance, Adverse events |
| Horby et al., 2020 [11] | Randomized, Open labelled trial | March 2020 to June 2020 (United Kingdom) | All Hospitalized COVID-19 patients | Total: 5040/4806LR: 1616/1394 SC: 3424/3412 |
LR: 66.0 (16.0)SC: 66.4 (15.8) | LR: 60.0SC: 61.0 | LR: DM (27.0) HD (25.0) SC: DM (28.0) HD (27.0) |
MV at randomization LR: (4.0)SR: (4.0) |
LR (400–100 mg) orally every 12 h for 10 days or until discharge | SC | 28 | Mortality |
| Hung et al., 2020 [12] | Randomized, Open labelled trial | Feb to March 2020 (China) | All Hospitalized COVID-19 patients | Total: 127/126LR: 41/40LR + IFB + RV: 86/86 |
LR: 52.0 (33.5–62.5)LR + IFB + RV: 51.0 (31.0–61.3) |
LR: 56.0LR + IFB + RV: 52.0 |
LR: DM (15.0) HT (32.0) LR + IFB + RV: DM (13.0) HD (27.0) |
Baseline NEWS2 score LR: 2 (2–2)LR + IFB + RV: 2 (1–2) |
LR (400–100 mg) orally every 12 h for 14 days | LR (400–100 mg) orally every 12 h, IFB 1 to 3 doses alternate day (8 MIU) SC, RV 400 mg every 12 h for 14 days | 30 | Mortality, Adverse events |
| Li et al., 2020 [13] | Randomized, Open labelled trial | Jan to April 2020** (China) | Mild-Moderate COVID-19 patients | Total: 86/86LR: 34/34UF: 35/35 SC: 17/17 |
LR: 50.7 (15.4)UF: 50.5 (14.6)SC: 44.3 (NS) | LR: 50.0UF: 45.7SC: 41.2 | Underlying chronic diseases LR: (20.6) UF: (14.3) SC: (35.3) |
Moderate cases LR: (82.4) UF: (94.3) SR: (82.4) |
LR (400–100 mg) orally every 12 h for 7–14 days | Umifenovir (200 mg) every 8 h for 7–14 days SC | 21 | Mortality, Virological clearance, Radiological improvement, Adverse events |
| Nazomi et al., 2020 [14] | Randomized, Open labelled trial | April to June 2020 (Iran) | All Hospitalized COVID-19 patients | Total: 100/100LR: 50/50 UF: 50/50 |
LR: 56.2 (14.8)UF: 56.6 (17.8) | LR: 54.0UF: 66.0 | LR: DM (26.0) HT (34.0) UF: DM (30.0) HT (44.0) |
Severe patients LR: (22.0) UF: (24.0) |
LR (400–100 mg) orally every 12 h for 10 days or until discharge Plus HCQ (400 mg) on Day 1 |
Umifenavir(200 mg) every 8 h for 7–14 days SC Plus HCQ (400 mg) on Day 1 |
30 | Mortality, Virological clearance, Radiological improvement, Adverse events |
| Pan et al., 2020 [15] | Randomized, Open labelled trial | March to October 2020 (30 countries of Africa, America, Asia, Europe) | Hospitalized COVID-19 patients | Total: LR: 1411/1399 SC: 1380/1372 |
LR: NSSC: NS | LR: 60.8SC: 58.4 | LR: DM (24.4) HD (20.6) SC: DM (23.6) HD (21.1) |
LR: Ventilated (8.0)SC: Ventilated (8.3) |
LR (400–100 mg) orally every 12 h for 14 days | SC | 28 | Mortality |
| Zheng et al., 2020 [16] | Randomized, Open labelled trial | NS (China) | All Hospitalized moderate to severe COVID-19 patients | Total: 89/89LR: 29/29NF: 30/30LR + NF: 30/30 |
LR: 37.0 (26.0–54.0)*NF: 46.5 (40.0–63.8)*LR + NF: 50.0 (37.8–62.8) |
LR: 41.4NF: 56.7LR + NF: 43.3 |
LR: DM (6.9) HT (3.3) NF: DM (10.0) HT (6.7) LR + NF DM (10.0) HT (10.0) |
Severe patients LR: (3.4)NF: (6.7)LR + NF: (6.7) |
LR (400–100 mg) orally every 12 h – Duration NS | NF: NF (20 μg) every 12 h inhalation – Duration NS LR + NF: LR (400–100 mg) orally every 12 h – Duration NS + NF (20 μg) every 12 h inhalation – Duration NS |
09 | Virological clearance, Adverse events |
**As per clinicaltrials.gov; LR: lopinavir-ritonavir; SC – supportive Care; NF: novaferon (recombinant anti-tumour and anti-virus protein); IFB: interferon beta; RV – ribavirin; UF – umifenovir; NS: not specified; DM – diabetes mellitus; CrVD – cerebrovascular disease; HT – hypertension; HD – heart disease; NEWS2 – national early warning score 2; MV – mechanical ventilation.
Cao et al. conducted an open labelled randomized study with a follow-up of 28 days. Of 199 randomized participants, 99 received lopinavir-ritonavir and 100 received standard care only. Participants in both groups were comparable at baseline for the age, gender, clinical features, laboratory values, comorbid conditions, National Early Warning Score 2 (NEWS2), days from illness to randomization and severity status. NEWS2 at admission is calculated based on respiration rate, oxygen saturation, systolic blood pressure, pulse rate and level of consciousness. Each parameter is scored from 0 to 3. The total possible score of NEWS2 ranges from 0 to 20. A higher score indicates greater clinical risk and the need for intensive monitoring. Both groups were comparable for the use of vasopressors, antibiotic agents and glucocorticoid therapy during the study period. However, the mean viral load was higher in patients who received lopinavir-ritonavir than those who received standard care alone (4.4 ± 2.0 vs. 3.7 ± 2.1) [10].
Horby et al. conducted an open labelled randomized study with a follow-up of 28 days. Of 5040 randomized participants, 1616 received lopinavir-ritonavir and 3424 received standard care only. Participants in both groups were comparable at baseline for the age, gender, ethnicity, comorbid conditions and requirement of respiratory support. The median duration of lopinavir-ritonavir treatment was 5 days (IQR: 2–8). Both groups were comparable for the use of azithromycin, dexamethasone, tocilizumab and convalescent plasma [11].
Hung et al. conducted an open labelled randomized trial with a follow-up of 30 days. Of 127 randomized participants, 41 assigned to receive lopinavir-ritonavir and 86 combined therapy (lopinavir-ritonavir, ribavirin and interferon beta-1b). Both groups were comparable at baseline for the age, gender, severity status, comorbidities, clinical presentations, radiological findings, days from illness to randomization, NEWS2 score and sequential organ failure assessment (SOFA) score and use of corticosteroids [12].
Li et al. conducted an open labelled, randomized study with a follow-up of 21 days. Of 86 randomized participants, 34 received lopinavir-ritonavir, 35 umifenovir and 17 standard care only. Participants in all three groups were comparable at baseline for the age, gender, clinical features, laboratory values, comorbid conditions, days from illness to treatment initiation, severity status and use of glucocorticoids [13].
Nozomi et al. conducted an open labelled, randomized study with a follow-up of 21 days. Of 100 randomized participants, 50 received lopinavir-ritonavir and 50 umifenovir. All participants received hydroxychloroquine 400 mg on day 1. Participants in all both groups were comparable at baseline for age, gender, clinical features, comorbid conditions and severity status [14].
Pan et al. conducted an open labelled, randomized study with a follow-up of 28 days. Of 2791 randomized participants, 1411 received lopinavir-ritonavir and1380 umifenovir. Participants in all both groups were comparable at baseline for age, gender, comorbid conditions, previous days in the hospital before randomization, baseline respiratory support and severity status [15].
Zheng et al. conducted an open labelled, randomized study with a follow-up of 9 days. Of 89 randomized participants, 29 received lopinavir-ritonavir, 30 novaferon and 30 combinations of lopinavir-ritonavir and novaferon. Participants in all both groups were comparable at baseline for age, gender, clinical features, comorbid conditions and severity status. The median time from symptom onset to randomization was longer in the combination group of noveferon and lopinavir-ritonavir than individual drug groups [16].
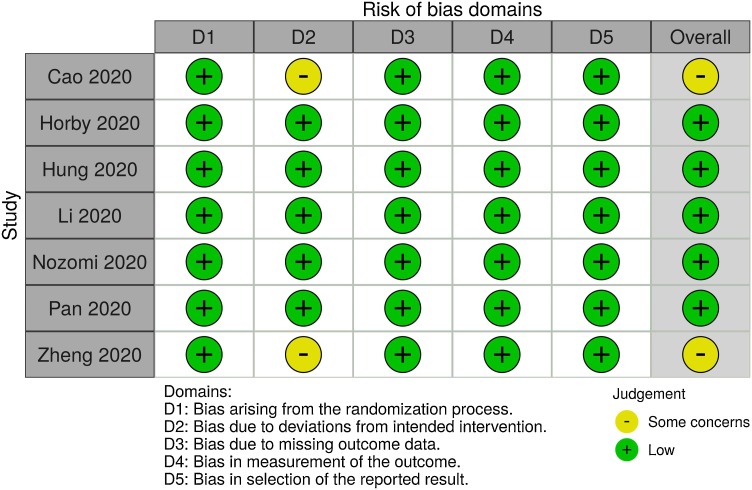
Risk of bias in included studies
The risk of bias assessment in individual randomized controlled trials is presented in Fig. 2 . Two randomized controlled trials studies were considered of having a ‘some concern’ as per ROB-II tool [10], [16]. The other studies were considered of having a ‘low’ risk of bias.
Fig. 2.
Traffic light plot showing risk of bias assessments for each individual study.
Efficacy outcomes
Mortality
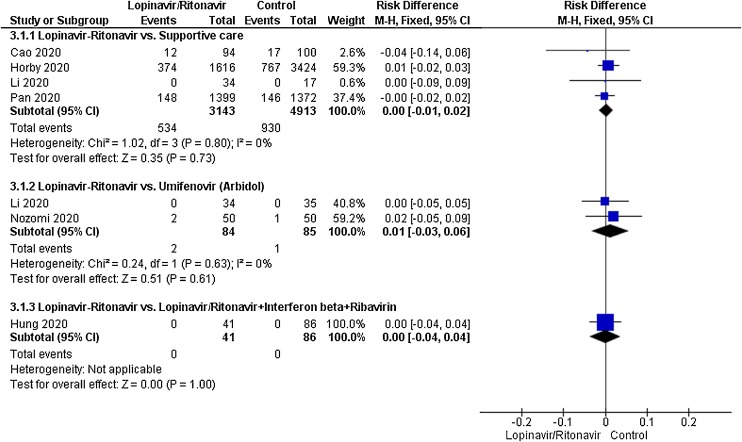
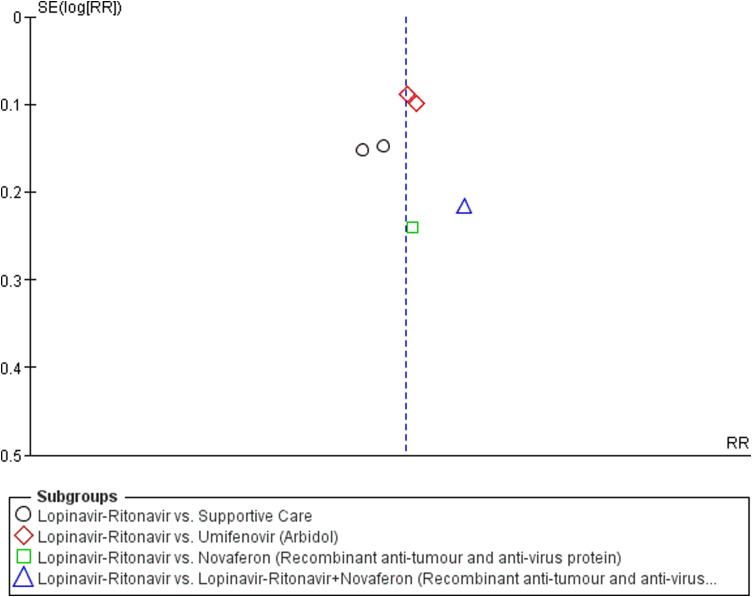
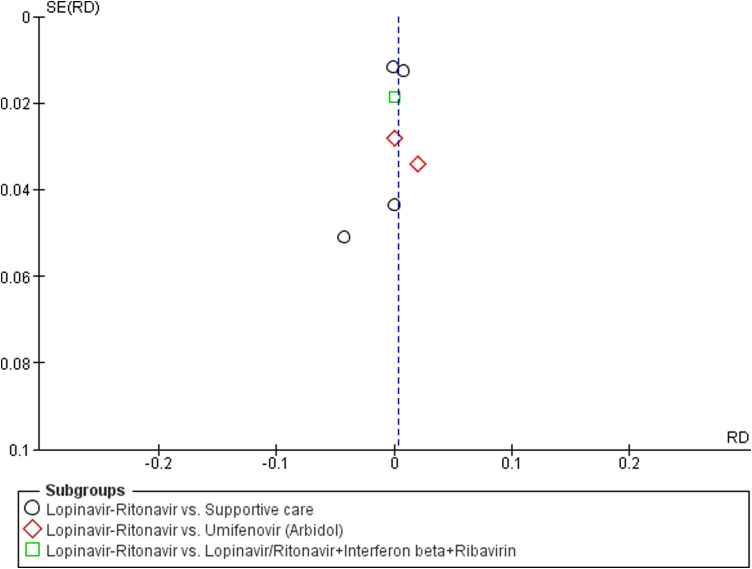
A total of six studies contributed to mortality data analyses. The control interventions were supportive care in 4 studies, umifenovir in 2 studies, novaferon in one stud and combined use of lopinavir-ritonavir, interferon-beta and ribavirin in 1 study. There was no significant difference in risk reduction between lopinavir-ritonavir and any of the control interventions in terms of mortality outcome (Fig. 3). An I 2 of 0% suggested a low degree of between-trial heterogeneity. The funnel plot was asymmetrical on visual inspection (Supplementary Figure File – Supplementary Figure 1a). A sensitivity analysis did not affect this outcome.
Fig. 3.
Meta-analytic summary of mortality outcome based on the type of control interventions through fixed effect model.
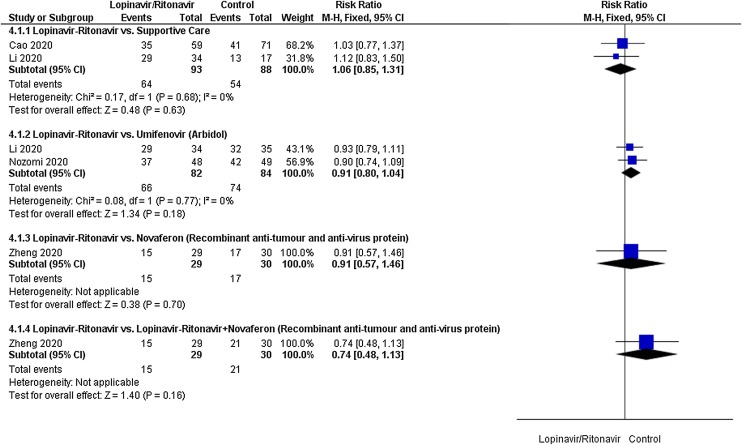
Virological cure
A total of four studies contributed to virological cure analyses. The control interventions were supportive care in 2 studies, umifenovir in 2 studies, novaferon in one stud and combined use of novaferon and lopinavir-ritonavir in 1 study. As shown in Fig. 4 , there was no significant difference between lopinavir-ritonavir and any of the control interventions in improving virological cure. An I 2 of 0% suggested a low degree of between-trial heterogeneity. The funnel plot was asymmetrical on visual inspection (Supplementary Figure File – Supplementary Figure 1b). A sensitivity analysis did not affect this outcome.
Fig. 4.
Meta-analytic summary of virologocal cure parameter based on the type of control interventions through fixed effect model.
Radiological improvement
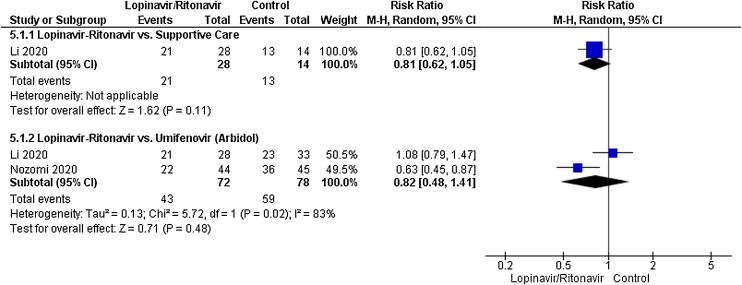
Two studies contributed to radiological improvement analyses. The control interventions were supportive care in 1 study and umifenovir in 2 studies. As shown in Fig. 5 , there was no significant difference between lopinavir-ritonavir and supportive care or umifenovir in improving virological cure. An I 2 of 83% suggested a high degree of between-trial heterogeneity among studies evaluating lopinavir-ritonavir and umifenovir.
Fig. 5.
Meta-analytic summary of radiological improvement parameter based on the type of control interventions through random effect model.
Safety outcomes
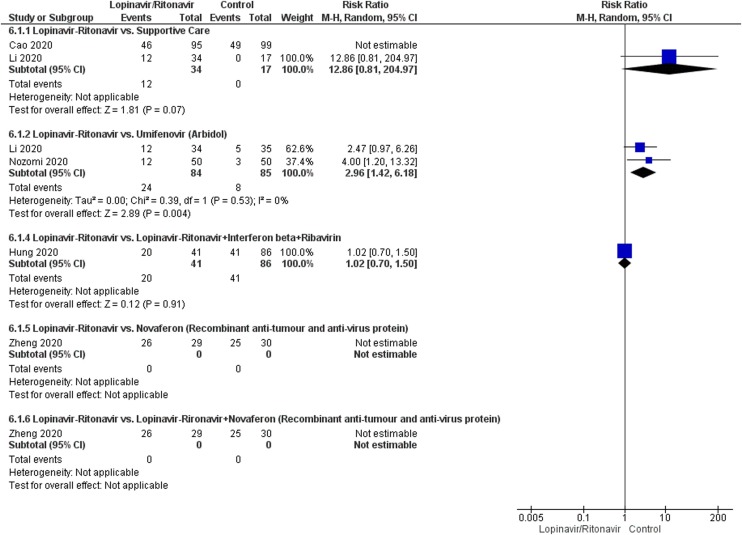
Adverse events
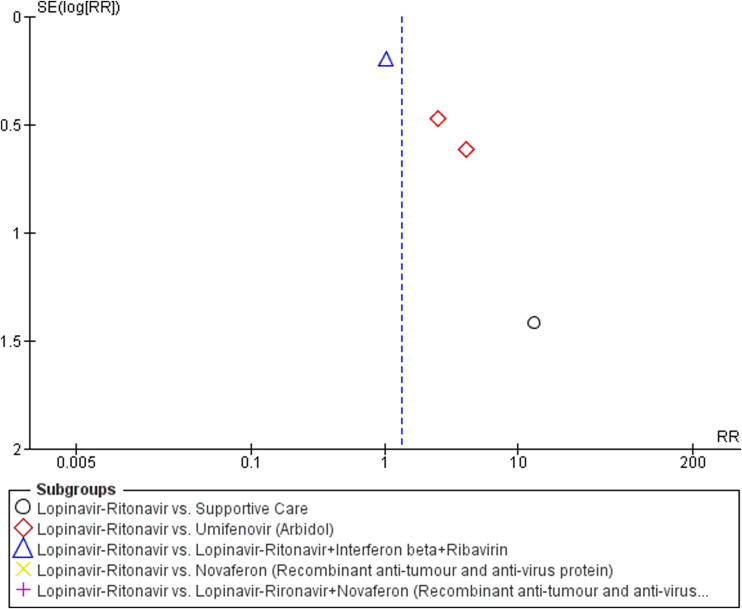
A total of five studies contributed to adverse event data analyses. The control interventions were supportive care in 2 studies, umifenovir in 2 studies, combined use of lopinavir-ritonavir, interferon-beta and ribavirin in 1 study, novaferon in one stud and combined use of lopinavir-ritonavir and novaferon in 1 study. As shown in Fig. 6 , we found no difference in adverse events in patients treated with lopinavir-ritonavir than supportive care [RR:2.59 (95% CI: 0.17, 38.90); I 2 = 75%]. Similarly, no difference was observed between lopinavir-ritonavir and novaferon or other combined intervention groups. However, we observed a significantly higher risk of adverse events in lopinavir-ritonavir treated patients than with the umifenovir [RR:2.96 (95% CI: 1.42, 6.18)]. An I 2 of 0% suggested a low degree of between-trial heterogeneity. The funnel plot was asymmetrical on visual inspection (Supplementary Figure File – Supplementary Figure 1c). A sensitivity analysis did not affect safety outcome.
Fig. 6.
Meta-analytic summary of adverse events based on the type of control interventions through random effect model.
Discussion
The pooled meta-analytic summary indicates a search for the better drug against COVID-19 should go beyond the lopinavir-ritonavir. We observed no benefits of lopinavir-ritonavir in the treatment of COVID-19. It did not affect mortality, virological cure and radiological improvement in COVID-19 patients. A similar trend was observed in the sensitivity analysis. We observed no difference in adverse events in patients treated with lopinavir-ritonavir as compared to control interventions.
Our findings emphasize the need to evaluate drug repurposing in the setting of randomized controlled trials before its widespread use [17]. In vitro efficacy of Lopinavir-ritonavir against COVID-19 is not reflected in clinical studies. This could be because of a lack of reaching enough minimum effective plasma concentration of lopinavir-ritonavir against SARS-CoV-2 in COVID-19 patients. Alvarez et al. observed that clinically tested dose of lopinavir-ritonavir (400 mg + 100 mg) would not achieve minimum effective concentration in 50% of patients [18]. Another reason could be due to the difference in protease enzyme present in HIV and coronavirus. In the case of HIV, the protease enzyme belongs to the aspartic protease family, whereas in coronavirus protease enzyme is of the cysteine protease family. Another important difference is the catalytic site – C2 symmetry, which is only present in the HIV protease enzyme [19]. The earlier evidence of the therapeutic utility of lopinavir-ritonavir was not robust against SARS and MERS [8]. In the case of SARS, lopinavir-ritonavir was studied in two retrospective studies (one with historical comparator) in combination with ribavirin [20], [21]. In the case of MERS, therapeutic evidence was based on two case reports in combination with ribavirin and interferon-alpha [22], [23].
Lopinavir-ritonavir was compared with four different treatment modalities in the included studies. The control interventions include supportive care, umifenovir, novaferon, novaferon + lopinavir-ritonavir and lopinavir-ritonavir + interferon beta 1b + ribavirin. All of them are repurposed drugs or their combinations. They are used in the treatment of COVID-19 based on in vitro data or an earlier experience with the out-brakes of influenza, SARS and MERS [4], [8], [24]. Their clinical evidence in COVID-19 is limited.
This study has several limitations. Our findings on lopinavir-ritonavir should be interpreted cautiously due to the inclusion of only open labelled randomized studies. The analysis of efficacy and safety outcomes are based on the small number of studies in each control intervention.
In conclusion, Lopinavir-ritonavir do not improve virological cure, radiological findings, mortality in COVID-19 patients. The findings need to be confirmed through a randomized double-blind controlled trial.
Funding
No funding sources.
Ethical approval
Not required.
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jiph.2021.03.015.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020;59:1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 5.Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang C.K., Seong M.W., Choi S.J., Kim T.S., Choe P.G., Song S.H. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J Intern Med. 2020;35:782–787. doi: 10.3904/kjim.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S.J., Yu K.M., Kim Y.I., Kim S.M., Kim E.H., Kim S.G. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio. 2020;11:e01114–e01120. doi: 10.1128/mBio.01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus – a possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I. A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 10.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Collaborative Group Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: anexploratory randomized controlled trial. Med. 2020;1:105–113. doi: 10.1016/j.medj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nojomi M., Yassin Z., Keyvani H., Makiani M.J., Roham M., Laali A. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect Dis. 2020;20:954. doi: 10.1186/s12879-020-05698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Solidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V. Repurposed antiviral drugs for Covid-19 – interim WHO solidarity trial results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. pp. NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng F., Zhou Y., Zhou Z.Z., Ye F., Huang B., Huang Y. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int J Infect Dis. 2020;99:84–91. doi: 10.1016/j.ijid.2020.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldenburg C.E., Doan T. Rigorous randomized controlled trial implementation in the era of COVID-19. Am J Trop Med Hyg. 2020;102:1154–1155. doi: 10.4269/ajtmh.20-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez J.C., Moine P., Davido B., Etting I., Annane D., Larabi I.A. Population pharmacokinetics of lopinavir/ritonavir in Covid-19 patients. Eur J Clin Pharmacol. 2020:1–9. doi: 10.1007/s00228-020-03020-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., De E., Clercq Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 20.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 21.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S. Group HUSS.Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanakis N., Tsiodras S., Haagmans B.L., Raj V.S., Pontikis K., Koutsoukou A. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. 2014;44:528–532. doi: 10.1016/j.ijantimicag.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antivir Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 24.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.