Abstract
Objective:
The objective of this study was to evaluate the in vitro performance of endodontic sealers in their freshly mixed and set forms.
Methods:
The commercially used endodontic sealers (AH Plus, Dia-ProSeal, GuttaFlow 2, and Pulpdent Root Canal Sealer) were investigated and the chemical structure of freshly mixed and set sealers were assessed with Fourier transform infrared spectroscopy (FTIR). The surface morphology and elemental analysis were assessed with a scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy. The pH and solubility analysis were performed and the cytotoxicity was done on extracts of freshly mixed and set materials using Alamar blue assay. One way ANOVA and Post Hoc Tukey analysis was used to do multiple comparison analysis of the mean values and standard deviation results through SPSS version 20 (IBM Software, NY, USA) for pH, solubility, and cytotoxicity analysis.
Results:
FTIR analysis revealed the structural pattern and the difference in freshly mixed and set samples was observed with the change in intensities of the peaks. The morphological pattern revealed the presence of micro/nano-particles with pores distributed throughout their structure. The sealer with the least solubility was AH Plus (0.10±0.01) followed by Dia-ProSeal (0.77±0.25), GuttaFlow 2 (1.88±0.82) and Pulpdent Root Canal Sealer (3.03±0.18). The solubility of AH plus was significantly lower (P<0.05) in comparison to GuttaFlow 2 and Pulpdent Root Canal Sealer. The highest pH (10.09±0.034) in the freshly mixed state and highest cytotoxicity in the freshly mixed (70.08±5.852) and set sealers (83.87±5.409) (P<0.05) at day 7 was observed in Dia-ProSeal. GuttaFlow 2 was the most biocompatible sealer in the set state and AH Plus was the most biocompatible sealer in the freshly mixed state at day 7.
Conclusion:
Clinically, the sealer is applied in fresh state, whereby this study signifies that which material is more biocompatible in fresh state and provides insight information to clinicians. AH Plus showed least solubility and cytocompatibility in fresh state compared to other groups.
Keywords: Endodontic sealers, cytotoxicity, morphology, pH, solubility
HIGHLIGHTS.
The morphological pattern revealed the presence of micro/nano-particles with pores distributed throughout their structure
The lowest solubility was indicated by AH Plus followed by Dia-ProSeal, GuttaFlow 2 and Pulpdent Root Canal Sealer.
The highest pH and cytotoxicity in fresh sealers and set sealers at day 7 was observed in Dia-ProSeal.
GuttaFlow 2 was the most biocompatible sealer in the set state and AH Plus was the most biocompatible sealer in the freshly mixed state.
INTRODUCTION
Root canal filling materials have been refined with the evolution of dentistry (1-3). Regardless of the advanced root canal materials like gutta percha and innovative methods, the seal is inadequate and impervious without the use of root canal sealers (4). The use of an endodontic sealer is obligatory to fill the space between the dentinal wall and the interface of the obturating cone (5). Root canal sealers not only fill the voids to a greater extent but also flow into the irregularities of a root canal and simultaneously in the accessory and lateral canals that cannot be sealed by gutta percha cones (6). Several root canal sealers are available in the market; zinc oxide eugenol based, glass ionomer, calcium hydroxide, calcium silicate, and methacrylate resin based and epoxy resin-based sealers are the most common (7).
During root canal filling, the root canal sealer can inevitably extrude through the apical foramen and extend into the periradicular tissues (8). This may result in clinical symptoms such as pain, swelling, dysesthesia, and paresthesia (9). The extrusion of root canal sealers can be detrimental to the proliferation of periradicular cells (10, 11). However, the extruded root canal sealer may or may not dissolve over time (12). Lateral and accessory canal may serve as a pathway for biological fluids and bacteria to enter into the root canals from periodontal tissues and can serve to degrade the sealer consequently leaching various components (13). Thus, the biological properties of a sealer play a crucial role as these sealers may come into contact with the periapical tissues subsequently stimulating the healing process and affecting the biological seal (14).
An ideal sealer should be biocompatible, bacteriostatic, should not show shrinkage on setting and it should not be soluble in tissue fluids whereby in case of extrusion from canal, it should be dissolved without causing any toxicity (5, 15). All root canal sealers exhibit some form of toxicity irrespective of their type (16). Therefore, cytotoxicity of sealers remains an issue even though newer sealers have been introduced due to their high biocompatibility (10). A previous study assessing the cytotoxicity of set endodontic sealers on human periodontal ligament cells suggested that silicone-based sealers have higher cell viability as compared to epoxy resin sealers and calcium silicate-based sealers (17). Little information is available on the biocompatibility of new sealers available in the market (18). In order to identify the functional groups present in the sealers that may be responsible for cytotoxicity of the endodontic sealers, Fourier Transform Infrared Spectroscopy (FTIR) can provide a reliable data to assess chemical structures (19, 20). Scanning electron microscopy (SEM) and the objective of elemental mapping using energy dispersive X-ray spectroscopy (EDX) is also another technique to reveal the elements in the constituents that may contribute to the cytotoxicity of these sealers (21, 22). This study aimed to evaluate the pH, solubility, structural pattern and in vitro cytotoxic effects of various endodontic sealers in their freshly mixed and set states. Whereby the cells were exposed to freshly mixed and set sealers to investigate the biocompatibility of commercially available sealers in both phases.
In clinical situations the root canal sealer is introduced into the root canal in a freshly mixed state; however, even after setting it may exert cytotoxic effects by releasing harmful constituents. Hence, the evaluation of biocompatibility in both the states is important. It is anticipated that it would help clinicians in making a better choice among the sealers during a clinical setting based on the results of biocompatibility. Furthermore, the effect of pH and solubility would determine their role in relation to biocompatibility.
MATERIALS AND METHODS
Prior to the start of experimental work, ethical approval was obtained from the institutional ethical committee (Ref. No. IIDC/IRC/2020/02/006). The purpose of this study was to compare four commonly available endodontic sealers. The composition of these commercial endodontic sealers is given in Table 1. Each endodontic sealer was mixed with sterile instruments according to the recommendations of the manufacturer. The freshly mixed samples were utilised immediately. The set samples were mixed and allowed to set for 24 hours before testing (23). A total of 120 samples were prepared from all four commercial sealers (n=30) group and were structurally, morphologically, physically, and biologically analysed.
TABLE 1.
Composition of commercial endodontic sealers
| Tested material | Manufacturer | Constituents |
|---|---|---|
| AH Plus | Dentsply, Konstanz, Germany | Paste A: Bisphenol-F epoxy resin, zirconium oxide, Bisphenol-A epoxy resin, silica, calcium tungstate, and Iron oxide pigments Paste B: Tricyclodecanediamine, dibenzyldiamine, aminoadamantane zirconium oxide, calcium tungstate, silicone oil, and silica |
| GuttaFlow 2 | Coltene, Langenau, Switzerland | Polydimethylsiloxane (PDMS), gutta-percha powder, zirconium dioxide, platinum catalyst, coloring agents, micro silver |
| Dia-ProSeal | DiaDent Group, Cheongju-si, Korea. | Base: Bisphenol-F epoxy resin, Bisphenol A-co-epichlorohydrin, zirconium oxide, siloxanes and silicones, calcium hydroxide, iron oxide, Catalyst: zirconium oxide, hexamethylenetetramine, siloxanes, silicones, calcium tungstate, calcium hydroxide |
| Pulpdent Root Canal Sealer | Pulpdent Corp. Watertown, U.S.A. | Powder: Zinc stearate, zinc oxide, barium sulphate, and calcium phosphate Liquid: Canada balsam and eugenol |
Fourier transform infrared spectroscopy (FTIR)
A comparative structural analysis of freshly mixed and set sealers (n=6) was performed with a FTIR spectrophotometer (Thermo Scientific Nicolet 6700, ThermoFisher Scientific, Waltham, United States) and spectra were obtained in a spectral range of 4000-500 cm-1 following the procedure mentioned previously (19).
Scanning electron microscopy (SEM)
Morphological and surface analysis of the set endodontic sealers was performed with SEM (Tescan Vega, Brno, Czech Republic). Teflon moulds (4 mm diameter and 1.5 mm height) were used to prepare samples. The sealers along with the moulds were stored at a relative humidity of 95% in a water bath. The temperature was maintained at 37ºC for 48 h. Later, samples were coated with gold sputter (Quoram Technologies, Lewes, UK) for 90 seconds and morphological assessment of these sealers was achieved with an accelerating voltage of 10-20 kV at various magnifications. In order to assess the morphology of the sealers closely, the images were taken at 500X, 1000X, 5000X, and 10,000X. Energy Dispersive X-ray (EDX) microanalyzer (Oxford Instruments, Abingdon, UK) was employed for the elemental mapping of the surface of set endodontic sealer samples.
pH analysis
Freshly mixed and set states of sealers (n=6) were used to evaluate the pH at 1.3 and 7 days with a SevenCompact pH meter S220 (Mettler Toledo, Columbus, United States) in order to establish a comparison. Prior to use, the pH meter was calibrated using buffer solutions (pH ~ 4, 7, and 10). The sealer samples were prepared in Teflon moulds with a 5 mm internal diameter and 5 mm height and pH was analysed according to the procedure mentioned earlier (24). The freshly mixed samples were immediately placed in 15 mL sterile capped centrifuge tubes (ThermoFisher Scientific, Waltham, United States) containing 7.5 mL distilled water and the pH of this water containing samples was measured after 1, 3 and 7 days. The set samples were kept in in 15 mL sterile capped centrifuge tubes (ThermoFisher Scientific, Waltham, United States) for one day at 37ºC to ensure complete setting of all the samples. After one day 7.5 mL of distilled water was added to these samples. The pH of this water containing samples was measured after 1, 3 and 7 days. To avoid any discrepancy and errors in the values of pH the centrifuge tubes containing the endodontic sealers and distilled water were slightly shaken before the readings were obtained.
Solubility analysis
The samples from each group (n=6) were prepared in 5 x 5 mm2 Teflon moulds and the solubility analysis was conducted as per ISO 6786 specifications (25). The endodontic sealers were mixed and manipulated according to the proportions specified by the manufacturer. Consequently, the mixed sealers were applied into the Teflon moulds with a 5 mm height and a 5 mm internal diameter. This was done in order obtain equal volumes of all the sealers and to establish a comparative evaluation.
This complete assembly containing sealers within the mould was stored in an incubator. The relative humidity in the incubator was maintained at 95% and the temperature was kept at 37ºC for a time period longer than the setting time by 100%. Removal of endodontic sealer samples from the cylindrical Teflon moulds was ensured after the completion of setting time. An analytical balance (Mettler Toledo, Columbus, United States) was utilised to weigh the removed samples thrice in order to avoid any discrepancies and errors. The mean weight for each of the endodontic sealer sample was recorded as W1. These sealer samples were subsequently placed in sterile 15 mL capped centrifuge tubes containing of 10 mL distilled water. These sealers were stored in the capped centrifuge tubes containing distilled water for a duration of 7 days. The samples were removed from the centrifuge tubes containing distilled water after the completion of 7 days. An absorbent paper and a desiccator (Thomas Scientific, Swedesboro, United States) was employed for the removal of excessive water from the samples in order to avoid error in the recorded values. With the help of the analytical balance (Mettler Toledo, Columbus, United States) the sample specimens were weighed again. Each sample was weighed three times and W2 was documented as the mean weight.
The following mathematical calculation was performed in order to achieve a solubility evaluation of each endodontic sealer specimen (26):
Solubility (%)=(W1-W2)/W1x100
Cytotoxicity analysis
The eluents of the endodontic sealers of each group (n=6) were prepared for equal volumes of both freshly mixed and set states according to ISO 10993-5 specifications (27). The samples were prepared in 5x5 mm2 Teflon moulds. To obtain the eluent of samples, the mixed endodontic sealers were stored for a duration of 24 h at 37ºC. Subsequently, Dulbecco’s modified Eagle’s medium (DMEM) was added as 1.25 cm2/mL to these samples. The freshly mixed samples after mixing were instantaneously placed in sterile tubes. The culture medium containing the samples was incubated at 37ºC for 24 h before the removal of extract. Extracts were prepared and generated for freshly mixed and set form of endodontic sealers to assess cytotoxicity. Six samples were prepared for each of the freshly mixed and set forms of endodontic sealers to assess cytotoxicity. Whereby, 100% concentrated extracts of sample groups with no dilution were used to assess the cytotoxicity of each group of sealer and were compared to a negative control group.
L929 fibroblasts (ATCC cell line CCL 1, NCTC clone 929) were utilised for analysing the cytotoxicity of endodontic sealers and were cultured as described previously (28).
The following mathematical equation was used to calculate the cell viability (29):
Cell viability (%)=(Mean Optical Density of Test Group/Mean Optical Density of Control Group)X100%
Alamar blue assay
Alamar blue assay was performed with an alamarBlue™ Cell Viabilty Reagent (ThermoFisher Scientific, Waltham, United States). Alamar blue assay was employed to evaluate cell proliferation and metabolic activity after exposure to the extract generated out of freshly mixed and set forms of sealers. The cells were seeded at 1x104 cells in a well after the cells were shifted into a 96 well plate from a 24 well plate (Sigma Aldrich, St. Louis, United States). The cells were incubated in this new well plate for a period of 24 h to allow the attachment. Subsequently, the cell culture medium was removed and replaced with 50 µL extracts of freshly mixed and set state of sealers. This was done for all of the wells in the 96 well plate except for the control group. The cells were incubated with these extracts for a time period of 1, 3, and 7 days. The morphological pattern of the cells was observed under an inverted microscope (Euromex, Arnhem, Netherlands) after 7 days. After the completion of each incubation period, the endodontic sealer extract in each well was replaced with 10% Alamar Blue Agent for 4 h and absorbance values were calculated from microplate reader at 590 nm. Each sealer extract was evaluated in triplicates. The cell viability of all the sealers was evaluated in comparison to the fixed cell viability of the control group that was 100%. The following mathematical equation was used to calculate the cell viability:
Cell viability (%)=(Mean Optical Density of Test Group/Mean Optical Density of Control Group)X100%
The cytotoxicity response of materials was rated as non-cytotoxic (cell viability >90%), mild (cell viability 60-90%), moderate (cell viability 30-60%) and severe (30%) (30).
Statistical analysis
One way ANOVA and Post Hoc Tukey analysis was used to do multiple comparison analysis of the mean values and standard deviation results through SPSS version 20 (IBM Software, NY, USA) for pH, solubility, and cytotoxicity analysis. The confidence interval was 95% and the statistical significance was considered at P<0.05.
RESULTS
Fourier transform infrared spectroscopy (FTIR)
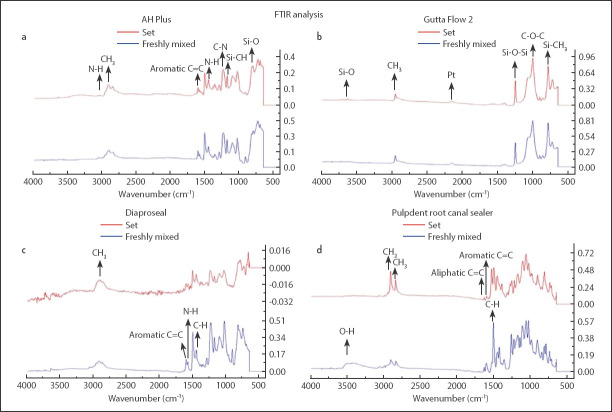
The characteristic structural peaks of freshly mixed and set samples of each group appeared in the FTIR analysis as shown in (Fig. 1 a-d). The changes in absorbance were observed after setting as tabulated in Table 2.
Figure 1.
(a) FTIR spectrum of freshly mixed and set AH Plus, (b) FTIR spectrum of freshly mixed and set GuttaFlow 2, (c) FTIR spectrum of freshly mixed and set Dia-ProSeal, (d) FTIR spectrum of freshly mixed and set Pulpdent Root Canal Sealer
TABLE 2.
Comparative change in spectral absorbance intensity of freshly mixed and set samples
| Groups | Freshly mixed | Set state Absorbance | |
|---|---|---|---|
| Wavenumber (cm-1) | Absorbance | ||
| AH Plus | |||
| N-H | 1456 | 0.239 | 0.208 |
| C-N | 1296 | 0.188 | 0.175 |
| C-N | 1239 | 0.137 | 0.310 |
| Si-O | 803 | 0.449 | 0.340 |
| Si-CH | 1183 | 0.248 | 0.345 |
| GuttaFlow 2 | |||
| CH3 (symmetric) | 2961 | 0.195 | 0.234 |
| Si-O-Si | 1257 | 0.422 | 0.505 |
| Si-O | 3643 | 0.088 | 0.129 |
| Dia-ProSeal | |||
| CH3 | 2920 | 0.103 | -0.011 |
| N-H | 1581 | 0.095 | 0.015, 0.015 |
| C=C (Aromatic) | 1605 | 0.122 | -0.015 |
| C-H | 1452 | 0.214 | -0.006 |
| C=C (bending peaks) | 825 | 0.471 | 0.007 |
| Si-O-Si | 1226 | 0.463 | |
| Pulpdent root canal sealer | |||
| O-H | 3420 | 0.096 | |
| C=C (Aliphatic) | 1637 | 0.048 | 0.094 |
| C=C (Aromatic) | 1606 | 0.095 | 0.121 |
| CH3 | 2913 | 0.135 | 0.463 |
| CH3 | 2843 | 0.116 | 0.351 |
| C-H | 1510 | 0.565 | 0.517, 0.499 |
| C-H | 1452 | 0.221 | 0.395 |
FTIR analysis of AH Plus
The comparative spectra of AH Plus showed an O-H band that appeared at 3500-3200 cm-1 and an N-H peak that appeared at 3028 cm-1. The peaks at 1604 cm-1 and 1508 cm-1 were assigned to aromatic groups, whereas the peak at 1456 cm-1 attributed to bending absorbance of N-H groups. The stretching vibration peaks of C-N appeared at 1290 cm-1 and 1239 cm-1. The peak at 1183 cm-1 was attributed to Si-CH due to the presence of silicone oil in AH Plus, while the epoxide band appeared at 1100-1030 cm-1. The peak at 803 cm-1 was attributed to Si-O. After setting, change in the intensity of peaks was observed, whereas, shifting and appearance of new peaks were not observed (Fig. 1a).
FTIR analysis of GuttaFlow 2
The FTIR analysis of freshly mixed GuttaFlow 2 revealed a peak of Si-O at 3643 cm-1. The asymmetric and symmetric stretching peak of CH3 appeared at 2961 cm-1 and 2820 cm-1 respectively. In the spectrum of the set sample, the peak of platinum was observed at 2156 cm-1. A peak of Si-O-Si was also visible at 1257 cm-1 and a peak of Si-CH3 was observed at 791 cm-1 in both freshly mixed and set GuttaFlow 2. A peak of C-O-C was also observed at 1126 cm-1 in both freshly mixed and set samples. The intensity of the peak observed for Si-O-Si at 1257 cm-1 was increased from 0.422 in the freshly mixed state to 0.505 in the set state (Fig. 1b).
FTIR analysis of Dia-ProSeal
The spectral analysis of freshly mixed Dia-ProSeal revealed a stretching peak of CH3 at 2920 cm-1. An aromatic peak of C=C and an N-H peak was observed at 1605 cm-1 and 1581 cm-1 respectively in the freshly mixed sample. This N-H peak was observed due to hexamethylenetetramine that is a constituent of Dia-ProSeal. A bending peak of C-H was observed at 1452 cm-1 in the spectrum of the unset sample. A peak of Si-O-Si was also observed in a freshly mixed sample at 1226 cm-1 due to the presence of siloxanes and silicones in this sealer. The spectrum also depicted a C=C bending peak at 825 cm-1. After setting, it was found that the intensity of the bending peak of the CH3 group was reduced from 0.103 to 0.011 due to the consumption of this group. The intensity of the aromatic peak of C-H at 1605 cm-1 was also reduced to 0.015 from 0.122 (Fig. 1c).
FTIR analysis of pulpdent root canal sealer
The freshly mixed samples of Pulpdent Root Canal Sealer revealed a stretching peak of the O-H group at 3500 cm-1. The asymmetric and symmetric stretching peaks of C-H were observed at 2913 cm-1 and 2843 cm-1 respectively. The aliphatic peak of C=C was observed at 1637 cm-1. Likewise, the aromatic peak of C=C was observed at 1606 cm-1 and the bending peaks of C-H were observed at 1510 cm-1 and 1452 cm-1.
After setting, the O-H peak completely disappeared due to the consumption of the group. The intensity of aliphatic and aromatic peaks of C=C was increased from 0.048 and 0.095 to 0.094 and 0.121 respectively. It was also revealed that the single bending peak of C-H (1510 cm-1) observed in the freshly mixed state was divided into two peaks (1505 cm-1 and 1536 cm-1) in the set state that was a consequence of consumption of C-H group that might be due to its reaction with the O-H group present in eugenol (Fig. 1d).
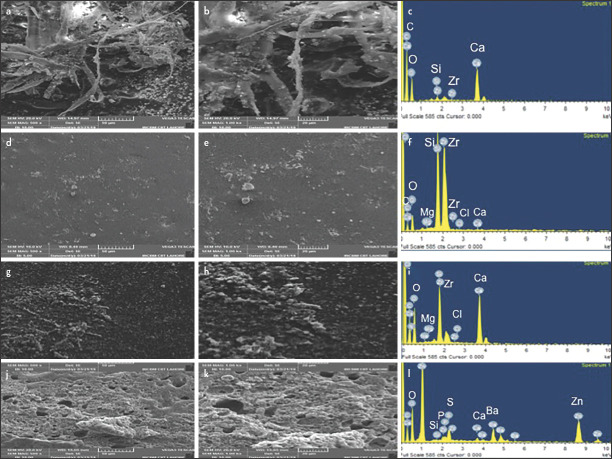
Scanning electron microscopy (SEM)
AH Plus
Surface analysis of set AH Plus revealed a fibrous polymeric structure (Fig. 2a, b). These fibers were distributed irregularly throughout this polymeric structure. It was observed that small-sized particles were attached to these fibers, whereby the size of these particles was in a range of 2-3 µm. Pores were also present in the polymeric structure of this sealer. EDX analysis of AH Plus displayed a high peak of Ca owing to the presence of calcium tungstate (Fig. 2c). Peaks of Zr and Si were also observed in this analysis due to the presence of ZrO2 and silica, respectively.
Figure 2.
SEM images of AH Plus (a) 500X and (b) 1000X show the fibrous structure and particles observed on fibrous structure (c) EDX spectrum of AH Plus confirms the presence of Ca and Zr, GuttaFlow2 (d) 500X, (e) 1000X images show nanoparticles on surface (f) EDX spectrum of GuttaFlow 2 shows elemental peaks of Ca, Zr, and Si, SEM images of Dia-ProSeal (g) 500X, (h) 1000X exhibit particles, whereby (i) respective EDX spectrum of Dia-ProSeal confirms the presence of Ca, Si, and Zr,, Pulpdent Root Canal Sealer images (j) 500X (k), 1000X show the porous structure and (l) Respective EDX spectrum of Pulpdent Root Canal Sealer shows the presence of Ca, C, Zr , O, P and Ba
GuttaFlow 2
Surface analysis of GuttaFlow 2 displayed a flat surface with smaller sized particles distributed throughout its structure (Fig. 2d, e). These particles were 250-200 nm in size and were uniformly distributed. No pores were observed in the structure of this sealer. EDX analysis revealed a high peak of Zr and Si due to the presence of zirconia nanoparticles and polydimethylsiloxane, respectively (Fig. 2f). Peaks of Ca, Cl, and Mg were also observed due to the presence of traces of these elements in its composition (Fig. 2f).
Dia-ProSeal
Surface analysis of Dia-Proseal revealed the presence of smaller sized particles in the polymeric structure (Fig. 2g, h). At higher magnifications, it was observed that these small-sized particles were fully embedded in this polymeric structure. However, these particles were much more evenly distributed as compared to the particles in AH Plus. These particles were much more spherical and the size of these particles was in a range of 300-1000 nm. The presence of pores in its structure was also confirmed in this analysis. The EDX analysis revealed the peak of Ca, Zr, Si, and N (Fig. 2i).
Pulpdent root canal sealer
SEM images exhibited an interconnecting matrix-like structure (Fig. 2j, k). These images unveiled the presence of different sized pores distributed throughout the structure. At higher magnifications, these images disclosed the presence of outgrowth of various sized particles that were present throughout the structure of this zinc oxide eugenol containing root canal sealer. In the EDX spectrum, an intense peak of Zn and Ba was observed and traces of Ca and P were present as calcium phosphate (Fig. 2l).
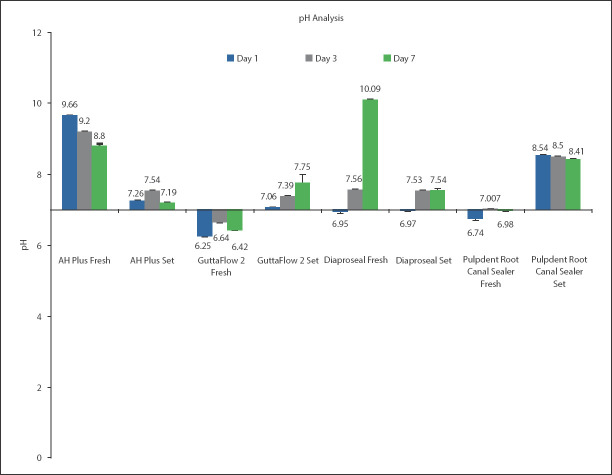
pH analysis
The pH of all freshly mixed and set samples was found to be different across all days. The only exception was observed for set Dia-ProSeal, whereby the comparison in pH was not significant between days 3 and 7 (Mean difference=-0.007) (P>0.05) (detailed description in supplemental files appendix 1). Statistically, non-significant difference was observed within groups at different time intervals. The comparative pH analysis of freshly prepared and set sample is given in Figure 3. The pH of AH Plus was basic in both fresh and set state, whereas, the pH of GuttaFlow 2 was acidic in fresh state and basic in set state . The pH of freshly mixed and set Dia-ProSeal was slightly acidic at day 1 and turned basic at days 3 and 7. The pH of freshly mixed Pulpdent Root Canal Sealer was acidic on day 1, whereas, it turned neutral on day 3 and was acidic at day 7, whereas, it was basic in set state (Fig. 3).
Figure 3.
pH of freshly mixed and set form of the same and different sealers at days 1, 3, and 7 (P<0.05 in same sealers at days 1,3 & 7) (P>0.05 at day 7 in comparison to set GuttaFlow 2 and Dia-Proseal) (P>0.05 at day 3 in comparison of set AH Plus and Dia-Proseal). The error bars denote standard deviation (SD)
Solubility analysis
The AH Plus group had the lowest solubility (0.10±0.01) followed by Dia-ProSeal (0.77±0.25), GuttaFlow 2 (1.88±0.82) and Pulpdent Root Canal Sealer (3.03±0.18). In the intergroup differences, the solubility change difference between groups is given in Table 3.
TABLE 3.
Solubility comparison between different root canal sealer materials; SE (Standard error)
| Material | Comparison material | Mean difference (SE) | P value |
|---|---|---|---|
| AH Plus | GuttaFlow 2 | -1.77(0.36) | 0.01 |
| Dia-Proseal | -0. 66 (0.36) | 0.48 | |
| Pulpdent Root Canal Sealer | -2.93 (0.36) | 0.001 | |
| Gutta Flow 2 | Dia-Proseal | 1.10 (0.36) | 0.13 |
| Pulpdent Root Canal Sealer | -1.16 (0.36) | 0.11 | |
| Dia-Proseal | Pulpdent Root Canal Sealer | -2.27 (0.36) | 0.001 |
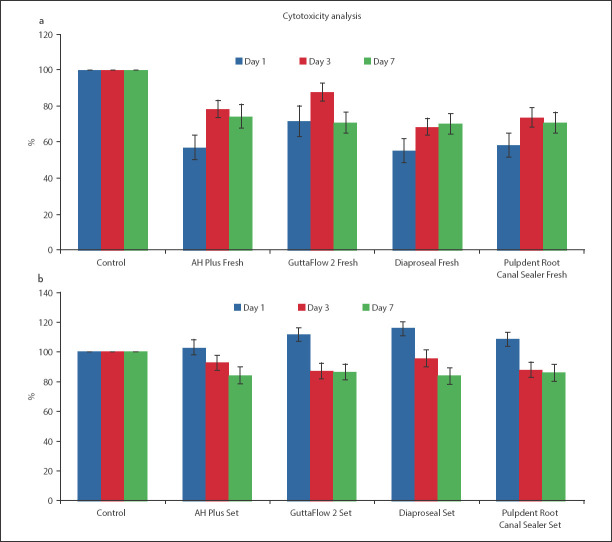
Cytotoxicity analysis
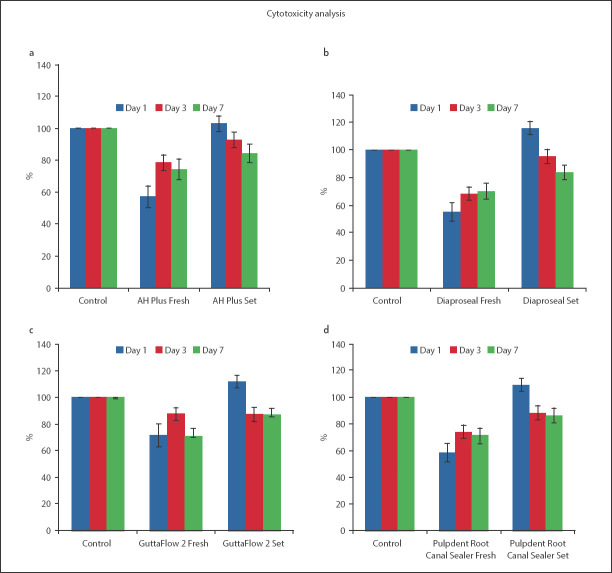
Fresh samples
There was no discernible trend in survival rates observed for AH Plus and GuttaFlow 2 group across days 1, 3, and 7 (Fig. 4a, b). The Dia-ProSeal samples indicated a clear trend in cell viability with time (Fig. 4c). Similar to the AH Plus and GuttaFlow 2 group the Pulpdent Root Canal Sealer group also had no discernible trend in cell viability across days 1, 3, and 7. For the AH Plus, GuttaFlow 2 and Pulpdent Root Canal sealer groups, the cell viability was maximum for day 3 (Fig. 4a, b, d). The cell viability for freshly mixed AH Plus, Dia-ProSeal, and Pulpdent Root Canal Sealer samples on day 1 was significantly lower from the cell viability on days 3 and 7 (P<0.05) (supplemental files appendix 3). Hence, these sealers were significantly more cytotoxic on day 1 as compared to that on days 3 and 7. However, the difference in cell viability for these three groups was not significant when compared between days 3 and 7. For GuttaFlow 2, the comparison in cell viability between days 1 and 7 was not significant (P>0.05) (supplemental files appendix 3). The cell viability between all other days was significant for GuttaFlow 2.
Figure 4.
Comparison of mean (SD) values of fibroblast survival rate of freshly mixed and set form of the same sealer with control group on days 1, 3 and 7 (a) AH plus (p<0.05 for freshly mixed sealer at day 1, 3, and 7) (P>0.05 for set sealer at day 1 and 3 and P<0.05 for day 7), (b) GuttaFlow 2 (P<0.05 for freshly mixed and set sealer at day 1, 3, and 7) , (c) Dia-Proseal (P<0.05 for freshly mixed sealer at day 1, 3, and 7) (P<0.05 for set sealer at day 1 and 7 and P>0.05 for day 3), and (d) Pulpdent Root Canal Sealer (P<0.05 for freshly mixed and set sealer at day 1, 3 and 7). The error bars denote standard deviation (SD)
However, no discernible trend in cell viability was observed for the other three groups across days 1, 3, and 7.
Set samples
For the set samples, the difference in cell viability between all days was significant for AH Plus and Dia-ProSeal samples (P<0.05) (supplemental files appendix 3). For GuttaFlow 2 and Pulpdent Root Canal Sealer samples, the only exception for a non-significant difference was between days 3 and 7 (P>0.05) (supplemental files appendix 3). A clear trend was observed for all set groups, with a decrease in cell viability as the days progressed. All samples depicted the maximum cell viability of fibroblast cells on day 1 and a minimum cell viability on day 7 (Fig. 4).
The comparison of cell viability rate between all freshly mixed sealers and their set forms revealed that their difference was statistically significant at day 1 (P<0.05) (supplemental files appendix 3) as shown in Figure 5.
Figure 5.
Comparison of mean (SD) values of fibroblasts survival rates for freshly mixed and set sealer samples on day 1, 3, and 7. The error bars denote standard deviation (SD)
The comparison of cell viability between freshly mixed AH Plus, Dia-Proseal and Pulpdent root canal sealer and their set forms revealed statistically significant difference at day 3 (P<0.05) as shown in Tab. A20 (supplemental files appendix 3). However, for GuttaFlow 2 the difference in cell viability for freshly mixed and set form was not statistically significant at day 3 (P>0.05) (supplemental files appendix 3). At day 7, the comparison of cell viability between all freshly mixed sealers and their set forms revealed that their difference was statistically significant (P<0.05) (supplemental files appendix 3).
The L929 fibroblasts used in this study had a normal elongated, fusiform or spindle shape. The scattered rounded cells indicated mitosis of the cells.
The morphogological pattern of fibroblast cells was same at days 1, 3, and 7. The representative images of the fibroblast cells after 7 days are shown in Figure 6.
Figure 6.
Morphology of cells in (a) Control, (b) AH Plus set, (c) AH Plus freshly mixed, (d) GuttaFlow 2 set, (e) GuttaFlow 2 freshly mixed, (f) Dia-ProSeal set, (g) Dia-ProSeal freshly mixed, (h) Pulpdent Root Canal Sealer set, and (i) Pulpdent Root Canal Sealer freshly mixed
DISCUSSION
This study evaluated the chemical structure, pH, solubility, and cytotoxicity of commercially used endodontic sealers in their freshly mixed and set states. The presence of certain cytotoxic chemical groups and elements in the structure of these sealers was revealed. This study also revealed the cytotoxicity of these sealers on the mouse fibroblasts confirming the cytotoxic nature of endodontic sealers.
The FTIR analysis of endodontic sealers can help in identifying some of the causative agents responsible for the cytotoxicity of the sealers. The amines in AH Plus are present in the form of aminoadamantane, dibenzyldiamine, and tricyclodecane-diamine in the constituents of this sealer.
Morphological analysis of set AH Plus with a scanning electron microscope revealed the presence of pores in the structure of this sealer. These pores can lead to a higher degradation of this sealer (31). It is expected that the higher degradation rate may release cytotoxic substances from this sealer that might be associated with this sealer. Many elements have been known to be toxic to cells in the human body. The EDX analysis of AH Plus revealed the presence of zirconium (Zr) in its composition and this element is known to be cytotoxic (22). The pH of freshly mixed AH Plus was alkaline in nature, whereas in set state it showed neutral pH, which was in accordance to previous study, where it was reported that the set AH Plus exhibited neutral behavior (32). However, moderate-mild toxicity was observed in freshly mixed state, whereas neutral behaviour in set state also supported cell proliferation. Furthermore, low solubility behaviour also supported cell viability. The moderate-mild toxicity might be due to presence of amine groups as amines have cytotoxic nature and can affect the proliferation of cells. Epoxy resin sealers are known to release amines and formaldehyde that are cytotoxic and can affect the growth and proliferation of human cells (10, 22, 33).
The FTIR analysis of freshly mixed GuttaFlow 2 revealed the peaks of Si-CH3, Si-O-Si, and CH3 at 791 cm-1, 1257 cm-1, and 2961 cm-1 respectively. These peaks in GuttaFlow 2 confirmed the presence of polydimethylsiloxane (PDMS) in the structure of this sealer. Previous studies suggested that PDMS has been used for biomedical application and is a biocompatible material (34). Therefore, this group showed cytocompability in this present study. This present study revealed that the freshly mixed samples of GuttaFlow 2 were acidic, whereas the set samples showed neutral pH. The cytotoxic behavior of freshly mixed GuttaFlow 2 in the present study is related to acidic pH. The presence of Zr may be another aggravating factor for cytotoxicity. However, set samples showed a cell viability of more than 90% with intact morphology that is incoherent due to the neutral pH of set samples.
The results associated with Dia-ProSeal revealed moderate to mild cytotoxicity, which could be due to the presence of amines, bisphenol epoxy, and Zr (22). Hence, the extrusion of root canal sealers out of the root canal and into periapical tissues should be avoided as they include potential cytotoxic elements. With a change in pH value, the cytotoxicity behaviour was changed from moderate to mild. The change in pH from acidic to alkaline was due to the presence of calcium hydroxide. This higher pH helps in neutralizing the acids produced by the osteoclasts. Bacterial membranes and their protein structures can also be destroyed by this high pH.
The cytotoxicity of AH plus and DiaProseal epoxy resin sealers could be due to presence of bisphenol A in their composition.
The spectral peaks of Pulpdent Root Canal Sealer confirmed the presence of eugenol. Eugenol is a known cytotoxic agent and is responsible for effecting the proliferation and viability of cells as it comes in contact with the cells (35). The porous structure of Pulpdent Root Canal Sealer might absorb physiological fluid, subsequently leading to leaching of ions. Therefore, initially set samples showed non-toxic behaviour, however, with the increase in time, the behaviour was mildly cytotoxic. The presence of Zr and Ba are additional aggravating factors. The pH of freshly mixed samples was acidic to neutral, however, the set samples indicated an alkaline behavior. The solubility analysis of Pulpdent Root Canal Sealer revealed that it had the highest solubility (3%) as compared to all the sealers. Previously, studies have concluded that after storage in water zinc oxide eugenol exhibits weight loss to a certain extent varying from lower than 1% to 7% (36).
Freshly mixed AH Plus, Dia-ProSeal, and Pulpdent Root Canal Sealer possess alkaline pH. Sealers possessing alkaline pH have better biocompatibility and antibacterial activity (32). A highly soluble sealer allows gap formation within the sealer and between the sealer and root dentin interface thereby allowing a pathway for leakage to and from the periapical tissues and the oral cavity (6).
A sealer with lower solubility and lower biocompatibility extruded into the periapical area may affect the repair of periapical tissues.
Similarly, a high pH also helps in the activation of alkaline phosphatase enzyme that helps in the process of mineralization (30). However, in this study, only Pulpdent Root Canal Sealer showed alkaline behaviour in set phase, whereby all other sealers were in alkaline phase for a short period of time. Therefore, it is difficult to say either they would be helpful in mineralization or not.
All the sealers investigated in this study fulfilled the ANSI/ADA specification 6876 and standard set by the International Standards Organization (specification No. 57) for solubility (25). This study revealed that all the sealers were mildly cytotoxic in their freshly mixed and set forms, where their cell viability was lower as compared to the control group. However, the cell viability was higher in the set forms of all the sealers. Among all the sealers Dia-ProSeal exhibited the lowest cell viability in both freshly mixed and set forms and set GuttaFlow 2 samples exhibited the highest cell viability and freshly mixed AH Plus exhibited the highest cell viability at day 7.
In a clinical setting, these results can be utilised to improve the selection of materials by the clinicians to achieve better outcomes in the clinical scenarios. This can also improve the predictability of the clinical results based on their physicochemical properties and biocompatibility. Based on the results from solubility analysis, the selection of an appropriate material may reduce the presence of voids in the root canal filling due to solubility ensuring a proper seal of the root canal system and achieving better results. Similarly, selection of sealers on the basis of their pH and solubility may improve biological response of the tissues rendering improved clinical outcomes.
CONCLUSION
The FTIR analysis of the endodontic sealers revealed the functional groups related to the cytotoxic constituents present in these sealers and helped in the identification of these materials. The surface morphology of all the root canal sealers in a set state was variable containing particles of various sizes. Dia-ProSeal and AH Plus in their freshly mixed state and Pulpdent Root Canal Sealer in its set state were highly alkaline. The lowest solubility was depicted by epoxy resin sealers (AH Plus and Dia-ProSeal). This study concluded that GuttaFlow 2 was the most biocompatible sealer in its set state and AH Plus was the most biocompatible sealer in its freshly mixed state after 7 days. All of the sealers investigated did not affect the morphology of the cells.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
Ethics Committee Approval: Ethical Committee of Islamic International Dental Hospital (Date: February 7, 2020 Number: IIDC/IRC/2020/02/006).
Peer-review: Externally peer-reviewed.
Financial Disclosure: No financial assistance was taken from any organization and company.
Authorship contributions: Concept – F.M., A.S.K.; Design – M.T.K., S.Z.S., A.S.K.; Supervision – F.M.; Funding - M.T.K., S.Z.S., A.S.K; Materials - M.T.K., F.S., A.M.; Data collection &/or processing – M.T.K., F.S., A.M.; Analysis and/or interpretation – M.T.K., F.S.; Literature search – M.T.K., A.M.; Writing – M.T.K., A.S.K.; Critical Review – A.S.K., F.M.
REFERENCES
- 1.Komabayashi T, Colmenar D, Cvach N, Bhat A, Primus C, Imai Y. Comprehensive review of current endodontic sealers. Dent Mater J. 2020;39(5):703–20. doi: 10.4012/dmj.2019-288. [DOI] [PubMed] [Google Scholar]
- 2.Baras BH, Melo MAS, Thumbigere-Math V, Tay FR, Fouad AF, Oates TW, et al. Novel Bioactive and Therapeutic root canal sealers with antibacterial and remineralization properties. Materials (Basel) 2020;13(5):1096. doi: 10.3390/ma13051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyagi S, Mishra P, Tyagi P. Evolution of root canal sealers:An insight story. Eur J Gen Dent. 2013;3(2):199–218. [Google Scholar]
- 4.Washio A, Morotomi T, Yoshii S, Kitamura C. Bioactive glass-based endodontic sealer as a promising root canal filling material without semisolid core materials. Materials (Basel) 2019;12(23):3967. doi: 10.3390/ma12233967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikly A, Jaafoura S, Kammoun D, Sahtout S. Sealing ability of endodontic cements:An invitro study. Int J Dent. 2020;2020:1–7. doi: 10.1155/2020/5862598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Haddad A, Che Ab Aziz ZA. Bioceramic-based root canal sealers:A review. Int J Biomateræ. 2016;2016:9753210. doi: 10.1155/2016/9753210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ŠimundićMunitić M, PoklepovićPeričić T, Utrobičić A, Bago I, Puljak L. Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers:A systematic review. PLoS One. 2019;14(10):e0223575. doi: 10.1371/journal.pone.0223575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubbe KL, de Oliveira KV, Coelho BS, Baratto-Filho F. AH Plus extrusion into periapical tissue:literature review of main related properties and report of clinical cases. RSBO. 2016;13(4):280–8. [Google Scholar]
- 9.Dalopoulou A, Economides N, Evangelidis V. Extrusion of root canal sealer in periapical tissues - report of two cases with different treatment management and literature review. Balk J Dent Med. 2017;21(1):12–8. [Google Scholar]
- 10.Jung S, Sielker S, Hanisch MR, Libricht V, Schäfer E, Dammaschke T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS One. 2018;13(3):e0194467. doi: 10.1371/journal.pone.0194467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed TI, Hossain MM, Susta FH. An uneventful effect of accidental extrusion of excess sealer on periradicular healing:Two case reports. Update Dent Coll. 2016;5(2):52–6. [Google Scholar]
- 12.Ricucci D, Rôças IN, Alves FR, Loghin S, Siqueira JF., Jr Apically Extruded Sealers:Fate and Influence on Treatment Outcome. J Endod. 2016;42(2):243–9. doi: 10.1016/j.joen.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Gomes BPFA, Herrera DR. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz Oral Res. 2018;32(suppl 1):e69. doi: 10.1590/1807-3107bor-2018.vol32.0069. [DOI] [PubMed] [Google Scholar]
- 14.Baldasso FE, Kopper PM, Morgental RD, Steier L, Figueiredo JA, Scarparo RK. Biological tissue response to a new formulation of a silicone based endodontic sealer. Braz Dent J. 2016;27(6):657–63. doi: 10.1590/0103-6440201600719. [DOI] [PubMed] [Google Scholar]
- 15.Jo SB, Kim HK, Lee HN, Kim YJ, Dev Patel K, Campbell Knowles J, et al. Physical properties and biofunctionalities of bioactive root canal sealers in vitro. Nanomaterials (Basel) 2020;10(9):1750. doi: 10.3390/nano10091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca DA, Paula AB, Marto CM, Coelho A, Paulo S, Martinho JP, et al. Biocompatibility of root canal sealers:A systematic review of in vitro and in vivo studies. Materials (Basel) 2019;12(24):4113. doi: 10.3390/ma12244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, Moraleda JM, Rodríguez-Lozano FJ. Cytotoxicity of GuttaFlow Bioseal GuttaFlow2, MTA Fillapex and AH Plus on human periodontal ligament stem cells. J Endod. 2017;43(5):816–22. doi: 10.1016/j.joen.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Giacomino CM, Wealleans JA, Kuhn N, Diogenes A. Comparative biocompatibility and osteogenic potential of two bioceramic sealers. J Endod. 2019;45(1):51–6. doi: 10.1016/j.joen.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Khan AS, Khalid H, Sarfraz Z, Khan M, Iqbal J, Muhammad N, et al. Vibrational spectroscopy of selective dental restorative materials. Appl Spectrosc Rev. 2016;52(6):507–40. [Google Scholar]
- 20.Donnermeyer D, Urban K, Bürklein S, Schäfer E. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application:epoxy resins and zinc oxide-eugenols. Int Endod J. 2020;53(5):690–7. doi: 10.1111/iej.13267. [DOI] [PubMed] [Google Scholar]
- 21.Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CE, et al. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45(5):419–28. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- 22.Sampaio FC, Alencar AH, Guedes OA, Veloso HH, Santos TO, Estrela C. Chemical elements characterization of root canal sealers using scanning electron microscopy and energy dispersive X-ray analysis. Oral Health Dent Manag. 2014;13(1):27–34. [PubMed] [Google Scholar]
- 23.Szczurko G, Pawińska M, Łuczaj-Cepowicz E, Kierklo A, Marczuk-Kolada G, Hołownia A. Effect of root canal sealers on human periodontal ligament fibroblast viability:ex vivo study. Odontology. 2018;106(3):245–56. doi: 10.1007/s10266-017-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges ÁH, Orçati Dorileo MC, Dalla Villa R, Borba AM, Semenoff TA, Guedes OA, et al. Physicochemical properties and surfaces morphologies evaluation of MTA FillApex and AH plus. ScientificWorldJournal. 2014;2014:589732. doi: 10.1155/2014/589732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Organization for Standardization. ISO 6876:Dental root sealing materials. Geneva: The Organization; 2001. [Accessed Mar 9 2021]. Available at: https://www.iso.org/standard/34965.html . [Google Scholar]
- 26.Song YS, Choi Y, Lim MJ, Yu MK, Hong CU, Lee KW, et al. In vitro evaluation of a newly produced resin-based endodontic sealer. Restor Dent Endod. 2016;41(3):189–95. doi: 10.5395/rde.2016.41.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biological evaluation of medical devices- Part 5:Tests for in vitro cytotoxicity. ISO 10993-5. 2009. [Accessed Mar 9 2021]. Available at: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:en .
- 28.Cannella V, Altomare R, Chiaramonte G, Di Bella S, Mira F, Russotto L, et al. Cytotoxicity evaluation of endodontic pins on L929 cell line. Biomed Res Int. 2019;2019:1–5. doi: 10.1155/2019/3469525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marczuk-Kolada G, Łuczaj-Cepowicz E, Pawińska M, Hołownia A. Evaluation of the cytotoxicity of selected conventional glass ionomer cements on human gingival fibroblasts. Adv Clin Exp Med. 2017;26(7):1041–5. doi: 10.17219/acem/64944. [DOI] [PubMed] [Google Scholar]
- 30.Colombo M, Poggio C, Dagna A, Meravini MV, Riva P, Trovati F, et al. Biological and physico-chemical properties of new root canal sealers. J Clin Exp Dent. 2018;10(2):e120–6. doi: 10.4317/jced.54548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yusop AH, Bakir AA, Shaharom NA, Abdul Kadir MR, Hermawan H. Porous biodegradable metals for hard tissue scaffolds:a review. Int J Biomater. 2012;2012:641430. doi: 10.1155/2012/641430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poggio C, Dagna A, Ceci M, Meravini MV, Colombo M, Pietrocola G. Solubility and pH of bioceramic root canal sealers:A comparative study. J Clin Exp Dent. 2017;9(10):e1189–94. doi: 10.4317/jced.54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. An in vitro study of the cytotoxicity of two root canal sealers. J Endod. 2000;26(4):228–9. doi: 10.1097/00004770-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Sheikh Z, Khan AS, Roohpour N, Glogauer M, Rehman IU. Protein adsorption capability on polyurethane and modified-polyurethane membrane for periodontal guided tissue regeneration applications. Mater Sci Eng C Mater Biol Appl. 2016;68:267–75. doi: 10.1016/j.msec.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Markowitz K, Moynihan M, Liu M, Kim S. Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surg Oral Med Oral Pathol. 1992;73(6):729–37. doi: 10.1016/0030-4220(92)90020-q. [DOI] [PubMed] [Google Scholar]
- 36.Poggio C, Arciola CR, Dagna A, Colombo M, Bianchi S, Visai L. Solubility of root canal sealers:a comparative study. Int J Artif Organs. 2010;33(9):676–81. doi: 10.1177/039139881003300914. [DOI] [PubMed] [Google Scholar]