Abstract
The coronavirus disease 2019 (COVID-19) and the imposed lockdowns in order to control the pandemic, had undoubtedly influenced the lifestyle of millions of people worldwide. The period of confinement, which was characterized by seizing most business activities and allowing only for e-classes at schools and universities, leading also to a lower physical activity, could have affected eating behaviors of people of all ages. In this study we aimed to investigate the impact of the first lockdown period (March–May 2020) on body weight (BW) and on body mass index (BMI) in both adults and adolescents (>16 years old). A systematic literature search was conducted in PubMed®, Scopus®, Web of Science® and EMBASE® databases and 36 observational (35 cross-sectional and one cohort) studies were included. BW and BMI changes after/during the lockdown period were examined. BW was stated as increased in a significant part of the individuals (11.1–72.4%), although a range of 7.2–51.4% of individuals reported weight loss. A significant higher BW was observed with a weighted mean between-group difference (WMD) 1.57 (95% CI 1.01 to 2.14) in the post-lockdown period compared to the before lockdown time and higher BMI, 0.31 WMD (95% CI, 0.17 to 0.45) was identified before the lockdown period. At variance with general trends, one study in older adults (>60 years old) notably reported a significant BW loss, suggesting a higher risk for lockdown-induced weight loss and potentially malnutrition in the elderly population. Overall increments in BW are an alarming effect of lockdown during the COVID-19 pandemic, leading to potential higher incidence of overweight, obesity and related health-risks as well as other noncommunicable diseases. Further studies are needed to assess potential group-specific impacts, with particular regard to weight gain in younger people and risk of weight loss, malnutrition and sarcopenia in older adults.
Keywords: BMI, COVID-19, Sars-Cov-2, Weight change, Weight gain, Weight loss
Abbreviations: BMI, Body mass index; BW, Body weight; COVID-19, Coronavirus disease 2019; CVD, Cardiovascular diseases; T2DM, Type 2 diabetes mellitus; MENA, Middle East and North Africa; NOS, Newcastle Ottawa Scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; USA, United States of America; WHO, World Health Organization; WMD, Weighted mean difference
1. Introduction
In December 2019 a severe acute respiratory syndrome caused by a novel coronavirus (SARS-COV-2) was first reported in Wuhan, China due to an outbreak of a severe pneumonia across the population [1]. Due to its rapid spread throughout the globe, the World Health Organization (WHO) after initially (end of January 2020) claiming this as a public health incidence of international concern, declared the coronavirus disease 2019 (COVID-19) on March, 11th 2020 as a pandemic [2]. The absence of effective drugs or vaccine at that time, led the Governments of more than 100 countries to enforce strict measures in their efforts to limit the transmission and control the spread of the disease [3]. Such measures included physical distancing with full or partial lockdowns, schools and universities closures, as well as discontinuation of most business' activities; so as by April 2020 more than a third of the population globally was under any kind of lockdown restriction [3]. During the confinement period, people were encouraged to telework and/or online learning and stay at their homes as much as possible [3]. Medical emergencies, essential work, limited physical activity and purchasing food were the main reasons for individuals to leave their homes [3]. All these crucial measures of self-isolation could have negative impact on people's life both on mental health and on lifestyle related behaviors [[4], [5], [6]]. Changes in daily routine, fear and anxiety of COVID-19 and absence from the work environment, school or university which are linked to boredom could lead either to binge/emotional eating [[7], [8], [9]], or to appetite loss [10]. Moreover, during the COVID-19 lockdown high prevalence of sleep disorders [11,12] as well as physical inactivity [13] which are also linked to imbalanced nutritional patterns for both adults and adolescents were identified [14,15]. In addition the dramatic increase in the food offer via online applications could potentially lead to high consumption of processed foods [16]. Some of the above-mentioned changes (i.e. higher inactivity, absence from the work environment or school, etc.) have similarities with the holiday season where people are more prone to weight gain [17,18], and therefore we aimed to examine the impact of that confinement period on body weight (BW) and Body Mass Index (BMI).
Nevertheless, little is known regarding BW changes during this health crisis worldwide (which is also combined with higher levels of anxiety and/or stress), although many observational studies have been conducted locally. According to our knowledge, this is the first combined systematic review and meta-analysis which investigates changes on BW and/or BMI of both adults and adolescents after and/or during the first COVID-19 lockdown period (March–May 2020) compared to before lockdown times.
2. Material and methods
This is a combined systematic review with a meta-analysis which was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [19] (Supplementary Table 1).
A systematic electronic search was conducted on PubMed®, Scopus®, Web of Science® and EMBASE® databases (up to December, 21st 2020) using the index Medical Subject Headings (MeSH) terms “BMI”, “weight change”, “weight gain”, “weight loss”, “covid-19”, “Sars-Cov-2” and the final search string was: ((BMI) OR (Weight change) OR (Weight gain) OR (weight loss)) AND (COVID-19) for PubMed® database and was modified accordingly for other research engines (Keywords and terms used for our search strategy can be found in Supplementary File 1).
All observational (prospective and retrospective cohorts, and cross-sectional) studies examining BW and BMI before and after/during the period of lockdown and/or percentage of BW change were eligible. The population of interest was adults and adolescents ≥16 years old who were under lockdown restrictions due to the COVID-19, during the pre-specified period of March–May 2020. Studies including data from children <16 years old, hospitalized subjects, symptomatic COVID-19 patients and individuals suffering from chronic diseases that could affect BW and/or BMI were excluded.
Records retrieved throughout literature search were input in a reference database (EndNote X7 for Windows, Thomson Reuters). After the removal of duplicates, remaining studies were assessed independently by two reviewers DB and MC for eligibility. Any disagreements on study selection were solved by consensus.
The process of data extraction from the included studies was independently conducted by two reviewers (DB and MC) using a previous specified excel form. All disagreements were solved by consensus. Data extracted from each study were country origin, number, age and sex of the examined population, type and the period of the survey. In addition, changes in BW were examined. Variables referred to BW changes were presented as percentages. Available data regarding BW and/or BMI before and after/during lockdown were extracted as mean ± SD and were included in our meta-analysis.
Assessment of the quality of included studies was performed by the two reviewers (DB, MC) using the modified Newcastle Ottawa Scale (NOS) for cross-sectional and cohort studies [20]. A protocol for this systematic review and meta-analysis was submitted in OSF® platform (https://osf.io/zg43y/).
The main outcome of our study was to examine changes in BW and BMI after/during the period of lockdown compared to the pre-lockdown period. Incomplete studies or studies not written in English or Spanish language were excluded.
In this study, a meta-analysis was performed for included studies which reported data regarding BW and BMI before and after/during lockdown. The random-effects in the meta-analytic model were used in order to estimate the various assessment effects in studies, due to high between-trial heterogeneity. Moreover, the inverse variance method was used to estimate weights of each study. Weighted mean between-group difference (WMD) was used because both BW and BMI outcomes were expressed by the same measurement scale (kg and kg/m2 accordingly). Chi-squared and I2 test were performed to assess statistical heterogeneity across studies (p-values <0.1 indicating existence of heterogeneity). I2 <25% indicated low degree of heterogeneity, 25–50% moderate heterogeneity and >50–70% showed significant heterogeneity. Subgroup analyses were conducted for both BW change and BMI outcomes in order to separate subjects >18 years old and with obesity, respectively. Sensitivity analysis was performed and aiming to ensure robustness of results studies with NOS rating <7 were excluded. Statistical analyses were performed using the Review Manager (RevMan, version 5.4).
3. Results
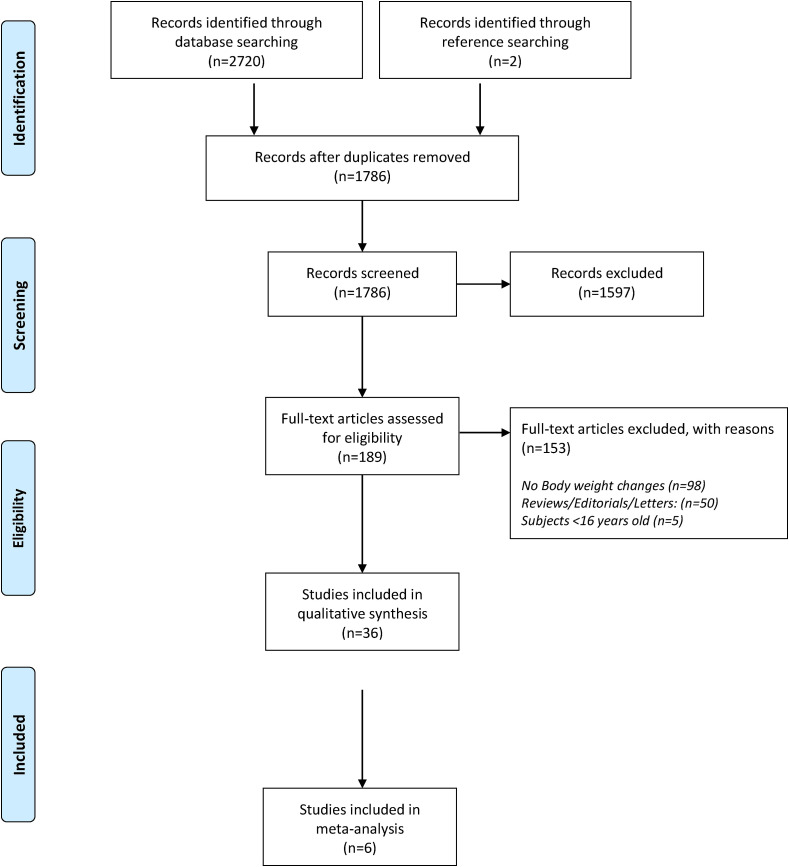
The eligibility process of studies included can been seen in our flow diagram in Fig. 1 . Total records identified through database searching were 2720 and two more studies were identified through references searching. After removing of duplicates (n = 936), 1786 studies were screened for eligibility. Irrelevant studies according to title and abstract, studies including children <16 years old and not observational studies were excluded. Finally 36 studies, in which changes on either BW or BMI were reported, were assessed as acceptable [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]] (35 cross-sectionals and one cohort) for our combined systematic review and meta-analysis. Characteristics of the included studies (country, period of survey, sex and age of participants) are presented in Table 1 . From those studies, two studies included both adults and adolescents [35,42] and for the remaining 34 studies the results referred only to adult population [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34],[36], [37], [38], [39], [40], [41],[43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]].
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of eligibility process of included studies.
Table 1.
Characteristics of included studies.
| Study ID (Country) | Period of survey | Type of survey | Subjects (female) | Agea | Assessment |
|---|---|---|---|---|---|
| Ahmed et al., 2020 (Iraq) | 23 April–23 June | Individual face-to-face interview | 765 (302) | 18–20: 1.17% 21–30: 45.62% 31–40: 27.18% 41–50: 15.68% 51–60: 5.09% 61–70: 3.92% |
BW change |
| Barrea et al., 2020 (Italy) | January–30 April | Interviews | 121 (78) | 44.9 ± 13.3 | BW and BMI before and after lockdown |
| Błaszczyk-Bębenek et al., 2020 (Poland) | 29 April–19 May | Online Questionnaire | 312 (200) | ≤24: 8.7% 25–65: 84.2% ≥66: 7.1% |
BW change |
| Cancello et al., 2020 (Italy) | 15 April–4 May | Online Questionnaire | 490 (410) | ≤30: 14.5% 31–60: 65.1% >60: 20.4% |
BW change |
| Carriedo et al., 2020 (Spain) | 21–24 March | Online Questionnaire | 1795 (1150) | 40.54 ± 15.68 | BW change |
| Cheikh Ismail et al., 2020 (MENA) | 15–29 April | Online Questionnaire | 2970 (2126) | 18-25: 29.6% 26-35: 26.4% 36-45: 22.5% 46-55: 14.9% >55: 6.6% |
BW change |
| Chopra et al., 2020 (India) | 15–30 August | Online Questionnaire | 995 (412) | 33.33 ± 14.5 | BW change |
| De Luis et al., 2020 (Spain) | 4–8 May | Telephone survey | 284 (211) [obese] | 60.4 ± 10.8 | BW change |
| Di Santo et al., 2020 (Italy) | 21 April–7 May | Telephone survey | 126 (102) | 74.29 ± 6.51 (≥60 years) | BW change |
| Dogas et al., 2020 (Croatia) | 25 April–5 May | Online Questionnaire | 3027 (1989) | 40.0 ± 2.83 | BW change |
| Dou et al. (a) 2020 (China) | 22–27 April | Online Questionnaire | 1615 | No info | BW change |
| Dou et al. (b) 2020 (USA) | 17–27 April | Online Questionnaire | 1329 | No info | BW change |
| Drywien et al., 2020 (Poland) | April–May | Online Questionnaire | 1769 (1769) | <30: 29.1% 30–39: 45.6% 40–49: 12% 50–59: 6.8% >60: 6.4% |
BW change |
| Elmacıoğlu et al., 2020 (Turkey) | 6–26 May | Online Questionnaire | 1036 (827) | 33 ± 0.5 | BW change |
| Federik et al., 2020 (Argentina) | June | Online Questionnaire | 788 (583) | 35.74 ± 14.82 | BW change |
| Fernandez-Rio et al., 2020 (Spain) | March–April | Online Questionnaire | 4379 (2667) | 16–84 years | BW change |
| Flanagan et al., 2020 (USA) | 3 April–3 May | Online Questionnaire | 5354 (1708) | No info | BW change |
| Ghosal et al., 2020 (India) | No info | Telephone survey | 100 | No info | BW change |
| Gomes et al., 2020 (Brazil) | 29 April–10 May | Online Questionnaire | 766 | 46.04 ± 3.01 | BW change |
| He et al., 2020 (China) | March | Online Questionnaire | 339 (181) | 37.04 ± 12.18 | BW before and after lockdown |
| Huber et al., 2020 (Germany) | March–April | Online Questionnaire | 1964 (1383) | 23.3 ± 4.0 | BW change |
| Ismail et al., 2020 (UAE) | March–April | Online Questionnaire | 1012 (768) | 18–25: 27.7% 26–35: 29.1% 36–45: 23.7% 46–55: 15.2% >55: 4.3% |
BW change |
| Jia et al., 2020 (China) | May | Online Questionnaire | 10,082 (7229) | 19.8 ± 2.3 (>16 years) | BW and BMI before and after lockdown |
| Keel et al., 2020 (USA) | 15–24 April | Online Questionnaire | 90 (79) | 19.45 ± 1.26 | BW change |
| Kriaucioniene et al., 2020 (Lithuania) | 14–28 April | Online Questionnaire | 2447 (2149) | 18–35: 40.1% 36–50: 36.7% ≥51: 23.2% |
BW change |
| Kumari et al., 2020 (India) | July | Online Questionnaire | 103 (50) | 35.92 ± 15.09 | BW change |
| Lopez-Moreno et al., 2020 (Spain) | 28 May–21 June | Online Questionnaire | 675 (472) | 39.1 ± 12.9 | BW and BMI before and after lockdown |
| Marchitelli et al., 2020 (Italy) | 25 April–10 May | Telephone survey | 63 (42) | 47.24 ± 14.3 | BW change |
| Martínez-de-Quel et al., 2020 (Spain) | 16–31 March | Online Questionnaire | 161 (60) | 35.0 ± 11.2 | BW before and after lockdown |
| Papandreou et al., 2020 a (Greece) | 23 April–May | Online Questionnaire | 839 (560) | 42.4 ± 11.7 | BW change |
| Papandreou et al., 2020 b (Spain) | 23 April–May | Online Questionnaire | 1002 (704) | 46.1 ± 13.3 | BW change |
| Pellegrini et al., 2020 (Italy) | 14–21 April | Telephone survey | 150 (116) [obese] | 47.9 ± 16.0 | BW and BMI before and after lockdown |
| Reyes-Olavarría et al., 2020 (Chile) | April | Online Questionnaire | 700 (528) | 31.2 ± 7.04 | BW change |
| Rodriguez-Perez et al., 2020 (Spain) | 20 March–10 April | Online Questionnaire | 7514 (5305) | <20: 3.0% 21–35: 34.0% 36–50: 31.6% 51–65: 25.7% >65: 5.7% |
BW change |
| Scarmozzino et al., 2020 (Italy) | 15 April | Online Questionnaire | 1929 (1292) | <20: 14.4% 21–35: 63.1% 36–50: 9.6% 51–65: 11.4% >65: 1.5% |
BW change |
| Sidor et al., 2020 (Poland) | 17 April–1 May | Online Questionnaire | 1097 (1043) | 27.7 ± 9.0 | BW change |
| Sinisterra Loaiza et al., 2020 (Spain) | 2–15 May | Online Questionnaire | 1350 (945) | 63.2 ± 8.1 | BW change |
| Zachary et al., 2020 (USA) | No info | Online Questionnaire | 173 (96) | 28.1 ± 12.5 | BW change |
MENA: Middle East and North Africa, USA: United States of America, UAE: United Arab Emirates.
Variables are presented as mean ± SD, percentage and/or (range).
Results of the quality assessment of the included studies, according to the NOS scale [20], can be found in Supplementary File 2. According to the NOS, 23 studies [24,25,[27], [28], [29],31,[33], [34], [35], [36], [37], [38], [39], [40],44,45,[47], [48], [49],51,53,54,56] were classified as satisfactory quality (5–6 stars), due to their low rating in the domain of exposure and the remaining 13 studies [[21], [22], [23],26,30,32,[41], [42], [43],46,50,52,55] were classified as good quality (7–8 stars).
A summary of results of BW changes can be found in Table 2 . In general, a tendency towards an increase in BW was reported in the majority of studies [[21], [22], [23], [24], [25], [26], [27], [28],[30], [31], [32], [33], [34], [35], [36], [37], [38], [39],[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]]. The highest increase of body weight (72.4%) was stated by Ahmed et al. including a population in Iraq [21]. In several studies, subjects who reported weight gain represented more than 30% of the total study population [[22], [23], [24],[26], [27], [28],[30], [31], [32], [33], [34],[37], [38], [39], [40], [41], [42],[44], [45], [46], [47], [48], [49], [50], [51],55]. For the rest of the included studies in which weight gain was more prevalent than weight loss, the range of the percentage reporting weight gain was 12.8–29.9% [25,35,36,43,[52], [53], [54],56]. Only in one study (from Italy) a significant part of the sample had weight loss after/during the lockdown period, but it has to be noted that in this all the participants were older than 60 years old [29].
Table 2.
Impact of the COVID-19 lockdown on body weight.
| Study ID (Country) | % Increased | % Decreased | % No changes | % Not measured/Unknown |
|---|---|---|---|---|
| Ahmed et al., 2020 (Iraq) | 72.4 | 27.6 | – | – |
| Błaszczyk-Bębenek et al., 2020 (Poland) | 45.9 | 21.7 | 32.4 | – |
| Cancello et al., 2020 (Italy) | 45.0 | 16.0 | 34.0 | 5.0 |
| Carriedo et al., 2020 (Spain) | 21.2 | 17.1 | 54.3 | 7.4 |
| Cheikh Ismail et al., 2020 (MENA) | 30.3 | 16.9 | 43.9 | 8.9 |
| Chopra et al., 2020 (India) | 31.5 | 13.8 | 47.9 | 6.8 |
| De Luis et al., 2020 (Spain) | 36.3 | – | 63.7 | – |
| Di Santo et al., 2020 (Italy) | 11.1 | 35.7 | 53.2 | – |
| Dogas et al., 2020 (Croatia) | 30.7 | No info | No info | No info |
| Dou et al. (a) 2020 (China) | 43.4 | 7.2 | 46.6 | 2.8 |
| Dou et al. (b) 2020 (USA) | 32.9 | 19.7 | 39.4 | 8.0 |
| Drywien et al., 2020 (Poland) | 33.6 | 19.1 | 48.3 | – |
| Elmacıoğlu et al., 2020 (Turkey) | 35.0 | 20.0 | 36.0 | 9.0 |
| Federik et al., 2020 (Argentina) | 31.1 | No info | No info | No info |
| Fernandez-Rio et al., 2020 (Spain) | 25.8 | 21.3 | 52.9 | – |
| Flanagan et al., 2020 (USA) | 27.3 | 17.3 | 55.4 | – |
| Ghosal et al., 2020 (India) | 40.0 | 19.0 | 41.0 | – |
| Gomes et al., 2020 (Brazil) | 32.9 | 19.4 | 44.9 | 2.8 |
| Huber et al., 2020 (Germany)b | 30.9 | 16.7 | 51.4 | 1.0 |
| Ismail et al., 2020 (UAE) | 31.0 | 20.9 | 40.1 | 8.0 |
| Keel et al., 2020 (USA) | 28.4 | 15.9 | 55.7 | – |
| Kriaucioniene et al., 2020 (Lithuania) | 31.5 | – | 68.5a | – |
| Kumari et al., 2020 (India) | 32.0 | 15.5 | 47.6 | 4.9 |
| Lopez-Moreno et al., 2020 (Spain) | 38.8 | 31.1 | 30.1a | – |
| Marchitelli et al., 2020 (Italy) | 49.2 | No info | No info | No info |
| Papandreou et al., 2020 (Greece) | 39.8 | No info | No info | No info |
| Papandreou et al., 2020 (Spain) | 38.4 | No info | No info | No info |
| Reyes-Olavarría et al. 2020 (Chile) | 35.0 | 15.6 | 49.4 | – |
| Rodriguez-Perez et al., 2020 (Spain) | 12.8 | – | 47.3 | 39.9 |
| Scarmozzino et al., 2020 (Italy) | 19.5 | – | 50.7 | 29.8 |
| Sidor et al., 2020 (Poland) | 29.9 | 18.6 | 38.0 | 13.5 |
| Sinisterra Loaiza et al., 2020 (Spain) | 44.0 | No info | No info | No info |
| Zachary et al., 2020 (USA) | 22.0 | 19.0 | 59.0 | – |
Variables are presented as mean ± SD, percentage and/or (range).
MENA: Middle East and North Africa, No info: No information, USA: United States of America, UAE: United Arab Emirates.
Percentage of stable and not measured body weight.
BMI changes.
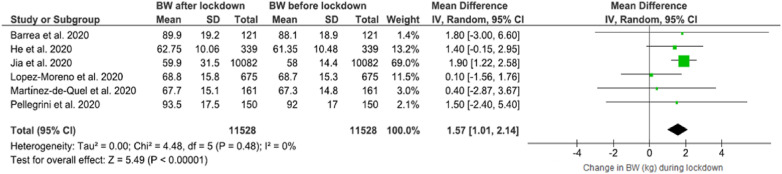
The forest plot showing BW changes can be found in Fig. 2 . Across six studies [22,39,42,46,48,50] in which mean BW before and after/during lockdown were reported, a significant higher BW was observed 1.57 WMD (95% CI 1.01 to 2.14) in the after lockdown period compared to the before lockdown time, without heterogeneity (I2 = 0%).
Fig. 2.
Forest plot of the impact of COVID-19 lockdown on body weight (BW) (N = 11,528, ≥16 years old).
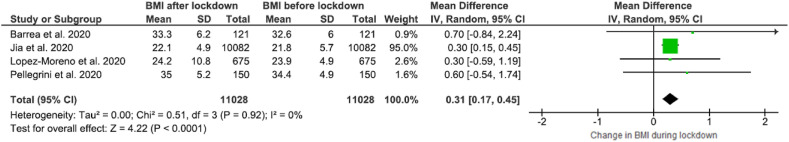
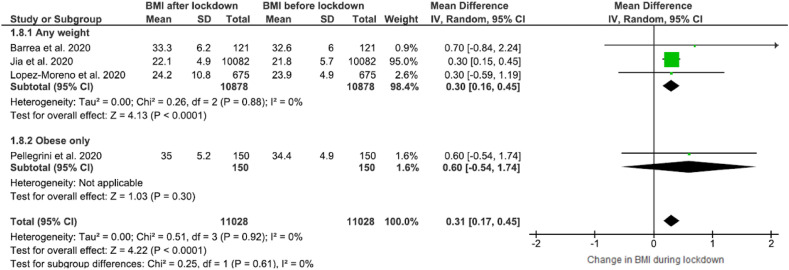
The forest plot showing BMI changes with respect to the impact of the COVID-19 lockdown can be found in Fig. 3 . Across the four studies included in this analysis [22,42,46,50], BMI was found increased after the lockdown period, 0.31 WMD (95% CI, 0.17 to 0.45) with low heterogeneity (I2 = 0%).
Fig. 3.
Forest plot of the impact of COVID-19 lockdown on BMI in adults (N = 11,028, ≥16 years old).
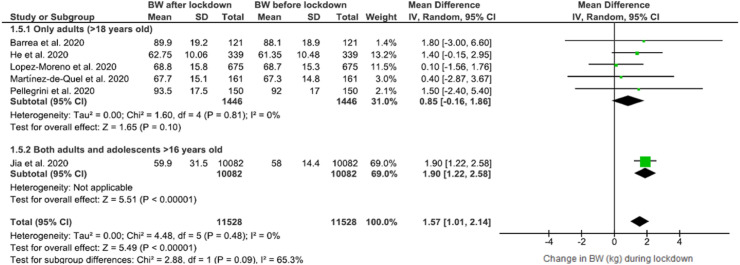
In order to explore the heterogeneity across the studies examined [22,39,42,46,48,50] BW before and after/during the lockdown period a subgroup analysis was performed according to subject age (Fig. 4 ). In studies in which individuals were adults (>18 years old) a tendency towards increase in BW was observed, 0.85 WMD (95% CI, −0.16 to 1.86), with low heterogeneity among studies, (I2 = 0%) but not as statistically significant as when adolescents were also included (Fig. 2).
Fig. 4.
Forest plot of the impact of COVID-19 lockdown on body weight (BW) (Age subgroup analysis).
Furthermore, subgroup analysis was performed according to the weight status of individuals where obese people constituted a separate subgroup. Results revealed an increase of BMI in individuals of any weight, 0.30 WMD (95% CI, 0.16 to 0.45) with low heterogeneity across the studies (I2 = 0) [22,42,46,50] (Fig. 5 ). Regarding BMI changes one more subgroup analysis was conducted according to subject age groups, which can be found in Supplementary Fig. 1. In the subgroup including only adults, a tendency towards increase in BMI (not statistically significant) was observed after the lockdown period 0.46 (95% CI, −0.18 to 1.10).
Fig. 5.
Forest plot of the impact of COVID-19 lockdown on BMI (subgroup analysis).
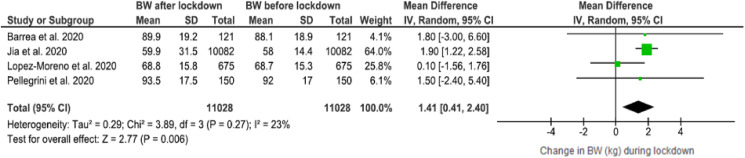
Sensitivity analysis was performed for BW changes including only the high quality studies [22,42,46,50] according to the NOS (rating >7). Results after removing the studies which were assessed as “satisfactory” [39,48], showed similarly statistically significant higher BW after the COVID-19 lockdown period, 1.41 WMD (95% CI, 0.41 to 2.40), with lower heterogeneity across the studies (I2 = 23%) (Fig. 6 ). Since all the studies presenting BMI changes were classified as “good quality”, an additional sensitivity analysis was not performed.
Fig. 6.
Sensitivity analysis (included studies with NOS score ≥7): Body weight changes after the COVID-19 lockdown period.
4. Discussion
According to our knowledge this is the first systematic review and meta-analysis which aimed to examine the impact of the COVID-19 first lockdown on BW. In total 59,711 subjects >16 years old from 32 countries around the world (Algeria, Argentina, Bahrain, Brazil, Chile, China, Croatia, Egypt, Germany, Greece, India, Iraq, Italy, Jordan, Lebanon, Libya, Lithuania, Morocco, Oman, Palestine, Poland, Qatar, Saudi Arabia, Spain, Sudan, Syria, Tunisia, Turkey, UAE, USA and Yemen) were included in our study.
Results of our systematic review regarding BW changes, reported that a range of 11.1–72.4% of individuals asked, stated an increased in BW after/during the lockdown period. The highest increase was observed in the study from Iraq in which the majority of the participants (45.6%) were 21–30 years old [21].
Different results could be seen even in studies coming from the same country. In 3 studies from Poland results in regard to BW gain varied between 29.9 and 45.9% of the participants, whereas the percentage of participants who stated BW loss did not differ significantly (18.6–21.7%) [23,32,54]. The fact that sample sizes differed between those three studies could be a possible explanation for the observed range of BW gain (312 in Błaszczyk-Bębenek et al. [23], 1769 in Drywien et al. [32] and 1097 subjects in Sidor and Rzymski [54]). In seven studies including Spanish population, differences in results could also be observed, ranging between 12.8–44% for reported BW gain [25,28,35,46,49,52,55] and 21.3–31.3% for reported BW loss [35,46]. Furthermore, in Italy a wide range of participants stated an increased in BW (19.5–49.2%) [24,47,53] and 16.0% stated BW decrease [24]. Variation was low in studies coming from the USA (22.0–32.9% for reported BW gain) and 15.9–19.7% for reported BW loss [31,36,43,56]. The lower percentage for reported BW came from a rather small (sample size 173 subjects) study which also did not share information of survey time [56].
The characteristics of the participants studied seem to be an important parameter for the results obtained. In particular, with regard to the highest increase observed in the study from Iraq, the majority of participants (45.6%) were 21–30 years old [21]. Similarly, in the study lead by de Luis et al. [28], including only obese subjects, more than one third (36.3%) of them stated a further increased BW during/after lockdown. This highlights that such group of the population faces a higher difficulty to control their BW during a lockdown as a result of the COVID-19 pandemic.
Τhe duration of lockdown at the time of the survey also seemed to affect the result of the studies. In the Italian study by Scarmozzino and Visioli [53] the survey was conducted only for one day, on April, 15th. Lockdown restrictions were implemented by the Italian government on March 9th [57], therefore the most likely explanation of the lower percentage (19.5%) reporting a BW increase in the study of Scarmozzino and Visioli [53] compared to the two other Italian studies (conducted from 15 April to 4 May [45%] and 25 April to 10 May [49.2%] respectively) [24,47], is that time interval between the lockdown initiation and the day of the survey was shorter. Moreover, in Spanish studies conducted between March and April an increase in BW was stated by fewer individuals (12.8–25.8%) [25,35,52] compared to studies conducted between end of April–June in which 36.3–44.0% of participants stated increase in their BW [28,46,55]. This could be explained, similar to the Italian studies, by the fact that the highest impact of lockdown could be seen when the population was examined at the end or after the lockdown period. Therefore, results from studies conducted later during, or shortly after the lockdown could have been more successful in capturing BW changes. Regarding results from India, a range of 31.5–40% of participants reported BW gain and 13.8–19.0% reported BW loss [27,37,45]. However the fact that the lockdown in India ended in May and surveys were conducted in July [45] and August [27] could have affected the accuracy of these results. Period of survey's conduction was not reported in the Indian study of Ghosal et al. [37].
Significant loss in BW was observed only in one study from Italy [29]. In particular, in this Italian study all the participants were ≥60 years old (mean age 74.3 ± 6.51) and 35.7% stated weight loss whereas only 11.1% of them gained BW [29]. These results could be an alarming sign of a risk of developing malnutrition in elderly [58]. Warnings regarding the risk of malnutrition in elderly in the time of COVID-19 lockdown have been published [59,60] but further efforts are needed in this filed.
Results from our meta-analysis showed that BW (Fig. 2) was statistically significantly higher [WMD 1.57 (95% CI 1.01 to 2.14)] after the first COVID-19 lockdown period and this result should not be disregarded. Previously published studies have demonstrated that lifestyle changes and overeating during the COVID-19 lockdown period could lead to an increase in obesity prevalence, with a proposed definition of “Covibesity” pandemic [61]. It is well known that obesity is related to several comorbidities such as type-2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), obstructive sleep-apnea and even cancer [62]. Moreover, obesity constitutes also a risk factor for COVID-19 infection [63,64] and may be related to more severe forms of the disease compared to non-obese patients [65].
In the subgroup including only subjects ≥18 years old, a higher BW after the lockdown period could be observed but was not less significant in comparison to the analysis including subjects ≥16 years old (Fig. 2, Fig. 4). A possible explanation could be that adolescents and youths were more prone to gain BW during the lockdown period. The clear reasons behind this are not clear, and only assumptions can be made. One potential explanation could be that the younger population was more physically active before the lockdown, and the related inactivity might have led to a higher positive energy balance. As an alternative, younger individuals could have engaged in even less healthy eating habits during the lockdown, with higher calorie intake. Further studies are clearly needed to test these hypotheses.
It may be partly surprising that BMI was statistically significantly higher after/during the lockdown period as stated in included studies (Fig. 3). Analysis of subgroups after excluding obese subjects (from the study of Pellegrini et al. [50]) (Fig. 5) did not modify this results, however in individuals <18 years old [42] (Suppl. Fig. 1) a tendency towards increase in BMI was observed but not statistically significant. More studies will be needed to more clearly dissect potential group-specific and overall impacts of lockdown on BMI.
Our study can be characterized by several strengths. First of all, this is the first systematic review which examined the impact of lockdown on BW changes globally, with several studies and countries to be included. Moreover, all studies included were classified as satisfactory or good quality studies according to the NOS (Suppl. Table 1). Among the limitations of this study is that in the majority of studies the sample examined was not representative of the country's population and this could affect the accuracy of our conclusions. In addition, relatively few studies were included in our meta-analysis despite their homogeneity. Moreover, the fact that surveys were conducted online or via telephone could have an impact on the reliability of results, as obviously data on BW and height (and therefore BMI) were self-reported. Lastly, only studies in English and Spanish were included and therefore published related studies in any other language are not part in our study.
5. Conclusions
The first COVID-19 lockdown affected the BW of subjects ≥16 years old globally. Significant portion of the participants stated that their BW was increased during lockdown, resulting in overall significant BW increments in the current meta-analysis. This overall effect is alarming due to the risk of overweight, obesity and their relevant comorbidities. Moreover, the BW loss observed in one study in older adults may be an alarming sign for lockdown-related risk of weight loss and malnutrition in older adults, and further research is required in this important population group. Awareness of the public with respect to the importance of balanced diet and physical activity in confinement situations should be enhanced [66] also considering that the risk of future lockdowns is unfortunately not absent.
Author distribution
DB and MC searched the databases; DB and MC wrote the paper; RB, SB, KW, JB and MC made the necessary recommendations; and DB, RB SB, KW, JB and MC revised the manuscript. All authors have read and approved the final version of manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.04.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloch S., Baloch M.A., Zheng T., Pei X. The coronavirus disease 2019 (COVID-19) pandemic. Tohoku J Exp Med. 2020;250(4):271–278. doi: 10.1620/tjem.250.271. [DOI] [PubMed] [Google Scholar]
- 3.Koh D. COVID-19 lockdowns throughout the world. Occup Med (Lond) 2020 doi: 10.1093/occmed/kqaa073. [DOI] [Google Scholar]
- 4.Clay J.M., Parker M.O. Alcohol use and misuse during the COVID-19 pandemic: a potential public health crisis? Lancet Public Health. 2020;5(5):e259. doi: 10.1016/S2468-2667(20)30088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S., Roy D., Sinha K., Parveen S., Sharma G., Joshi G. Impact of COVID-19 and lockdown on mental health of children and adolescents: a narrative review with recommendations. Psychiatry Res. 2020;293:113429. doi: 10.1016/j.psychres.2020.113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laguna L., Fiszman S., Puerta P., Chaya C., Tárrega A. The impact of COVID-19 lockdown on food priorities. Results from a preliminary study using social media and an online survey with Spanish consumers. Food Qual Prefer. 2020;86:104028. doi: 10.1016/j.foodqual.2020.104028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moynihan A.B., van Tilburg W.A., Igou E.R., Wisman A., Donnelly A.E., Mulcaire J.B. Eaten up by boredom: consuming food to escape awareness of the bored self. Front Psychol. 2015;6:369. doi: 10.3389/fpsyg.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yannakoulia M., Panagiotakos D.B., Pitsavos C., Tsetsekou E., Fappa E., Papageorgiou C., et al. Eating habits in relations to anxiety symptoms among apparently healthy adults. A pattern analysis from the ATTICA Study. Appetite. 2008;51(3):519–525. doi: 10.1016/j.appet.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Mestre Z.L., Melhorn S.J., Askren M.K., Tyagi V., Gatenby C., Young L., et al. Effects of anxiety on caloric intake and satiety-related brain activation in women and men. Psychosom Med. 2016;78(4):454–464. doi: 10.1097/PSY.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen A.J., Tran T., Hammarberg K., Kirkman M., Fisher J., Group C.-R.I.R. Poor appetite and overeating reported by adults in Australia during the coronavirus-19 disease pandemic: a population-based study. Public Health Nutr. 2020:1–7. doi: 10.1017/S1368980020003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janati Idrissi A., Lamkaddem A., Benouajjit A., Ben El Bouaazzaoui M., El Houari F., Alami M., et al. Sleep quality and mental health in the context of COVID-19 pandemic and lockdown in Morocco. Sleep Med. 2020;74:248–253. doi: 10.1016/j.sleep.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto J., van Zeller M., Amorim P., Pimentel A., Dantas P., Eusébio E., et al. Sleep quality in times of Covid-19 pandemic. Sleep Med. 2020;74:81–85. doi: 10.1016/j.sleep.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constandt B., Thibaut E., De Bosscher V., Scheerder J., Ricour M., Willem A. Exercising in times of lockdown: an analysis of the impact of COVID-19 on levels and patterns of exercise among adults in Belgium. Int J Environ Res Public Health. 2020;17(11):4144. doi: 10.3390/ijerph17114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crispim C.A., Zalcman I., Dáttilo M., Padilha H.G., Edwards B., Waterhouse J., et al. The influence of sleep and sleep loss upon food intake and metabolism. Nutr Res Rev. 2007;20(2):195–212. doi: 10.1017/S0954422407810651. [DOI] [PubMed] [Google Scholar]
- 15.Manz K., Mensink G.B.M., Finger J.D., Haftenberger M., Brettschneider A.-K., Lage Barbosa C., et al. Associations between physical activity and food intake among children and adolescents: results of KiGGS Wave 2. Nutrients. 2019;11(5):1060. doi: 10.3390/nu11051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi S. The pandemic has more than doubled food-delivery apps' business. Now what? 2020. https://www.marketwatch.com/story/the-pandemic-has-more-than-doubled-americans-use-of-food-delivery-apps-but-that-doesnt-mean-the-companies-are-making-money-11606340169 Available from:
- 17.Díaz-Zavala R.G., Castro-Cantú M.F., Valencia M.E., Álvarez-Hernández G., Haby M.M., Esparza-Romero J. Effect of the holiday season on weight gain: a narrative review. J Obes. 2017;2017:2085136. doi: 10.1155/2017/2085136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelan S., Wing R.R., Raynor H.A., Dibello J., Nedeau K., Peng W. Holiday weight management by successful weight losers and normal weight individuals. J Consult Clin Psychol. 2008;76(3):442–448. doi: 10.1037/0022-006X.76.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., et al. The Ottawa Hospital Research Institute; 2016. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 21.Ahmed H.O. The impact of social distancing and self-isolation in the last corona COVID-19 outbreak on the body weight in Sulaimani governorate- Kurdistan/Iraq, a prospective case series study. Ann Med Surg (2012) 2020;59:110–117. doi: 10.1016/j.amsu.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrea L., Pugliese G., Framondi L., Di Matteo R., Laudisio D., Savastano S., et al. Does Sars-Cov-2 threaten our dreams? Effect of quarantine on sleep quality and body mass index. J Transl Med. 2020;18(1) doi: 10.1186/s12967-020-02465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Błaszczyk-Bębenek E., Jagielski P., Bolesławska I., Jagielska A., Nitsch-Osuch A., Kawalec P. Nutrition behaviors in Polish adults before and during COVID-19 lockdown. Nutrients. 2020;12(10) doi: 10.3390/nu12103084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancello R., Soranna D., Zambra G., Zambon A., Invitti C. Determinants of the lifestyle changes during covid-19 pandemic in the residents of northern Italy. Int J Environ Res Public Health. 2020;17(17):1–14. doi: 10.3390/ijerph17176287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carriedo A., Cecchini J.A., Fernandez-Rio J., Mendez-Gimenez A. Resilience and physical activity in people under home isolation due to COVID-19: a preliminary evaluation. Ment Health Phys Act. 2020;19:100361. doi: 10.1016/j.mhpa.2020.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheikh Ismail L., Osaili T.M., Mohamad M.N. 2020. Assessment of eating habits and lifestyle during coronavirus pandemic in the MENA region: a cross-sectional study; pp. 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopra S., Ranjan P., Singh V., Kumar S., Arora M., Hasan M.S., et al. Impact of COVID-19 on lifestyle-related behaviours – a cross-sectional audit of responses from nine hundred and ninety-five participants from India. Diabetes Metab Syndr. 2020;14(6):2021–2030. doi: 10.1016/j.dsx.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Luis Roman D.A., Izaola O., Primo Martin D., Gomez Hoyos E., Torres Torres B., Lopez Gomez J.J. Efecto del confinamiento por COVID-19 sobre la ganancia de peso corporal autorreportada en una muestra de pacientes obesos, Effect of lockdown for COVID-19 on self-reported body weight gain in a sample of obese patients. Nutr Hosp. 2020;37(6):1093–1295. doi: 10.20960/nh.03307. [DOI] [PubMed] [Google Scholar]
- 29.Di Santo S.G., Franchini F., Filiputti B., Martone A., Sannino S. The effects of COVID-19 and quarantine measures on the lifestyles and mental health of people over 60 at increased risk of dementia. Front Psychiatr. 2020;11:578628. doi: 10.3389/fpsyt.2020.578628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dogas Z., Kalcina L.L., Dodig I.P., Demirovic S., Madirazza K., Valic M., et al. The effect of COVID-19 lockdown on lifestyle and mood in Croatian general population: a cross-sectional study. Croat Med J. 2020;61(4):309–318. doi: 10.3325/cmj.2020.61.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou Z., Stefanovski D., Galligan D., Lindem M., Rozin P., Chen T., et al. 2020. The COVID-19 pandemic impacting household food dynamics: a cross-national comparison of China and the U.S. [Google Scholar]
- 32.Drywien M.E., Hamulka J., Zielinska-Pukos M.A., Jeruszka-Bielak M., Gornicka M. The COVID-19 pandemic lockdowns and changes in body weight among Polish women. A cross-sectional online survey PLifeCOVID-19 study. Sustainability. 2020;12(18) [Google Scholar]
- 33.Elmacıoğlu F., Emiroğlu E., Ülker M.T., Özyılmaz Kırcali B., Oruç S. 2020. Evaluation of nutritional behaviour related to COVID-19; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Federik M.A., Calderon C., Degastaldi V., Duria S.A., Monsalvo C., Pinto M., et al. Dietary habits and COVID. Descriptive analysis during social isolation in Argentina. Nutr Clín Diet Hosp. 2020;40(3):84–91. [Google Scholar]
- 35.Fernandez-Rio J., Cecchini J.A., Mendez-Gimenez A., Carriedo A. Weight changes during the COVID-19 home confinement. Effects on psychosocial variables. Obes Res Clin Pract. 2020;14(4):383–385. doi: 10.1016/j.orcp.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Flanagan E.W., Beyl R.A., Fearnbach S.N., Altazan A.D., Martin C.K., Redman L.M. The impact of COVID-19 stay-at-home orders on health behaviors in adults. Obesity (Silver Spring, Md) 2020 doi: 10.1002/oby.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosal S., Arora B., Dutta K., Ghosh A., Sinha B., Misra A. Increase in the risk of type 2 diabetes during lockdown for the COVID19 pandemic in India: a cohort analysis. Diabetes Metab Syndr. 2020;14(5):949–952. doi: 10.1016/j.dsx.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes C.M., Favorito L.A., Henriques J.V.T., Canalini A.F., Anzolch K.M.J., de Carvalho Fernandes R., et al. Impact of COVID-19 on clinical practice, income, health and lifestyle behavior of Brazilian urologists. Int Braz J Urol. 2020;46(6):1042–1071. doi: 10.1590/S1677-5538.IBJU.2020.99.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He M., Xian Y., Lv X., He J., Ren Y. Changes in body weight, physical activity, and lifestyle during the semi-lockdown period after the outbreak of COVID-19 in China: an online survey. Disaster Med Public Health Prep. 2020:1–6. doi: 10.1017/dmp.2020.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber B.C., Steffen J., Schlichtiger J., Brunner S. Altered nutrition behavior during COVID-19 pandemic lockdown in young adults. Behav Sci (Basel, Switzerland) 2020:1–10. doi: 10.1007/s00394-020-02435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ismail L.C., Osaili T.M., Mohamad M.N., Al Marzouqi A., Jarrar A.H., Abu Jamous D.O., et al. Eating habits and lifestyle during COVID-19 lockdown in the United Arab Emirates: a cross-sectional study. Nutrients. 2020;12(11) doi: 10.3390/nu12113314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia P., Zhang L., Yu W., Yu B., Liu M., Zhang D., et al. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS) Int J Obes. 2020;45(3):695–699. doi: 10.1038/s41366-020-00710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keel P.K., Gomez M.M., Harris L., Kennedy G.A., Ribeiro J., Joiner T.E. Gaining “The Quarantine 15:” Perceived versus observed weight changes in college students in the wake of COVID-19. Int J Eat Disord. 2020;53(11):1801–1808. doi: 10.1002/eat.23375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kriaucioniene V., Bagdonaviciene L., Rodríguez-Pérez C., Petkeviciene J. Associations between changes in health behaviours and body weight during the covid-19 quarantine in Lithuania: the Lithuanian covidiet study. Nutrients. 2020;12(10):1–9. doi: 10.3390/nu12103119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumari A., Ranjan P., Vikram N.K., Kaur D., Sahu A., Dwivedi S.N., et al. A short questionnaire to assess changes in lifestyle-related behaviour during COVID 19 pandemic. Diabetes Metab Syndr. 2020;14(6):1697–1701. doi: 10.1016/j.dsx.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Moreno M., Lopez M.T.I., Miguel M., Garces-Rimon M. Physical and psychological effects related to food habits and lifestyle changes derived from covid-19 home confinement in the Spanish population. Nutrients. 2020;12(11):1–17. doi: 10.3390/nu12113445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchitelli S., Mazza C., Lenzi A., Ricci E., Gnessi L., Roma P. Weight gain in a sample of patients affected by overweight/obesity with and without a psychiatric diagnosis during the covid-19 lockdown. Nutrients. 2020;12(11):1–12. doi: 10.3390/nu12113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez-de-Quel Ó., Suárez-Iglesias D., López-Flores M., Pérez C.A. Physical activity, dietary habits and sleep quality before and during COVID-19 lockdown: a longitudinal study. Appetite. 2020;158 doi: 10.1016/j.appet.2020.105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papandreou C., Arija V., Aretouli E., Tsilidis K.K., Bulló M. Comparing eating behaviours, and symptoms of depression and anxiety between Spain and Greece during the COVID-19 outbreak: cross-sectional analysis of two different confinement strategies. Eur Eat Disord Rev. 2020;28(6):836–846. doi: 10.1002/erv.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pellegrini M., Ponzo V., Rosato R., Scumaci E., Goitre I., Benso A., et al. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):1–11. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes-Olavarría D. Positive and negative changes in food habits, physical activity patterns, and weight status during COVID-19 confinement: associated factors in the Chilean population. Science (New York, NY) 2020;17(15) doi: 10.3390/ijerph17155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Perez C., Molina-Montes E., Verardo V., Artacho R., Garcia-Villanova B., Guerra-Hernandez E.J., et al. Changes in dietary behaviours during the COVID-19 outbreak confinement in the Spanish COVIDiet study. Nutrients. 2020;12(6) doi: 10.3390/nu12061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarmozzino F., Visioli F. Covid-19 and the subsequent lockdown modified dietary habits of almost half the population in an Italian sample. Obesity (Silver Spring, Md) 2020;9(5) doi: 10.3390/foods9050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sidor A., Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12(6) doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinisterra Loaiza L.I., Vazquez Belda B., Miranda Lopez J.M., Cepeda A., Cardelle Cobas A. Food habits in the Galician population during confinement for COVID-19. Nutr Hosp. 2020;37(6):1190–1196. doi: 10.20960/nh.03213. [DOI] [PubMed] [Google Scholar]
- 56.Zachary Z., Brianna F., Brianna L., Garrett P., Jade W., Alyssa D., et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14(3):210–216. doi: 10.1016/j.orcp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berardi C., Antonini M., Genie M.G., Cotugno G., Lanteri A., Melia A., et al. The COVID-19 pandemic in Italy: policy and technology impact on health and non-health outcomes. Health Policy Technol. 2020;9(4):454–487. doi: 10.1016/j.hlpt.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans C. Malnutrition in the elderly: a multifactorial failure to thrive. Perm J. 2005;9(3):38–41. doi: 10.7812/tpp/05-056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hugo B.U. 2020. Dietician warns malnutrition of elderly could increase during COVID-19 pandemic. [Google Scholar]
- 60.Sounding the alarm about the rising risk of malnutrition among older people during lockdown. 2020. [Google Scholar]
- 61.Khan M.A., Moverley Smith J.E. “Covibesity,” a new pandemic. Obes Med. 2020;19:100282. doi: 10.1016/j.obmed.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fruh S.M. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):S3–S14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jayawardena R., Jeyakumar D.T., Misra A., Hills A.P., Ranasinghe P. Obesity: a potential risk factor for infection and mortality in the current COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(6):2199–2203. doi: 10.1016/j.dsx.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., et al. Obesity – a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caussy C., Wallet F., Laville M., Disse E. Obesity is associated with severe forms of COVID-19. Obesity (Silver Spring, Md) 2020;28(7):1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr (Edinburgh, Scotland) 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.