To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been associated with the development of autoimmune processes.[1], [2], [3], [4] Molecular mimicry has been suggested as a potential mechanism for these associations.1 In an in vitro study, Vojdani et al. 5 showed that antibodies against the spike protein S1 of SARS-CoV-2 had high affinity against the following human tissue proteins: transglutaminase 3, transglutaminase 2, anti-extractable nuclear antigen, nuclear antigen, and myelin basic protein. As this is the same viral protein that the vaccine mRNA codes for, it is plausible that these vaccines may unmask autoimmune diseases in predisposed patients. Recently, several cases of immune thrombocytopenia (ITP) developing days after COVID-19 vaccination, have been reported to the Vaccine Adverse Event Reporting System (VAERS), reinforcing the possibility of vaccine-induced autoimmunity.6
We have recently treated a 35-year-old Caucasian female in her third month postpartum, who developed autoimmune hepatitis after COVID-19 vaccination. During pregnancy, she was diagnosed with gestational hypertension and started on labetalol 100 mg bid. C-section was performed without any complications, and patient was discharged from the hospital on labetalol for blood pressure control. She resumed her job as a healthcare provider in mid-December, and received her first dose of Pfizer-BioNTech COVID-19 vaccine on January 4th. After 1 week, she started developing generalized pruritus, then choluria, and finally noticed jaundice, presenting to the emergency room on day +13 after COVID-19 vaccination.
She had a normal physical exam, except for scleral icterus, jaundice and palpable hepatomegaly. In the emergency room, laboratories were significant for: bilirubin 4.8 mg/dl, AST 754 U/L, ALT 2,001 U/L, alkaline phosphatase 170 U/L, and ammonium 61 mg/dl. Laboratory results were negative for hepatitis A, B, and C, Epstein-Barr virus (EBV), cytomegalovirus (CMV), herpes simplex virus (HSV) type 1 and 2, and HIV. At the time of submission, HEV had not been tested. Antinuclear antibody (ANA) was positive (1:1,280; homogeneous pattern). Double-stranded DNA antibodies were also positive (1:80). Other antibodies (i.e. anti-mitochondrial, anti-smooth muscle, liver-kidney microsomal, antineutrophil cytoplasmic antibodies) were negative. Total IgG was 1,081 mg/dl (normal range: 694–1,618 mg/dl). Ceruloplasmin, transferrin saturation, alpha-1-antitrypsin, TSH, and serum protein electrophoresis were all normal. Abdominal ultrasound with Doppler reported hepatomegaly without cirrhotic morphology, and no intra- or extra-hepatic biliary dilation.
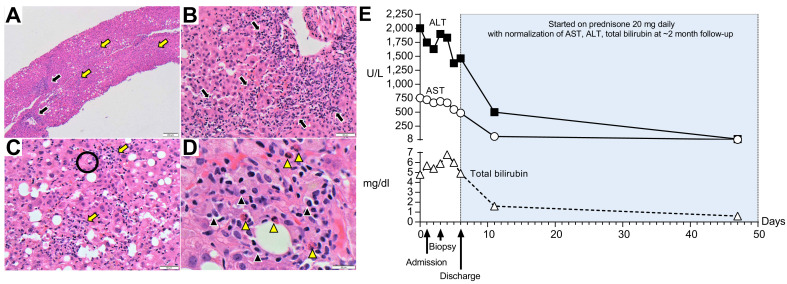
Endoscopic ultrasound showed no evidence of biliary lithiasis or biliary dilation, and transduodenal liver biopsies were obtained. In Fig. 1 A–D we have included the slides of her liver biopsy and a full description of the histology. Liver biopsy was consistent with drug/toxin related liver injury, autoimmune hepatitis or infectious related etiologies. A PAS-D stain, CMV & HSV 1/2 immunohistochemical stains, EBV by in situ hybridization (EBER-ISH) and Grocott methenamine special stain for fungi were all negative. Other than the COVID-19 vaccine and labetalol, no other drugs, herbal supplements or toxins were reported by the patient. The Revised Original Score for autoimmune hepatitis pretreatment was 18 (results >15 suggest definite autoimmune hepatitis). Fig. 1E summarizes plasma ALT, AST, and total bilirubin over time, before and after treatment with prednisone 20 mg daily.
Fig. 1.
Histological (H&E stain) and biochemical findings.
(A) Low-magnification (40x) shows pan-lobular hepatitis (black arrows: portal inflammation and yellow arrows: lobular inflammation). (B) Medium-magnification images (200x) show a portal tract with an intense lymphoplasmacytic infiltrate effacing the interface with rosette formation and (C) lobular activity with scattered hepatocyte necrosis (Black circle: acidophilic bodies). (D) At high magnification (600x), the inflammation consists primarily of lymphocytes with plasma cells (black arrowheads) and eosinophils (yellow arrowheads). (E) Trends of plasma ALT, AST and total bilirubin over time.
To our knowledge, this is the first reported episode of autoimmune hepatitis developing post-COVID-19 vaccination, raising concern regarding the possibility of vaccine-induced autoimmunity. As causality cannot be proven, it is possible that this association is just coincidental. However, severe cases of SARS-CoV-2 infection are characterized by an autoinflammatory dysregulation that contributes to tissue damage.1 As the viral spike protein appears to be responsible for this,1 , 5 it is plausible that spike-directed antibodies induced by vaccination may also trigger autoimmune conditions in predisposed individuals. In support of this, several cases of ITP have been reported days after COVID-19 vaccination.6
Several atypical features of her presentation deserve further discussion. First, immunoglobulin G levels were not elevated as typically reported for autoimmune hepatitis. However, Hartl et al. recently reported that ~10% of patients with autoimmune hepatitis had normal immunoglobulin G levels at presentation.7 Second, histology revealed the presence of eosinophils, which are more commonly seen with drug or toxin induced liver injury. However, they can be found in cases of autoimmune hepatitis.8 It is also possible that we could be in the presence of a vaccine-related drug-induced liver injury with features of autoimmune hepatitis, as previously described for nitrofurantoin or minocycline.9 In line with this, the patient has already started a prednisone taper, as patients with well documented drug-induced AIH do not typically show relapses after steroid discontinuation.10 Finally, symptoms developed 6 days after vaccination, which instinctively appears as a short period of time. However, latency periods after vaccination of just days have been observed in prior reports.6 , 11
In summary, autoimmune hepatitis developed in a healthy 35-year-old female in her third month postpartum. Whether there exists a causal relationship between COVID-19 vaccination and the development of autoimmune hepatitis remains to be determined. We are hopeful that this manuscript will not discourage healthcare providers from getting and prescribing COVID-19 vaccines, but that it will raise awareness about potential side effects that will likely emerge as we continue to vaccinate more people. Only long-term follow-up of large cohorts of patients receiving the vaccine will answer the question as to whether it increases the risk of autoimmune conditions. Until then, healthcare providers are encouraged to remain vigilant.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
FB: Patient care, writing of the manuscript, and revision of the final version of the manuscript. SAD: Pathology reading, and revision of the final version of the manuscript. MD: Patient care, and revision of the final version of the manuscript. DMF: Patient care, and revision of the final version of the manuscript.
Conflict of interests
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.04.003.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zulfiqar A.A., Lorenzo-Villalba N., Hassler P., Andres E. Immune thrombocytopenic purpura in a patient with covid-19. N Engl J Med. 2020;382:e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with covid-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain-barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol. 2021 Feb 19 doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartl J., Miquel R., Zachou K., Wong G.W., Asghar A., Pape S., et al. Features and outcome of AIH patients without elevation of IgG. JHEP Rep. 2020;2:100094. doi: 10.1016/j.jhepr.2020.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiniakos D.G., Brain J.G., Bury Y.A. Role of histopathology in autoimmune hepatitis. Dig Dis. 2015;33(Suppl 2):53–64. doi: 10.1159/000440747. [DOI] [PubMed] [Google Scholar]
- 9.de Boer Y.S., Kosinski A.S., Urban T.J., Zhao Z., Long N., Chalasani N., et al. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–112. doi: 10.1016/j.cgh.2016.05.043. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornsson E.S., Bergmann O., Jonasson J.G., Grondal G., Gudbjornsson B., Olafsson S. Drug-induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017;15:1635–1636. doi: 10.1016/j.cgh.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Agmon-Levin N., Kivity S., Szyper-Kravitz M., Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009;18:1198–1204. doi: 10.1177/0961203309345730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.