Abstract
Recent reports have highlighted rare, and sometimes fatal, cases of cerebral venous sinus thrombosis (CVST) and thrombocytopenia following the Vaxzevria vaccine. An underlying immunological mechanism similar to that of spontaneous heparin-induced thrombocytopenia (HIT) is suspected, with the identification of antibodies to platelet factor-4 (PF4), but without previous heparin exposure. This unusual mechanism has significant implications for the management approach used, which differs from usual treatment of CVST. We describe the cases of two young males, who developed severe thrombocytopenia and fatal CVST following the first dose of Vaxzevria. Both presented with a headache, with subsequent rapid neurological deterioration. One patient underwent PF4 antibody testing, which was positive. A rapid vaccination programme is essential in helping to control the COVID-19 pandemic. Hence, it is vital that such COVID-19 vaccine-associated events, which at this stage appear to be very rare, are viewed through this lens. However, some cases have proved fatal. It is critical that clinicians are alerted to the emergence of such events to facilitate appropriate management. Patients presenting with CVST features and thrombocytopenia post-vaccination should undergo PF4 antibody testing and be managed in a similar fashion to HIT, in particular avoiding heparin and platelet transfusions.
Keywords: Neurology, Stroke, Cerebrovascular disease, COVID-19, Vaccine, Cerebral venous sinus thrombosis, Thrombocytopenia, Immunology
1. Introduction
Recent data published by the European Medicines Agency have highlighted 18 cases of cerebral venous sinus thrombosis (CVST), the majority (67%) of which had associated thrombocytopenia, following vaccination with Vaxzevria (previously named COVID-19 Vaccine AstraZeneca), amongst over 20 million recipients of this vaccine (European Medicines Agency, 2021). More recently, the Medicines and Healthcare products Regulatory Agency (MHRA) have reported 22 cases of CVST with associated thrombocytopenia following vaccination with Vaxzevria, amongst 18.1 million recipients in the UK (Medicines and Healthcare Products Regulatory Agency, 2021).
The majority of the cases reported thus far have involved female patients under the age of 55 and occurred between 4 and 16 days after vaccination. Because it is a rare event and data are still being collected, it is currently not certain if the rate of CVST associated with the COVID-19 vaccine is higher than the background rate, which is 1.32–1.57/100,000/year (Coutinho et al., 2012, Devasagayam et al., 2016). The mortality from vaccine-associated CVST appears higher than expected at 29–33% (European Medicines Agency, 2021, Medicines and Healthcare Products Regulatory Agency, 2021, Paul-Ehrlich-Institut, 2021), as opposed to the usual mortality of approximately 4.4% (Haghighi et al., 2012).
Typical laboratory features include a platelet count < 100 x109/L, raised D-dimers, and an inappropriately low fibrinogen. A causal link has not been proven, but there is a growing consensus amongst thrombosis experts that the underpinning pathophysiological mechanism is similar to that of spontaneous heparin-induced thrombocytopenia (HIT), in which, in the absence of recent heparin exposure, antibodies target a complex of platelet factor-4 (PF4) and heparin, and activate cellular FcγIIA receptors on platelets, inducing a prothrombotic cascade together with thrombocytopenia (Greinacher et al., 2021, British Society for Haematology, 2021, Arepally, 2017, Gesellschaft für Thrombose und Hämostaseforschung, 2021).
This discovery is of particular salience given that the management of CVST typically involves anticoagulation with heparin and treating any underlying secondary causes (Ferro et al., 2017), while the management of thrombocytopenia can include platelet transfusions. The treatment of spontaneous HIT, however, requires the avoidance of both heparin and platelet transfusions, utilisation of alternative non-heparin-based anticoagulants (Arepally, 2017), and intravenous immunoglobulin (Warkentin, 2019).
Case 1
A 32-year-old male with no known medical history or regular medication developed a thunderclap headache and subsequent left-sided incoordination and hemiparesis several hours later. This was nine days following first dose of the Vaxzevria vaccine (Table 1 ).
Table 1.
Summary of the demographics, clinical features, laboratory investigations, neuroimaging findings, and treatment of the two cases. “-” denotes that the test was not performed, owing to no samples being available. *Patient was being weaned off budesonide for suspected autoimmune hepatitis, as the diagnosis was subsequently revised to primary sclerosing cholangitis, based on immunological and histological results.
| Case 1 | Case 2 | ||
|---|---|---|---|
| Age, Gender (M/F), Ethnicity | 32, M, White | 25, M, White | |
| Past medical history | Nil | Primary sclerosing cholangitis, Migraines | |
| Regular medications | Nil | Ursodeoxycholic acid, Budesonide,*Sumatriptan, Amitriptyline | |
| Family history of autoimmune or clotting disorder | No | No | |
| Smoking history | Ex-smoker | Smoker | |
| Time of onset after vaccination (days) | 9 | 6 | |
| Initial symptom | Thunderclap headache | Meningitic headache | |
| Other symptoms | Left hemiparesis, left-sided incoordination | Photophobia, vomiting, petechial rash, gum bleeding, left hemiparesis, left hemisensory loss | |
| Deterioration during admission | Seizures, reduced GCS, decerebrate posturing, dilated unreactive pupils | Seizures, agitation, decerebrate posturing, reduced GCS | |
| Lab parameters | Platelets on admission (x109/L; 150–450) | 30 | 19 |
| Haemoglobin on admission (x109/L; 133–167) | 146 | 148 | |
| Fibrinogen (g/L; 1.5–4.5) | 1.4 | 1.3 | |
| Bilirubin (µmol/L; 3–20) | 9 | 9 | |
| Lactate dehydrogenase (IU/L; 〈2 4 0) | – | 192 | |
| Blood film | Thrombocytopenia, no cell fragments | Thrombocytopenia, no cell fragments | |
| Antiphospholipid antibodies | – | Negative | |
| ADAMTS-13 | – | Normal | |
| Paroxysmal nocturnal haemoglobinuria test | – | Negative | |
| Thrombophilia DNA | – | Factor V Leiden: heterozygous for the c.1601G > A (p.Arg534Gln) variant | |
| Platelet factor-4 antibodies | – | Positive | |
| Creatinine on admission (µmol/L; 45–120) | 71 | 58 | |
| CRP on admission (mg/L; <5) | 47 | 4 | |
| SARS-CoV2 PCR | Negative | Negative | |
| Imaging | Sinuses involved | Superior sagittal sinus | Superior sagittal sinus |
| Cortical veins involved | Diffuse; prominently involving the right superficial anastomotic vein | Diffuse | |
| Clot burden | Heavy; significant venous expansion | Heavy; significant venous expansion | |
| Subarachnoid haemorrhage | Mainly cortical | Cortical and basal cisterns | |
| Intraparenchymal haemorrhage | Extensive (venous distribution) | Extensive (venous distribution) | |
| Treatment | Low molecular weight heparin | No | No |
| Unfractionated heparin | No | Yes | |
| Steroids | No | Dexamethasone 40 mg once daily | |
| Intravenous immunoglobulin | No | Yes (1 g/kg) | |
| Platelet transfusions | No | Yes | |
Neuroimaging revealed superior sagittal sinus and cortical vein thrombosis and significant cortical oedema with small areas of parenchymal and subarachnoid haemorrhage. (Fig. 1 A). Blood tests confirmed a severe thrombocytopenia (platelet count of 30 x109/L on presentation with a nadir of 7 x109/L), and an inappropriately low fibrinogen, with no schistocytes on blood film, a normal lactate dehydrogenase, and normal coagulation. Thus, there was no evidence of microangiopathic haemolytic anaemia (MAHA) or disseminated intravascular coagulopathy (DIC).
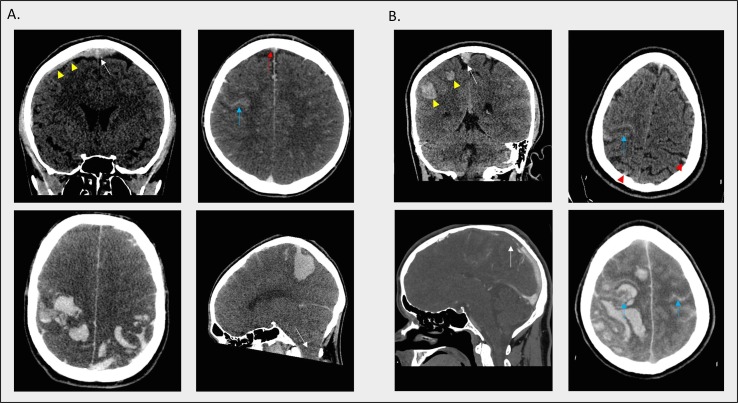
Fig. 1.
A. Upper panel: volumetric coronal non-contrast CT brain study (left image) and volumetric axial CT venogram brain study (right image) was performed at the time of hospital admission. There is a heavy burden of clot expanding the middle to anterior third of the superior sagittal sinus, seen as an area of hyperdensity in the non-contrast study (white arrow in left image) and as a filling defect in the contrast study (red arrow in right image). There is extension into the superficial cortical venous system, most prominently the right superficial anastomotic vein which is seen to be thrombosed and dilated in the non-contrast study (yellow arrowheads in left image). There is significant cortical oedema with sulcal crowding. There is subarachnoid haemorrhage along the right central sulcus and a small subcortical intraparenchymal haematoma inferomedial to this (blue arrow in right image). Lower panel: volumetric axial and sagittal non-contrast CT brain imaging 3 days post-admission demonstrates extensive parietal haemorrhage in a typical venous distribution with evidence of cerebellar tonsillar descent (white arrow in right image). B. Upper panel: volumetric coronal and axial non-contrast CT brain imaging at time of hospital admission demonstrates large volume clot within the superior sagittal sinus (white arrow in left image) and its supplying cortical venous tributaries (examples shown with red arrowheads in right image). In addition, there are focal areas of venous haemorrhage in the subcortical parietal lobes (yellow arrowheads in left image), and subarachnoid haemorrhage along the right central and post-central sulci (blue arrow in right image). Lower panel: Volumetric sagittal and axial CT venogram study performed 3 hours later highlights a large filling defect in the anterior two thirds of the superior sagittal sinus (white arrow in left image). Extensive parenchymal acute haemorrhage is seen in the right frontal and parietal lobes with marked oedema and midline shift. There is also extensive subarachnoid haemorrhage within the frontoparietal convexity sulci bilaterally (examples shown with blue arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
No specific haematological or immunological treatments were administered for this case, as his neurological condition had deteriorated rapidly. His Glasgow Coma Score (GCS) fell from 15 to 4 over 3 hours, and he developed generalised tonic-clonic seizures and decerebrate posturing, necessitating intubation and ventilation, after which his pupillary responses deteriorated and became fixed and dilated despite repeated doses of mannitol and hypertonic saline.
Repeat imaging showed further haemorrhagic progression and signs of significant cerebral oedema, midline shift, and cerebellar herniation (Fig. 1A). Brainstem death was subsequently confirmed, and ventilator support withdrawn.
Case 2
A 25-year-old male with a background of primary sclerosing cholangitis (PSC) and migraines presented with a four-day history of worsening headache with photophobia, neck stiffness, and visual disturbances, associated with a non-blanching petechial rash over his lower limbs and bleeding of his gums. This was six days following first dose of the Vaxzevria vaccine. Subsequently, he developed left hemiparesis and hemisensory loss, and focal motor seizures (Table 1).
Neuroimaging revealed superior sagittal sinus thrombosis with extension into the cortical veins, and haemorrhage in lobar and subarachnoid locations (Fig. 1B). Blood tests indicated thrombocytopenia (platelet count of 19 x109/L on presentation with a nadir of 17 x109/L) and low fibrinogen, with no evidence of MAHA or DIC.
He was treated with intravenous unfractionated heparin, platelet transfusions, intravenous dexamethasone, intravenous immunoglobulin, and intravenous levetiracetam.
Unfortunately, his neurology continued to deteriorate over 2 h with worsening headache and right focal motor seizures, followed by a reduced GCS and decerebrate posturing, necessitating sedation and intubation. Repeat neuroimaging revealed extensive bilateral frontoparietal intraparenchymal and subarachnoid haemorrhages with midline shift (Fig. 1B). Formal testing off sedation confirmed brainstem death and he was taken off ventilator support.
Further assays revealed the presence of PF4 antibodies, and a factor V Leiden heterozygous c. 1601G > A (p.Arg534GIn) variant.
2. Conclusion
There have been more than 120 million cases of COVID-19 infection, and more than 2 million deaths reported globally, highlighting the importance of an effective vaccination programme as the most powerful way of limiting illness and death due to the pandemic (World Health Organisation, 2021). Based on the current information available, and in light of the reported rarity of CVST and severe thrombocytopenia following COVID-19 vaccination, the benefits of vaccination outweigh the potential risks. Furthermore, the risk of thrombosis with COVID-19 infection itself is high, especially if admitted to intensive care, highlighting further benefits of vaccination. However, as this is a rapidly evolving situation, strict monitoring of numbers is needed to determine an accurate incidence of cases.
An interesting observation is the preponderance of thrombosis occurring in the cerebral venous sinuses, more so than elsewhere in the body. Classical HIT, although being a highly prothrombotic condition, does not preferentially present with CVST. Also, of note, neuroimaging detected high clot burden in both cases, with a large amount of parenchymal and subarachnoid haemorrhage. Further studies are required to determine why this may be the case.
We emphasise the importance of neurologists and other clinicians being aware of this rare, but serious, and potentially fatal, complication and its manifestations. In our centre, we recommend considering this phenomenon in patients with a persisting or severe headache, and/or focal neurology, seizures, a platelet count of <100 x109/L, and COVID-19 vaccination in the preceding 28 days.
Regarding the management of our cases, the first case presented early in the course of the vaccination programme. Whilst a Yellow Card Notification was submitted to the MHRA, there were no other reports available at that time, and no obvious reason to strongly suspect a causal link to the vaccine. Regarding the second case, again, this was early in terms of reports of such events. The PF4 antibodies had just been reported, and so testing was performed posthumously.
With the knowledge that such events are due to a HIT-like phenomenon, it is of great importance that up-to-date management recommendations are circulated. In suspected cases, first-line imaging should involve a plain CT head with an additional CT venogram as necessary, alongside laboratory testing of full blood count, reticulocyte count, blood film, PT, APTT, fibrinogen, D-dimer, LDH, antiphospholipid screen, paroxysmal nocturnal haemoglobinuria screen, and ADAMTS-13. Finally, an urgent serum sample for PF4 antibodies ELISA should be sent. Urgent discussion with colleagues in haematology of course is essential in every case.
Neurologists should be aware that management recommendations crucially differ from routine treatment of CVST, requiring clinicians to avoid heparin in all forms (i.e. unfractionated heparin, even for line flushes, or LMWH e.g. enoxaparin). For purposes of anticoagulation, non-heparin agents are recommended, such as argatroban. Platelet transfusions should be avoided. The administration of intravenous immunoglobulin is recommended (1 g/kg, which can be given in divided doses over two days).
Going forward, identification of risk factors would be useful in stratifying patients and further limiting this risk. Although earlier reports have been predominantly in females, our cases illustrate two young males who have been affected. There is ongoing research looking into the potential impacts of smoking and use of the oral contraceptive pill. Although our cases are anecdotal, one was a current smoker, and was subsequently found to harbour a Factor V Leiden mutation that would have increased his baseline risk of venous thrombosis. PSC might have indicated an underlying autoimmune tendency. Of note, PSC is not in itself a recognised risk factor for CVST, and the patient did not have inflammatory bowel disease (which can predispose to CVST) in association.
In conclusion, as a medical community, we must be quick to respond to emerging knowledge regarding this phenomenon, so that patients receive optimal management (Ng Kee Kwong et al., 2020, Paterson et al., 2020, Benger et al., 2020). We also emphasise the importance of close collaboration between neurology and haematology when managing challenging cases of CVST with thrombocytopenia.
Patient consent: Consent from the next of kin was obtained for both cases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We acknowledge the patients and their families and friends, and the referring clinicians.
Contributors.
PRM and SAM: Drafted the initial manuscript. PRM, LKS, and SAM: Provided clinical care. MB: Provided the radiological images. JC and RA: Provided haematology expertise clinically and on the manuscript. LKS: Provided supervision. All authors: Revised the manuscript critically for important intellectual content.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Arepally G.M. Heparin-induced thrombocytopaenia. Blood. 2017;129(21):2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benger M., Williams O., Siddiqui J., Sztriha L. Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series. Brain Behav. Immun. 2020;88:940–944. doi: 10.1016/j.bbi.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Society for Haematology: A message from BSH President, Professor Adele Fielding - March 2021. https://b-s-h.org.uk/about-us/news/a-message-from-bsh-president-professor-adele-fielding-march-2021 (accessed 29 March 2021).
- Coutinho J.M., Zuurbier S.M., Aramideh M., Stam J. The incidence of cerebral venous thrombosis: A cross-sectional study. Stroke. 2012;43(12):3375–3377. doi: 10.1161/STROKEAHA.112.671453. [DOI] [PubMed] [Google Scholar]
- Devasagayam S., Wyatt B., Leyden J., Kleinig T. Cerebral Venous sinus thrombosis incidence is higher than previously thought. A retrospective population-based study. Stroke. 2016;47(9):2180–2182. doi: 10.1161/STROKEAHA.116.013617. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency: Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – COVID-19 Vaccine AstraZeneca (Other viral vaccines). [Published on 24 March 2021]. https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf (accessed 5 April 2021).
- Ferro J.M., Bousser M.-G., Canhão P., et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis. Eur. Stroke J. 2017;2(3):195–221. doi: 10.1177/2396987317719364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesellschaft, für Thrombose und, Hämostaseforschung. Updated GTH statement on vaccination with the AstraZeneca COVID-19 vaccine, as of March 22, 2021. https://gth-online.org/wp-content/uploads/2021/03/GTH_Stellungnahme_AstraZeneca_engl._3_22_2021.pdf (accessed 29 March 2021).
- Greinacher A, Thiele T, Warkentin T, et al. A prothrombotic thombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination/. [Preprint; published on 28 March 2021]. https://doi: 10.21203/rs.3.rs-362354/v1 (accessed 29 March 2021).
- Haghighi A.B., Edgell R.C., Cruz-Flores S., Feen E., Piriyawat P., Vora N., Callison R.C., Alshekhlee A. Mortality of cerebral venous-sinus thrombosis in a large national sample. Stroke. 2012;43(1):262–264. doi: 10.1161/STROKEAHA.111.635664. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare products Regulatory Agency: Coronavirus vaccine - weekly summary of Yellow Card reporting. [Published on 1 April 2021]. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed 5 April 2021).
- Ng Kee Kwong K.C., Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: A neurological perspective. J. Clin. Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R.W. Paterson R.L. Brown L. Benjamin R. Nortley S. Wiethoff T. Bharucha D.L. Jayaseelan G. Kumar R.E. Raftopoulos L. Zambreanu V. Vivekanandam A. Khoo R. Geraldes K. Chinthapalli E. Boyd H. Tuzlali G. Price G. Christofi J. Morrow P. McNamara B. McLoughlin S.T. Lim P.R. Mehta V. Levee S. Keddie W. Yong S.A. Trip A.J.M. Foulkes G. Hotton T.D. Miller A.D. Everitt C. Carswell N.W.S. Davies M. Yoong D. Attwell J. Sreedharan E. Silber J.M. Schott A. Chandratheva R.J. Perry R. Simister A. Checkley N. Longley S.F. Farmer F. Carletti C. Houlihan M. Thom M.P. Lunn J. Spillane R. Howard A. Vincent D.J. Werring C. Hoskote H.R. Jäger H. Manji M.S. Zandi The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings 143 10 2020 2020 3104 3120. [DOI] [PMC free article] [PubMed]
- Paul-Ehrlich-Institut: Latest News - 30 March 2021. https://www.pei.de/EN/service/press/latest-news/latest-news-node.html (accessed 5 April 2021).
- Warkentin T.E. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: A review. Expert Rev. Hematol. 2019;12(8):685–698. doi: 10.1080/17474086.2019.1636645. [DOI] [PubMed] [Google Scholar]
- Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID-19 subcommittee on safety signals related to the AstraZeneca COVID-19 vaccine. https://www.who.int/news/item/19-03-2021-statement-of-the-who-global-advisory-committee-on-vaccine-safety-(gacvs)-covid-19-subcommittee-on-safety-signals-related-to-the-astrazeneca-covid-19-vaccine (accessed 29 March 2021).