Abstract
Background
Few small studies have described hospital-acquired infections (HAIs) occurring in patients with COVID-19.
Research Question
What characteristics in critically ill patients with COVID-19 are associated with HAIs and how are HAIs associated with outcomes in these patients?
Study Design and Methods
Multicenter retrospective analysis of prospectively collected data including adult patients with severe COVID-19 admitted to eight Italian hub hospitals from February 20, 2020, through May 20, 2020. Descriptive statistics and univariate and multivariate Weibull regression models were used to assess incidence, microbial cause, resistance patterns, risk factors (ie, demographics, comorbidities, exposure to medication), and impact on outcomes (ie, ICU discharge, length of ICU and hospital stays, and duration of mechanical ventilation) of microbiologically confirmed HAIs.
Results
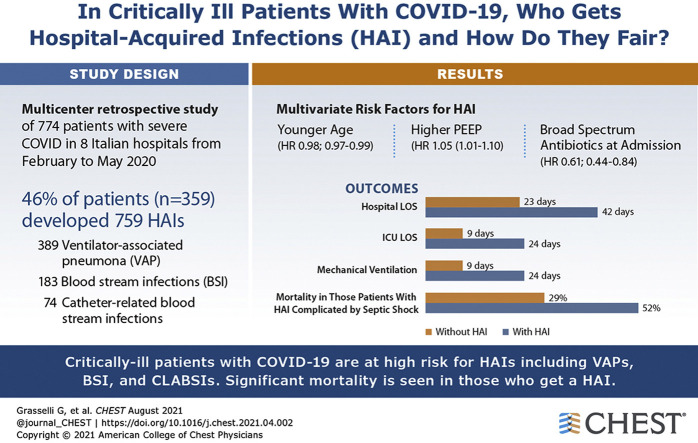
Of the 774 included patients, 359 patients (46%) demonstrated 759 HAIs (44.7 infections/1,000 ICU patient-days; 35% multidrug-resistant [MDR] bacteria). Ventilator-associated pneumonia (VAP; n = 389 [50%]), bloodstream infections (BSIs; n = 183 [34%]), and catheter-related BSIs (n = 74 [10%]) were the most frequent HAIs, with 26.0 (95% CI, 23.6-28.8) VAPs per 1,000 intubation-days, 11.7 (95% CI, 10.1-13.5) BSIs per 1,000 ICU patient-days, and 4.7 (95% CI, 3.8-5.9) catheter-related BSIs per 1,000 ICU patient-days. Gram-negative bacteria (especially Enterobacterales) and Staphylococcus aureus caused 64% and 28% of cases of VAP, respectively. Variables independently associated with infection were age, positive end expiratory pressure, and treatment with broad-spectrum antibiotics at admission. Two hundred thirty-four patients (30%) died in the ICU (15.3 deaths/1,000 ICU patient-days). Patients with HAIs complicated by septic shock showed an almost doubled mortality rate (52% vs 29%), whereas noncomplicated infections did not affect mortality. HAIs prolonged mechanical ventilation (median, 24 days [interquartile range (IQR), 14-39 days] vs 9 days [IQR, 5-13 days]; P < .001), ICU stay (24 days [IQR, 16-41 days] vs 9 days [IQR, 6-14 days]; P = .003), and hospital stay (42 days [IQR, 25-59 days] vs 23 days [IQR, 13-34 days]; P < .001).
Interpretation
Critically ill patients with COVID-19 are at high risk for HAIs, especially VAPs and BSIs resulting from MDR organisms. HAIs prolong mechanical ventilation and hospitalization, and HAIs complicated by septic shock almost double mortality.
Trial Registry
ClinicalTrials.gov; No.: NCT04388670; URL: www.clinicaltrials.gov
Key Words: COVID-19, critical care, hospital-acquired infections, SARS-CoV-2
Abbreviations: BSI, blood stream infection; HAI, hospital-acquired infection; IMV, invasive mechanical ventilation; IQR, interquartile range; LOS, length of stay; MDR, multidrug-resistant; VAP, ventilator-associated pneumonia
Graphical abstract
FOR EDITORIAL COMMENT, SEE PAGE 387
Patients affected by SARS-CoV-2 infection can demonstrate COVID-19, which is associated with a high rate of hospitalization, admission to the ICU,1 and death.2 For several weeks, starting February 20, 2020, Italy was the epicenter of the first COVID-19 outbreak in the Western world.3 In two previous studies,1 , 4 we reported that critically ill patients with COVID-19 primarily were relatively old men with several comorbidities (eg, hypertension, diabetes, COPD) who experienced severe respiratory failure and needed invasive mechanical ventilation (IMV) in almost 90% of the cases. Comorbidities, immune suppression associated with SARS-CoV-2 infection5 , 6 and with the critical illness per se, use of immunomodulators (particularly steroids), and the frequent need for invasive life support procedures predispose patients with COVID-19 to a high risk of hospital-acquired infections (HAIs). To date, few studies have described HAIs during COVID-19 illness, mostly without a specific focus on patients in the ICU.10, 11, 12, 13, 14, 7, 8, 9 This retrospective, single-nation, multicenter study aimed to evaluate the incidence, microbial cause, resistance patterns, risk factors, and impact on outcome of HAI in a large cohort of patients with COVID-19 admitted to the ICU.
Methods
This was a retrospective analysis of prospectively collected data of all consecutive patients with COVID-19 admitted to the ICUs of eight Italian hub hospitals (e-Appendix 1) from February 20, 2020, through May 20, 2020. Follow-up ended on July 23, 2020. The participating centers shared the following management approaches for HAIs: (1) routine antibiotic prophylaxis was not recommended, (2) stress ulcer and DVT prophylaxes were provided, (3) ventilator-associated pneumonia (VAP) bundles15 were applied, and (4) no selective digestive decontamination was used. The participating centers shared the same policy for microbiological surveillance: routine surveillance cultures for bacterial and fungal infections (perineal and nasal swabs for multidrug-resistant (MDR) bacteria, tracheal aspirate, and urine cultures) were obtained at ICU admission and then at least once weekly, whereas further microbiological examinations were performed in the presence of a clinical or laboratory suspicion of infection.
The study was approved by the ethical committee of the promoting center (Comitato Etico Milano Area 2; Protocol: 0008489) and by the local ethical committees and was preregistered at clinicaltrials.gov (Identifier: NCT04388670). Written informed consent was waived because of the retrospective nature of the analysis.
All consecutive patients with laboratory-confirmed SARS-CoV-2 infection (positive reverse-transcription polymerase chain reaction results)16 admitted to the participating ICUs were considered for inclusion. Exclusion criteria were: (1) age < 18 years, (2) ICU length of stay (LOS) < 24 h, (3) nosocomial COVID-19, (4) bacterial coinfections at ICU admission, and (5) reason for ICU admission different from COVID-19.
The following patient data were collected at admission: demographics; weight, height, and BMI; comorbidities stratified according to Charlson Comorbidity Index; immunocompromised status (ie, chronic immunosuppressive therapies, active hematologic or solid malignancies, autoimmune diseases); hypertension; diabetes; Sequential Organ Failure Assessment score; Pao2 to Fio2 ratio; arterial blood gas analyses; ventilatory settings (ie, positive end expiratory pressure, respiratory rate, tidal volume, and plateau pressure); and blood examination results (ie, complete blood count, bilirubin, creatinine, lactate dehydrogenase, C-reactive protein, D-dimers, ferritin, and IL-6). We recorded the use of remdesivir, hydroxychloroquine, lopinavir and ritonavir, corticosteroids (low dosage, < 2 mg/kg/die; high dosage, > 2 mg/kg/die of methylprednisolone or equivalents), anakinra, and tocilizumab and assessed the use of renal replacement therapy, extracorporeal membrane oxygenation, and pronation. Finally, we assessed the antibiotics administered before ICU admission and then before and during each infectious episode. For the analysis, fluoroquinolones, third-generation or later cephalosporins, and carbapenem were defined as broad-spectrum antibiotics. The following outcomes were recorded: survival at ICU and hospital discharge, ICU and hospital LOS, and duration of IMV.
Infections were identified and recorded considering all microbiologic isolates obtained during the ICU course, independently reviewed and classified in light of the available clinical, laboratory, and radiographic data by dedicated intensivists (one for each center) and infectious disease specialists (one for each center), following international guidelines.17 , 18 The timeframe for diagnosing HAIs was limited to the ICU stay, without follow-up after ICU discharge. Infections were considered as ICU acquired infections whether they occurred ≥ 48 h from ICU admission. Furthermore, a specialized intensivist (V. S.) and an infectious disease specialist (A. B.) from the promoting center were available to support other participating centers’ decisions. The following HAIs were diagnosed: VAP, hospital-acquired pneumonia, catheter-associated urinary tract infection, bloodstream infection (BSI), catheter-related bloodstream infection, Clostridioides difficile colitis, suspected or proven invasive candidiasis, and invasive pulmonary aspergillosis (e-Table 1, e-Appendix 1).19 , 20 VAPs were classified further as early onset (ie, occurring < 5 days from intubation) or late onset (ie, ≥ 5 days from intubation). Viral infections were not included in the analysis. Finally, for every infectious episode, the presence of sepsis or septic shock21 was recorded.
We defined as MDR all microorganisms resistant to at least one agent in three or more antimicrobial classes of agents22 or the microorganisms with specific antibiotic resistance mechanisms (eg, methicillin-resistant Staphylococcus aureus). Each antibiotic susceptibility testing was analyzed, and resistance patterns for each antimicrobial agent were classified. Of note, the presence of extended-spectrum β-lactamases and carbapenemases in surveillance specimens was tested directly either with phenotypic assays (growth-based assays or immunochromatographic methods) or with molecular tests, depending on the clinical workflow of the microbiology laboratory. Conversely, the antimicrobial resistance profile of bacterial strains isolated from other clinical specimens was either tested (immunochromatographic or molecular tests) or deduced as per clinical practice.
Statistical Analyses
Descriptive statistics were produced for demographic, clinical, and laboratory characteristics of patients. Mean and SD (or, in case of skewed distribution, median and interquartile range [IQR]) are reported for continuous variables, and number and percentages are reported for categorical variables.
Groups were compared with parametric or nonparametric tests, according to data distribution, for continuous variables and with Pearson χ 2 test (or Fisher exact test when appropriate) for categorical variables. The crude incidence rate per 1,000 patient-days of ICU stay and relative 95% CIs were calculated. All the infectious episodes, including multiple infectious episodes for each patient, were considered. The analysis time scale was the time since ICU admission until the date of ICU discharge. Time at risk of ICU HAIs was from ICU admission to HAIs, death, or discharge from ICU. Risk factors for HAI were explored through multilevel Weibull regression models, with random intercepts for hospital and patient, with drugs as time-dependent variables.
Univariate and multivariate models were fitted; variable selection strategy for multivariate models was clinically relevant variables, not colinear (ie, ρ < 0.30), < 10% missing data, with no further selection. Competing risk analysis was used to estimate the cumulative incidence of HAIs, with death as a competing event. Patients were censored at discharge from ICU. In this analysis, only the first HAI was included. Fine and Gray competing risk regression models were used to assess independent risk factors for HAIs; subhazard ratios and their corresponding 95% CIs are reported. Death was considered a competing event for an infection developing. Univariate and multivariate models were fitted, with the same analysis strategy as described. As exploratory subgroup analyses, the distributions of microorganisms identified in HAIs were calculated by the type of infection. Also, mortality risk in VAP, BSI, and other relevant subgroups was assessed. Other secondary analyses included risk factors for HAI resulting from MDR pathogens.
All tests were two-sided, and P < .05 was chosen to indicate statistical significance. JMP version 11 software (SAS Institute) and Stata version 16.1 software (StataCorp) were used for statistical analysis. Additional details on statistical analysis are provided in e-Appendix 1.
Results
From February 20, 2020, through May 20, 2020, 813 patients were admitted to the participating ICUs for COVID-19 pneumonia. No single case of nosocomial-acquired COVID-19 was documented. After excluding 39 patients, 774 patients were included in the analysis (e-Fig 1).
Patient characteristics at ICU admission are summarized in Table 1 . Median age was 62 years (IQR, 54-68 years), and 77% were men. Median Pao2 to Fio2 ratio was 123 mm Hg (IQR, 90-174 mm Hg), and IMV was necessary in 89% of the patients. The median hospital and ICU LOSs were 29 days (IQR, 17-47 days) and 14 days (IQR, 8-26 days). IMV lasted a median of 14 days (IQR, 8-26 days). Renal replacement therapy and extracorporeal membrane oxygenation were used in 72 patients (9%) and 20 patients (2.5%), respectively.
Table 1.
Patient Characteristics at ICU Admission, Comorbidities, and Therapies Used and Univariate Risk Factors for Infection
| Variable | Total (N = 774) | Available Observations | Infected (n = 359 [46%]) | Not Infected (n = 415 [54%]) | P Value | HR (95% CI) |
|---|---|---|---|---|---|---|
| Sex, female | 177 (23) | 774 (100) | 74 (21) | 103 (25) | .762 | 0.97 (0.81-1.16) |
| Age, y | 62 (54-68] | 774 (100) | 62 (54-68) | 62 (54-69) | .290 | 0.99 (0.98-1.01) |
| BMI, kg/m2 | 28 (25-31) | 713 (92) | 28 (25-31) | 28 (25-31) | .452 | 1.00 (0.99-1.02) |
| Charlson’s Comorbidity Index | 2 (1-3) | 774 (100) | 2 (1-3) | 2 (1-3) | .242 | 0.97 (0.93-1.02) |
| Immunologic comorbiditya | 91 (12) | 774 (100) | 34 (9) | 57 (14) | .027 | 0.74 (0.56-0.97) |
| Hypertension | 350 (45) | 774 (100) | 170 (47) | 180 (44) | .562 | 1.04 (0.90-1.21) |
| Diabetes | 130 (17) | 774 (100) | 57 (16) | 73 (18) | .461 | 1.08 (0.88-1.33) |
| SOFA score | 4 (3-5) | 755 (98) | 4 (3-5) | 4 (3-5) | .080 | 0.95 (0.90-1.00) |
| Nonrespiratory SOFA score | 1 (1-2) | 755 (98) | 1 (1-2) | 1 (1-2) | .027 | 0.88 (0.80-0.98) |
| SAPS II score | 37 (30-44) | 774 (100) | 38 (32-44) | 36 (30-44) | .988 | 0.99 (0.99-1.01) |
| APACHE II score | 10 (7-12) | 774 (100) | 9 (7-12) | 10 (7-13) | .867 | 1.00 (0.98-1.01) |
| Pao2 to Fio2 ratio, mm Hg | 123 (90-174) | 750 (97) | 119 (87-161) | 127 (93-182) | .337 | 0.99 (0.98-1.00) |
| Pao2 to Fio2 ratio | ||||||
| > 200b | 118 (15) | … | 46 (13) | 72 (17) | … | … |
| < 100 and ≥ 200b | 382 (49) | … | 180 (50) | 202 (49) | .626 | 0.94 (0.75-1.18) |
| ≤ 100b | 250 (32) | … | 122 (34) | 128 (31) | .602 | 0.94 (0.74-1.19) |
| Respiratory rate, beats/min | 22 (18-28) | 726 (94) | 22 (18-28) | 22 (18-27.25) | .582 | 0.99 (0.98-1.00) |
| TV/PBW, mL/kg | 6.8 (6.2-7.7) | 433 (56) | 6.9 (6.2-7.7) | 6.8 (6.1-7.8) | .397 | 1.04 (0.96-1.13) |
| PEEP, cm H2O | 12 (10-14) | 706 (91) | 12 (10-14) | 10 (10-14) | .207 | 1.02 (0.99-1.05) |
| Plateau pressure, cm H2O | 24 (22-26) | 447 (58) | 24 (22-27) | 24 (21-26) | .091 | 1.02 (0.99-1.05) |
| pH, units | 7.41 (7.34-7.46) | 755 (98) | 7.40 (7.32-7.46) | 7.42 (7.35-7.47) | .471 | 0.74 (0.32-1.68) |
| ≥ 7.35 and < 7.45 | 301 (39) | … | 137 (38) | 164 (40) | … | … |
| < 7.25c | 41 (5) | … | 25 (7) | 16 (4) | .595 | 1.08 (0.81-1.45) |
| ≥ 7.25 and < 7.35c | 188 (24) | … | 102 (28) | 86 (21) | .203 | 1.13 (0.94-1.35) |
| ≥ 7.45c | 225 (29) | … | 89 (25) | 136 (33) | .688 | 1.04 (0.86-1.26) |
| Paco2, mm Hg | 42 (36-51) | 753 (97) | 44 (36-53) | 41 (35-48) | .019 | 1.01 (1.00-1.01) |
| WBC count, 103/mm3 | 8.68 (6.24-11.7) | 762 (98) | 9.1 (6.6-12.48) | 8.38 (6.12-11.29) | .796 | 1.00 (0.99-1.01) |
| Neutrophil count, 103/mm3 | 7.26 (5.02-10.33) | 685 (88) | 7.91 (5.63-11) | 6.8 (4.81-9.78) | .127 | 1.01 (0.99-1.03) |
| Lymphocyte count, 103/mm3 | 0.7 (0.47-1) | 646 (83) | 0.7 (0.48-1) | 0.7 (0.44-1) | .491 | 0.99 (0.95-1.03) |
| Neutrophil to lymphocyte ratio | 10 (5.9-17.1) | 646 (83) | 10.6 (6-17.4) | 9.1 (5.83-16.8) | .525 | 1.00 (0.99-1.01) |
| Platelet count, 103/mm3 | 241 (180-314) | 761 (98) | 232 (179-307) | 245 (181-316) | .638 | 1.00 (0.99-1.01) |
| Serum bilirubin, mg/dL | 0.7 (0.5-1) | 739 (95) | 0.7 (0.5-1.1) | 0.7 (0.5-0.9) | .543 | 0.97 (0.88-1.07) |
| INR | 1.19 (1.1-1.29) | 513 (67) | 1.19 (1.09-1.29) | 1.18 (1.1-1.29) | .308 | 0.78 (0.48-1.26) |
| Creatinine, mg/dL | 0.86 (0.69-1.08) | 758 (98) | 0.82 (0.7-1.06) | 0.89 (0.67-1.1) | .118 | 0.92 (0.82-1.02) |
| LDH, units/L | 458 (356-600) | 657 (85) | 469 (368-597.5) | 448 (340-606.25) | .183 | 1.00 (0.99-1.01) |
| D-dimer, ng/mL | 1,201 (517-4,215) | 593 (77) | 1,663 (610-6,160) | 1,039 (447-3,013.5) | .284 | 1.00 (0.99-1.01) |
| C-reactive protein, mg/dL | 14.3 (6-23) | 679 (88) | 14.9 (7.07-24.6) | 13.2 (5.2-20.65) | .930 | 1.00 (0.99-1.01) |
| Procalcitonin, ng/mL | 0.4 (0.2-1.1) | 458 (59) | 0.4 (0.2-1.2) | 0.3 (0.19-0.9) | .993 | 1.01 (0.98-1.02) |
| Ferritin, ng/mL | 1,437 (822-2,472) | 210 (37) | 1,573 (1,026-2,553) | 1,213 (639-1,994) | .403 | 1.00 (0.99-1.01) |
| IL-6, ng/L | 200 (82-755) | 156 (21) | 166 (72-304) | 277 (93-1,206) | .942 | 1.00 (0.99-1.01) |
| Antibiotic therapy | … | 774 (100) | … | … | … | … |
| No antibiotic | 240 (31) | … | 130 (36) | 110 (27) | … | … |
| Narrow spectrumd | 294 (38) | … | 130 (36) | 164 (40) | .559 | 0.94 (0.75-1.17) |
| Broad spectrumd | 240 (31) | … | 99 (28) | 141 (34) | .038 | 0.82 (0.68-0.99) |
| Corticosteroidse | ||||||
| High dose | 36 (5) | 774 (100) | 17 (5) | 19 (5) | .347 | 0.90 (0.72-1.12) |
| Low dose | 171 (22) | 774 (100) | 80 (22) | 91 (22) | .023 | 0.66 (0.46-0.94) |
| Anakinrae | 89 (12) | 774 (100) | 52 (14) | 37 (9) | .821 | 0.94 (0.58-1.54) |
| Tocilizumabe | 187 (24) | 774 (100) | 85 (24) | 102 (25) | .099 | 2.12 (0.87-5.16) |
Data are presented as No. (%) of included patients or as median (interquartile range), unless otherwise indicated. APACHE = Acute Physiology and Chronic Health Evaluation; HR = hazard ratio; INR = international normalized ratio; LDH = lactate dehydrogenase; NC = not calculable; PBW = predicted body weight; PEEP = positive end expiratory pressure; SAPS = Simplified Acute Physiology Score; SOFA = Sequential Organ Failure Assessment; TV = tidal volume.
dvs no antibiotic.
Including chronic immunosuppressive therapies, active hematologic malignancies, neoplastic diseases, and autoimmune diseases.
vs Pao2 to Fio2 ratio of > 200.
vs pH of ≥ 7.35 and < 7.45.
At least 24 h before infection.
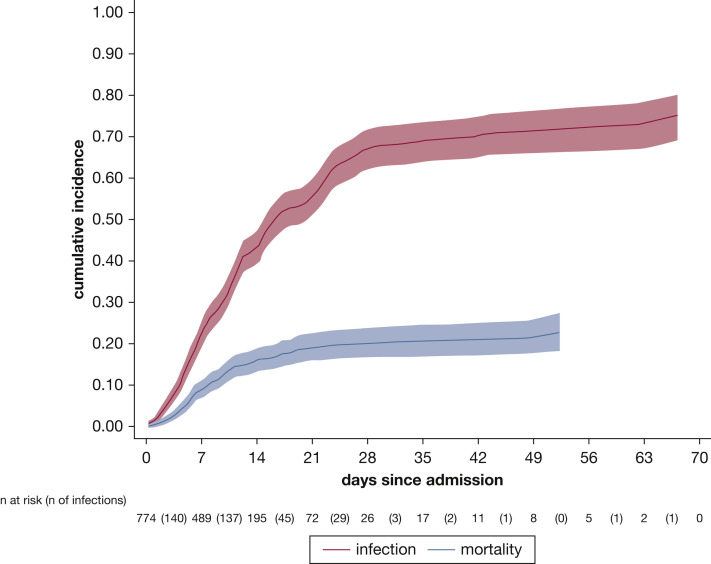
Three hundred fifty-nine patients (47%) demonstrated a total of 759 microbiologically confirmed HAIs during the ICU course, corresponding to a median of 1 episode (IQR, 0-3 episodes) per patient (range, 0–9 episodes) and an incidence rate of the first HAI of 44.7 infections/1,000 ICU patient-days. The first HAI occurred after a median of 12 days (IQR, 8-18 days) from hospital admission, 8 days (IQR, 5-12 days) from ICU admission, and 7 days (IQR, 5-12 days) from intubation. Of note, none of the 82 nonintubated patients demonstrated an HAI during ICU stay. The probability of an infection developing increased rapidly with the number of days since admission after considering the competing event of death (Fig 1 ).
Figure 1.
Line graph showing cumulative incidence function of infection and death.
Univariate analysis comparing clinical characteristics of infected and noninfected patients is presented in Table 1. In the multivariate analysis, variables independently associated with infection were age, positive end expiratory pressure, and broad-spectrum antibiotic treatment at admission (Table 2 ). Notably, the multilevel Weibull regression model showed that higher age was associated with a lower risk of infection. This association was absent at Fine and Gray analysis, which accounts for the competing risk of death (e-Table 2).
Table 2.
Multivariate Risk Factors Associated With Infection
| Variable | Total (N = 774) | Infected (n = 359 [46%]) | Not Infected (n = 415 [54%]) | P Value | HR (95% CI) |
|---|---|---|---|---|---|
| Sex, female | 177 (23) | 74 (21) | 103 (25) | .668 | 1.06 (0.80-1.41) |
| Age, y | 62 (54-68) | 62 (54-68) | 62 (54-69) | .004 | 0.98 (0.97-0.99) |
| Nonrespiratory SOFA score | 1 (1-2) | 1 (1-2) | 1 (1-2) | .108 | 0.91 (0.81-1.02) |
| Pao2 to Fio2 ratio | |||||
| > 200 | 118 (15) | 46 (13) | 72 (17) | … | … |
| ≥ 200 and < 100a | 382 (49) | 180 (50) | 202 (49) | .762 | 1.06 (0.74-1.50) |
| ≤ 100a | 250 (32) | 122 (34) | 128 (31) | .196 | 1.28 (0.88-1.87) |
| PEEP, cm H2O | 12 (10 -14) | 12 (10 -14) | 10 (10 -14) | .021 | 1.05 (1.01-1.10) |
| pH | |||||
| ≥ 7.35 and < 7.45 | 301 (39) | 137 (38) | 164 (40) | … | … |
| < 7.25b | 41 (5) | 25 (7) | 16 (4) | .716 | 1.07 (0.73-1.58) |
| ≥ 7.25 and < 7.35b | 188 (24) | 102 (28) | 86 (21) | .519 | 0.92 (0.70-1.19) |
| ≥ 7.45b | 225 (29) | 89 (25) | 136 (33) | .309 | 0.85 (0.62-1.16) |
| Antibiotic therapyc | |||||
| No antibiotic | 240 (31) | 130 (36) | 110 (27) | … | … |
| Narrow spectrumd | 294 (38) | 130 (36) | 164 (40) | .121 | 0.78 (0.56-1.07) |
| Broad spectrumd | 240 (31) | 99 (28) | 141 (34) | .002 | 0.61 (0.44-0.84) |
| Anakinrae | 89 (12) | 52 (14) | 37 (9) | .799 | 0.93 (0.53-1.62) |
| Tocilizumabe | 187 (24) | 85 (24) | 102 (25) | .425 | 1.60 (0.50-5.12) |
| Corticosteroidse | |||||
| High dose | 36 (5) | 17 (5) | 19 (5) | .822 | 1.07 (0.58-1.97) |
| Low dose | 171 (22) | 80 (22) | 91 (22) | .178 | 0.80 (0.57-1.11) |
Data are presented as No. (%) of the included patients or median (interquartile range), unless otherwise indicated. HR = hazard ratio; PEEP = positive end expiratory pressure; SOFA = Sequential Organ Failure Assessment.
vs Pao2 to Fio2 ratio of > 200.
vs pH of ≥ and < 7.45.
At ICU admission.
vs no antibiotic.
At least 24 h before infection.
No association was observed between the use of immunomodulating drugs and infections. The timing and clinical characteristics of patients treated with immunomodulators is shown in e-Table 3. Two hundred forty patients (31%) did not receive antibiotics at admission, 534 patients (69%) received at least one antibiotic at admission, and of these, 240 patients received broad-spectrum antibiotics (e-Table 4). e-Table 5 presents antibiotic use before , during, and after the infectious episodes.
The cause and incidence of each infection type are detailed in Table 3 . VAP was the most common infection (389/759 [51%]), followed by BSI (257/759 [34%]). Sixty-four percent of VAP episodes were caused by gram-negative bacteria (especially Enterobacterales), whereas S aureus was the most frequent gram-positive agent causing VAPs (110/389 [28%]). HAIs were the result of MDR bacteria in 38% (272/723) of all infectious episodes. In particular, 55% of S aureus isolates were methicillin-resistant, whereas gram-negative bacteria were producers of extended-spectrum β-lactamase and carbapenemases in 19% and 42% of patients, respectively (e-Table 6). e-Table 7 shows the univariate risk factors for MDR HAIs. We observed that among patients treated with antibiotics at ICU admission (n = 229/359 infected patients [63%]), 93 (40%) had an MDR HAI, whereas among patients not receiving antibiotics at ICU admission (n = 130/359 patients [36%]), only 40 (ie, 31%) had a MDR HAI, with a hazard ratio of 1.014 (95% CI, 0.777-1.324) and P = .918. e-Table 8 presents the microbiologic agents causing early (n = 35 [9%]) vs late (n = 354 [91%]) VAP. Late-onset VAP mostly was the result of gram-negative bacteria, whereas gram-positive bacteria more commonly caused early VAP. We observed only 16 episodes of hospital-acquired pneumonia. Invasive pulmonary aspergillosis and invasive candidiasis occurred in 17 and 17 patients, respectively. Finally, 2 cases of C difficile colitis were documented.
Table 3.
Microorganisms of the Nosocomial Infections Resulting From a Bacterial Agent (N = 723)a
| Variable | VAP | BSI | CRBSI | UTI | HAP | Overall | MDR |
|---|---|---|---|---|---|---|---|
| Included patients | 389 (50) | 183 (24) | 74 (10) | 60 (8) | 17 (2) | 723 (44) | 272 (35) |
| Incidence, No. infections/1,000 ICU patient-days | 26.03 (23.57-28.76)b | 11.71 (10.13-13.54) | 4.74 (3.78-5.95) | 3.84 (2.98-4.95) | 1.09 (0.68-1.75) | 42.78 (39.78-46.01) | 16.05 (14.25-18.07) |
| Gram-staining microorganisms | |||||||
| Gram-positive microorganisms | 140 (36) | 97 (54) | 40 (54) | 33 (55) | 7 (40) | 317 (44) | 138 (51) |
| S. aureus | 110 (28) | 26 (14) | 11 (16) | 4 (24) | 151 (21) | 83 (31) | |
| Enterococcus species | 21 (5) | 45 (25) | 18 (24) | 33 (55) | … | 117 (16) | 29 (11) |
| Coagulase-negative staphylococci | … | 21 (12) | 9 (12) | … | … | 30 (4) | 24 (9) |
| Streptococcus pneumoniae | 3 (1) | 1 (1) | … | 1 (5) | 5 (1) | 1 (1) | |
| Other | 6 (2) | 5 (3) | 1 (1) | … | 2 (11) | 14 (2) | 2 (1) |
| Gram-negative microorganisms | 249 (64) | 86 (46) | 34 (46) | 27 (45) | 10 (60) | 406 (56) | 133 (49) |
| Pseudomonas aeruginosa | 85 (21) | 17 (9) | 6 (8) | 8 (13) | 3 (18) | 119 (16) | 34 (12) |
| Enterobacterales (other) | 53 (14) | 30 (17) | 13 (18) | 4 (7) | 1 (6) | 101 (14) | 29 (11) |
| Klebsiella species | 43 (11) | 11 (5) | 5 (7) | 3 (5) | 1 (6) | 63 (9) | 25 (9) |
| Escherichia coli | 31 (8) | 9 (5) | 1 (1) | 12 (20) | 1 (6) | 54 (8) | 18 (7) |
| Acinetobacter baumannii | 6 (2) | 10 (6) | 5 (7) | … | 21 (3) | 19 (7) | |
| Other | 31 (8) | 9 (4) | 4 (5) | … | 4 (24) | 48 (7) | 8 (3) |
Data are presented as No. (%) of the subgroup or crude rate (95% CI). BSI = blood stream infection; CRBSI = catheter-related blood stream infection; HAP = hospital-acquired pneumonia; MDR = multidrug resistant; UTI = urinary tract infection; VAP = ventilator-associated pneumonia.
Excluding two C. difficile infections.
Incidence (No. of infections/1,000 ventilation days).
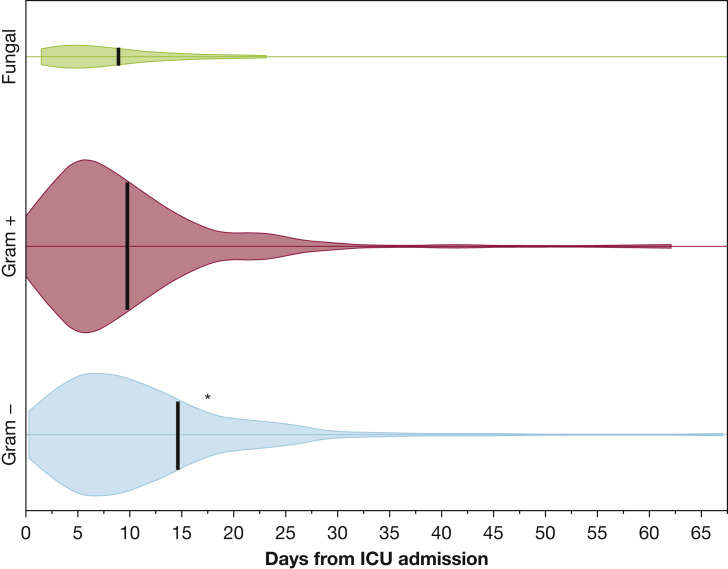
Figure 2 shows the onset time of the different HAIs. Infections resulting from gram-negative bacteria occurred later than infections resulting from gram-positive and fungi (median, 15 days (IQR, 9-26 days) vs 10 days (IQR, 6-18 days) and 9 days (IQR, 5-20 days) from ICU admission; P < .001 and P = .014, respectively). Onset times did not differ among VAP, urinary tract infection, BSI, and catheter-related BSI (e-Table 9) and between MDR and non-MDR infections (e-Table 10).
Figure 2.
Kernel density plot (violin plot) showing infection onset time. Black tick marks represent median onset time. ∗P < .05 vs gram-positive and fungal infections.
Sepsis and septic shock were documented in 168 of 759 (22%) and 161 of 759 (21%) of all infectious episodes, respectively. Gram-negative and gram-positive bacteria were the causative agents in similar proportions (ie, 53% v. 46%), and VAP was the most frequent HAI associated with septic shock (ie, 54% of the overall septic shock events). Gram-negative and gram-positive agents caused septic shock in 23.6% and 18.0% of the HAIs, respectively.
Complete follow-up of the patients until ICU discharge was achieved. Two hundred thirty-four of 774 patients (30%) died during the ICU stay. The ICU death rate was 15.3 deaths/1,000 ICU patient-days. Only 8 nonintubated patients (9%) died during the ICU stay. On July 23, of the 540 patients discharged alive from the ICU, 474 patients (87.7%) had been discharged from hospital, 25 patients (4.6%) had died, and 41 patients (7.5%) were still hospitalized. Overall in-hospital mortality was 33% (259/774). Infected patients showed similar ICU mortality (31.5%) compared with noninfected patients (29.1%; P = .483). Patients with at least one infection who experienced septic shock (98/774 [13%]) showed higher ICU mortality (52%) as compared with noninfected patients (415/774 [54%]; mortality, 29%), patients with infection (168/774 [22%]; mortality, 21%), and patients with sepsis (93/774 [12%]; mortality, 28%; P < .001). The same mortality risk (ie, 31%) was observed in patients with infections resulting from MDR bacteria (n = 135) and in those with infections resulting from non-MDR bacteria (n = 224; P = .903). Finally, patients who experienced infectious complications had a significantly longer duration of IMV (median, 24 days [IQR, 14-39 days] vs 9 days [IQR, 5-13 days]; P < .001), ICU LOS (median, 24 days [IQR, 16-41 days] vs 9 days [IQR, 6-14 days]; P = .003), and hospital LOS (42 days [IQR, 25-59 days] vs 23 days [IQR, 13-34 days]; P < .001).
Discussion
In this study, we analyzed the epidemiologic and etiologic factors and impact on outcomes of ICU-acquired infections in a large cohort of critically ill patients with COVID-19. The incidence of infectious complications was very high, with almost half of the patients experiencing at least one infectious episode during the ICU stay. Specifically, 14 days after ICU admission, the probability of having an infection was more than 40%.
Previous reports documented a frequency of HAIs in patients with COVID-19 ranging from 10% to 45%,23, 24, 25, 26 but provided limited information about microbial cause and impact on outcomes. Giacobbe et al,8 in a small cohort (n = 78) of critically ill patients, reported an incidence of BSI of 47 episodes (95% CI, 35-63 episodes) per 1,000 patient-days, mainly resulting from S aureus, Enterococcus species, and coagulase-negative Staphylococci. He at al,7 in a single-center study of a mixed patient population (ie, 33% with critical disease and 66% with severe disease), described the microbial cause of 65 HAIs and showed that patients with HAIs had a higher mortality rate.
Our work provides a detailed description of infectious complications in critically ill patients with COVID-19. Infections occurred relatively early (about 1 week after intubation), and their frequency increased with extended ICU stays. The most common infections were VAP resulting from Enterobacterales, whereas S aureus was the most frequent gram-positive organism. Bloodstream infections accounted for one-quarter of all HAIs and were caused almost equally by gram-positive and gram-negative bacteria. This rate of BSIs was significantly higher than that reported in the largest study published so far in the general population of ICU patients27 and higher even than that observed in patients undergoing extracorporeal membrane oxygenation for refractory respiratory failure.28 Another key finding with potentially important clinical implications is that MDR bacteria caused about one-third of the infectious episodes. Although patients were treated in ICUs where methicillin-resistant S aureus prevalence was reported previously to be low,29 this organism accounted for more than 50% of all S aureus infections. A high incidence of methicillin-resistant S aureus infections has been reported also in patients with severe H1N1 influenza, possibly favored by excessive mucus production, impaired mucociliary clearance, and epithelial cell breakdown.30 However, because our ICUs were overwhelmed by an unexpected number of critically ill patients and personnel from different wards had to be recruited to surge the ICU capacity, the high incidence of infections resulting from MDR germs may be attributed at least in part to suboptimal adherence to the standard infection control policies. In addition, infections by MDR bacteria may have been favored by the selective pressure of antibiotic therapies.
Regarding the use of antimicrobial prophylaxis, the recent Surviving Sepsis Campaign Guidelines suggest, with a weak level of evidence, the use of empiric antibacterial agents in patients with COVID-19 and respiratory failure. As expected, a high rate of the current patients (68%) already were receiving broad-spectrum antibiotic treatment (usually with cephalosporins, fluoroquinolones, or both) before ICU admission. The policy of all participating ICUs is to interrupt empiric antimicrobial treatments if culture results at admission are negative for bacterial coinfections. Notably, only 8 patients (1%) in this study demonstrated a coinfection at ICU admission, and a low rate of coinfections (8%) also was reported in a recent metanalysis.31 Even if we observed an apparently protective effect of broad-spectrum antibiotics at ICU admission on the occurrence of infection, our study was not designed specifically to evaluate this aspect. Thus, this observation should be taken with caution; given the high rate of infections resulting from MDR organisms, recommending routine antimicrobial prophylaxis with broad-spectrum antibiotics does not seem justified in our setting.
At the primary Weibull multilevel analysis, we observed older patients to have a lower risk of death. This counterintuitive association is reasonably the result of the immortal time bias; that is, older patients have a higher risk of death and die before an HAI develops. Accordingly, the secondary Fine and Gray competing risk analysis, which accounts for the competing risks of death and infection, did not confirm such an association.
Treatment with the IL-1 receptor antagonist anakinra, corticosteroids, or tocilizumab was not associated with an increased risk of secondary infections. However, this finding must be interpreted with caution for the following reasons: (1) treatment was not undertaken according to a protocol and the indications differed among centers; (2) anakinra and tocilizumab were administered on a compassionate basis to the most severe patients, possibly introducing a selection bias; and (3) significant variability occurred in dosing and timing, especially for corticosteroids. Because the study design does not allow ruling out the effect of these confounders, our findings should be considered as merely explorative, and no definitive conclusions can be drawn about the association of treatment with immunomodulators and the risk of secondary infections.
Infected and noninfected patients showed similar mortality. We did not analyze the factors associated with mortality because a specific study design and causal inferencing to disentangle multiple covariates would have been required. Nevertheless, HAIs complicated by septic shock almost doubled mortality, and overall, HAIs were associated with significantly increased mechanical ventilation and ICU and hospital LOS. However, we cannot exclude a cause-and-effect relationship between the duration of IMV or ICU LOS and the risk of infection.
Our study has several limitations. First, because it is a retrospective analysis of data collected primarily for clinical reasons in one of the regions most severely hit by the pandemic, not all data were available for all patients. Second, no standard management approach was undertaken during the study period across different centers. In particular, the antibiotic strategy and medical treatment were not uniform among the centers. Third, because we included in the analysis exclusively microbiologically confirmed infections, we may have underestimated the incidence of infectious episodes, neglecting some difficult-to-diagnose infections (eg, invasive aspergillosis) or infections characterized by low-yield cultures (eg, culture samples obtained while the patients were receiving an antibiotic treatment). Fourth, a formal blind revision of chest radiography was not performed. However, an intensivist and an infectious disease specialist from the promoting center were available to adjudicate independently the diagnosis of HAI. Fifth, the study was conducted in a single western European country with a high incidence of MDR infection. Hence, it may be improper to generalize and extrapolate the study findings to the worldwide population of patients with COVID-19. Also, sample size was dictated by contingencies, and the study was not designed or powered to detect the effect of HAIs on mortality; thus, all results should be viewed as hypothesis generating only. Finally, the study design does not allow a comparison between the cohort of patients with COVID-19 with that of patients with ARDS of a different cause, and we cannot draw any conclusion about a causal association between COVID-19 and an increased risk of HAIs.
Interpretation
Critically ill patients with COVID-19 are at high risk of HAIs, especially VAP and BSIs, frequently caused by MDR bacteria. Patients with HAIs complicated by shock showed almost double mortality, and infected patients experienced prolonged IMV and hospitalization. Clinicians should make every effort to implement protocols for surveillance and prevention of infectious complications.
Take-home Points.
Study Question: What characteristics of critically ill patients with COVID-19 are associated with HAIs and how are HAIs associated with outcomes in these patients?
Results: Among 774 included patients, 359 patients (46%) demonstrated 759 HAIs, 35% of them caused by MDR bacteria. VAP (50%), BSIs (34%), and catheter-related BSIs (10%) were the most frequent HAIs. Variables independently associated with infection were age, positive end expiratory pressure, and treatment with broad-spectrum antibiotics at admission. Mortality during ICU stay was 30%. Patients with HAIs complicated by septic shock showed almost doubled mortality (52% vs 29%), whereas noncomplicated infections did not affect mortality.
Interpretation: Critically ill patients with COVID-19 are at high risk of HAIs, in particular VAPs and BSIs resulting from MDR organisms. HAIs prolong mechanical ventilation and hospitalization, and HAIs complicated by septic shock almost double mortality.
Acknowledgments
Author contributions: G. G. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, and no important aspects of the study have been omitted. G. G., V. S., L. A., and A. G. conceived and designed the analysis; L. S. performed statistical analyses; A. G. and S. L. contributed to data management; D. M., M. B., E. B., P. B., N. B., I. C., S. L. C., D. F., G. Fi., A. F., M. F., M. Gr., M. M., A. Me., P. M., A. Mu., S. R., F. S., and T. T. collected and analyzed the data; G. B., G. D. P., G. M., M. A., M. C., G. Fo., R. F., M. Gi., M. Ran., P. V., M. Rav., and A. P. contributed in data analysis, interpretation of the data, and drafting the manuscript. All authors reviewed and approved the manuscript for submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. G. reports personal fees and nonfinancial support from Getinge, personal fees and nonfinancial support from Biotest, personal fees from Thermofisher, grants and personal fees from Fisher&Paykel, and personal fees from Draeger Medical outside the submitted work. M. C. reports personal fees from Edwards Lifesciences, personal fees from Directed Systems, and personal fees from Cheetah Medical outside the submitted work. A. P. reports personal fees from Maquet, personal fees from Novalung/Xenios, personal fees from Baxter, and personal fees from Boehringer Ingelheim outside the submitted work. A. Mu. received travel expenses and registration for meetings, congresses, and courses and lecture fees from Vygon. M. A. received an unrestricted research grant from GE and Toray and participated to boards for Pfizer and Fisher and Paykel. None declared (V. S., D. M., L. S., L. A., M. B., G. B., E. B., P. B., N. B., I. C., S. L. C., G. D. P., D. F., G. Fi., A. F., M. F., M. Gr., A. G., S. L., M. M., A. Me., G. M., P. M., S. R., F. S., T. T., G. Fo., R. F., M. Gi., M. Ran., P. V., M. Rav., A. G., A. B.).
Data sharing statement: The authors commit to making the relevant anonymized patient level data available on reasonable request.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Grasselli and Scaravilli contributed equally to this manuscript.
FUNDING/SUPPORT: This work was partly funded by the Italian Ministry of Health, grant Ricerca Finalizzata [Grant COVID-2020-12371675: “COVID19: epidemiological, clinical, genetic, and social determinants of infection and disease progression”].
Supplementary Data
References
- 1.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Greco M., Zanella A. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with coronavirus. 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y., Li W., Wang Z., Chen H., Tian L., Liu D. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol. 2020;41(8):982–983. doi: 10.1017/ice.2020.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacobbe D.R., Battaglini D., Ball L. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):1–8. doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langford B.J., So M., Raybardhan S. Bacterial coinfection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y., Xu D., Fu S. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):1–10. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudoignon E., Caméléna F., Deniau B. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin Infect Dis. 2020;72(5):905–906. doi: 10.1093/cid/ciaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Vidal C., Sanjuan G., Moreno-García E. Incidence of coinfections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ripa M., Galli L., Poli A. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2020;27(3):451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatt P.J., Shiau S., Brunetti L. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis. 2020;53(1):151–159. doi: 10.1093/cid/ciaa1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papazian L., Klompas M., Luyt C.E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019 [published online ahead of print June 16, 2020]. Clin Infect Dis. 10.1093/cid/ciaa760 [DOI]
- 17.Torres A., Niederman M.S., Chastre J. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 18.Manian F.A. IDSA guidelines for the diagnosis and management of intravascular catheter-related bloodstream infection. Clin Infect Dis. 2009;49(11):1770–1771. doi: 10.1086/648113. [DOI] [PubMed] [Google Scholar]
- 19.Pappas P.G., Kauffman C.A., Andes D. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hage C.A., Carmona E.M., Epelbaum O. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2019;200(5):535–550. doi: 10.1164/rccm.201906-1185ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes A., Evans L.E., Alhazzani W. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 22.Magiorakos A.P., Srinivasan A., Carey R.B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent J.-L., Rello J., Marshall J. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 28.Grasselli G., Scaravilli V., Bella S.Di. Nosocomial infections during extracorporeal membrane oxygenation. Crit Care Med. 2017;45(10):1726–1733. doi: 10.1097/CCM.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 29.Malacarne P., Boccalatte D., Acquarolo A. Epidemiology of nosocomial infection in 125 Italian intensive care units. Minerva Anestesiol. 2010;76(1):13–23. [PubMed] [Google Scholar]
- 30.Nickol M., Ciric J., Falcinelli S., Chertow D., Kindrachuk J. Characterization of host and bacterial contributions to lung barrier dysfunction following coinfection with 2009 pandemic influenza and methicillin resistant Staphylococcus aureus. Viruses. 2019;11(2):116. doi: 10.3390/v11020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawson T.M., Moore L.S.P., Zhu N. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.