Abstract
Background
To this end, the influence of COVID-19 on pregnant women and their neonates is not completely clear. Therefore, the main aim of this study is to investigate maternal and neonatal clinical outcomes with confirmed COVID-19 infection. Besides, it investigates the likelihood of vertical transmission of COVID-19 infection from pregnant women to their neonates.
Methods
A retrospective descriptive study was conducted in three medical centers during the period from March to November 2020. Data were collected from the available medical records in the respective hospitals using a standardized questionnaire on maternal and neonatal clinical outcomes. All pregnant women with confirmed COVID-19 infection across the three hospitals and their neonates were eligible to participate in this study. Descriptive statistics were presented as a median and interquartile range (IQR) or frequencies and percentages as appropriate using SPSS 24.0 software.
Results
This study has identified a total of 288 pregnant women with confirmed COVID-19 infection over the study period of a median age of 30 years and median GA at diagnosis 38 weeks (IQR: 39 -33) as well as 27% of them were obese (n = 78). The majority of pregnant women were symptomatic with cough (n = 92, 31.9%) being the most frequent COVID-19 symptom followed by fever and dyspnea (n = 36, 12.5%). Two-hundred and four pregnant delivered (70.84%) and caesarean sections were prevalent among 35.8% of them. The most common adverse pregnancy outcome was premature (n = 31, 15.5%), followed by fetal distress (n = 13, 6.5%), preeclampsia (n = 4, 2.0%), and one pregnant woman died. The laboratory results exhibit that temperature higher than 38 (n = 27), leukopenia (n = 19), neutropenia (n = 54), ALT (n = 12), AST (n = 31), and thrombocytopenia (n = 35) were less frequent among pregnant women while lymphopenia (n = 126), hemoglobin levels lower than 13.0 (n = 218), deceased albumin levels (n = 195) were most frequent among them. However, a small proportion of pregnant women were admitted to the ICU (3.8%). The most frequent maternal treatments were antibiotics (n = 81), antiviral (n = 49), and corticosteroid (n = 24). Of 204 neonates, four had died and all the remaining neonates were alive. The median gestational age at delivery was 39 weeks (IQR: 35–40). Most neonates had normal laboratory results. However, 14 had lymphopenia (7.0%), 22 had neutropenia (11.0%), and 11 had thrombocytopenia (5.5%). Four infants had low hemoglobin levels of less than 13.0 (2.0%) and 81 had hyperbilirubinemia (e.g., total bilirubin of higher than 23; 40.5%). Approximately less than one-half of neonates required admission to the NICU (n = 86, 43%), 7% of them required respiratory support of mechanical ventilation, and none of them get infected with COVID-19 disease.
Conclusion
This multicenter study suggests that the majority of pregnant women had mild or moderate disease symptoms. Nevertheless, this study did not find any evidence of possible vertical transmission of COVID-19 infection from mothers to their babies. This study may provide a baseline for further studies focusing on investigating long-term maternal and neonate's outcomes and possible vertical transmission of COVID-19 from mothers to their newborn babies.
Keywords: Pregnancy, Maternal, Neonates, COVID-19, Clinical symptoms
Introduction
The viral respiratory infection associated with the novel coronavirus disease (COVID-19) that was firstly emerged in Wuhan in China in December 2019 has spread out rapidly among people worldwide. This disease caused a global health crisis and it has been confirmed as a pandemic and as a Public Health Emergency of International Concern (PHEIC) by the World Health Organization (WHO) [1], [2], [3]. Positive confirmed cases mostly develop symptoms including fever, dry cough, headache, and fatigue or tiredness, loss of smell, and taste. In severe cases, patients may develop loss of breath, confusion, loss of appetite, and pneumonia [4].
Pregnant women are being considered at a high risk of getting viral respiratory infections and having symptomatic of severe pneumonia because of the changes in their physiological characteristics including immune and cardiopulmonary systems that arise during pregnancy [5], [6]. The earlier viral infectious diseases such as the Middle East respiratory syndrome coronavirus (MERS) and the Severe Acute Respiratory Syndrome coronavirus (SARS), and influenza suggest that pregnant women are particularly susceptible to adverse outcomes such as admission to an intensive care unit (ICU) and death [7], [8], [9].
There is a growing debate regarding the clinical outcomes of maternal and neonates with confirmed COVID-19 [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. The study of Chen et al. was among the first exploratory studies that assessed the clinical outcomes and possibility of vertical transmission to their neonates among nine pregnant with COVID-19 disease in Wuhan [10]. Various studies have shown that most pregnant women have mild to moderate COVID-19 symptoms while a small proportion of them have developed severe symptoms including high temperature and pneumonia. However, almost all previous studies did not detect any possible vertical transmission to the neonates. A recent systematic review and meta-analysis indicated that approximately less than one-third (i.e., 31.1%) of pregnant women were admitted to ICU and 2.7% have died while about 11.3% of newborn infants admitted to ICU and 2.2% of them experienced perinatal death. However, no cases of transmission from mothers to their fetus were detected over the study period [11]. High body mass index and maternal age and pre-existing comorbidities were found among the most common risk factors to manifest severe COVID-19. The rates of preterm birth were higher among COVID-19 infected pregnant women than non-infected pregnant women with COVID-19 [13]. Various systematic reviews indicated that pregnant and non-pregnant women shared almost similar symptoms of COVID-19 and the likelihood of vertical transmission from infected mothers to their neonates was rare [14], [15]. A study by Ferrazzi et al. found that only three out of 42 neonates were positively infected with COVID-19 who were born to mothers with COVID-19 in Italy [16]. Even though there are enormous studies on the effects of COVID-19 infection on pregnant women, newborns, and the possibility of vertical transmission, there is no strong and sufficient data to provide evidence regarding these effects [14].
With this current COVID-19 outbreak, the current knowledge concerning the effects of COVID-19 on pregnant women and their neonates is still unclear and scarce. Therefore, this study aims to investigate the COVID-19 clinical manifestations and outcomes of maternal and neonates as well as exploring the possibility of vertical transmission of COVID-19 from mothers to their new-borns.
Materials and methods
Study design and setting
This is a retrospective cohort and multicenter study of positively COVID-19 infected pregnant women who delivered at the designed three selected hospitals located in Riyadh city in Saudi Arabia during the period from March 2020 to November 2020. The hospitals are King Fahad Medical City (KFMC), Al-Yammamah hospital (AYH), and Imam Abdulrahman Al-Faisal hospital (AIAAFH). The study population comprises all pregnant women with COVID-19 who delivered and not delivered at the respective hospitals, and their neonates from March 2020 to November 2020 are included in the study, which is retrospectively selected as a study sample. All deliveries across hospitals were carefully revised and all cases that had positive PCR test results were included along with their neonates in the sample. Thus, the final sample consists of 288 pregnant women and their respective neonates (Four patients had twins). All pregnant women and their neonates were triaged according to the WHO protocol and guidelines [42], [43]. PCR samples were taken from neonates to diagnose them for COVID-19 infection in each respective hospital. In KFMC, PCR samples were taken from neonates after 12 and 36 h of delivery while in other hospitals they were taken after 24 and 48 h of delivery. All pregnant women with confirmed covid-19 and their neonates would be included in the study and fit the inclusion criteria.
Data collection
Data were collected from the available medical records in the respective hospitals using a standardized Data collection sheet on maternal and neonatal clinical outcomes during the period from March to November 2020. The investigators reported all pregnant women and their neonates successively observed who were eligible to participate in this study as follows:
-
•
Maternal characteristics including age, GA at admission, pregnancy/birth, DM, hypertension, gestational age, COVID-19 symptoms, respiratory support, mode of delivery, corticosteroid received, antibiotic, antiviral, lab results, ICU admission, and mortality.
-
•
Neonatal characteristics including PCR result, days from birth to COVID-19 detection, sex, neonatal death, birth weight, GA at delivery, 1-min and 5-min APGAR scores, respiratory support, PCR result 1st, and 2nd sample, lab results, and mortality.
Ethical consideration and data protection
For confidentiality, the data will be assured that all information provided will be treated in strict confidentiality and will be used solely for research purposes. All forms will be stored in a locked file cabinet in a locked office and will be erased on completion of the research. To assure anonymity the name will not be written, or any identifying information in any part of the research. The study has been approved by the research committee in King Fahad Medical City.
Statistical analysis
This study utilized SPSS 24.0 software to perform all statistical analysis. All continuous variables were presented as a median and interquartile range (IQR) as appropriate. All categorical variables were presented as frequencies and percentages.
Results
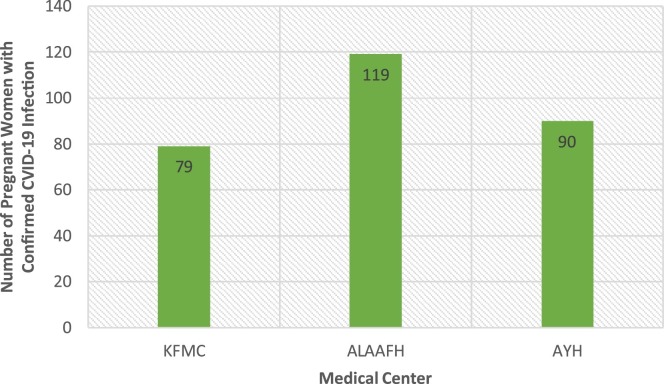
The current study has identified 288 pregnant women with confirmed COVID-19 infection from the three hospitals distributed as King Fahad Medical City (KFMC), Al-Yammamah hospital (AYH), and Imam Abdulrahman Al-Faisal hospital (AIAAFH) as indicated in Fig. 1 .
Fig. 1.
Distribution of the sample by medical center.
The median age of pregnant women included in the current study was 30 years (IQR: 35–26) and the median gestational age at diagnosis was 38 weeks (IQR: 39–33). Less than one-third of pregnant women were obese (n = 78, 27.1%). Thirteen patients had anemia pre-existing disease (4.5%), nine patients had hypothyroidism (3.1%), seven patients had diabetes mellitus, chronic hypertension, and asthma (2.4%), and one patient had cardiac and chronic lung diseases (0.3%). Most pregnant women developed COVID-19 symptoms (n = 247, 85.8%) in which the most prevalent symptom was cough (n = 92, 31.9%) followed by fever on admission and dyspnea (n = 36, 12.5%). The majority of participants did not need respiratory support (n = 176, 61.1%). However, about 9.4% of pregnant women need respiratory support of face mask (n = 27), 3.8% need nasal cannula (NC) support (n = 11), 2.1% need High flow nasal cannula (HFNC) and mechanical ventilator support (n = 6), and 1.4% need continuous positive airway pressure (CPAP) support (n = 4) (Table 1 ).
Table 1.
Maternal characteristics with COVID-19 infection.
| Age (years) | 30.0 (35–26) |
| Gestational age at diagnosis in weeks | 38 (39–33) |
| Comorbid conditions | N (%) |
| Diabetes Mellitus (DM) | 7 (2.4%) |
| Chronic Hypertension (HTN) | 7 (2.4%) |
| Asthma | 7 (2.4%) |
| Hypothyroidism | 9 (3.1%) |
| Cardiac disease | 1 (0.3%) |
| Chronic lung disease | 1 (0.3%) |
| Obesity | 78 (27.1%) |
| Anemia | 13 (4.5%) |
| COVID-19 symptoms | |
| Fever on admission | 36 (12.5%) |
| Fever after admission | 8 (2.7%) |
| Cough | 92 (31.9%) |
| Sore throat | 33 (11.5%) |
| Dyspnea/chest pain | 36 (12.5%) |
| Fatigue | 17 (5.9%) |
| Myalgia | 2 (0.7%) |
| Malaise | 8 (2.8%) |
| Diarrhea | 8 (2.8%) |
| Nausea or vomiting | 7 (2.4%) |
| Asymptomatic | 41 (14.2%) |
| Delivered | |
| Yes | 204 (70.8%) |
| No | 84 (29.2%) |
| Number of fetuses | |
| Single | 200 (98%) |
| Twin | 4 (2%) |
| Mode of delivery | |
| Viginal | 131 (64.2%) |
| Cesearean (C-section) | 73 (35.8%) |
| Adverse pregnancy outcomes | |
| Premature or preterm delivery | 31 (15.5%) |
| Prolonged rupture of membrane (PROM) | 16 (8%) |
| Fatal distress | 13 (6.5%) |
| Preeclampsia | 4 (2.0%) |
| Mortality | 1 (0.5%) |
| Maternal Respiratory Support Needed | |
| No support | 176 (61.1%) |
| NC | 11 (3.8%) |
| HFNC | 6 (2.1%) |
| Face mask | 27 (9.4%) |
| CPAP | 4 (1.4%) |
| Mechanical ventilator | 6 (2.1%) |
| Missing | 58 (20.1%) |
The majority of pregnant women were delivered (n = 204, 70.8%) and with single fetus (n = 200, 98%). Viginal delivery was prevalent among approximately less than two-thirds of delivered women (n = 131, 64.2%). The most common adverse pregnancy outcome was premature (n = 31, 15.5%), followed by fetal distress (n = 13 (6.5%), Preeclampsia (n = 4, 2.0%), and one pregnant woman died (Table 1).
The laboratory test results of pregnant women are presented in Table 2 . Eight pregnant women had pneumonia revealed from a chest x-ray diagnosis (2.8%). About 9.4% of patients had a temperature higher than 38 (n = 27). Nineteen pregnant women had leukopenia (6.6%), 54 had neutropenia (18.6%) and 126 had lymphopenia (43.8%). Elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were observed in 4.2% (n = 12) and 10.8% (n = 31), respectively. Thrombocytopenia (i.e., <150) was detected among 12.2% of pregnant women (n = 35) and higher platelet count (i.e., >450) was detected among 2.4% of them (n = 7). The majority of patients had hemoglobin levels lower than 13.0 (n = 218, 75.7%) and had decreased albumin levels (n = 195, 67.7%).
Table 2.
Maternal laboratory tests and treatment.
| CT | 8 (2.8%) |
| Temp > 38 | 27 (9.4%) |
| Wight blood cells (WBC) | |
| <4000 | 19 (6.6%) |
| 4000–12000 | 179 (62.2%) |
| >12000 | 44 (15.3%) |
| Missing | 46 (15.9%) |
| Lymphocyte | |
| <20% | 126 (43.8%) |
| 20–40% | 88 (30.5%) |
| >40% | 6 (2.1%) |
| Missing | 68 (23.6%) |
| Neutrophils | |
| <40% | 54 (18.6%) |
| 40–75% | 136 (47.2) |
| >75% | 45 (15.6) |
| Missing | 54 (18.6) |
| Platelet count | |
| <150 | 35 (12.2%) |
| 150–450 | 226 (78.5%) |
| >450 | 7 (2.4%) |
| Missing | 20 (6.9%) |
| Hemoglobin | |
| <13.0 | 218 (75.7%) |
| ≥13.0 | 28 (9.7%) |
| Missing | 42 (14.6%) |
| Alanine aminotransferase (ALT) | |
| <7 | 20 (6.9%) |
| 7–40 | 220 (76.4%) |
| >40 | 12 (4.2%) |
| Missing | 36 (12.5%) |
| Aspartate aminotransferase (AST) | |
| <13 | 18 (6.2) |
| 13–35 | 203 (70.5%) |
| >35 | 31 (10.8%) |
| Missing | 36 (12.5) |
| Albumin | |
| <40 | 195 (67.7%) |
| 40–55 | 35 (12.2%) |
| ≥55 | 0 (0.0%) |
| Missing | 58 (20.1%) |
| Treatment and Medication | |
| Corticosteroid | 24 (8.3%) |
| Antibiotics | 81 (28.1%) |
| Antiviral | 49 (17.0%) |
| ICU admission | 11 (3.8%) |
| Length of stay in hospital (LOS) in days | 3 (4–1) |
The most common maternal treatment was antibiotics (n = 81, 28.1%). Forty-nine patients were given antiviral (17.0%) and 24 were given corticosteroid (8.3%). Eleven pregnant women were admitted to the ICU (3.8%). Of 11 patients admitted to the ICU, one patient developed almost all COVID-19 symptoms and was in the 25th week of gestational age, and 10 pregnant women had cough and chest pain (pneumonia) and were between 28 and 36 weeks’ gestational age. The median length of stay in the hospital was three days (IQR: 4–1) and all patients discharged except one patient had died (Table 2).
Neonatal clinical outcomes are shown in Table 3 . There were 200 neonates were born for 200 pregnant women and 4 fetal deaths observed during the period of the study. The median gestational age at delivery was 39 weeks (IQR: 35–40) and the majority of neonates had normal birth weight (2500–4000 g, n = 170, 85.0%), had 1-min Apgar score higher than 5 (n = 182, 91.0%), and had 5-min Apgar score higher than 5 (n = 195, 97.5%). The laboratory test results indicate that no neonate had leukopenia, 14 had lymphopenia (7.0%), 22 had neutropenia (11.0%), and 11 had thrombocytopenia (5.5%). Four infants had low hemoglobin levels of less than 13.0 (2.0%) and 81 had hyperbilirubinemia (e.g., total bilirubin of higher than 23; 40.5%).
Table 3.
Neonates clinical outcomes of mothers infected with COVID-19.
| Neonate outcome | |
| Alive | 200 (98.0%) |
| Fetal death | 4 (2.0) |
| Neonatal death | 0 (0.0%) |
| Gestational age at delivery (weeks) | 39 (35–40) |
| Birth weight (in grams) | |
| <2500 g | 29 (14.5%) |
| 2500–4000 g | 170 (85.0%) |
| >4000 g | 1 (0.5%) |
| 1-min Apgar score | |
| <5 | 18 (9.0%) |
| ≥5 | 182 (91.0%) |
| 5-min Apgar score | |
| <5 | 5 (2.5%) |
| ≥5 | 195 (97.5%) |
| Neonatal lab test | |
| WBC | |
| <4000 | 0 (0.0%) |
| 4000–12000 | 95 (47.5%) |
| >12000 | 105 (52.5%) |
| Lymphocyte | |
| <20% | 14 (7.0%) |
| 20–40% | 142 (71.0%) |
| >40% | 44 (22.0%) |
| Neutrophils | |
| <40% | 22 (11.0%) |
| 40–75% | 174 (87.0%) |
| >75% | 4 (2.0%) |
| Platelet count | |
| <150 | 11 (5.5%) |
| 150–450 | 186 (93%) |
| >450 | 3 (1.5%) |
| Hemoglobin | |
| <13.0 | 4 (2.0%) |
| 13.0–17.5 | 58 (29.0%) |
| ≥17.5 | 138 (69.0%) |
| Total bilirubin | |
| <23 | 119 (59.5%) |
| ≥23 | 81 (40.5%) |
| COVID-19 test result | All negative |
| Neonatal complications and treatment | |
| NICU admission | 86 (43.0%) |
| IUGR | 3 (1.5%) |
| Length of Stay (days) | 8 (13–7) |
| Corticosteroid or antiviral | 0 (0.0%) |
| Antibiotics | 62 (31.0%) |
| Mechanical ventilator support needed | 14 (7.0%) |
Neonatal complications were observed among some neonates where 86 of them were admitted to the NICU (43.0%), three of them had IUGR (1.5%), and 14 neonates required respiratory support of mechanical ventilation (7.0%). The most common treatment given to neonates was antibiotics (n = 62, 31.0%) and all of them were discharged with a median length of stay of 8 days interquartile range (IQR: 13–7) (Table 3). The COVID-19 PCR test results after 12 h or 24 h of delivery and 36 or 48 h of delivery were all negative. Therefore, none of the neonates had confirmed COVID-19 infection, and thus no evidence was found of vertical transmission from mothers to their neonates during the study period (Table 3).
Discussion
The current study aims to investigate the clinical characteristics and outcomes of pregnant women and neonates with COVID-19 who were admitted into three different hospitals located in Riyadh, Saudi Arabia over the period from March 2020 to November 2020. Therefore, this study retrospectively includes all pregnant women with COVID-19 infection who were either delivered or continued pregnancy resulted in a multicenter sample of 288 patients and 200 neonates over the study period. This study is among the first studies conducted in Saudi Arabia that differed from other overseas preliminary reports and studies in which it includes to some extent a large cohort of pregnant women and neonates [10], [17], [22], [23], [29], [32], [37].
The results from the present study indicate that the majority of pregnant women with COVID-19 infection were symptomatic, 85.8%. The most common COVID-19 symptoms among pregnant women were cough (31.9%) and fever and dyspnea (12.5%), which is congruent with most previous research and reports [12], [14], [15], [17], [22], [23], [29], [32], [37], [38], [41]. A retrospective cohort study in the New York City hospital in the United States found that dry cough, fever, and myalgias at the presentation were among the most common COVID-19 symptoms among pregnant women [38]. A most recent systematic review and meta-analysis found that the most observed complaints by pregnant women were fever (76%; 95% C.I is [57%–90%]) and cough (38%; 95% C.I is [28%–47%]) [12]. Also it has been reported that fever, cough, and dyspnea were among the most common symptoms of COVID-19 at presentation plus fatigue, and myalgia [14], [15]. Those symptoms were also common among infected pregnant women in Kuwait [41]. Other less common symptoms were sore throat, fatigue, myalgia, malaise, diarrhea, and vomiting [12], [15], [38], [41]. However, a systematic review of sixty studies indicated that about 43.5–92.0% of pregnant women were asymptomatic [21], [34]. The current study indicates that the majority of pregnant women were hospitalized due to Covid-19 disease during their 3rd trimester (i.e., median GA at presentation was 38 weeks), which consistent with the findings of most overseas studies [10], [12], [27], [30], [37], [38]. However, the study in Kuwait showed that most pregnant women were hospitalized at a median gestational age of 29 weeks in addition to some Chinese studies which showed that the average gestational age at presentation was 30 weeks [18], [41], [42]. The most prevalent comorbidity conditions were obesity (27.1%) and anemia (4.5%). The study conducted in the New York City hospital found obesity and mild intermittent asthma as the most prevalent comorbidity conditions of pregnant women [38].
Maternal laboratory tests indicate that the majority of pregnant women had hemoglobin levels lower than 13.0. However, lymphopenia was observed among 43.8% of pregnant women. Neutropenia, thrombocytopenia, higher body temperature (i.e., >38), and leukopenia were observed among 18.6%, 12.2%, 9.4%, and 6.6% of them, respectively. In addition, increased concentration of AST and ALT were observed among 10.8% and 4.2% among patients, respectively. Hemoglobin levels were in the normal range among the majority of pregnant women. The findings are consistent with previous research and reports but the prevalence of laboratory tests was variant [10], [12], [14], [15], [41], [44], [45]. More specifically, lymphopenia was common not only among pregnant women with COVID-19 infection but also among the general population with confirmed COVID-19 infection [34], [46], [47]. Moreover, more than two-thirds of pregnant women in the current study delivered, and only four of them had twin fetuses. Cesarean section and vaginal modes were observed as methods of delivery where the last mode being most prevalent among delivered women (64.2%). This finding is consistent with previous findings as these delivery methods were observed in previous research [12], [14], [15], [17], [22], [23], [29], [32], [37], [38], [41]. A recent systematic review and meta-analysis indicated that the prevalence of cesarean section was detected in several studies and ranged from 66.7% to 100% of pregnant women where the pooled percentage of cesarean section was 88% [12]. Another systematic review found that cesarean section was presented among 78.1% of pregnant women with confirmed COVID-19 infection [14]. In Wuhan Children's Hospital, Kuwait, and New York City Center hospital, cesarean section was carried out among 78.8%, 47.8%, and 44.4% of pregnant women, respectively [37], [38], [41]. The most frequent adverse pregnancy outcomes and complications were premature (15.5%), fetal distress (6.5%), preeclampsia (2.0%), and one maternal death (0.5%) for which these complications had occurred among pregnant women who were in less than 37 weeks of gestational age. The current study is in line with previous findings that found that premature, fetal distress, preeclampsia, placenta praevia, uterine rupture, gestational diabetes, hypertension, fetal asphyxia, stillbirth, abortion, and maternal death were being among the broad adverse pregnancy outcomes [10], [12], [13], [14], [15], [16], [18], [20], [21], [34], [38], [41], [45], [48].
Following maternal treatment, patients were mostly medicated with antibiotics, antivirals, and corticosteroid medications. Furthermore, a small proportion of pregnant women required respiratory support and admission to the ICU (3.8%) and mostly they have developed symptoms of pneumonia and were between 28 and 36 weeks’ gestational age. Almost all of them have been discharged from the hospital with 3 days median length of stay except one patient had died. Our findings are in line with previous research findings, which provide evidence of low ICU admission and very low mortality rates among pregnant women [10], [12], [13], [14], [15], [18], [21], [34], [38], [41], [45], [48]. A recent systematic review and meta-analysis revealed that the proportion of admission to ICU was 4% in several studies [12]. Likewise, the pooled percentage of admission to ICU among pregnant women was 13% [13]. Another systematic review found that 4.7% of pregnant women were required admission to the ICU [14]. Mortality rates were extremely low as indicated in several studies and most studies did not report any deaths [12], [13], [14], [15], [41], [45], [49]. Recent studies have shown that the ICU admission and mortality rates among pregnant women are comparable with the general population infected with COVID-19 disease [26], [50]. Therefore, maternal COVID-19 clinical outcomes are almost similar to those among general individuals with confirmed COVID-19 infection [51]. Of 200 newborns included in the current study, there were 15.5% of them are born preterm and the median gestational age at delivery was 39 weeks. Similarly, a retrospective descriptive study in Kuwait and a prospective cohort study in the United Kingdom found that about 26.6% and 66% of newborns were preterm infants, respectively, which is higher than our findings [41], [50]. The pooled percentage of preterm babies was 23%, which higher than our proportion [12]. The median gestational age at delivery of 38 weeks in these studies [41], [50]. However, a systematic review shows that the average gestational age at delivery ranged from 28 to 41 weeks [14]. This study also shows that a small proportion of newborns had birth weights less than 2500–4000 g, which congruent with previous studies [10], [12], [14], [15], [41], [44], [45]. Almost all neonates had 1-min and 5-min Apgar scores of higher than 5 meaning that almost all of them did not require assistance for establishing breathing at 1-min and 5-min of age [52]. We found almost similar results to those revealed in various studies for which these studies indicated that both 1-min and 5-min Apgar scores were 7–9 and 8–10, respectively [10], [14], [20], [36], [38], [41], [44], [45], [53], [54].
The laboratory tests indicate that lymphopenia, neutropenia, thrombocytopenia, and low hemoglobin levels were less frequently prevalent among neonates. Meanwhile, neonatal jaundice was prevalent among 40.5% of neonates in this study. However, no leukopenia among them was observed. A retrospective study assessed clinical outcomes of 10 neonates born to mothers with confirmed COVID-19 infection in China indicated that there is a chance of adverse perinatal complications such as thrombocytopenia, fetal distress, and premature as a result of abnormal liver function [23]. Some studies indicated that fever, pneumonia, lymphopenia, respiratory distress syndrome, low birth weight, and rapid heart rate were among the frequent neonatal complications who were born for mothers of confirmed COVID-19 infection [10], [23], [37], [55]. Meanwhile, another retrospective study conducted in Wuhan did not find any evidence of these neonatal complications [54]. The rate of neonatal admission to the ICU was somewhat high (43%) and the IUGR was 1.5% of neonates. There were no neonatal deaths and the median length of stay was 8 days but there were four cases of fetal deaths. A study in Italy showed that adverse neonatal outcomes were preterm birth (26%) as a result of worsening maternal COVID-19 symptoms and spontaneous labor [16]. A recent report in China showed that about 47% of all newborns were transferred to the NICU and there was one neonatal death due to severe neonatal asphyxia [26]. Moreover, a cohort study in the United States indicates that about 63.6% of neonates were transferred to the NICU and no neonatal death was observed [56]. The current study also finds that antibiotic medication was given to 31% of neonates and about 7% of neonates needed mechanical ventilation support but this was probably due to the neonatal complications of preterm delivery at lower than 37 weeks not due to COVID-19 infection [12].
All alive newborns in this study were not infected with COVID-19 disease and we did not find any evidence of vertical transmission from mothers with confirmed COVID-19 infection to neonates. This finding is congruent with most previous studies that showed little evidence of possible vertical transmission [23], [27], [38]. A systematic review and meta-analysis show that the pooled percentage of infected neonates with COVID-19 disease was 6% indicate newborns are at low risk of infection [12]. In Kuwait, only 2 out of 185 neonates got infected for infected mothers with COVID-19 [41]. Nevertheless, the current knowledge on vertical transmission of COVID-19 from mothers to their neonates in most review studies is inconclusive [12], [14], [15]. Therefore, further studies are needed to provide strong evidence regarding vertical transmission potentials.
The current study adds further knowledge to the currently available evidence regarding clinical outcomes among maternal who are infected with COVID-19 and the infection risk among their neonates and mostly the current findings are almost consistent with previous research reports. To the best of the authors’ knowledge, this is the first-ever exploratory multicenter study conducted in Saudi Arabia that focused on assessing the clinical outcomes of COVID-19 disease among pregnant women and the possible vertical transmission to their neonates.
This study, however, is subject to some potential limitations. First, the lack of full medical records exhibited by the presence of some missing values limits our attempts to investigate some potential maternal and neonatal complications. Second, this is a descriptive study and thus we were not able to draw some inferences in terms of testing and comparing two groups. For example, testing whether there are significant differences in clinical outcomes among pregnant and non-pregnant with confirmed COVID-19 infection. The generalizability of this study should be interpreted with caution since the medical records had a loss of follow-up over the study period and the prevalence of disease may differ greatly according to the geographic regions especially in those with low disease prevalence due to heterogeneity in health conditions across regions and therefore the results may not be generable to all regions or medical centers. Therefore, further studies are needed to provide in-depth state of art of COVID-19 outcomes among maternal and neonates at the national level.
Conclusion
This is a multicenter study carried out in three different hospitals located in Riyadh, Saudi Arabia. This study revealed that the majority of pregnant women had mild or moderate disease symptoms. Nevertheless, this study did not find any evidence of possible vertical transmission of COVID-19 infection from mothers to their babies. The findings of this study shed light on some important interventions and implications concerning improving the health systems and may insist on some policy interventions for disease prevention control strategies during this current pandemic.
Finally, this study may provide a baseline for further studies focusing on investigating long-term maternal and neonate's outcomes and possible vertical transmission of COVID-19 from mothers to their newborn babies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors have no conflicts of interest to declare
Ethical approval
This study was a retrospective study. Ethical approval was obtained from the Ethics Review Board of King Fahad Medical City, Riyadh, Saudi Arabia. The confidentiality of the data was maintained during the study.
Acknowledgments
The authors would like to thanks the Research Centre, King Fahad Medical City, Riyadh, Saudi Arabia, for providing support in preparing this manuscript.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19). Situation report – 45. Geneva, Switzerland: World Health Organization. <https://www.who.int/docs/default-source/coronaviruse/situationreports/20200305-sitrep-45-covid-19.pdf?sfvrsn=ed2ba78b_4>; 2020.
- 2.WHO. Coronavirus disease 2019 (COVID-19) Situation Report – 77 [Internet]. Geneva. <https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200406-sitrep-77-covid-19.pdf?sfvrsn=21d1e632_2>; 2020.
- 3.WHO. Rolling updates on coronavirus disease (COVID-19) [Internet]. Coronavirus disease update: events as they happen. <https://www.who.int/emergencies/diseases/novelcoronavirus-2019/events-as-they-happen>; 2020 [cited 25.03.20].
- 4.WHO. What are the symptoms of COVID-19? [Internet]. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19 [accessed October 2020].
- 5.Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 6.Naccasha N., Gervasi M.T., Chaiworapongsa T., Berman S., Yoon B.H., Maymon E. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185(5):1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 7.Assiri A., Abedi G.R., Al Masri M., Bin Saeed A., Gerber S.I., Watson J.T. Middle East respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63(7):951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siston A.M., Rasmussen S.A., Honein M.A., Fry A.M., Seib K., Callaghan W.M. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):1–14. doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capobianco G., Sadri L., Aliberti S., Mondoni M., Piana A., Dessole F. COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:543–558. doi: 10.1016/j.ejogrb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020:370. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal, and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashraf M.A., Keshavarz P., Hosseinpour P., Erfani A., Roshanshad A., Pourdast A. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157. [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG. 2020 doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144(7):799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 18.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X. 2020. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhidin S., Moghadam Z.B., Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019-nCoV: a systematic review. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 21.Pettirosso E., Giles M., Cole S., Rees M. COVID-19 and pregnancy: a review of clinical characteristics, obstetric outcomes, and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60(5):640–659. doi: 10.1111/ajo.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lokken E.M., Walker C.L., Delaney S., Kachikis A., Kretzer N.M., Erickson A. Clinical characteristics of 46 pregnant women with a SARS-CoV-2 infection in Washington State. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.London V., McLaren R., Jr., Atallah F., Cepeda C., McCalla S., Fisher N. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. 2020;37(10):991. doi: 10.1055/s-0040-1712164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020;2(3):100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J., Guo J., Fan C., Juan J., Yu X., Li J. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Lu J.Y., Min W. Analysis of pregnancy outcomes of pregnant women during the epidemic of new coronavirus pneumonia in Hubei [J/OL] Chin J Obstet Gynecol. 2020:55. [Google Scholar]
- 28.Pierce-Williams RAM, Burd J, Felder L. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol 10 [Epub ahead of print 08.05.20]. [DOI] [PMC free article] [PubMed]
- 29.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P., Wang X., Liu P., Wei C., He B., Zheng J. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020:104356. doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan S., Peng L., Siddique R., Nabi G., Xue M., Liu J. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. 2020;41(6):748–750. doi: 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehan A., Venkatesh A., Girish M. COVID-19 in pregnancy: risk of adverse neonatal outcomes. J Med Virol. 2020 doi: 10.1002/jmv.25959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith V., Seo D., Warty R., Payne O., Salih M., Chin K.L. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15(6):e0234187. doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui Y., Tian M., Huang D., Wang X., Huang Y., Fan L. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221(11):1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-center, descriptive study. Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panahi L., Amiri M., Pouy S. Clinical characteristics of COVID-19 infection in newborns and pediatrics: a systematic review. Arch Acad Emerg Med. 2020;8(1):e50. [PMC free article] [PubMed] [Google Scholar]
- 40.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11(1):1–10. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayed A., Embaireeg A., Benawadh A., Al-Fouzan W., Hammoud M., Al-Hathal M. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth. 2020;20(1):1–9. doi: 10.1186/s12884-020-03461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis; 2020 (February). pii:ciaa200. [DOI] [PMC free article] [PubMed]
- 43.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. <https://wwwwhoint/docs/default-source/coronaviruse/clinical-management-of-novel-covpdf?sfvrsn=bc7da517_2>; 2020
- 44.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect; 2020. pii: S0163-4453(20) 30109-2. [DOI] [PMC free article] [PubMed]
- 46.Yang H., Hu B., Zhan S., Yang L.Y., Xiong G. Effects of severe acute respiratory syndrome coronavirus 2 infections on pregnant women and their infants. Arch Pathol Lab Med. 2020;144(10):1217–1222. doi: 10.5858/arpa.2020-0232-SA. [DOI] [PubMed] [Google Scholar]
- 47.Shi L., Wang Y., Yang H., Duan G., Wang Y. Laboratory abnormalities in pregnant women with novel coronavirus disease 2019. Am J Perinatol. 2020;37(10):1070. doi: 10.1055/s-0040-1712181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X., Sun R., Chen J., Xie Y., Zhang S., Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynecol Obstet. 2020 doi: 10.1002/ijgo.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: a national population-based cohort study. BMJ 2020:369. [DOI] [PMC free article] [PubMed]
- 51.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apgar V. A proposal for a new method of evaluation of the newborn. Classic Pap Crit Care. 1952;32(449):97. doi: 10.1213/00000539-195301000-00041. [DOI] [Google Scholar]
- 53.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E. Maternal death due to COVID-19 disease. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y.T., Liu J., Xu J.J., Chen Y.F., Yang W., Chen Y. Neonatal outcome in 29 pregnant women with COVID-19: A retrospective study in Wuhan, China. PLoS Med. 2020;17(7):e1003195. doi: 10.1371/journal.pmed.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierce-Williams R.A., Burd J., Felder L., Khoury R., Bernstein P.S., Avila K. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020:100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]