Abstract
Objectives:
Elevated neutrophil gelatinase–associated lipocalin (NGAL) is a promising marker for severe acute pancreatitis (SAP) and multiple organ failure, suggesting systemic and local contributions during pancreatitis. We investigated the role of NGAL locally on acinar cell biology.
Methods:
Western blot, reverse transcriptase–polymerase chain reaction, and immunohistochemistry analysis were performed to analyze the levels of NGAL receptors, apoptotic and regeneration markers, and 4-hydroxynonenal (4HNE) levels, 3-[4,5-Dimethylthiazole-2-yl]-2, 5-diphenyltetrazolium bromide assay, and annexin V/propidium iodide staining were used to evaluate cell viability, and effect on endothelial cells was accessed by endothelial permeability assay.
Results:
Cerulein treatment at 20 μM for 12 hours significantly reduced acinar cell viability by 40%, which was rescued by NGAL at 800 and 1600 ng/mL concentrations, observed during mild and SAP, respectively. Mechanistically, NGAL significantly reduced the levels of reactive oxygen species and 4HNE adduct formation in a 24p3R-dependent manner and upregulated the expression of acinar cell regeneration markers, like CDK-2, CDK-4, and C-myc. However, SAP levels of NGAL significantly increased endothelial permeability and downregulated the levels of ZO-1, and cerulein treatment in NGAL knockout mice showed increased levels of 4HNE adducts.
Conclusions:
Neutrophil gelatinase–associated lipocalin rescues intracellular reactive oxygen species during pancreatitis and promotes survival and regeneration of acinar cells.
Keywords: pancreatitis, NGAL, acinar cells, endothelial cells, reactive oxygen species
Acute pancreatitis (AP) is a reversible inflammatory disorder of the pancreas initiated by damage to the pancreatic parenchyma that may range from mild to severe conditions. According to the Revised Atlanta Classification 2012, AP is classified into 3 categories: mild acute pancreatitis (MAP), moderately severe acute pancreatitis (SAP), and SAP based on persistent organ failure (>48 hours).1 Mild AP is a self-limiting condition, which starts resolving throughout 48 to 72 hours. On the other hand, SAP is accompanied by disseminated intravascular coagulation and persistent multiple organ failure (MOF) with significant mortality.2 Damage to pancreatic parenchyma due to alcohol, smoking, bile acid reflex, and idiopathic reasons leads to acinar cell death by apoptosis or necrosis. Two important biochemical pathways, endoplasmic reticulum (ER) stress and reactive oxygen species (ROS), are responsible for acinar cell apoptosis.3 The factors leading to increased ER stress cause irrepressible damage to acinar cells, accompanied by perturbation of Ca++ signaling, high levels of ROS, and release of cytochrome c, leading to local inflammatory response.3,4 Excessive ROS generation promotes apoptosis due to adduct formation and damage to the lipid bilayer, proteins, and DNA molecules. However, at lower levels, ROS prevents necrosis and reduces the severity of AP.5
Our previous study identified significantly elevated serum levels of neutrophil gelatinase–associated lipocalin (NGAL) during the first 48 hours of SAP onset and demonstrated it as an early predictor of MOF, suggesting its local and systemic contribution to the disease pathology.6 Neutrophil gelatinase–associated lipocalin is a protease-resistant, acute-phase extracellular protein, secreted by neutrophils, with multifaceted roles in inflammatory disorders.7 There are 2 known receptors/transporters for NGAL: 24p3R (SLC22A17) and low-density lipoprotein–related protein 2 (LRP2).8,9 The 24p3R expression is high in the brain, prostate, kidney, and choroid plexus.8 Conversely, LRP2 is predominantly expressed in the kidney, placenta, and thyroid and plays a critical role in the uptake of lipoprotein, sterols, and hormones.9 However, the expression and function of these receptors, as well as NGAL, have never been investigated in context to pancreatic acinar cells and AP severity. Neutrophil gelatinase–associated lipocalin expression is negligible in the normal pancreas; however, akin to other inflammatory disorders, NGAL expression increases early during the onset of AP and remains persistently elevated in SAP, correlating with MOF and fatality in patients with AP.6 In this study, we evaluated the contribution of increased NGAL levels locally on the pancreatic acinar cells to comprehend its mechanistic involvement in acinar cell biology using the concentrations of NGAL observed in MAP and SAP conditions (Supplemental Fig. 1A, http://links.lww.com/MPA/A829). Our results demonstrate that NGAL, through its receptor 24p3R, rescues pancreatic acinar cells from cerulein-induced cell death by reducing intracellular ROS levels. Moreover, NGAL provides regeneration and proliferative advantage to acinar cells in the resolution phase, early during pancreatic injury.
MATERIALS AND METHODS
Cell Culture and Reagents
Mouse pancreatic acinar cells (266.6, ATCC: CRL-2151; American Type Culture Collection, Manassas, Va) and human and mouse microvascular endothelial cells hMEC-1 and mBMEC [gifted by Dr Matthew C. Zimmerman, University of Nebraska Medical Center (UNMC), Omaha, Neb], respectively, were cultured under standard tissue culture conditions using Dulbecco's Modified Eagle Medium (Sigma, St. Louis, Mo) supplemented by 10% fetal bovine serum (Sigma), penicillin G, and streptomycin. Cerulein (cat. no. C9026), 2′,7′-dichlorofluorescein diacetate (DCFDA) (cat. no. D6883), and N-acetylcysteine (NAC; cat. no. A0737) were purchased from Sigma. The recombinant human (cat. no. LCN2-4930H) and mouse NGAL (cat. no. 50060-M08H) were purchased from Sino Biological (Radnor, Pa). A list of primer sequences and antibodies used is provided in Supplemental Tables 1 and 2 (http://links.lww.com/MPA/A829), respectively. The 24p3R was overexpressed (construct cat. no. MR226001, Origene, Rockville, Md) in HEK293 cells. The 24p3R short hairpin RNA was cloned in pRFP-C-RS vector (cat. no. TR30014, Origene) and used for transient silencing of 24p3R in 266.6 cells using the Turbofect transfection reagent (cat. no. R0531, ThermoFisher Scientific, Waltham, Mass).
RNA Isolation and Reverse Transcriptase–Polymerase Chain Reaction
RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR) was done as previously described.10 Briefly, total RNA was isolated using the Qiagen RNeasy kit (Qiagen, Germantown, Md), reverse transcribed to generate complementary DNA. The complementary DNA was diluted to a final concentration of 10 ng/μL and used to evaluate the expression of mouse or human 24p3R, LRP2, and XBP1 genes.
In Vitro Growth Analysis
Pancreatic acinar cell growth at indicated treatments was analyzed using 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide assay.11 Briefly, 5 × 103 of 266.6 cells/well were seeded in 96-well plates and treated with 20 μM cerulein or dimethyl sulfoxide (DMSO) control either alone or in combination with MAP (800 ng/mL) or SAP (1600 ng/mL) concentration for recombinant mouse NGAL for 12 hours. Ten microliters per well of 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide reagent (5 mg/mL) was added and incubated for 4 hours at 37°C. After removing the media, 100 μL DMSO was added to dissolve the formazan crystals, and absorbance was read at 570 nm.
Western Blotting
Western blot analysis was performed as described previously.10 The blots were quantified using ImageJ software (version 1.52s, National Institutes of Health [Besthesda, Md] and Laboratory for Optical and Computational Instrumentation, University of Wisconsin [Madison, Wis]).
Annexin V/Propidium Iodide Staining
Acinar cells (3 × 106) were seeded per 100-mm plate.12 Cells were treated with 20 μM cerulein or DMSO control either alone or in combination with MAP (800 ng/mL) or SAP (1600 ng/mL) concentration for recombinant mouse NGAL for 12 hours. Cells were washed and resuspended in 1× binding buffer and treated with 5 μL each of annexin V and propidium iodide (PI) for 15 minutes at room temperature under dark conditions. An additional 400 μL of 1× binding buffer was added in each tube and immediately analyzed by flow cytometry.
Animals and Generation of AP Model
The NGAL−/− [NGAL knockout (KO)] mice,13 gifted by Dr Shizuo Akira at Osaka University, Osaka, Japan, and wild-type (WT) animals were maintained in the UNMC animal house facility. All experimental procedures were performed by the approval and guidelines from the Institutional Animal Care and Use Committee. The AP was induced by repeated 8 hourly intraperitoneal saline or cerulein (75 μg/Kgbw) injections in 8- to 10-week-old WT and NGAL KO mice for 2 alternate days, as per the “staggered” protocol,14 and the mice were killed 12 hours after the last injection.
Immunohistochemistry and Immunofluorescence Analysis
Immunofluorescence (IF) and immunohistochemistry (IHC) assays were performed as described previously.15 Acinar cells seeded on poly-l-lysine–coated coverslips were treated as indicated for 12 hours. Cells were blocked and incubated with 10 μg/mL of 24p3R antibody overnight (antibody validation; Supplemental Fig. 1B, http://links.lww.com/MPA/A829) at 4°C. The next day, cells were thoroughly washed and counterstained with goat anti-rabbit Alexa488 secondary antibody (1:400). Coverslips were mounted on glass slides using VECTASHIELD-containing DAPI for nuclear staining (Vector Laboratories, Burlingame, Calif). For IHC, the pancreatic tissue sections, 5 μm thick, were backed overnight at 58°C, progressively rehydrated, and antigens were retrieved using citrate buffer (pH 6.0) for 15 minutes. Tissue sections were blocked with 2.5% horse serum and incubated with 24p3R antibody overnight at 4°C. The next day, tissue sections were washed and incubated with horseradish peroxidase-conjugated secondary for 1 hour, washed, and developed using 3,3′-diaminobenzidine for 2 minutes at room temperature. Slides were counterstained with hematoxylin, washed, and dehydrated before mounting. The Histoscore (H-score) for IHC was calculated based on intensity and the area of staining.16
Measurement of ROS Generation
The ROS levels were analyzed by staining with DCFDA dye.17 Briefly, 5 × 105 266.6 cells were seeded in 6-well plates and treated either with 20 μM cerulein alone or in combination with MAP or SAP concentration for the recombinant mouse NGAL for 12 hours. The cells were incubated with 1 μM final concentration of DCFDA dye for 30 minutes at room temperature, and fluorescence was analyzed using EVOS FL Auto imaging system (ThermoFisher Scientific).
Endothelial Permeability Assay
The effect of NGAL on endothelial permeability was analyzed using previously established protocol.18 Briefly, 1.5 × 105 human monovascular endothelial cells were seeded onto Matrigel-coated 12-well hanging inserts twice after 24-hour interval to form a uniform monolayer. The upper chamber containing 20 μg/mL fluorescein isothiocyanate albumin in DMEM was treated with MAP and SAP concentrations of NGAL, taking glucose and untreated wells as positive and negative controls, respectively. At indicated time points, 10 μL media from the lower chamber of every treatment was transferred to 96-well black clear bottom plates (cat. no. 3603, Costar, Sigma), and the fluorescence intensity was measured at excitation and emission wavelengths of 485 and 528 nm, respectively.
Statistical Analysis
The statistical significance of the data was calculated using a 2-tailed Student t test and one-way analysis of variance. Immunofluorescence images were quantified by using ImageJ software. All the values are represented as the mean standard error. P values less than 0.05 were considered as statistically significant.
RESULTS
Pancreatic Acinar Cells Express NGAL Receptor 24p3R
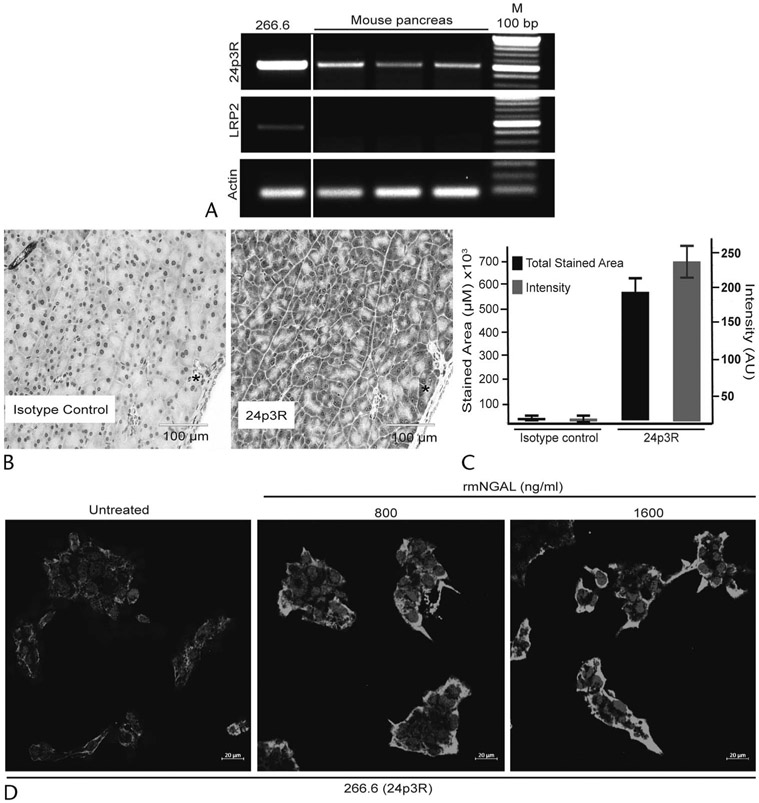
There are 2 well-characterized transporters/receptors for NGAL: 24p3R and LRP2.8,9 We first evaluated the expression of these receptors in the mouse pancreas and the acinar cell line 266.6. The RT-PCR analysis demonstrated a higher expression of 24p3R compared to LRP2 both, in the mouse acinar cell line and mouse pancreas (Fig. 1A). Similarly, IHC analysis of the normal mouse pancreas showed expression of 24p3R primarily in the acinar compartments (Figs. 1B, C). Interestingly, treatment of 266.6 cells with rmNGAL increased 24p3R expression, as demonstrated by Western blot (Supplemental Fig. 1C, http://links.lww.com/MPA/A829) and IF analyses (Fig. 1D). Together, these data suggested that high levels of NGAL could potentially influence pancreatic acinar cell biology during injury or inflammatory response through 24p3R.
FIGURE 1.
Pancreatic acinar cells express high levels of the NGAL receptor 24p3R. A, Reverse transcriptase PCR analysis performed using RNA isolated from a murine acinar cell line (266.6) and pancreas demonstrated the higher expression of NGAL receptor 24p3R compared with LRP2. The white space separates the expression in a cell line (266.6) and mouse pancreas. B, Immunohistochemistry analysis showed the strong expression of 24p3R in murine pancreatic acinar cells. Scale bar, 100 μm. C, Quantification of intensity and total stained area from the randomly selected areas for 24p3R in the murine pancreas (n = 6). D, Effect on 24p3R expression upon treatment with the previously identified MAP (800 ng/mL) and SAP (1600 ng/mL) rmNGAL concentrations using immunofluorescence analysis. Scale bar, 20 μm.
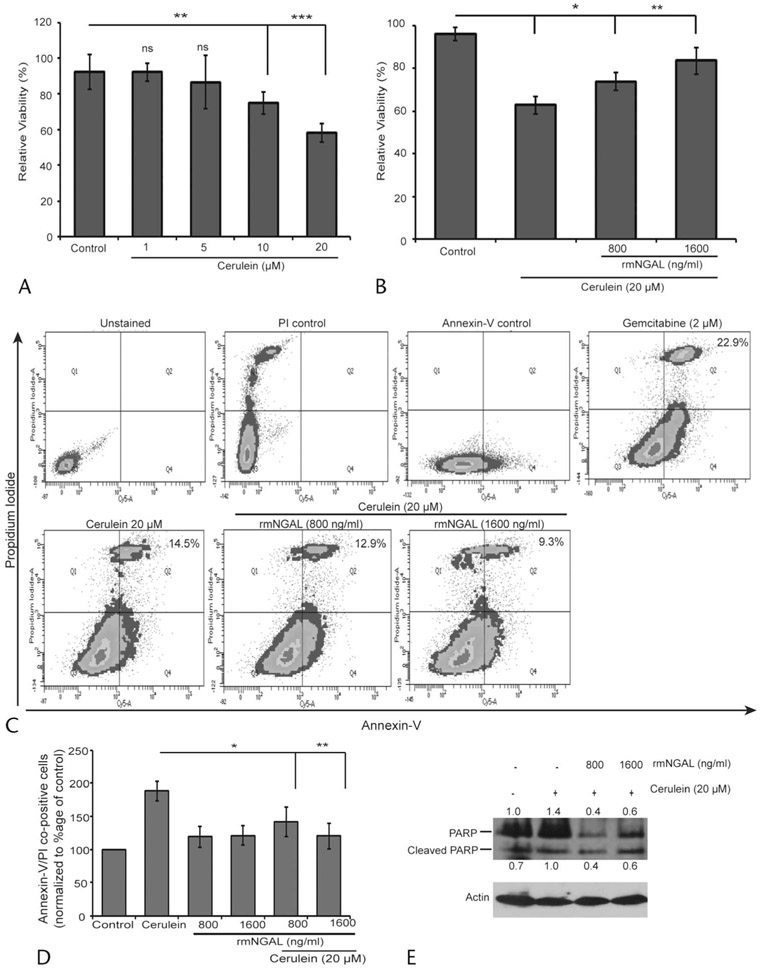
NGAL Rescues Cerulein-Induced Mouse Pancreatic Acinar Cell Death
Cerulein treatment disturbs the Ca++ balance, resulting in premature activation of proteases that induce acinar cell death in vitro (Supplemental Fig. 1D, http://links.lww.com/MPA/A829).19 Keeping this in mind, we investigated the effect of NGAL at concentrations observed during MAP (800 ng/mL) and SAP (1600 ng/mL)6 on cerulein-induced acinar cell death. First, we observed that cerulein in a dose-dependent manner reduced acinar cell viability up to 40% (P = 0.0001) after 12 hours of treatment at 20 μM (Fig. 2A, Supplemental Fig. 2, http://links.lww.com/MPA/A829). The rmNGAL treatment at both MAP (P = 0.01) and SAP (P = 0.001) concentrations significantly rescued acinar cells from cerulein-induced cell death (Fig. 2B). Furthermore, the reduction in percentage of annexin V/PI co-positive cells upon rmNGAL treatment confirmed its role in rescuing cerulein-induced acinar cell death (Figs. 2C, D). Neutrophil gelatinase–associated lipocalin itself at both concentrations showed no change in annexin V/PI co-positive acinar cells. For further validation, we analyzed poly(ADP-ribose) polymerase cleavage as a marker for cerulein-induced acinar cell death. Cerulein treatment–induced PARP expression and cleavage were substantially reduced in the presence of rmNGAL, further supporting our observation that NGAL rescues acinar cells from apoptosis (Fig. 2E). Collectively, the data suggested that rmNGAL protects mouse pancreatic acinar cells from cerulein-induced apoptosis.
FIGURE 2.
Neutrophil gelatinase–associated lipocalin rescues cerulein-induced cell death in mouse acinar cells. A, MTT assay for evaluating cerulein dose response in pancreatic acinar cell viability during a 12-hour treatment. B, Viability of acinar cells in the presence of 20 μM cerulein along with 800 and 1600 ng/mL levels of rmNGAL for 12 hours as measured by MTT assay. C, D, Effect of rmNGAL in preventing cerulein-induced acinar cell death as demonstrated by annexin V/PI live dead staining. E, The rmNGAL blocks cerulein-induced acinar cell death by modulating PARP expression as demonstrated by Western blot analysis. *P < 0 0.05, **P < 0.01, ***P < 0.001, ns, not significant.
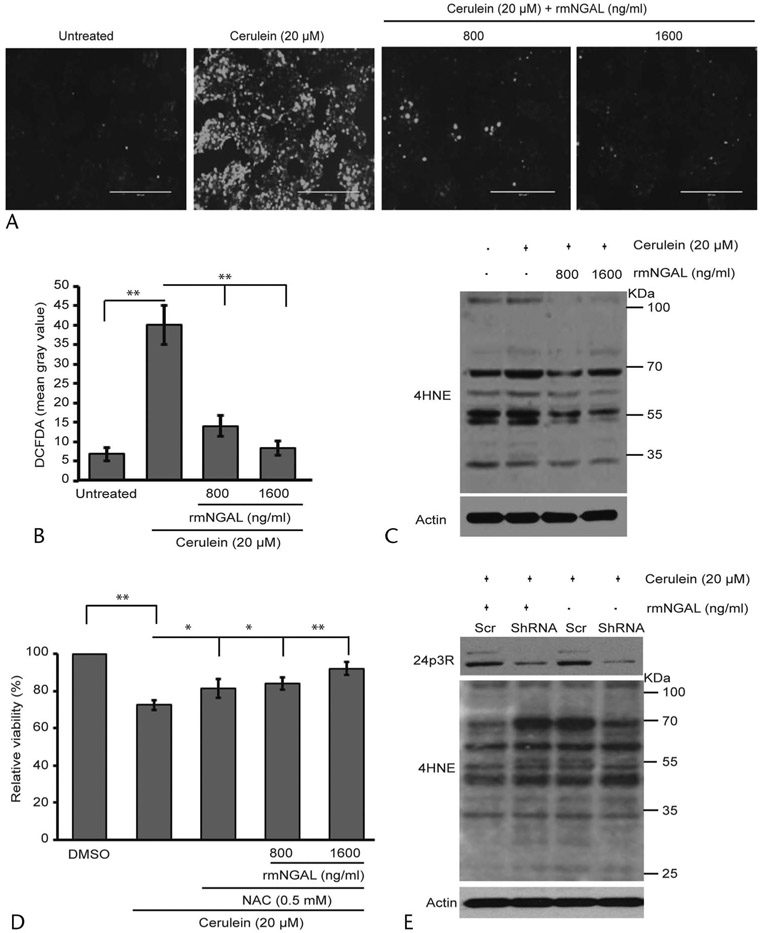
NGAL Significantly Reduces the Levels of Cerulein-Induced ROS
The major mechanisms of cerulein-induced acinar cell death include the generation of ROS and increased ER stress. We investigated the possible mechanisms through which NGAL prevents cerulein-induced acinar cell death. The cells were treated for 12 hours either with cerulein alone, or in combination with 800 or 1600 ng/mL of rmNGAL, and stained using DCFDA dye for intracellular ROS detection. Interestingly, the addition of NGAL significantly reduced the intracellular ROS levels induced by cerulein treatment (Figs. 3A, B). Notably, most of the cerulein-generated ROS were undetectable after rmNGAL treatment at 1600 ng/mL, suggesting that NGAL can significantly reduce the ROS-mediated cellular damage. Increased oxidative stress induces the formation of lipid peroxidation adducts such as 4-hydroxynonenal (4HNE). Therefore, we investigated the formation of 4HNE adducts in the presence and absence of rmNGAL. The Western blot analysis demonstrated increased 4HNE adduction of cellular proteins upon cerulein treatment, which was markedly reduced in the presence of rmNGAL (Fig. 3C). In addition, rmNGAL alone reduced the levels of 4HNE adducts in the untreated acinar cells, further suggesting the ability of NGAL in targeting cellular oxidative stress. To confirm the role of NGAL in protecting acinar cells from ROS-mediated damage the cells were treated either in the presence of 0.5 mM NAC alone or in combination with rmNGAL. An addition of 800 ng/mL of rmNGAL in combination with NAC showed concomitant rescue of acinar cell viability as compared to NAC treatment alone, suggesting that NGAL and NAC may act through a common pathway. However, rmNGAL at higher concentrations (1600 ng/mL) showed some additive effect in combination with 0.5 mM NAC on acinar cell viability (Fig. 3D). To ascertain that the effect of NGAL is mediated through its receptor expressed on acinar cells, we transiently silenced 24p3R in 266.6 cells and treated them with rmNGAL. Consistently, the receptor proficient cells treated in combination with rmNGAL showed the reduced formation of 4HNE adducts compared with the cells treated with cerulein alone. However, NGAL treatment failed to decrease the levels of 4HNE adducts after 24p3R knockdown using short hairpin RNA (shRNA), suggesting the involvement of 24p3R in reducing the cerulein-induced ROS in acinar cells (Fig. 3E). We also investigated the modulation in ER stress through CHOP and XBP1 expression. Unlike tunicamycin-treated cells, cerulein treatment (20 μM) for a short period of 12 and 24 hours had a marginal effect on protein and mRNA levels of CHOP (Supplemental Fig. 3A, http://links.lww.com/MPA/A829) and XBP1 (Supplemental Fig. 3B, http://links.lww.com/MPA/A829), respectively, as shown by a previous study.20 Taken together, the NGAL-mediated impact on acinar cell viability is primarily through a reduction in the levels of ROS in the acinar cell.
FIGURE 3.
Neutrophil gelatinase–associated lipocalin prevents acinar cell death by scavenging cerulein-induced ROS. A, Pancreatic acinar cells were treated with cerulein alone, or in combination with 800 or 1600 ng/mL of rmNGAL for 12 hours and stained with DCFDA for the detection of intracellular ROS. Highly fluorescent 2′,7′-dichlorofluorescein (DCF) production from DCFDA after ROS-mediated oxidation was measured by fluorescence microscopy. Scale bar, 10 μm. B, Quantification of fluorescence intensity from different treatment groups from (A). C, Effect of cerulein alone or in combination with NGAL treatment on ROS induced 4HNE adducts, as assessed by Western blot analysis. D, MTT assay demonstrates the effect of rmNGAL in rescuing acinar cells from death by scavenging cerulein-induced intracellular ROS along with 0.5 mM NAC, a known ROS scavenger. E, Effect of 24p3R silencing on the formation of 4HNE adducts due to increased ROS levels after treatment with cerulein. *P < 0.05, **P < 0.01. Scr, scrambled; ShRNA, short hairpin RNA.
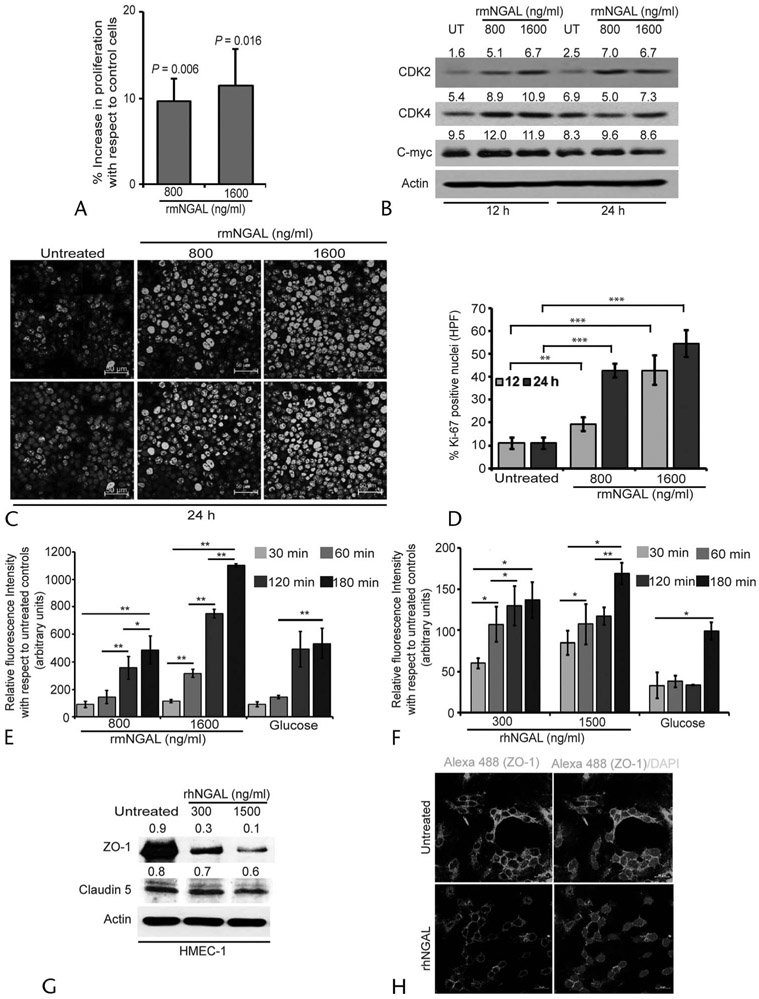
NGAL Affects the Expression of Cellular Regeneration Markers and Endothelial Permeability
Considering the role of NGAL in reducing oxidative stress and lipid peroxidation levels, we investigated its function in repair and regeneration of acinar cells during the resolution phase of inflammation. The MTT assay after 12 hours of rmNGAL treatment demonstrated that the acinar cells treated with both 800 and 1600 ng/mL showed increased overall growth with respect to the control cells (Fig. 4A). Furthermore, increasing concentrations of rmNGAL increased the expression of acinar cell regeneration markers such as CDK2, CDK4, and C-myc (Fig. 4B). Furthermore, rmNGAL treatment significantly increased the expression and nuclear localization of Ki-67 as observed by IF, suggesting the increased proliferative potential of acinar cells after treatment with NGAL (Figs. 4C, D).
FIGURE 4.
Neutrophil gelatinase–associated lipocalin treatment promotes mouse acinar cell regeneration and alters endothelial permeability. A, Effect of rmNGAL on acinar cell growth as analyzed by MTT assay following treatment with MAP or SAP concentration for rmNGAL for 12 hours. B, Expression profile of acinar cell regeneration markers, CDK2, CDK4, and C-myc after treatment with rmNGAL at different time points. C, Expression of Ki-67 in acinar cells treated with MAP (800 ng/mL) and SAP (1600 ng/mL) levels of rmNGAL. D, Quantification of the percentage of acinar cells with Ki-67 nuclear staining after 12 and 24 hours demonstrated that rmNGAL treatment promotes proliferation of acinar cells. **P < 0.01, ***P < 0.001. Scale bar, 50 μm. E and F, Murine and human endothelial cells, mBMEC and hMEC-1, respectively, were used to evaluate the role of NGAL on endothelial permeability at indicated time points. High glucose levels (20 mmol/L)21 have been shown to increase endothelial permeability and, therefore, used as positive control. *P < 0.05, **P < 0.01. G and H, Effect of rhNGAL on the expression of tight junction proteins, ZO-1, and claudin 5 as assessed by Western blot (G) and IF experiments (H). Scale bar, 20 μm. UT, untreated.
As our previous study demonstrated the association between persistent NGAL levels with MOF, we investigated the ability of MAP and SAP concentrations of NGAL on endothelial permeability, which is the early event leading to MOF. To test this, we performed an in vitro endothelial permeability assay at early time points of 30, 60, 120, and 180 minutes, using human and mouse rNGAL. Human MEC1 and murine BMEC cell monolayers were treated with rhNGAL and rmNGAL, and the levels of FITC-albumin leakage into the lower chamber through endothelial monolayers were analyzed (Figs. 4E, F). Interestingly, unlike untreated controls, NGAL, especially at SAP concentrations, significantly increased the endothelial permeability by 7-fold within 120 minutes of treatment, suggesting the contribution of NGAL in a rapid increase in endothelial cell permeability. Furthermore, there was a significant reduction in the expression of tight junction proteins such as ZO-1 and claudin 5 within 12 hours of NGAL treatment, supporting the role of NGAL in compromising endothelial barrier integrity (Figs. 4G, H). Collectively, the data suggest that NGAL provides regeneration advantage locally to the pancreatic acinar cells by increasing their proliferation and survival during resolution of AP; however, persistent and increased systemic NGAL levels during SAP led to a rapid and robust increase in endothelial permeability, which might facilitate early events leading to MOF.
Reduced Oxidative Stress and 4HNE Adduct Formation in the Pancreas of NGAL Knockout Mice During AP
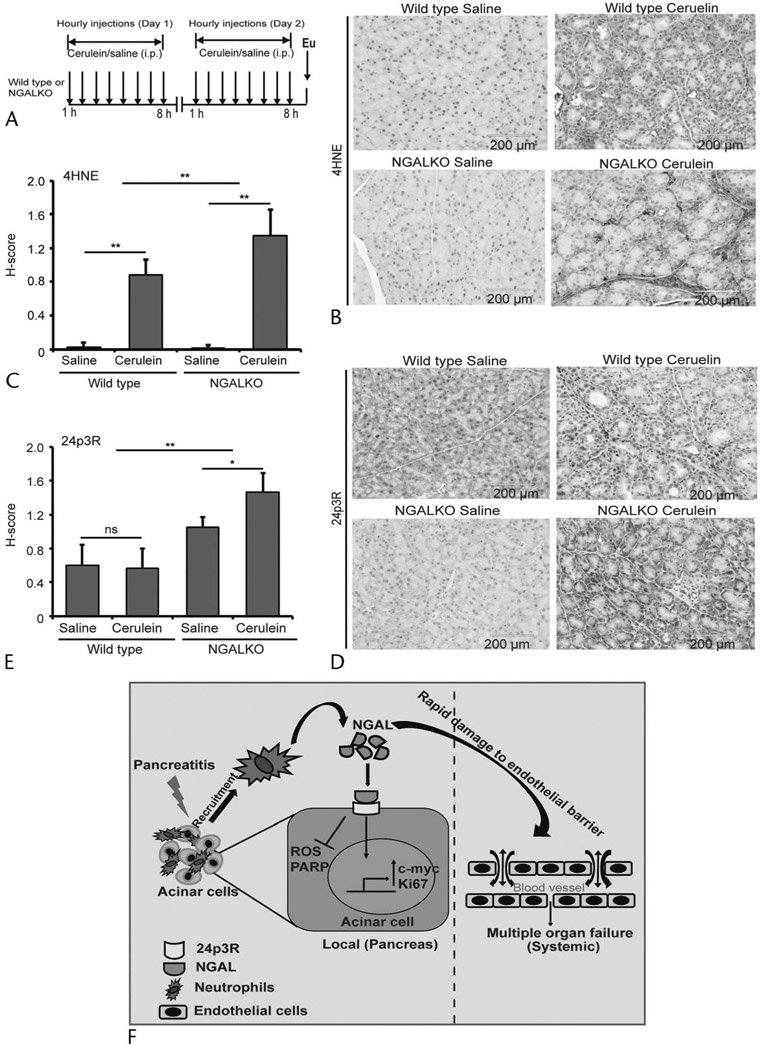
To evaluate the ROS-mediated mechanistic contribution of NGAL in acinar cell biology, we treated NGAL KO animals with cerulein following a protocol outlined in Figure 5A. The pancreatic tissues from both saline- and cerulein-treated WT and NGAL KO mice were stained for 24p3R expression and 4HNE adduct formation. As compared with the WT cerulein-treated mice, there was a significant increase in 4HNE adduct formation in the NGAL KO pancreas, as represented by significantly higher H-score calculated based on expression and intensity (Figs. 5B, C). Interestingly, we found that there was a significant increase in 24p3R expression in cerulein-treated NGAL KO mice compared with cerulein-treated WT controls (Figs. 5D, E). This increase in receptor expression might be due to higher ROS levels, which remain elevated in the absence of NGAL. Collectively, the data suggest that NGAL could significantly reduce the ROS-mediated damage to proteins and DNA in pancreatic acinar cells during insult and inflammation.
FIGURE 5.
Neutrophil gelatinase–associated lipocalin KO increases ROS levels and 24p3R expression in the AP model. A, The method followed to induce AP in WT and NGAL KO mice by a series of intraperitoneal cerulein injections. B and D, Immunohistochemistry for the extent of 4HNE adduct formation (B) and 24p3R expression (D) on pancreatic tissues from WT and NGAL KO mice either treated with cerulein or vehicle control. C and E, Average H-score (n = 6) for the intensity and expression of 4HNE (C) and 24p3R (E), respectively, in cerulein- and saline-treated WT and NGAL KO mice. F, Schematic representation of NGAL-mediated rescue and regeneration of acinar cells by reducing the intracellular ROS locally at the site of inflammation. However, persistently high levels of NGAL in serum increase endothelial permeability systemically, resulting in drops in blood pressures and subsequent induction of MOF. *P < 0.05, **P < 0.01. i.p., intraperitoneal; ns, not significant.
DISCUSSION
Among gastrointestinal nonmalignant pathologies, AP is a leading cause of hospital admissions and mortality. The systemic inflammatory response syndrome early during the onset of AP and MOF, followed by infection and secondary MOF, significantly contributes to the overall mortality. Therefore, early identification of severity and appropriate therapeutic intervention are expected to substantially improve the outcome of patients with AP. However, the etiology of the AP is poorly understood, and the molecular players are inadequately defined. Previous studies from our laboratory have shown that persistently high levels of NGAL significantly correlated with SAP and MOF.6 As MOF is a systemic disorder, we anticipated that NGAL secreted during pancreatic inflammation has systemic (vascular) effects leading to MOF; however, its role, locally, in acinar cell biology has never been evaluated. Therefore, in this study, we primarily focused on the contribution of NGAL in acinar cell biology using serum levels of NGAL observed during MAP and SAP. Concurrently, using these concentrations of NGAL, we evaluated the endothelial barrier integrity for early time points as these events initiate early during the onset of the symptoms of AP. Our study showed that NGAL rescues acinar cell death by efficiently reducing cerulein-induced oxidative stress at both the previously defined MAP (800 ng/mL) and SAP (1600 ng/mL) concentrations of NGAL.6 However, there was an insignificant effect on ER stress markers, CHOP and XBP1, suggesting that NGAL predominantly acts by reducing the ROS levels in acinar cells. Oxidative stress during AP has been shown to modulate various cellular events, including increased inflammatory cytokine expression, recruitment of inflammatory cells, and promoting tissue damage.22 Studies have shown that low levels of ROS (1–10 μM) induce acinar cell death by apoptosis, whereas the higher levels (0.5–1 mM) promote necrosis by mitochondrial dysfunction.5
The role of ROS in acinar cell death has previously been demonstrated in experimental murine models of AP and human and mouse acinar cell cultures.23 The gallstone-mediated obstruction is a common cause of pancreatitis due to bile reflux into the pancreatic duct. The bile acid, taurolithocholic acid, has been shown to increase ROS levels in the acinar cells in the Ca++-dependent manner, followed by mitochondrial dysfunction.24 Studies have further demonstrated that the pharmacological stimulation of NAD(P)H: quinone oxidoreductase (NQO1) by Dunnione25 and inhibition of ASK1 by NQDI-126 attenuate the acinar cells apoptosis and reduce the severity of the AP. The NQDI-1, in addition to ROS, reduced the expression of necrosis-related proteins, RIP3 and pMLKL, therefore preventing the acinar cell necrosis. Concurrently, the decrease in the glutathione (GSH) depletion positively correlates with the extent of the pancreatic insult during AP and the probiotics supplements, which increase the synthesis of GSH and reduce the oxidative stress and severity of AP.27,28 The contribution of oxidative stress in acinar cell death was further reinforced by the decrease in biochemical and ultrastructural damages in the acinar cells when cerulein-treated animals were coadministered superoxide dismutase and catalase as supplements.29 Based on the overwhelming evidence of the involvement of ROS in acinar cell damage and severity, the antioxidant therapies have been clinically evaluated. The combined administration of selenium, methionine, vitamins C and E, and β-carotene improved the symptoms of recurrent AP in a placebo-controlled clinical trial.23 Another clinical trial has reported faster recovery in patients with AP after intravenous administration of high-dose vitamin C compared with patients with low levels of vitamin C.30 Similarly, a high dose of allopurinol, as an antioxidant therapy, reduced the incidence of AP in postendoscopic retrograde cholangiopancreatography patients in a prospective double-blinded, placebo-controlled trial.31 Neutrophil gelatinase–associated lipocalin is involved in iron transport through 24p3R, and studies have shown that iron overload leads to the development of chronic pancreatitis. Furthermore, dysregulation of the hepcidin:ferroportin axis leads to iron accumulation in pancreatic acinar cells, leading to oxidative damage, degeneration, and failure of the exocrine pancreas.32 Therefore, in future studies, we will evaluate the role of NGAL in maintaining iron homeostasis to prevent acinar cell death during iron overload.
Acinar cell death is followed by the regeneration of the pancreatic parenchyma to restore its normal function during the resolution phase. The cerulein hyperstimulation murine model of AP demonstrates that pancreatic inflammation resolves within 7 days of cerulein-induced damage to the pancreatic parenchyma.33 Multiple studies have demonstrated that molecules like periostin, Bmi1, endogenous cholecystokinin, and pathways, including NOTCH34 and hedgehog,35 are involved in exocrine pancreas regeneration after cerulein-induced pancreatitis.36-38 Our study demonstrated that NGAL facilitates acinar cell regeneration and proliferation by increasing the expression of CDK2, CDK4, and C-myc. Furthermore, nuclear localization of Ki-67 after NGAL treatment suggests its role in restoring the damaged parenchyma.
Akin to pancreatic acinar cells, human and murine endothelial cells also express high levels of NGAL receptor, 24p3R. Neutrophil gelatinase–associated lipocalin is an established marker of acute renal injury, and elevated monomeric NGAL levels are associated with chronic kidney disease due to tubulointerstitial damage.39 Similarly, in the brain, NGAL increases blood-brain barrier permeability by reducing the expression of tight junction proteins after ischemic stroke.40 Normally, adherens and tight junctions maintain endothelial barrier function, controlling the vascular integrity and flow of paracellular fluids or electrolyte.41-43 Importantly, MOF in AP is followed by various hemodynamic abnormalities like septic shock, infections, and translocation of pathogenic endotoxins from the gut lumen.44 Studies suggest that increased endothelial permeability contributes to the vascular breakdown, systemic complications, edema, and, finally, organ dysfunction.45,46 Our study demonstrated that an early increase in NGAL levels leads to a rapid and robust increase in endothelial permeability, especially at 1600 ng/mL, a level of NGAL observed in patients with MOF. Furthermore, NGAL at 1600 ng/mL significantly reduced the expression of 2 tight junction molecules, ZO-1 and claudin-5, suggesting that the persistently elevated levels of NGAL progressively compromise endothelial permeability and increase the risk of MOF as the severity of AP worsens.
In conclusion, our data suggest that the initial rise in serum NGAL levels during AP is an early event in rescuing pancreatic acinar cells from oxidative stress and simultaneously providing a proliferative and regenerative advantage locally to stressed-out acinar cells. However, persistent NGAL elevation leads to a breach in endothelial permeability systemically, which increases the risk of MOF (Fig. 5F).
Supplementary Material
Acknowledgments
This study was partly supported by funding from the National Institutes of Health (P01CA217798, U01 CA210240, U01 CA200466, R01 CA206444, R21 AA026428, and R01 CA228524).
Footnotes
S.K.B. is a cofounder of Sanguine Diagnostics and Therapeutics Inc. The other authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 2.Tsuji Y, Takahashi N, Tsutomu C. Pancreatic perfusion CT in early stage of severe acute pancreatitis. Int J Inflam. 2012;2012:497386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrera K, Stanek A, Okochi K, et al. Acinar cell injury induced by inadequate unfolded protein response in acute pancreatitis. World J Gastrointest Pathophysiol. 2018;9:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugea A, Waldron RT, French SW, et al. Drinking and driving pancreatitis: links between endoplasmic reticulum stress and autophagy. Autophagy. 2011;7:783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong JA, Cash NJ, Ouyang Y, et al. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018;293:8032–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty S, Kaur S, Muddana V, et al. Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am J Gastroenterol. 2010;105:2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langelueddecke C, Roussa E, Fenton RA, et al. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PloS One. 2013;8:e71586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thévenod F, Fels J, Lee WK, et al. Channels, transporters and receptors for cadmium and cadmium complexes in eukaryotic cells: myths and facts. Biometals. 2019;32:469–489. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Das S, Rachagani S, et al. NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene. 2015;34:4879–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 12.Lakshmanan I, Batra SK. Protocol for apoptosis assay by flow cytometry using annexin V staining method. Bio Protoc. 2013;3:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng X, Lin H, Ding T, et al. Lipocalin 2 is required for BCR-ABL–induced tumorigenesis. Oncogene. 2008;27:6110–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris JP 4th, Cano DA, Sekine S, et al. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Torres MP, Kaur S, et al. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene. 2015;34:2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud H, Mahmoud O, Layasadat K, et al. Dexamethasone effects on Bax expression in the mouse testicular germ cells. Folia Histochem Cytobiol. 2009;47:237–241. [DOI] [PubMed] [Google Scholar]
- 17.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. [DOI] [PubMed] [Google Scholar]
- 18.Martins-Green M, Petreaca M, Yao M. An assay system for in vitro detection of permeability in human “endothelium”. Methods Enzymol. 2008;443:137–153. [DOI] [PubMed] [Google Scholar]
- 19.Kim H. Cerulein pancreatitis: oxidative stress, inflammation, and apoptosis. Gut Liver. 2008;2:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Dror K, Birk R. Oleic acid ameliorates palmitic acid–induced ER stress and inflammation markers in naive and cerulein-treated exocrine pancreas cells. Biosci Rep. 2019;39:BSR20190054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hempel A, Maasch C, Heintze U, et al. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81:363–371. [DOI] [PubMed] [Google Scholar]
- 22.Yu JH, Kim H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J Gastroenterol. 2014;20:17324–17329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Criddle DN. Reactive oxygen species, Ca(2+) stores and acute pancreatitis; a step closer to therapy? Cell Calcium. 2016;60:180–189. [DOI] [PubMed] [Google Scholar]
- 24.Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–2125. [DOI] [PubMed] [Google Scholar]
- 25.Shen A, Kim HJ, Oh GS, et al. Pharmacological stimulation of NQO1 decreases NADPH levels and ameliorates acute pancreatitis in mice. Cell Death Dis. 2018;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie X, Yuan C, Yin L, et al. NQDI-1 protects against acinar cell necrosis in three experimental mouse models of acute pancreatitis. Biochem Biophys Res Commun. 2019;520:211–217. [DOI] [PubMed] [Google Scholar]
- 27.Lüthen R, Niederau C, Grendell JH. Intrapancreatic zymogen activation and levels of ATP and glutathione during caerulein pancreatitis in rats. Am J Physiol. 1995;268:G592–G604. [DOI] [PubMed] [Google Scholar]
- 28.Lutgendorff F, Trulsson LM, van Minnen LP, et al. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1111–G1121. [DOI] [PubMed] [Google Scholar]
- 29.Guice KS, Miller DE, Oldham KT, et al. Superoxide dismutase and catalase: a possible role in established pancreatitis. Am J Surg. 1986;151:163–169. [DOI] [PubMed] [Google Scholar]
- 30.Du WD, Yuan ZR, Sun J, et al. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003;9:2565–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsinelos P, Kountouras J, Chatzis J, et al. High-dose allopurinol for prevention of post-ERCP pancreatitis: a prospective randomized double-blind controlled trial. Gastrointest Endosc. 2005;61:407–415. [DOI] [PubMed] [Google Scholar]
- 32.Lunova M, Schwarz P, Nuraldeen R, et al. Hepcidin knockout mice spontaneously develop chronic pancreatitis owing to cytoplasmic iron overload in acinar cells. J Pathol. 2017;241:104–114. [DOI] [PubMed] [Google Scholar]
- 33.Boggs K, Wang T, Orabi AI, et al. Pancreatic gene expression during recovery after pancreatitis reveals unique transcriptome profiles. Sci Rep. 2018;8:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siveke JT, Lubeseder-Martellato C, Lee M, et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. [DOI] [PubMed] [Google Scholar]
- 35.Fendrich V, Esni F, Garay MV, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hausmann S, Regel I, Steiger K, et al. Loss of periostin results in impaired regeneration and pancreatic atrophy after cerulein-induced pancreatitis. Am J Pathol. 2016;186:24–31. [DOI] [PubMed] [Google Scholar]
- 37.Jurkowska G, Grondin G, Morisset J. Involvement of endogenous cholecystokinin in pancreatic regeneration after cerulein-induced acute pancreatitis. Pancreas. 1992;7:295–304. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda A, Morris JP 4th, Hebrok M. Bmi1 is required for regeneration of the exocrine pancreas in mice. Gastroenterology. 2012;143:821–831.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nickolas TL, Forster CS, Sise ME, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82:718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin M, Kim JH, Jang E, et al. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2014;34:1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. [DOI] [PubMed] [Google Scholar]
- 42.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. [DOI] [PubMed] [Google Scholar]
- 43.Zihni C, Mills C, Matter K, et al. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. [DOI] [PubMed] [Google Scholar]
- 44.Visconti M, Rabitti PG, Uomo G, et al. [The multiple-organ failure syndrome in acute pancreatitis. Its pathogenesis and treatment]. [Article in Italian]. Recenti Prog Med. 1995;86:81–85. [PubMed] [Google Scholar]
- 45.Kumar P, Shen Q, Pivetti CD, et al. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldenberg NM, Steinberg BE, Slutsky AS, et al. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3:88ps25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.