Abstract
For patients with the acute respiratory distress syndrome (ARDS), ventilation strategies that limit end-expiratory derecruitment and end-inspiratory overdistension are the only interventions to have significantly reduced the morbidity and mortality. For this reason, the use of high-frequency oscillatory ventilation (HFOV) was considered to be an ideal protective strategy, given its reliance on very low tidal volumes cycled at very high rates. However, results from clinical trials in adults with ARDS have demonstrated that HFOV does not improve clinical outcomes. Recent experimental and computational studies have shown that oscillation of a mechanically heterogeneous lung with multiple simultaneous frequencies can reduce parenchymal strain, improve gas exchange, and maintain lung recruitment at lower distending pressures compared to traditional ‘single-frequency’ HFOV. This review will discuss the theoretical rationale for the use of multiple oscillatory frequencies in ARDS, as well as the mechanisms by which it may reduce the risk for ventilator-induced lung injury.
Current Opinion in Physiology 2021, 21:36–43
This review comes from a themed issue on Physiology of the diseased lung
Edited by Alexander Larcombe and Peter Noble
For a complete overview see the Issue
Available online 20th April 2021
https://doi.org/10.1016/j.cophys.2021.03.006
2468-8673/© 2021 Elsevier Ltd. All rights reserved.
Introduction
Of the many pathologies associated with respiratory failure, the acute respiratory distress syndrome (ARDS) is perhaps the most devastating in terms of outcome. Respiratory failure from ARDS is associated with mortality approaching 40% [1]. Survivors may also be burdened with substantial morbidity, including long-term physical and mental health impairments [2]. ARDS thus imposes significant burdens on public health resources worldwide, and only minimal improvements in outcomes have occurred over recent decades [3]. Risks for developing ARDS include a diverse range of predisposing factors and initiating insults, such as aspiration, trauma, sepsis, pneumonia, inhalation injury, blood product transfusion, or burns. Regardless of etiology, the syndrome results in a progressive deterioration of lung function towards a final common pathway: hypoxemic respiratory failure characterized by alveolar flooding, derecruitment, reduced compliance, increased shunt fraction, and increased dead space. A key pathologic feature of ARDS is the heterogeneous structural derangements to the lung tissues, arising from inflammation, edema, surfactant dysfunction, and fibroproliferation [4••].
Endotracheal intubation and supportive conventional mechanical ventilation (CMV) remain the mainstays of treatment for the early management of ARDS. However CMV may exacerbate existing lung injury, due to cyclic, intratidal overdistention (volutrauma) and repeated, asynchronous opening and closing of airspaces with each inflation (atelectrauma). The mechanical stresses associated with these phenomena, as well as the temporal rates at which they are applied [5], result in the release of cytokines and other inflammatory mediators (biotrauma) that can further exacerbate the existing injury [6,7]. This ventilator-induced lung injury (VILI) is thus a direct result of the mechanical heterogeneity of injured parenchyma, leading to maldistribution of ventilation and corresponding impairments in gas exchange. Since ventilation distribution in ARDS is governed by a heterogeneous distribution of regional mechanics, the most appropriate distending pressure, ventilation frequency, or tidal volume for one lung region may not necessarily be the same for another, even within the same patient. This conundrum of minimizing injurious stretch, while maintaining life-supporting gas exchange, is central to the ventilator management of these complex patients [8].
Ventilation strategies that limit this end-expiratory derecruitment and end-inspiratory overdistension are the only interventions to have significantly reduced the morbidity and mortality of ARDS, using low tidal volume (V T) or driving pressure (ΔP) to reduce inspiratory overdistention [9,10], and appropriate levels of positive end-expiratory pressure (PEEP) to limit end-expiratory opening and closing [11•]. Such ‘protective’ ventilation strategies, however, may result in significant hypoventilation of the injured lung. Increasing respiratory rate is the only means available to increase CO2 removal during CMV, but may be largely ineffective given the increased dead space and ventilation-to-perfusion mismatch associated with ARDS [12]. Adjustments to V T, ΔP, or PEEP based on such criteria provide little insight into how such interventions impact regional gas transport in the injured lung, or how to customize ventilator management for the pathophysiology of an individual patient. For example, optimal PEEP for a given patient depends much more on the unique pattern of injury and the amount of recruitable lung [13], rather than on oxygenation alone [14]. Moreover, there may still exist focal regions of high stress and strain within the lung, despite apparently modest distending pressures transduced at the airway opening [15,16••]. Thus the ability to improve, if not optimize, non-injurious ventilation in patients with ARDS is a consideration not only for improving in clinical outcomes, but also for appropriate management of scarce resources.
The rise and fall of high frequency oscillatory ventilation in ARDS
High-frequency oscillatory ventilation (HFOV) is an alternative form of mechanical ventilation utilizing tidal volumes smaller than anatomic dead space, 10-fold to 50-fold higher respiratory frequencies than CMV, and high instantaneous flows. Compared to CMV which relies on convective transport as the dominant mechanism for gas exchange [17], HFOV relies on several different mechanisms, including turbulence, pendelluft, asymmetric velocity profiles, Taylor dispersion, molecular diffusion, collateral ventilation, and cardiogenic mixing [18,19]. The relative contributions for any of these gas transport mechanisms are highly dependent on the frequency and amplitude of oscillation, the branching morphometry of the airway tree, as well as the size and mechanical properties of the lung [20,21•]. HFOV thus appears to achieve many of the goals of a lung protective strategy, utilizing appropriate mean airway pressures to sustain recruitment, and small tidal volumes to limit overdistention. Consequently, HFOV was initially thought to be panacea for ARDS and VILI [22, 23, 24]. Despite some initial promising results [25, 26, 27, 28], subsequent large randomized clinical trials and meta-analyses indicated that HFOV did not reduce mortality in adults with ARDS [29, 30, 31]. Moreover, HFOV has not been shown to be superior to CMV in preterm or low birth weight infants [32]. This failure of HFOV to reduce mortality suggested suboptimal, if not injurious, ventilation of the injured lung, potentially arising from variable (and unpredictable) effects of frequency, amplitude, and mean airway pressure [33, 34, 35]. The primary determinant of ventilation distribution in the injured lung is the distribution of regional mechanical properties of the airways and parenchyma, such as local resistance, inertance, and elastance [33]. Even within the same patient, the most appropriate distending pressure, ventilation frequency, or flow/volume amplitude for one region of the lung may not necessarily be the same for another [36]. When the lung exhibits such spatial mechanical heterogeneity, local ventilation distribution becomes highly frequency-dependent [20,21•,34,37,38], and the most effective frequency for optimal gas exchange in a given region will vary depending on its local mechanical properties [33,35]. Such frequency-dependence of regional ventilation may also lead to regional hyperinflation and/or derecruitment of the lung in the setting of mechanically heterogeneous disease [33,35,39,40]. Consistent with this notion, both computational and experimental studies have demonstrated that oscillatory ventilation at a single high frequency results in some regions of the lung being underventilated and subjected to the risk of atelectrauma, while other regions are overventilated and at risk for volutrauma [21•,33,34,41]. Such data indicate that small amplitude volume oscillation at a single, arbitrary frequency is not suitable for maintaining effective gas transport and exchange throughout a spatially heterogeneous lung. In addition, the use of very high mean airway pressures during HFOV may impair venous return and cardiac output, with implications for end-organ perfusion [42].
Oscillatory ventilation revisited: the use of multiple simultaneous frequencies
Strategies to optimize CMV or HFOV based on arbitrary targets for V T, ΔP, PEEP, or mean airway pressure neglect the important influence of regional mechanical heterogeneity on ventilation distribution [33, 34, 35,43]. Recent studies have proposed that mechanical function and gas exchange in the lung can be significantly improved if volume oscillations are applied at multiple frequencies simultaneously, rather than at a single high frequency, due to more even distribution of ventilation to different regions in accordance with local mechanical properties [44, 45, 46, 47, 48]. This unique modality has been termed Multi-Frequency Oscillatory Ventilation (MFOV), as a natural extension of HFOV. MFOV is specifically designed to complement the heterogeneity of the injured lung, by relying on the local mechanical impedances of the airways and parenchyma, which can selectively filter out flows of ‘less-desirable’ frequencies, while simultaneously allowing flows at frequencies more ‘optimal’ for a particular region to participate in gas exchange (Figure 1 ). With further adjustments in oscillatory volume amplitude and mean airway pressure, MFOV may improve gas exchange in the injured lung while minimizing the detrimental effects of cyclic alveolar overdistention and derecruitment [44]. MFOV attempts to exploit mechanical heterogeneity in the lung, by the design and implementation of flow waveforms with spectral content more appropriate for ARDS. Accordingly, MFOV challenges current paradigms for ventilator management in ARDS that seek to reduce the influence of regional mechanical heterogeneity on gas exchange and exacerbation of injury [49]. However, its use requires a sophisticated, higher order understanding of the interplay of regional mechanics, ventilation distribution, and gas exchange. Such understanding has been made possible by recent experimental and computer simulation studies.
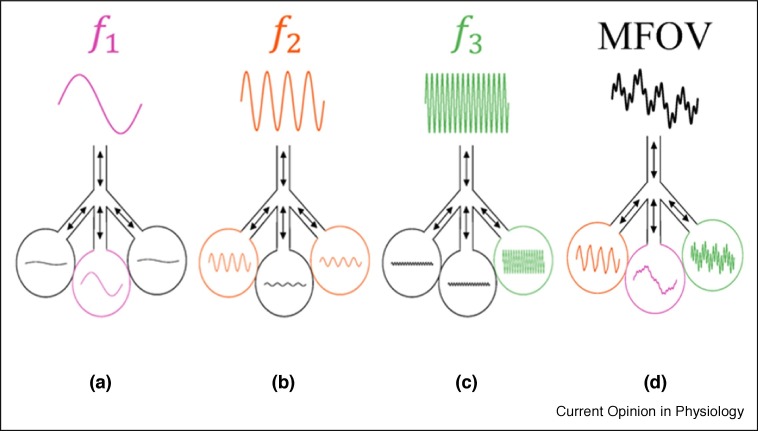
Figure 1.
Schematic of the concept of multifrequency oscillatory ventilation (MFOV) using a three compartment lung model. Each lung unit preferentially receives convective flows at different oscillatory frequencies, depending on its compliance (or elastance). One unit prefers lower frequencies (a), while the others prefer intermediate (b) or higher (c) frequencies. When these distinct oscillatory frequencies are simultaneously combined into a spectrally broadband MFOV waveform (d), each compartment can selectively ‘filter out’ its non-preferential frequencies.
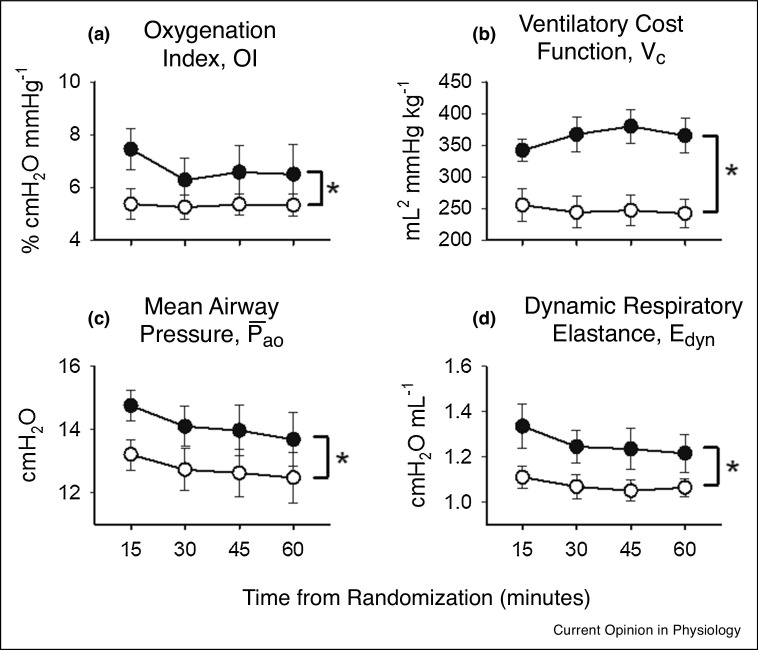
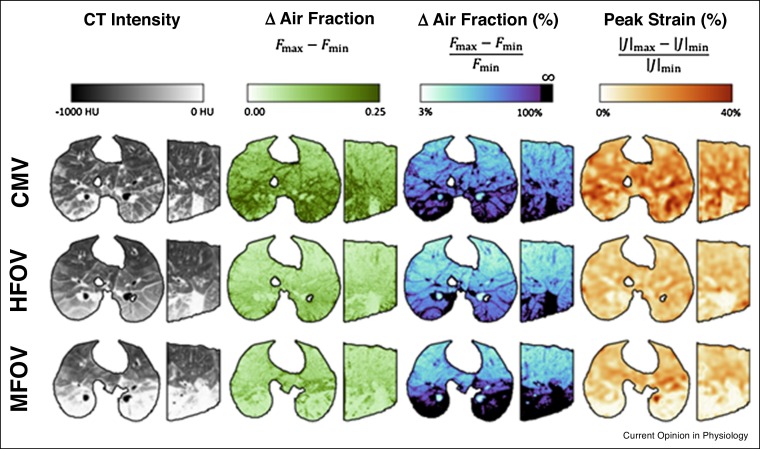
A previous study in preterm lambs demonstrated that a generic MFOV waveform, generated by a commercially available hybrid pediatric ventilator-oscillator, could achieve significantly better oxygenation (Figure 2 -a) and more efficient CO2 elimination (Figure 2-b) compared to traditional single-frequency HFOV in preterm lambs [44]. These improved indices of gas exchange could also be maintained with significantly lower mean airway pressures (Figure 2-c), possibly indicating that the additional frequencies in the MFOV waveform enhanced lung recruitment. Moreover, respiratory system elastance was also significantly lower during MFOV compared to HFOV (Figure 2-d), consistent with enhanced lung recruitment at lower distending airway pressures. These data therefore indicate that MFOV can be a more efficient ventilatory modality in preterm lungs compared to traditional HFOV, and can maintain lung recruitment at lower mean airway pressures. More recently in a dynamic CT imaging study of porcine lung injury [47], both HFOV and MFOV improved gas exchange efficiency and reduced intratidal variations in regional strain compared to CMV (Figure 3 ). These results also indicated that parenchymal strain during oscillatory ventilation was regionally heterogeneous and dependent on frequency. MFOV also significantly reduced the average regional intratidal strain throughout the injured lung compared to either CMV or HFOV, and reduced the spatial gradients of strain compared to CMV.
Figure 2.
Summary of (a) oxygenation index OI, (b) ventilator cost function VC, (c) mean airway pressure , and (d) dynamic respiratory elastance Edyn versus time in thirteen lambs randomly assigned to receive traditional single-frequency HFOV (closed symbols) or MFOV (open symbols) in a crossover design study. OI was computed as , where denotes the fraction of inspired O2 and PaO2 arterial O2 tension. Vc was computed as , where denotes the root mean square of the airway volume waveform, denotes the arterial CO2 tension, and Wt denotes subject weight. *Significant difference between SFOV and MFOV modalities, using two-way repeated-measures ANOVA with Tukey Honest Significant Difference criterion. All data are expressed as mean ± standard error. Modified from Ref. [44], with permission.
Figure 3.
Example transverse and sagittal maps of original CT Hounsfield intensities, absolute and relative changes (Δ) in air fraction, and peak strain for a representative porcine subject with acute lung injury induced with oleic acid. Changes in air fraction F are computed based on Hounsfield intensities, assuming air and tissues have intensities — 1000 HU and 0 HU, respectively. Peak strain is computed based on the change in the Jacobian determinant |J| of the image registration deformation matrix. See Ref. [47] for details.
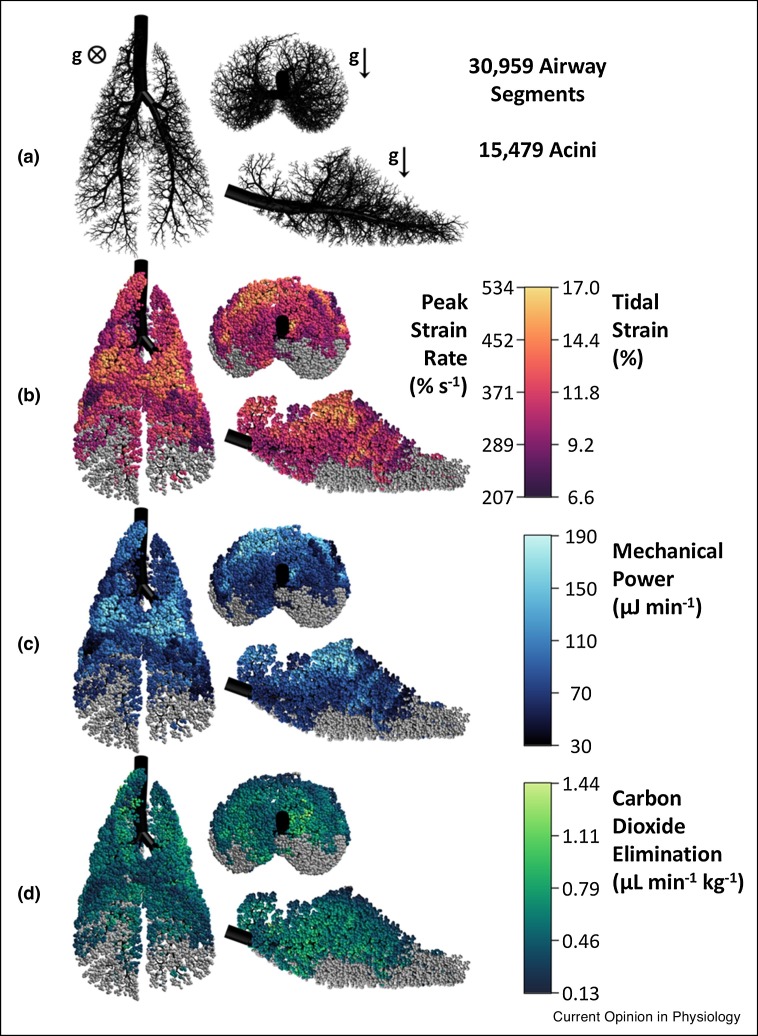
Consistent with the premise for MFOV, computer simulations in three-dimensional canine, porcine, and human lungs have demonstrated that ventilation distribution and CO2 elimination is spatially clustered [20,21•,45,50], and highly dependent on both the degree of heterogeneity as well as oscillatory frequency (Figure 4 ). Such regional and frequency-dependent differences in gas exchange support the notion that MFOV is ideally suited for the heterogeneously injured lung. More importantly, reduced heterogeneity in the distributions of ventilation and parenchymal strain can be achieved by the superposition of multiple simultaneous oscillatory frequencies, potentially reducing the risk for VILI compared to traditional single-frequency HFOV [45]. It is also theoretically possible that lung-protective MFOV waveforms can be specifically ‘tuned’ to mechanically heterogeneous lungs of individual patients [46], with sufficiently low flow/volume amplitudes to reduce parenchymal overdistention, while still being inflated with appropriate distending pressures to reduce cyclic recruitment/derecruitment. However, this likely would require more advanced imaging techniques [47].
Figure 4.
Computational model of a porcine lung with heterogeneous injury. The airway tree is shown in black (a), while the colored acini denote tidal strain (b), mechanical power (c), and CO2 elimination (d) throughout the model during oscillatory ventilation at 10 Hz and 46.7 mL. These oscillatory settings resulted in 5.5 mL min−1 kg−1 of total CO2 elimination for the whole model. Derecruited acini are shown in gray (∼39% of all acini in the model). Direction of gravity g is into the page (⊗) or toward the bottom of the page (↓) as indicated. Modified from Ref. [46], with permission.
Conclusions
In the light of recent clinical trials, debates on the merits of oscillatory ventilation as management strategy for ARDS has been tempered [51, 52, 53], even though it may still hold promise for patients with severe hypoxemia [54]. Perhaps this indicates a premature abandonment of this very unique, albeit nonintuitive, ventilatory modality in adults [55], before a sufficient understanding of its physiologic risks and benefits could be achieved. Nonetheless, there remains considerable room for improvement in the delivery of oscillatory ventilation in patients [56,57]. By taking advantage of the unique relationship between oscillatory frequency and ventilation distribution in the mechanically heterogeneous lung, MFOV may hold promise as a protective oscillatory ventilation strategy in ARDS and other forms of acute respiratory failure. However, its eventual use in patients will require further preclinical studies to understand its potential applications in other pathophysiologies relevant to ARDS. For example, exacerbations of asthma, COPD, or pneumonia also manifest themselves in a very spatially heterogeneous manner throughout the lung. Thus MFOV may also have implications for the management of acute respiratory failure from other etiologies. The possibility that MFOV can more efficiently penetrate ‘difficult-to-reach’ regions of the lung also has implications for the optimal delivery of aerosols and drugs, such as beta agonists, steroids, or even inhaled volatile anesthetics [58,59]. Moreover, the ability to enhance gas exchange at lower mean airway pressures may make MFOV a more appropriate ventilator modality in patients with impaired cardiac function [60], or in pathologies for which a structurally weakened parenchyma increases the risk barotrauma [61,62]. Whether other therapeutic interventions that are known to be efficacious in ARDS, such as prone positioning [63], confer additional lung protection when used in combination with MFOV is of course only speculative at this point. Thus while MFOV may have potential to change current ventilator management in critically ill patients, there remain fundamental questions regarding the mechanisms by which it improves gas exchange and mechanical function. It is also unclear if there are limits on the degree of lung mechanical heterogeneity for which MFOV can still maintain protective ventilation and efficacious gas exchange [33,45,46,64]. The preliminary experimental and computational studies presented in this review suggest that MFOV has potential to reduce the risk of VILI compared to CMV or traditional HFOV, at least based on short term physiologic and mechanical metrics. The potential for MFOV or other oscillatory modalities to reduce the morbidity and mortality associated with ARDS must of course await future clinical trials.
Funding
This work was supported in part by the Department of Anesthesia at the University of Iowa Hospital and Clinics and National Institutes of Health award R41 HL140640.
Conflict of interest statement
Dr Kaczka is a co-founder and shareholder of OscillaVent, Inc., and is a co-inventor on a patent involving multi-frequency oscillatory ventilation.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Herridge M.S., Tansey C.M., Matte A., Tomlinson G., Diaz-Granados N., Cooper A., Guest C.B., Mazer C.D., Mehta S., Stewart T.E., et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3.Rezoagli E., Fumagalli R., Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5:282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review on ARDS and current strategies for management.
- 5.Protti A., Maraffi T., Milesi M., Votta E., Santini A., Pugni P., Andreis D.T., Nicosia F., Zannin E., Gatti S., et al. Role of strain rate in the pathogenesis of ventilator-induced lung edema. Crit Care Med. 2016;44:e838–e845. doi: 10.1097/CCM.0000000000001718. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfuss D., Ricard J.D., Saumon G. On the physiologic and clinical relevance of lung-borne cytokines during ventilator-induced lung injury. Am J Respir Crit Care Med. 2003;167:1467–1472. doi: 10.1164/rccm.200206-611CP. [DOI] [PubMed] [Google Scholar]
- 7.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 8.Amini R., Herrmann J., Kaczka D.W. Intratidal overdistention and derecruitment in the injured lung: s simulation study. IEEE Trans Biomed Eng. 2017;64:681–689. doi: 10.1109/TBME.2016.2572678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Acute Respiratory Distress Syndrome Network, Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Amato M.B., Meade M.O., Slutsky A.S., Brochard L., Costa E.L., Schoenfeld D.A., Stewart T.E., Briel M., Talmor D., Mercat A., et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 11•.Bates J.H.T., Gaver D.P., Habashi N.M., Nieman G.F. Atelectrauma versus volutrauma: a tale of two time-constants. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting perspective on the important contributions of volutrauma and atelectrama in VILI using a computational model.
- 12.Nuckton T.J., Alonso J.A., Kallet R.H., Daniel B.M., Pittet J.F., Eisner M.D., Matthay M.A. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L., Caironi P., Cressoni M., Chiumello D., Ranieri V.M., Quintel M., Russo S., Patroniti N., Cornejo R., Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 14.Brower R.G., Lanken P.N., MacIntyre N., Matthay M.A., Morris A., Ancukiewicz M., Schoenfeld D., Thompson B.T., The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 15.Mead J., Takishima T., Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 16••.Gaver D.P., 3rd, Nieman G.F., Gatto L.A., Cereda M., Habashi N.M., Bates J.H.T. The POOR get POORer: a hypothesis for the pathogenesis of ventilator-induced lung injury. Am J Respir Crit Care Med. 2020;202:1081–1087. doi: 10.1164/rccm.202002-0453CP. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting hypothesis that VILI begins in isolated lung regions in which alveolar leak leads to surfactant dysfunction and increased regional tissue stress.
- 17.Venegas J.G., Hales C.A., Strieder D.J. A general dimensionless equation of gas transport by high-frequency ventilation. J Appl Physiol. 1986;60:1025–1030. doi: 10.1152/jappl.1986.60.3.1025. [DOI] [PubMed] [Google Scholar]
- 18.Pillow J.J. High-frequency oscillatory ventilation: mechanisms of gas exchange and lung mechanics. Crit Care Med. 2005;33(3 Suppl):S135–S141. doi: 10.1097/01.ccm.0000155789.52984.b7. [DOI] [PubMed] [Google Scholar]
- 19.Chang H.K. Mechanisms of gas transport during ventilation by high-frequency oscillation. J Appl Physiol. 1984;56:553–563. doi: 10.1152/jappl.1984.56.3.553. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann J., Tawhai M.H., Kaczka D.W. Regional gas transport in the heterogeneous lung during oscillatory ventilation. J Appl Physiol (1985) 2016;121:1306–1318. doi: 10.1152/japplphysiol.00097.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Herrmann J., Lilitwat W., Tawhai M.H., Kaczka D.W. High-frequency oscillatory ventilation and ventilator-induced lung injury: size does matter. Crit Care Med. 2020;48:e66–e73. doi: 10.1097/CCM.0000000000004073. [DOI] [PMC free article] [PubMed] [Google Scholar]; Computational modeling study to describe how heterogeneous ventilation distribution during HFOV can increase with lung size, potentially explaining why HFOV may be more injurious in adult patients compared to neonates.
- 22.Ferguson N.D., Slutsky A.S. High-frequency ventilation is the optimal physiological approach to ventilate ARDS patients. J Appl Physiol. 2008;104:1230–1231. doi: 10.1152/japplphysiol.01226.2007. [DOI] [PubMed] [Google Scholar]
- 23.Hager D.N. High-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Curr Opin Anaesthesiol. 2012;25:17–23. doi: 10.1097/ACO.0b013e32834ea57b. [DOI] [PubMed] [Google Scholar]
- 24.Ip T., Mehta S. The role of high-frequency oscillatory ventilation in the treatment of acute respiratory failure in adults. Curr Opin Crit Care. 2012;18:70–79. doi: 10.1097/MCC.0b013e32834f1805. [DOI] [PubMed] [Google Scholar]
- 25.Derdak S. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adult patients. Crit Care Med. 2003;31:S317–S323. doi: 10.1097/01.CCM.0000057910.50618.EB. [DOI] [PubMed] [Google Scholar]
- 26.Derdak S. High-frequency oscillatory ventilation for adult acute respiratory distress syndrome: a decade of progress. Crit Care Med. 2005;33(3 Suppl):S113–S114. doi: 10.1097/01.ccm.0000155787.26548.4c. [DOI] [PubMed] [Google Scholar]
- 27.Derdak S., Mehta S., Stewart T.E., Smith T., Rogers M., Buchman T.G., Carlin B., Lowson S., Granton J. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 28.Sud S., Sud M., Friedrich J.O., Meade M.O., Ferguson N.D., Wunsch H., Adhikari N.K. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. Br Med J. 2010;340 doi: 10.1136/bmj.c2327. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson N.D., Cook D.J., Guyatt G.H., Mehta S., Hand L., Austin P., Zhou Q., Matte A., Walter S.D., Lamontagne F., et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 30.Young D., Lamb S.E., Shah S., MacKenzie I., Tunnicliffe W., Lall R., Rowan K., Cuthbertson B.H., Group O.S. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 31.Maitra S., Bhattacharjee S., Khanna P., Baidya D.K. High-frequency ventilation does not provide mortality benefit in comparison with conventional lung-protective ventilation in acute respiratory distress syndrome: a meta-analysis of the randomized controlled trials. Anesthesiology. 2015;122:841–851. doi: 10.1097/ALN.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 32.Cools F., Offringa M., Askie L.M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD000104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colletti A.A., Amini R., Kaczka D.W. Simulating ventilation distribution in heterogenous lung injury using a binary tree data structure. Comput Biol Med. 2011;41:936–945. doi: 10.1016/j.compbiomed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen J.L., Frantz I.D., 3rd, Fredberg J.J. Heterogeneity of mean alveolar pressure during high-frequency oscillations. J Appl Physiol. 1987;62:223–228. doi: 10.1152/jappl.1987.62.1.223. [DOI] [PubMed] [Google Scholar]
- 35.Amini R., Kaczka D.W. Impact of ventilation frequency and parenchymal stiffness on flow and pressure distribution in a canine lung model. Ann Biomed Eng. 2013;41:2699–2711. doi: 10.1007/s10439-013-0866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dreyfuss D., Ricard J.D., Gaudry S. Did studies on HFOV fail to improve ARDS survival because they did not decrease VILI? On the potential validity of a physiological concept enounced several decades ago. Intensive Care Med. 2015;41:2076–2086. doi: 10.1007/s00134-015-4062-0. [DOI] [PubMed] [Google Scholar]
- 37.Allen J.L., Fredberg J.J., Keefe D.H., Frantz I.D. Alveolar pressure magnitude and asynchrony during high-frequency oscillations of excised rabbit lungs. Am Rev Respir Dis. 1985;132:343–349. doi: 10.1164/arrd.1985.132.2.343. [DOI] [PubMed] [Google Scholar]
- 38.Fredberg J.J., Keefe D.H., Glass G.M., Castile R.G., Frantz I.D., III Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. J Appl Physiol. 1984;57:788–800. doi: 10.1152/jappl.1984.57.3.788. [DOI] [PubMed] [Google Scholar]
- 39.Cha E.J., Chow E., Chang H.K., Yamashiro S.M. Lung hyperinflation in isolated dog lungs during high-frequency oscillation. J Appl Physiol (1985) 1988;65:1172–1179. doi: 10.1152/jappl.1988.65.3.1172. [DOI] [PubMed] [Google Scholar]
- 40.Venegas J.G., Fredberg J.J. Understanding the pressure cost of ventilation: why does high-frequency ventilation work? Crit Care Med. 1994;22(9 Suppl):S49–S57. doi: 10.1097/00003246-199422091-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lehr J.L., Butler J.P., Westerman P.A., Zatz S.L., Drazen J.M. Photographic measurement of pleural surface motion during lung oscillation. J Appl Physiol. 1985;59:623–633. doi: 10.1152/jappl.1985.59.2.623. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra A., Drazen J.M. High-frequency oscillatory ventilation on shaky ground. N Engl J Med. 2013;368:863–865. doi: 10.1056/NEJMe1300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen J.L., Frantz I.D., 3rd, Fredberg J.J. Regional alveolar pressure during periodic flow. Dual manifestations of gas inertia. J Clin Invest. 1985;76:620–629. doi: 10.1172/JCI112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaczka D.W., Herrmann J., Zonneveld C.E., Tingay D.G., Lavizzari A., Noble P.B., Pillow J.J. Multifrequency oscillatory ventilation in the premature lung: effects on gas exchange, mechanics, and ventilation distribution. Anesthesiology. 2015;123:1394–1403. doi: 10.1097/ALN.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrmann J., Tawhai M.H., Kaczka D.W. Parenchymal strain heterogeneity during oscillatory ventilation: why two frequencies are better than one. J Appl Physiol (1985) 2018;124:653–663. doi: 10.1152/japplphysiol.00615.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann J., Tawhai M.H., Kaczka D.W. Strain, strain rate, and mechanical power: an optimization comparison for oscillatory ventilation. Int J Numer Method Biomed Eng. 2019;35 doi: 10.1002/cnm.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrmann J., Gerard S.E., Shao W., Hawley M.L., Reinhardt J.M., Christensen G.E., Hoffman E.A., Kaczka D.W. Quantifying regional lung deformation using four-dimensional computed tomography: a comparison of conventional and oscillatory ventilation. Front Physiol. 2020;11:14. doi: 10.3389/fphys.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ort V., Kabelková E. Better distribution of minute ventilation and lung energy load in multi-frequency ventilation in neonatal inhomogeneous lungs: mathematical simulation. 2019 E-Health and Bioengineering Conference (EHB); Iasi, Romania; 2019. pp. 1–4. [Google Scholar]
- 49.Santini A., Mauri T., Dalla Corte F., Spinelli E., Pesenti A. Effects of inspiratory flow on lung stress, pendelluft, and ventilation heterogeneity in ARDS: a physiological study. Crit Care. 2019;23:369. doi: 10.1186/s13054-019-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrmann J., Tawhai M.H., Kaczka D.W. Computational modeling of primary blast lung injury: implications for ventilator management. Mil. Med. 2019;184(Suppl._1):273–281. doi: 10.1093/milmed/usy305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sklar M.C., Fan E., Goligher E.C. High-frequency oscillatory ventilation in adults with ARDS: past, present, and future. Chest. 2017;152:1306–1317. doi: 10.1016/j.chest.2017.06.025. Epub 2017 July. [DOI] [PubMed] [Google Scholar]
- 52.Vincent J.L. High-frequency oscillation in acute respiratory distress syndrome. The end of the story? Am J Respir Crit Care Med. 2017;196:670–671. doi: 10.1164/rccm.201703-0475ED. [DOI] [PubMed] [Google Scholar]
- 53.Mentzelopoulos S.D., Malachias S., Vrettou C., Zakynthinos S.G. Meta-analysis of high-frequency oscillation in acute respiratory distress syndrome and accuracy of results. Anesthesiology. 2016;124:246–247. doi: 10.1097/ALN.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 54.Meade M.O., Young D., Hanna S., Zhou Q., Bachman T.E., Bollen C., Slutsky A.S., Lamb S.E., Adhikari N.K.J., Mentzelopoulos S.D., et al. Severity of hypoxemia and effect of high-frequency oscillatory ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:727–733. doi: 10.1164/rccm.201609-1938OC. [DOI] [PubMed] [Google Scholar]
- 55.Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., Adhikari N.K.J., Amato M.B.P., Branson R., Brower R.G., et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J. Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Pacheco N., Sanchez-Luna M., Chimenti-Camacho P., Santos-Gonzalez M., Palau-Concejo P., Tendillo-Cortijo F. Use of very low tidal volumes during high-frequency ventilation reduces ventilator lung injury. J Perinatol. 2019;39:730–736. doi: 10.1038/s41372-019-0338-5. [DOI] [PubMed] [Google Scholar]
- 57.Liu S., Yi Y., Wang M., Chen Q., Huang Y., Liu L., Xie J., Zhou D., Qiu H. Higher frequency ventilation attenuates lung injury during high-frequency oscillatory ventilation in sheep models of acute respiratory distress syndrome. Anesthesiology. 2013;119:398–411. doi: 10.1097/ALN.0b013e31829419a6. [DOI] [PubMed] [Google Scholar]
- 58.Mondoñedo J.R., McNeil J.S., Amin S.D., Herrmann J., Simon B.A., Kaczka D.W. Volatile anesthetics and the treatment of severe bronchospasm: a concept of targeted delivery. Drug Discov Today Dis Models. 2015;15:43–50. doi: 10.1016/j.ddmod.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amin S.D., Majumdar A., Frey U., Suki B. Modeling the dynamics of airway constriction: effects of agonist transport and binding. J Appl Physiol. 2010;109:553–563. doi: 10.1152/japplphysiol.01111.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Angriman F., Ferreyro B.L., Donaldson L., Cuthbertson B.H., Ferguson N.D., Bollen C.W., Bachman T.E., Lamontagne F., Adhikari N.K.J. The harm of high-frequency oscillatory ventilation (HFOV) in ARDS is not related to a high baseline risk of acute cor pulmonale or short-term changes in hemodynamics. Intensive Care Med. 2020;46:132–134. doi: 10.1007/s00134-019-05806-8. Epub 2019 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGuinness G., Zhan C., Rosenberg N., Azour L., Wickstrom M., Mason D.M., Thomas K.M., Moore W.H. High incidence of barotrauma in patients with COVID-19 infection on invasive mechanical ventilation. Radiology. 2020 doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsolaki V., Zakynthinos G.E., Makris D. The ARDSnet protocol may be detrimental in COVID-19. Crit Care. 2020;24:351. doi: 10.1186/s13054-020-03081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 64.Herrmann J., Gerard S.E., Reinhardt J.M., Hoffman E.A., Kaczka D.W. Regional gas transport during conventional and oscillatory ventilation assessed by xenon-enhanced computed tomography. Ann Biomed Eng. 2021 doi: 10.1007/s10439-021-02767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]