Abstract
Glucosamine (GlcN) is commonly used as a dietary supplement to promote cartilage health in humans. We previously reported that GlcN could induce autophagy in cultured mammalian cells. Autophagy is known to be involved in the prevention of various diseases and aging. Here, we showed that GlcN extended the lifespan of the nematode Caenorhabditis elegans by inducing autophagy. Autophagy induction by GlcN was demonstrated by western blotting for LGG-1 (an ortholog of mammalian LC3) and by detecting autophagosomal dots in seam cells by fluorescence microscopy. Lifespan assays revealed that GlcN-induced lifespan extension was achieved with at least 5 mM GlcN. A maximum lifespan extension of approximately 30 % was achieved with 20 mM GlcN (p<0.0001). GlcN-induced lifespan extension was not dependent on the longevity genes daf-16 and sir-2.1 but dependent on the autophagy-essential gene atg-18. Therefore, we suggest that oral administration of GlcN could help delay the aging process via autophagy induction.
Keywords: anti-aging, atg-18, autophagy, Caenorhabditis elegans, glucosamine, lifespan
Abbreviations
FUdR, 5-fluoro-2′-deoxyuridine; GFP, green fluorescent protein; GPI, glycosylphosphatidylinositol; LC3, microtubule-associated protein 1 light chain 3; mTOR, mammalian target of rapamycin; NGM, nematode growth medium; PE, phosphatidylethanolamine.
INTRODUCTION
Glucosamine (2-amino-2-deoxy-D-glucose, GlcN) is the constitutional unit of chitosan and chitin, which are produced in nature by arthropods, fungi, and cephalopods. GlcN is industrially manufactured for dietary supplements by the hydrolysis of crustacean exoskeletons, which are mainly composed of chitin. GlcN has been reported to effectively prevent and treat osteoarthritis in humans1),2) and have a positive effect on skin aging in a clinical study.3) On the other hand, a meta-analysis has reported the ineffectiveness of GlcN on articulation.4) Therefore, the anti-osteoarthritis effect of GlcN remains controversial. Thus far, many people have taken GlcN as a dietary supplement. According to a large-scale epidemiological study on consumers of various dietary supplements, use of GlcN was reported to associate with decreased total mortility.5) However, the underlying molecular mechanism of the longevity effect of GlcN remains unclear.
Autophagy is a cellular process that nonspecifically degrades cytosolic components. An autophagic membrane engulfs parts of the cytosol containing proteins and organelles and is fused with the lysosome to degrade inner components.6) Autophagy is known to play critical roles in various cellular processes such as the response to starvation,7) the prevention of bacterial infection,8),9) antigen presentation,10) neural development,11) glycogen degradation,12) and lipid metabolism.13) In contrast to autophagy induced in response to environmental signals, basal level autophagy may also have important functions, which include protein quality control and cellular anti-aging functions.14),15) Therefore, the induction of autophagy by drugs has attracted attention as a promising anti-aging approach.16) Several autophagy inducers have been reported to ameliorate the toxicity of polyglutamine-expanded huntingtin and related proteinopathies16) and extend the lifespan of animal models.17)
Caenorhabditis elegans is a common animal model for lifespan assays. The lifespan of C. elegans is known to be mediated by several proteins.18) DAF-16, an ortholog of mammalian FOXO transcription factors, is regulated by TOR signaling and can affect lifespan.19) In addition, SIR-2.1 (NAD+-dependent protein deacetylase), an ortholog of mammalian SIRT1, is a longevity-related protein.20) A study found that resveratrol could extend the lifespan of nematodes and fruit flies in a SIR-2.1/Sir2-dependent manner without reducing fertility.21) Aging could also be mediated by autophagy and autophagy-related genes.22)
Previously, we reported that GlcN supplementation within a physiological concentration range could strongly induce autophagy in mammalian cells.23) To investigate the autophagy-inducing effect of GlcN on aging, we selected the nematode C. elegans as a model. C. elegans is commonly used as an aging model and for autophagy studies.22),24) Here, we demonstrated the autophagy-inducing activity of GlcN in the nematode. In addition, at least 5 mM GlcN extended the lifespan of the nematode in an autophagy-dependent manner.
MATERIALS AND METHODS
Chemicals.
GlcN hydrochloride was purchased from Nacalai Tesque, INC. (Kyoto, Japan). Other chemicals (not mentioned in the following sections) were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Strains.
The C. elegans strains N2 (wild type), DA2123 (asIs2122[lgg-1::GFP + rol(su1006)], and atg-18(gk378) were provided by the Caenorhabditis Genetic Center (CGC). The strains daf-16(mgDf50) and sir-2.1(ok434) were provided from Prof. Eisuke Nishida (Kyoto University, Kyoto, Japan).25) Escherichia coli OP50 was used as a food source for the nematodes.26)
C. elegans culture and preparation of L1 larvae.
C. elegans was maintained at 20 °C on nematode growth medium (NGM) (2.0 % agar, 0.5 % peptone, 50 mM NaCl, 25 mM potassium phosphate buffer pH 6.0, 1 mM CaCl2, 1 mM MgSO4, and 5 µg/mL cholesterol) with E. coli OP50 as food source.26) The eggs of C. elegans were collected by treating egg-bearing adult worms with alkaline hypochlorite solution, and they were shaken in S basal medium at 20 °C for 20–24 h to prepare synchronized first-stage larvae (L1).27)
Western blotting.
Worms were lysed in 20 mM Tris/HCl buffer (pH 7.4) containing 1.0 % Triton X-100, 150 mM NaCl, and cOmplete™ Protease Inhibitor Cocktail (F. Hoffmann-La Roche AG, Basel, Switzerland). After centrifugation at 12,000 rpm for 10 min, the supernatants were obtained. Protein concentration was measured using BCA Protein Assay Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Supernatants containing 20 µg of proteins were separated by a 15 % SDS-PAGE gel under reducing conditions and blotted onto a PVDF membrane. The membrane was blocked with 5.0 % bovine serum albumin (Nacalai Tesque) for the detection of phosphorylated proteins and 1.0 % skim milk (Wako Pure Chemical Industries) for other proteins. The primary antibody was mouse anti-GFP IgG (1/1,000; Sigma-Aldrich Corporation, St. Louis, MO, USA). The secondary antibody was horseradish peroxidase-conjugated anti-mouse IgG (1/4,000; MEDICAL & BIOLOGICAL LABORATORIES CO., LTD., Nagoya, Japan). Detection of the target proteins was carried out using West Pico Chemiluminescent Kit (Thermo Fisher Scientific) and LAS Image Analyzer (Fuji Film Corporation, Tokyo, Japan).
Fluorescence microscopy.
One drop of 10 mM sodium azide (in M9 buffer; 6.0 g/L Na2HPO4, 3.0 g/L KH2PO4, 5.0 g/L NaCl, 0.25 g/L MgSO4・7H2O) was placed onto a glass slide followed by a drop of M9 buffer containing nematodes, and a cover glass was placed on top. The transgenic worms expressing GFP::LGG-128) on the glass slide were analyzed using a fluorescence microscope (Olympus IX-70; Olympus Corporation, Tokyo, Japan). The GFP dots of approximately 100 seam cells were counted for each worm group.
Lifespan assay.
Lifespan assays were carried out at 20 °C.27) L1 nematodes were transferred to culture plates containing NGM with E. coli OP50. 5-Fluoro-2′-deoxyuridine (FUdR) was added (50 µM, final concentration) to the NGM plates to prevent progeny growth. Then, 50 synchronized animals (10 or 25 animals/plate) were placed on NGM plates containing GlcN with UV-killed E. coli OP50. Control worms were incubated in medium without GlcN. In each assay, 2 plates (50 animals) were used for the same condition. The numbers of live and dead animals were counted every second day under a microscope based on their movement. Each assay was repeated twice except for the assays using N2 adults (30 mM GlcN), atg-18 mutants, and sir-2.1 mutants. The survival curves were determined using the Kaplan-Meier method, and survival differences were tested for significance using the log-rank test. Data are expressed as the mean ± standard error (SE).
RESULTS
Autophagy induction in C. elegans by GlcN.
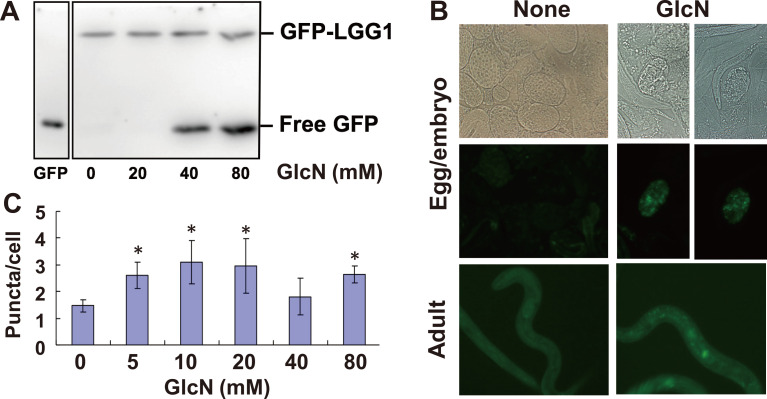
In a previous study, we found that GlcN could strongly induce autophagy in mammalian cells such as HeLa and COS cells.23) For the detection of autophagy induction, microtubule-associated protein 1 light chain 3 (LC3) is one of the most suitable marker proteins in mammalian cells.29) When autophagy is induced, cytosolic LC3-I is covalently attached to phosphatidylethanolamine (PE) on the autophagosomal membrane to form LC3-II. Membrane-anchored LC3-II is involved in autophagosomal membrane elongation, and those on the inner membrane will be degraded in autolysosomes. To assess the autophagy-inducing activity of GlcN in C. elegans, we used transgenic worms expressing LGG-1 (an ortholog of mammalian LC3) fused with GFP. The preliminary results revealed that growth inhibition was observed by microscopic analysis when GlcN was more than 80 mM (data not shown). Therefore, GFP::LGG-1 worms were grown with 0–80 mM GlcN for 96 h and harvested, and the lysates were analyzed by western blotting using anti-GFP antibody (Fig. 1A). PE-conjugated GFP::LGG-1, the autophagosomal membrane-anchored form, was hardly detected by SDS-PAGE because of the small difference in migration between PE-conjugated and -unconjugated GFP::LGG-1. However, the accumulation of protease-resistant free GFP could be detected, which might be transferred into autolysosomes via autophagy activation and released from PE-conjugated GFP::LGG-1 by lysosomal protease. Therefore, the level of GFP would correspond to the degree of autophagy induction. In this assay, we detected free GFP with 40–80 mM GlcN but not 20 mM GlcN. Next, autophagosome formation was analyzed by fluorescence microscopy using the same transgenic animals, which were continuously cultured in the presence of 40 mM GlcN. GFP-positive dots were observed in both the GlcN-treated eggs/embryos and adults but not in the control worms (Fig. 1B). In adult worms, seam cells arranged as longitudinal rows on the left and right sides of the body were used for counting autophagosomes.30) Approximately 100 cells were analyzed by fluorescence microscopy. A significant increase (p<0.05) in the number of GFP-positive dots was observed in the cytosol of the seam cells of worms grown with 5–80 mM GlcN (Fig. 1C). A similar pattern was observed with 40 mM GlcN; however, it was not significant. Taken together, these results suggest that 5–80 mM GlcN significantly induced autophagy activation. Inconsistency in autophagy induction levels and required GlcN concentration in Figs. 1A–1C might be due to differences in cell types and detection methods.
Fig. 1. Autophagy induction by GlcN in C. elegans.
(A) Transgenic worms expressing GFP::LGG-1 were grown with 0–80 mM GlcN for 96 h and harvested. GFP::LGG-1 and free GFP were detected by western blotting using anti-GFP antibody. (B) Autophagosome formation was analyzed by fluorescence microscopy. Left column, control; right column, worms grown in medium containing 40 mM GlcN. Upper row, Nomarski images of eggs/embryos; middle row, fluorescent images of eggs/embryos; bottom row, fluorescent images of adults. (C) The number of GFP-positive dots in the cytosol of seam cells was determined. Approximately 100 seam cells were analyzed. Data represent the mean ± standard deviation. Asterisks indicate significant differences compared with the control (p<0.05) by Student's t-test.
Lifespan extension of C. elegans by GlcN.
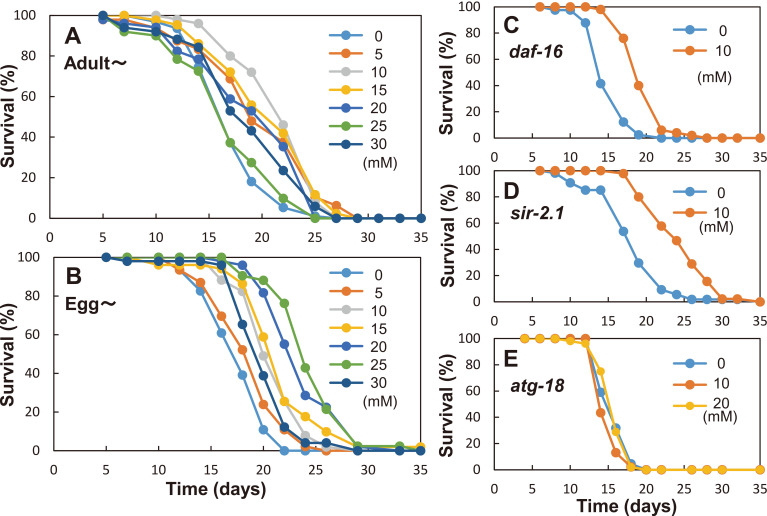
To assess the effect of GlcN on the lifespan of nematodes, synchronized young adults of the wild-type strain were treated with FUdR to prevent oviposition. They were grown in the presence of 0–30 mM GlcN, and their lifespan was measured (Fig. 2A). In the presence of 5–20 mM GlcN, the mean lifespan was significantly increased (p<0.01) compared with that of the control. The same experiments were independently performed twice, and the results are shown in Table 1. In these experiments, 15 mM GlcN was the most effective, demonstrating a lifespan extension of 22 %. A higher GlcN concentration (25–30 mM) was less effective for lifespan extension. In addition, we investigated the effect of GlcN on the total lifespan from the larval stage. Eggs were hatched in GlcN-containing medium. Then, the larvae were grown on the same plate, and their lifespan was measured. In this assay, 10–25 mM GlcN significantly increased the lifespan (p<0.0001), and a maximum lifespan extension of around 30 % was achieved with 20–25 mM GlcN (Fig. 2B, Table 1). Overall, 10–20 mM GlcN was found to be the most effective for the lifespan extension of C. elegans.
Fig. 2. Survival curves of C. elegans grown with GlcN.
(A) Young adults of the wild-type strain (N2) were grown with GlcN (0–30 mM). (B) Larvae of the wild-type strain (N2) were grown with GlcN (0–30 mM). (C) The daf-16 mutant worms were grown with or without 10 mM GlcN. (D) The sir-2.1 mutant worms were grown with or without 10 mM GlcN. (E) The atg-18 mutant worms were grown with or without 10–20 mM GlcN.
Table 1.
Summary and statistical analysis of C. elegans lifespan assays.
| Strain | GlcN(mM) | Mean lifespan± SE (days) | Extension(%) | Number of worms | p-value(vs. control) |
| N2 (starting from adult) | 0 | 19.0 ± 1.5 | 100 | ||
| 5 | 22.1 ± 1.8 | 16 | 100 | <0.001 | |
| 10 | 22.3 ± 0.1 | 17 | 100 | <0.0001 | |
| 15 | 23.2 ± 2.1 | 22 | 100 | <0.001 | |
| 20 | 21.2 ± 1.6 | 12 | 100 | <0.01 | |
| 25 | 18.4 ± 0.1 | –3 | 50 | NS | |
| 30 | 20.1 ± 0.1 | 6 | 50 | <0.01 | |
| N2 (starting from egg) | 0 | 18.2 ± 0.5 | 100 | ||
| 5 | 19.7 ± 1.0 | 8 | 100 | NS | |
| 10 | 22.0 ± 0.9 | 21 | 100 | <0.0001 | |
| 15 | 21.8 ± 0.1 | 20 | 100 | <0.0001 | |
| 20 | 23.8 ± 0.1 | 31 | 100 | <0.0001 | |
| 25 | 23.7 ± 1.0 | 30 | 100 | <0.0001 | |
| 30 | 20.3 ± 0.1 | 12 | 100 | <0.01 | |
| daf-16(mgDf50) | 0 | 15.5 ± 0.3 | 100 | ||
| 10 | 18.9 ± 1.0 | 22 | 100 | <0.0001 | |
| sir-2.1(ok434) | 0 | 18.4 ± 0.1 | 50 | ||
| 10 | 24.4 ± 0.1 | 33 | 50 | <0.0001 | |
| atg-18(gk378) | 0 | 15.9 ± 0.1 | 50 | ||
| 10 | 15.2 ± 0.1 | –4 | 50 | NS | |
| 20 | 16.0 ± 0.1 | 1 | 50 | NS |
The p-value (vs. control) was calculated by the log-rank test. NS, not significant.
Lifespan extension of C. elegans via an autophagy-dependent mechanism.
Thus far, several molecules involved in longevity, such as DAF-16 and SIR-2.1, have been identified in C. elegans.18) The former is the sole ortholog of the FOXO transcription factors of mammals, and the latter is an NAD+-dependent histone deacetylase. To determine whether the lifespan extension effect of GlcN is mediated via pathways involving these molecules, we used daf-16 and sir-2.1 mutants. These mutants were grown from eggs with or without 10 mM GlcN. The lifespan of both daf-16 and sir-2.1 mutants was significantly increased (p<0.0001) in the presence of GlcN (Figs. 2C and 2D, Table 1). These results indicated that GlcN-induced lifespan extension was independent of DAF-16 and SIR-2.1. Next, we investigated whether lifespan extension is induced by GlcN in an autophagy-dependent manner. The autophagy-defective atg-18 mutant has been reported to be short-lived, and it does not accumulate LGG-1 at the embryonic stage.31) The lifespan of the atg-18 mutant was not affected by 10–20 mM GlcN (Fig. 2E, Table 1). Taken together, these results clearly indicated that GlcN-induced lifespan extension was dependent on autophagy but not dependent on common longevity pathways that involve DAF-16 and SIR-2.1.
DISCUSSION
Thus far, the most common strategy for promoting longevity is calorie restriction. An appropriate level of calorie restriction has been experimentally demonstrated to elongate the lifespan of various animal models such as rhesus monkeys, mice, fruit flies, and nematodes.32) On the other hand, the intake of certain exogenous factors has also been reported to induce longevity.33) These longevity-inducing factors include resveratrol, rapamycin, spermidine, and 2-deoxy-glucose. Most of these factors and calorie restriction have been found to induce autophagy; thus, autophagy has been recognized as one of the most important cellular mechanisms that prevent aging.34),35) Resveratrol, a polyphenol found in red wine, can induce autophagy by activating sirtuin, an NAD+-dependent histone deacetylase.32) Rapamycin, an antibiotic produced by actinomycete, can directly inhibit TOR, which negatively regulates autophagy.32) Spermidine, a type of polyamine, can also induce autophagy through histone acetyltransferase inhibition in a dependent or independent manner.36) 2-Deoxy-glucose is a well known inhibitor of glycolysis that can deplete cellular ATP and act as a calorie restriction mimetic to induce autophagy.37),38)
We previously reported that GlcN could induce autophagy in mammalian cells via an mTOR-independent signaling pathway.23) In this study, we demonstrated for the first time that GlcN induced autophagy in the adults and embryos of C. elegans. We also found that GlcN supplementation extended the lifespan of the nematode. Recently, another group reported the longevity effect of GlcN on nematodes and mice.39) They suggest that the effect was caused by impaired glucose metabolism; however, the involvement of autophagy was not discussed. Here, we clearly showed that the longevity effect of GlcN required an autophagy gene, atg-18. However, unlike functional food factors and calorie restriction, the longevity genes sir-2.1 and daf-16 were not required for GlcN-induced lifespan extension.
GlcN is known to be incorporated into cells via glucose transporters and metabolized to UDP-GlcNAc through GlcN-6-phosphate.40) UDP-GlcNAc is used for the biosynthesis of O-linked GlcNAc, N-glycan, and GPI-anchor. Among these, O-linked GlcNAc is important modification of intracellular proteins required for regulating insulin signaling. Therefore, a high concentration of intracellular UDP-GlcNAc may induce autophagy. The increased synthesis of N-glycan precursors can also improve protein homeostasis and extend the lifespan of C. elegans.41)
An excess amount of GlcN-6-phosphate is metabolized to fructose-6-phosphate (an intermediate of glycolysis) and ammonia by glucosamine-6-phosphate deaminase. Intracellular ammonia has been reported to induce autophagy via UNC-51-like kinase 1 (ULK1)/ULK2-independent pathways.42) We found that mammalian cells cultured with ammonia induced autophagy in an mTOR-independent manner (unpublished data). However further studies would be needed to elucidate the autophagy-inducing mechanism of GlcN in terms of UDP-GlcNAc and/or ammonia.
Recently, non-metabolizable D-allulose, one of the rare hexoses, has been reported to extend the lifespan of nematodes.43) Similar to GlcN and 2-deoxy-glucose, D-allulose enters into cells through glucose transporters and inhibits glycolysis, inducing the metabolism of stored fat and mitochondrial respiration via AMP-activated protein kinase (AMPK). Increased respiration can cause the temporary formation of reactive oxygen species, leading to increased anti-oxidative enzyme activity, oxidative stress resistance, and survival rates.43),44) Orally administrated GlcN has also been reported to affect carbohydrate metabolism and reduce body fat in rodents,45) and it could contribute to enhanced oxidative stress resistance followed by AMPK activation.39) Therefore, the mechanism of the anti-aging effect of GlcN may be partially similar to that of D-allulose and 2-deoxy-glucose.
GlcN is widely consumed as a dietary supplement to promote cartilage health; however, its efficacy remains controversial. A large-scale epidemiological study on the long-term intake of various dietary supplements has revealed that the use of GlcN or chondroitin could significantly reduce mortality.5) Despite its limitations, the results of this study were consistent, to some extent, with those of the epidemiological study. Therefore, GlcN may exert anti-aging effects by inducing autophagy in humans.
ACKNOWLEDGMENTS
We thank Prof. Eisuke Nishida (Kyoto University, Kyoto, Japan) for the valuable advice and for providing the daf-16(mgDf50) and sir-2.1(ok434) strains. Furthermore, we thank Prof. Masashi Sato (Kagawa University, Kagawa, Japan) for the helpful discussion. This study was supported by JSPS KAKENHI grants 24580179 and 15K07448 (to H.A.). This study was also partially supported by the Sasakawa Scientific Research Grant from The Japan Science Society (to T.S.).
The authors declare no conflicts of interest. However, Tomoya Shintani is an employee of Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan).
REFERENCES
- 1).Reginster J.Y., Deroisy R., Rovati L.C., Lee R.L., Lejeune E., Bruyere O., Giacovelli G., Henrotin Y., Dacre J.E., and Gossett C.: Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. . Lancet, 357, 251–256 (2001) [DOI] [PubMed] [Google Scholar]
- 2).Kanzaki N., Ono Y., Shibata H., and Moritani T.: Glucosamine-containing supplement improves locomotor functions in subjects with knee pain a randomized, double-blind, placebo-controlled study. . Clin. Interv. Aging, 10, 1743(2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Gueniche A. and Castiel-Higounenc I.: Efficacy of glucosamine sulphate in skin ageing: results from an ex vivo anti-ageing model and a clinical trial. Skin Pharmacol. Physiol., 30, 36–41 (2017). [DOI] [PubMed] [Google Scholar]
- 4).Wandel S., Juni P., Tendal B., Nuesch E., Villiger P.M., Welton N.J., Reichenbach N.J., and Trelle S.: Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ, 341, 4675–4675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Pocobelli G., Kristal A.R., Patterson R.E., Potter J.D., Lampe J.W., Kolar A., Evans I., and White E.: Total mortality risk in relation to use of less-common dietary supplements. Am. J. Clin. Nutr., 91, 1791–1800 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Klionsky D.J. and Emr S.D.: Autophagy as a regulated pathway of cellular degradation. Science, 290, 1717–1721 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Nakatogawa H., Suzuki K., Kamada Y., and Ohsumi Y.: Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol., 10, 458–467 (2009). [DOI] [PubMed] [Google Scholar]
- 8).Orvedahl A. and Levine B.: Autophagy in mammalian antiviral immunity. Curr. Top. Microbiol. Immunol., 335, 267–285 (2009). [DOI] [PubMed] [Google Scholar]
- 9).Virgin H.W. and Levine B.: Autophagy genes in immunity. Nat. Immunol., 10, 461–470 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).English L., Chemali M., Duron J., Rondeau C., Laplante A., Gingras D., Alexander D., Leib D., Norbury C., Lippé R., and Desjardins M.: Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol., 10, 480–487 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., and Mizushima N.: The role of autophagy during the early neonatal starvation period. Nature, 23, 1032–1036 (2004). [DOI] [PubMed] [Google Scholar]
- 12).Kotoulas O.B., Kalamidas S.A., and Kondomerkos D.J.: Glycogen autophagy in glucose homeostasis. Pathol. Res. Pract., 202, 631–638 (2006). [DOI] [PubMed] [Google Scholar]
- 13).Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., and Czaja1 M.J.: Autophagy regulates lipid metabolism. Nature, 458, 1131–1135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Mizushima N.: Physiological functions of autophagy. Curr. Top. Microbiol. Immunol., 335, 71–84 (2009). [DOI] [PubMed] [Google Scholar]
- 15).Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., and Mizushima N.: Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature, 441, 885–889 (2006). [DOI] [PubMed] [Google Scholar]
- 16).Sarkar S., Ravikumar B., Floto R.A., and Rubinsztein D.C.: Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ., 16, 46 (2008). [DOI] [PubMed] [Google Scholar]
- 17).Ntsapi C. and Loos B.: Caloric restriction and the precision-control of autophagy: a strategy for delaying neurodegenerative disease progression. Exp. Gerontol., 83, 97–111 (2016). [DOI] [PubMed] [Google Scholar]
- 18).Kenyon C., Chang J., Gensch E., Rudner A., and Tabtiang R.: A C. elegans mutant that lives twice as long as wild type. Nature, 366, 461 (1993). [DOI] [PubMed] [Google Scholar]
- 19).Robida-Stubbs S., Glover-Cutter K., Lamming D.W., Mizunuma M., Narasimhan S.D., Neumann-Haefelin E., Sabatini D.M., and Blackwell T.K.: TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab., 15, 713–724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Tissenbaum H.A. and Guarente L.: Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature, 410, 227 (2001). [DOI] [PubMed] [Google Scholar]
- 21).Wood J.G., Rogina B., Lavu S., Howitz K., Helfand S.L., Tatar M., and Sinclair D.: Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature, 430, 686–689 (2004). [DOI] [PubMed] [Google Scholar]
- 22).Rubinsztein D.C., Marin G., and Kroemer G.: Autophagy and aging. Cell, 146, 682–695 (2011). [DOI] [PubMed] [Google Scholar]
- 23).Shintani T., Yamazaki F., Katoh T., Umekawa M., Matahira Y., Hori S., Kakizuka A., Totani K., Yamamoto K., and Ashida H.: Glucosamine induces autophagy via an mTOR-independent pathway. Biochem. Biophys. Res. Commun., 391, 1775–1779 (2010). [DOI] [PubMed] [Google Scholar]
- 24).Zhang H., Chang J.T., Guo B., Hansen M., Jia K., Kovács A.L., Kumsta C., Lapierre L.R., Legouis R., Lin L., Lu Q., Meléndez A., O'Rourke E.J., Sato K., Sato M., Wang X., and Wu F.: Guidelines for monitoring autophagy in Caenorhabditis elegans. Autophagy, 11, 9–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Honjoh S., Yamamoto T., Uno M., and Nishida E.: Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature, 457, 726–730 (2009). [DOI] [PubMed] [Google Scholar]
- 26).Brenner S.: The genetics of Caenorhabditis elegans. Genetics, 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Lewis J.A. and Fleming J.T.: Basic culture methods. Methods Cell Biol., 48, 3–29 (1995). [PubMed] [Google Scholar]
- 28).Kang C. and Avery L.: Systemic regulation of autophagy in Caenorhabditis elegans. Autophagy, 5, 565–566 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Klionsky D.J., Abeliovich H., Agostinis P., Agrawal D.K., Aliev G., Askew D.S., Baba M., Baehrecke E.H., Bahr B.A., Ballabio A., Bamber B.A., et al.: Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy, 4, 151–175 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Meléndez A., Tallóczy Z., Seaman M., Eskelinen E.L., Hall D.H., and Levine B.: Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science, 301, 1387–1391 (2003). [DOI] [PubMed] [Google Scholar]
- 31).Sato M. and Sato K.: Degradation of paternal mitochondria. Science, 37, 1141–1144 (2011). [DOI] [PubMed] [Google Scholar]
- 32).Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K., Criollo A., Galluzzi L., Malik S.A., Vitale I., Michaud M., Madeo F., Tavernarakis N., and Kroemer G.: Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis., 1, e10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Longo V. D., Antebi A., Bartke A., Barzilai N., Brown-Borg H.M., Caruso C., Curiel T.J., de Cabo R., Franceschi C., Gems D., Ingram D.K.et al.: Interventions to slow aging in humans: are we ready? Aging Cell, 14, 497–510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Hansen M., Chandra A., Mitic L.L., Onken B., Driscoll M., and Kenyon C.: A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet., 4, e24 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Madeo F., Pietrocola F., Eisenberg T., and Kroemer G.: Caloric restriction mimetics: towards a molecular definition. Nat. Rev. Drug Discov., 13, 727–740 (2014). [DOI] [PubMed] [Google Scholar]
- 36).Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., Fussi H., et al.: Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol., 11, 1305–1314 (2009). [DOI] [PubMed] [Google Scholar]
- 37).Ravikumar B., Stewart A., Kita H., Kato K., Duden R., and Rubinsztein D. C.: Raised intracellular glucose concentrations reduce aggregation and cell death caused by mutant huntingtin exon 1 by decreasing mTOR phosphorylation and inducing autophagy. Hum. Mol. Genet., 12, 985–994 (2003). [DOI] [PubMed] [Google Scholar]
- 38).Wang Q., Liang B., Shirwany N.A., and Zou M.H.: 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One, 6, e17234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Weimer S., Priebs J., Kuhlow D., Groth M., Priebe S., Mansfeld J., Merry T.L., Dubuis S., Laube B., Pfeiffer A.F., Schulz T.J., Guthke R., Platzer M., Zamboni N., Zarse K., and Ristowa M.: D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat. Commun., 5, 3563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Giacco F. and Brownlee M.: Oxidative stress and diabetic complications. Circ. Res., 107, 1058–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Denzel M.S., Storm N.J., Gutschmidt A., Baddi R., Hinze Y., Jarosch E., Sommer T., Hoppe T., and Antebi A.: Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell, 156, 1167–1178 (2014). [DOI] [PubMed] [Google Scholar]
- 42).Cheong H. and Lindsten T.: Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc. Natl. Acad. Sci., 108, 11121–11126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Shintani T., Sakoguchi H., Yoshihara A., Izumori K., and Sato M.: D-Allulose, a stereoisomer of D-fructose, extends Caenorhabditis elegans lifespan through a dietary restriction mechanism: a new candidate dietary restriction mimetic. Biochem. Biophys. Res. Commun., 493, 1528–1533 (2017). [DOI] [PubMed] [Google Scholar]
- 44).Schulz T.J., Zarse K., Voigt A., Urban N., Birringer M., and Ristow M.: Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab., 6, 280–293 (2007). [DOI] [PubMed] [Google Scholar]
- 45).Barrientos C., Racotta R., and Quevedo L.: Glucosamine attenuates increases of intraabdominal fat, serum leptin levels, and insulin resistance induced by a high-fat diet in rats. Nutr. Res., 30, 791–800 (2010). [DOI] [PubMed] [Google Scholar]