Abstract
Salmon cartilage proteoglycan fractions have recently gained favor as ingredients of functional food and cosmetics. An optimal hot water method to extract proteoglycan from salmon cartilage has recently been developed. The extracted cartilage includes hyaluronan and collagen in addition to proteoglycan as counterparts that interact with each other. In this study, biochemical analyses and atomic force microscopical analysis revealed global molecular images of proteoglycan in the hot water extract. More than seventy percent of proteoglycans in this extract maintained their whole native structures. Hyaluronan purified from the hot water extract showed a distribution with high molecular weight similar to hyaluronan considered to be native hyaluronan in cartilage. The current data is evidence of the quality of this hot water cartilage extract.
Keywords: proteoglycan, glycosaminoglycan, chondroitin sulfate, hyaluronan, domain structure, atomic force microscopy
Abbreviations
PG,proteoglycan; ChS, chondroitin sulfate; GlcUA, glucuronic acid; Gal, galactose; Xyl, xylose; GAG, glycosaminoglycan; KS, keratan sulfate; G1 domain, globular domain 1; G2 domain, globular domain 2; G3 domain, globular domain 3; HA, hyaluronan; EGF, epidermal growth factor; GdnHCl, guanidine hydrochloride; HABP, HA binding protein; AFM, atomic force microscopy.
INTRODUCTION
Proteoglycan (PG) in cartilage consists almost entirely of aggrecan in salmon as well as in mammals.1),2) Aggrecan is composed of a core protein and multiple chondroitin sulfate (ChS) chains covalently linked to the core protein through the common glucuronic acid-galactose-galactose-xylose-serine (GluUAβ1-3Galβ1-3Galβ1-4Xylβ-O-Ser) linkages.3) The core protein of aggrecan is composed of functional domains including glycosaminoglycan (GAG) domains, i.e. a ChS domain and a keratan sulfate (KS) domain, and globular domains 1, 2, and 3 (G1, G2, and G3).4) The G1 domain, located at the amino terminus, interacts with hyaluronan (HA) and link protein, anchoring the extracellular matrix. The G3 domain, at the carboxyl terminus, includes epidermal growth factor (EGF)-like module(s). Salmon aggrecan was demonstrated to have all functional domains as in the case with mammals, except a KS domain.2) We also characterized the global molecular structure of salmon aggrecan.5) Hereafter, in this paper, the term “PG” refers to aggrecan.
Functions of salmon PG fractions have been studied in parallel with performing the structural analysis, although there are differences in efficacy according to the preparation methods of the PG fractions. Effects such as immune modulation, epidermal growth factor (EGF)-like activity on cultured cells, and anti-angiogenesis activity have been shown.6),7),8),9),10),11),12) In animal experimental models, therapeutic or prophylactic efficacy for colitis, photoaging, autoimmune encephalomyelitis, arthritis, and type 2 diabetes have been demonstrated.13),14),15),16),17),18),19),20) Salmon cartilage PG fractions are now used as ingredients of functional foods and cosmetics.
In PG preparation for industrial uses, simple and low cost methods are required and the obtained extract needs to be safe and preferably includes an intact, undenatured form of PG. Recently, an optimal hot water method to extract PG from salmon cartilage has been developed.21),22) In this study, we analyzed the PG and HA in the hot water extract from salmon cartilage in order to elucidate their molecular structure.
MATERIALS AND METHODS
Materials.
Salmon cartilage tips were purchased from Nihon Yakuhin (Saga, Japan). DEAE-Sephacel and Sepharose CL-4B were from GE Healthcare Japan (Tokyo, Japan). Mouse monoclonal antibody, 12/21/1-C-6, which recognizes the G1 domain of rat chondrosarcoma PG, was obtained from the Developmental Studies Hybridoma Bank of the University of Iowa (USA). Rabbit polyclonal antibody against the synthetic peptide for the internal sequence of human aggrecan G3 domain (2277DGHPMQFENWRPNQPDN2293), corresponding to the peptide sequence in the salmon aggrecan G3 domain (1182DGSPLGFENWRPNQPDN1198)2), was purchased from Affinity BioReagents, Inc. (Golden, USA). Rabbits were immunized with synthetic peptide for an EGF-like module of salmon nasal cartilage aggrecan (1069RDLCEPNQCGTGTCSVQDGI1088, which is at the N-terminal region of the G3 domain) to generate anti-salmon aggrecan EGF (Immuno-Biological Laboratories Co., Ltd., IBL, Fujioka, Japan).5) Peroxidase-conjugated goat anti-rabbit immunoglobulins as well as peroxidase-conjugated rabbit anti-mouse immunoglobulins were purchased from Dako Japan (Tokyo, Japan). Actinase E (protease from S. griseus) and cellulase (from A. niger, EC 3.2.1.4) were from Kaken Pharmaceutical Co. (Tokyo, Japan) and Sigma-Aldrich (St. Louis, USA), respectively. Chondroitin ABC lyase (from P. vulgaris, EC 4.2.2.4) and unsaturated disaccharide standards were obtained from Seikagaku Biobusiness Co. (Tokyo, Japan). All analytical grade reagents were obtained from commercial sources.
Preparation of hot water PG extract from salmon cartilage.
Hot water PG extract of salmon cartilage was prepared according to the methods previously reported by Kato et al.21) In brief, 10 g (wet weight) of cartilage tips were suspended in 50 mL of water and incubated for 4 h at 90 °C with stirring. After cooling, the sample was centrifuged at 5,000 × G for 30 min at 4 °C, and then the supernatant was collected by suction filtration using one sheet of Whatman filter paper No. 1 (GE Healthcare Japan). Pellets were resuspended in 5 mL of water, and centrifuged at 5,000 × G for 30 min at 4 °C. The second supernatant was combined with the initial supernatant as a hot water extract. The total volume of the resulting extract was 81 mL (377.5 mg of uronic acid, 200.1 mg of protein). This preparation was performed in the absence of protease inhibitors).
Purification of PG and HA from hot water extract of salmon cartilage.
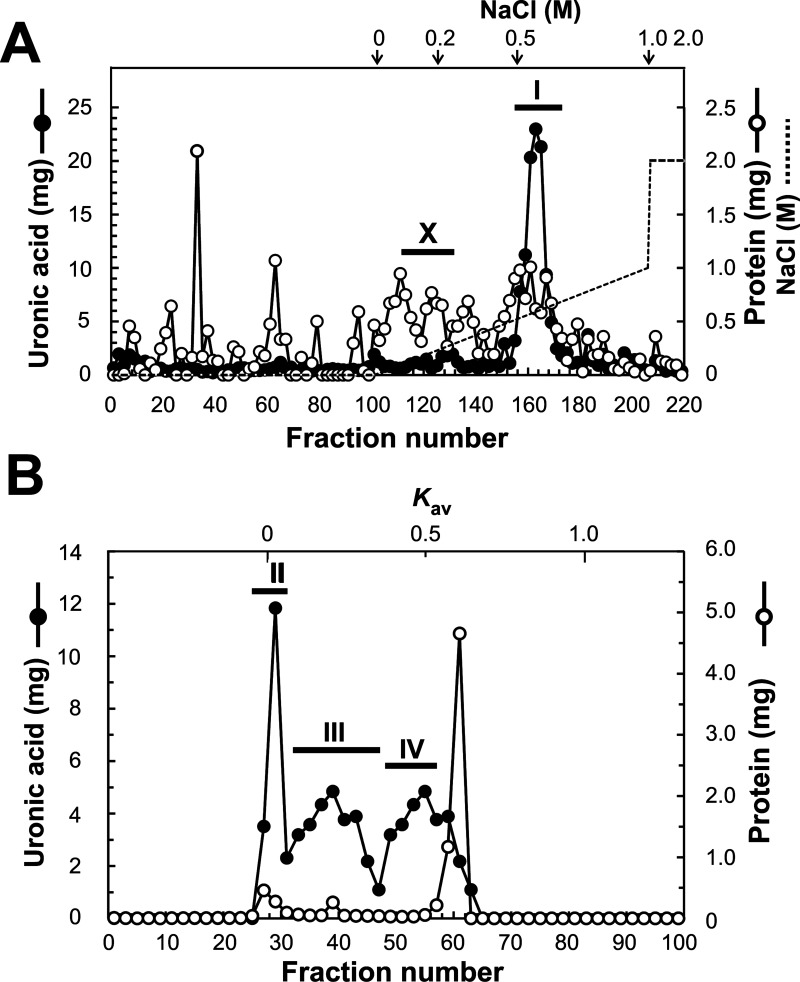
PG was purified in the same manner as in our previous report,5) in the presence of protease inhibitors (10 mM ethylenediaminetetraacetic acid, 5 mM benzamidine hydrochloride, 10 mM N-ethylmaleimide, and 1 mM phenylmethylsulfonyl fluoride). First, the hot water extract of salmon cartilage (139.8 mg by uronic acid) was separated by ion exchange DEAE-Sephacel column chromatography (1.8 cm φ × 15 cm) using the following eluents: 7 M urea in 50 mM Tris-HCl buffer (pH 7.4) with a linear gradient (0 to 1.0 M) of NaCl followed by 2.0 M of NaCl in the same buffer, at a flow rate of 0.8 mL/min. Nine-milliliter fractions were then collected. Fractions positive both for uronic acid and protein, and whose elution position corresponded to around 0.5 M NaCl were pooled as PG having ChS chains (ChS-PG) (Frs. 154–176 named pool I, in Fig. 1A). Fractions whose elution position corresponded to around 0.2 M NaCl were pooled as HA (Frs. 112–132 named pool X, in Fig. 1A). Pools I and X were desalted and concentrated by ultrafiltration, then pool I was further fractionated by gel filtration Sepharose CL-4B column chromatography (1.8 cm φ× 110 cm) using 4 M guanidine hydrochloride (GdnHCl) in 50 mM sodium acetate buffer (pH 6.0) as an eluent at a flow rate of 0.33 mL/min. Fractions (3.3 mL) were collected and uronic acid positive fractions were pooled as PG: pool II, Frs. 25–31; pool III, Frs. 37–41; pool IV, Frs. 53–57 (Fig. 1B). Pools were desalted and concentrated by ultrafiltration to be available for analysis.
Fig. 1. DEAE-Sephacel and Sepharose CL-4B column chromatography of hot water extract of salmon cartilage.
Hot water extract of salmon cartilage was fractionated by DEAE-Sephacel chromatography (A) followed by Sepharose CL-4B chromatography (B). Broken lines indicate the gradient curves of NaCl and arrows the specific NaCl concentrations. Solid circles, uronic acid content detected by carbazole sulfuric acid method; open circles, protein content detected by the method of Bradford (A) or protein content by monitoring the UV absorbance at 280 nm (B). Bars with Roman numerals indicate the fractions combined for recovery of material as: pool I (Frs. 154–176), pool II (Frs. 25–31), pool III (Frs. 37–41), pool IV (Frs. 53–57), and pool X (Frs. 112–132). Pool I from DEAE column chromatography was further fractionated by Sepharose CL-4B column chromatography and the resulting pools II, III, and IV were recovered and analyzed. Pool X was collected for the analysis of HA.
Analytical methods.
The carbazole sulfuric acid method23) was used to determine uronic acid content. The method of Bradford24) or monitoring the UV absorbance at 280 nm were performed to determine protein content.
Size analysis of purified PGs and HA was performed using HPLC according to our previous report.5),25)
GAG chains for the unsaturated disaccharide composition analysis were prepared by treating PG with actinase E, followed by cellulase (from A. niger) using its endo-β-xylosidase activity.26) The GAGs were exhaustively digested with chondroitin ABC lyase and then their unsaturated disaccharide compositions were determined by HPLC.27),28)
HA Assay Kit (PG Research, Tokyo, Japan) was used to measure HA content by ELISA-like assay using HA binding protein (HABP) according to the manufacturer’s instructions. The absorbance at 450 nm in each well was measured by a microplate spectrophotometer xMarkTM (Bio-Rad Laboratories, Inc. Tokyo, Japan). Size analysis of HA was performed per the method we reported previously.5) Briefly, pool X recovered by DEAE-Sephacel chromatography (Fig. 1A) was concentrated and fractionated by Shodex OHpak SB-804 HQ gel filtration column and HA content in each fraction (1.0 mL/fraction) was measured, then plotted as a chromatogram. HA purified from 4 M GdnHCl-extract of salmon cartilage5) was used as the size distribution control, which reflects native cartilage HA.
HPLC analyses.
HPLC analysis utilized a Hitachi ELITE LaChrom system with a model L-2420 UV-VIS detector and model L-2490 RI detector (Hitachi HighTechnologies, Tokyo, Japan).
Size was estimated with a Shodex OHpak SB-805 HQ column (8.0 mm φ × 300 mm, Shodex, Showa Denko K. K., Kawasaki, Japan) for PGs and a Shodex OHpak SB-804 HQ column (8.0 mm φ × 300 mm, Shodex, Showa Denko) for HA, both with 0.2 M NaCl as an eluent. Elution was performed at 40°C with a flow rate of 1.0 and 0.5 mL/min, respectively. Pullulans (Mr = 78.8 × 104, 40.4 × 104, 21.2 × 104, 11.2 × 104, 4.73 × 104, 2.28 × 104, 1.18 × 104, and 0.59 × 104, Shodex, Showa Denko) were used as size standards for the analysis of PGs, and HAs (Mr = 190 × 104, 80 × 104, 30 × 104, 10 × 104, and 4.1 × 104), courtesy of Denki Kagaku Kogyo (Tokyo, Japan) were used for the analysis of HA. HA octasaccharide (Mr=1623.6) and unsaturated HA disaccharide (Mr = 379.3) that we prepared previously29) were used as low molecular weight size standards. UV absorbance as well as differential refractive index (RI) were used to monitor the eluate. Details of our procedures for pretreatment of PG before HPLC to analyze core protein and GAG chains can be found in our previous report.5)
Unsaturated disaccharides were analyzed on a YMC-Pack Polyamine II column (4.6 mm φ × 250 mm, YMC Co., Kyoto, Japan). A linear gradient of NaH2PO4 from 16 to 478 mM over 60 min with a flow rate of 1.0 mL/min at 30 °C was used to elute disaccharides, which were monitored by UV absorbance at 232 nm.
Dotblot analysis using antibodies specific for domains of aggrecan.
As in our previous report, reduced-alkylated PG (0.2 μg of protein), was blotted to a PVDF membrane (Millipore, Billerica, USA) with a slot blotter (Scie-Plas, Cambridge, UK), followed by analysis by probing with antibodies against aggrecan G1 domain, aggrecan G3 domain, or salmon aggrecan EGF-like module.5)
AFM imaging.
As we previously reported5) atomic force microscopy (AFM) imaging was performed in the same manner as Yeh et al.30) and Ng et al.31) with the exception of using a Nanocute AFM system (SII NanoTechnology, Tokyo, Japan) with SSS-NCH probes (Nano World, Neuchâtel, Switzerland).
RESULTS AND DICSUSSION
Purified PG and HA from hot water extract of salmon cartilage.
PG was purified from hot water extract of salmon cartilage by DEAE chromatography followed by CL-4B chromatograpy (Fig. 1). In DEAE column chromatography, fractions 112–132 were collected as pool X, which is expected to contain HA (Fig. 1A). Combined fractions 154–176 of DEAE column chromatography were expected to contain ChS-PG, and they were collected as pool I (Fig. 1A). Pool I was further fractionated by CL-4B column chromatography and three uronic acid positive peaks and two protein positive peaks were detected. The last peak detected by absorbance at 280 nm was considered to be mainly due to the absorption of N-ethylmaleimide used as one of the protease inhibitors. After CL-4B column chromatography, that is, the final step of purification, recovery of PG was calculated as 86.1 %, based on the uronic acid content; percentage of the total uronic acid content of the three peaks, 120.4 mg, to initial uronic acid content, 139.8 mg, before DEAE column chromatography. Purification fold was also calculated as 26.6, based on the protein content; total protein content of the two peaks, 2.79 mg, to initial protein content, 74.1 mg. A major part (73.6 %) of PGs was eluted within the former two uronic acid positive peaks, where the high molecular PGs from 4 M GdnHCl-extract always elute.5) Fractions 25–31 (pool II), 37–41 (pool III), and 53–57 (pool IV), corresponding to the top of each peak, were collected as PG (intact or fragmented) and used for the following analyses.
Molecular size distribution of purified PG from hot water extract of salmon cartilage.
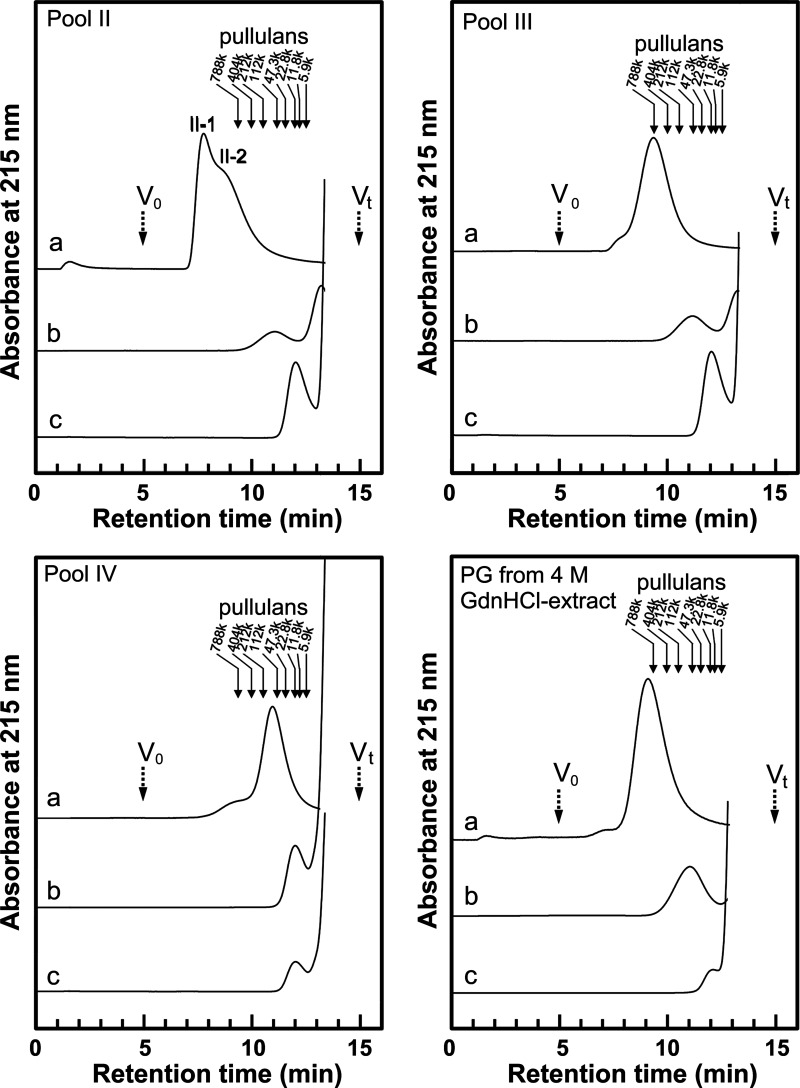
Determining the absolute molecular size distribution of high molecular weight PGs such as aggrecan, or even its GAG moiety, is impossible as there are no appropriate size standards because of GAG heterogeneity. To estimate relative size distributions of PGs in hot water extract (whole molecule, core protein, and single GAG sizes) we performed gel filtration HPLC with pullulans as size standards (Fig. 2). Table 1 shows relative size distributions (A–C) and the values (D, E) calculated from them. For comparison, salmon PG purified from 4 M GdnHCl-extract, which we studied previously,5) was analyzed simultaneously with pools II, III, and IV. We calculated the numbers of GAG chains (E) in the PGs using data, (A), (B), (C), from Table 1 as follows: First, the total Mr of the GAG chains was obtained by subtracting the estimated Mr of core protein (B) from the estimated Mr of the non-treated PG (A). Then, the numbers of GAG chains in the PGs were obtained by dividing the total Mr of the GAG chains by Mr of one GAG chain (C). Thus, the numbers of GAG chains in the PG is shown: (A)−(B)/(C), i.e., (MrPG−Mrcore)/MrGAG. Pools II and III from hot water extract, as well as PGs from 4 M GdnHCl-extract showed similar size distributions of core protein and GAG. However, size distribution of non-treated PGs in pool II was larger than those of PGs in pool III and 4 M GdnHCl-extract, therefore, the number of GAGs of PGs in pool II were calculated to be greater than those in the latter. It is conceivable that the size distributions of non-treated PGs observed in HPLC sometimes do not reflect the actual size distribution of PG monomers because of PG aggregation. Especially, in pool II, PGs may be partially aggregated into a peak (II-1) with a shoulder (II-2) in a high molecular area. Although PGs (non-treated and core protein) in pool IV were detected in remarkably smaller molecular sizes than those in pools II, III, and 4 M GdnHCl-extract, the size distribution of GAGs was equivalent to the latter. The number of GAG chains in PGs in pool IV were much less than those of the other pools.
Fig. 2. Gel filtration HPLC of PGs purified from hot water extract of salmon cartilage.
PGs in pools II, III, and IV (Fig. 1), and PG purified from 4 M GdnHCl-extract were analyzed by Shodex OHpak SB-805 HQ gel filtration column before treatment (a) or after treatment with LiOH followed by chondroitin ABC lyase (b) or after treatment with actinase E (c). Solid arrows indicate the elution positions of size standards of pullulan with defined approximate molecular weight. Dashed arrows indicate the void volume (V0) and total column volume (Vt). II-1 and II-2 indicate the peak and the shoulder, respectively.
Table 1.
Molecular size estimation of PGs purified from hot water extract (A, B, C) and the number of repeating disaccharide units (D) and the number of GAG chains calculated from them (E).
| Calculated molecular weight | PG from hot water extract |
PG from 4 M GdnHCl-extractc |
||
|---|---|---|---|---|
| Pool II | Pool III | Pool IV | ||
| (A) Non-treated | 700,000–8,600,000 II-1 (4,100,000*)d II-2 (1,520,000*) |
35,000–7,500,000 (810,000*) |
1,000–533,000 (138,000*) |
85,000–5,300,000 (1,100,000*) |
| (B) Deglycosylateda (core protein) |
2,750–540,000 (117,000*) |
2,700–650,000 (100,000*) |
N.D.–147,000 (23,700*) |
3,750–2,000,000 (123,000*) |
| (C) Peptide-degradedb (GAG chain) |
N.D.–112,000 (23,100*) |
N.D.–130,000 (23,100*) |
160–142,000 (23,500*) |
50–159,000 (20,000*) |
| (D) Number of repeating disaccharide units | N.D.–280.2 (56.5**) |
N.D.–325.5 (56.5**) |
N.D.–355.7 (57.5**) |
N.D.–398.5 (48.7**) |
| (E) Number of GAG chains | 72.0–N.D. II-1 (172.4**) 72.0–N.D. II-2 (60.7**) |
52.7–N.D. (30.7**) |
2.7–N.D. (4.9**) |
20.8–1562.5 (48.9**) |
PG samples were analyzed by gel filtration HPLC using a Shodex OHpak SB-805 HQ column. Various sizes of pullulan were used as size standards. PG, proteoglycan. Pools II, III, and IV were obtained in CL-4B chromatography (see Fig. 1). aDeglycosylation by LiOH treatment followed by chondroitin ABC lyase digestion. bPeptide-degradation by treatment with Actinase E. cThe same sample as the one used in Carbohydr. Polym., 103, 538–549 (2014).5) dII-1 and II-2 are indicated in Fig. 2. *Molecular sizes of material eluting at the peak position and **the values calculated from them.
Sugar chain analyses of purified PG from hot water extract of salmon cartilage.
Previously our cDNA analysis showed that salmon PG has no KS domain.2) Also, possible monosaccharide components of KS were detected in only one-tenth of those in bovine PG.5) Therefore, we only performed unsaturated disaccharide composition analysis after chondroitin ABC lyase to focus on the ChS structure. Similar compositions were seen in pools II, III, and IV from hot water extract, and PG from 4 M GdnHCl-extract (Table 2) although that in pool IV showed slightly less nonsulfated disaccharide units (ΔDi-0S), indicating that in cartilage from the same species, hot water extraction little affects the composition of sugar chains.
Table 2.
Unsaturated disaccharide compositions of GAG moiety of PG purified from hot water extract.
| Unsaturated disaccharide |
Unsaturated disaccharide (%) | |||
|---|---|---|---|---|
| PG from hot water extract | PG from 4 M GdnHCl-extract |
|||
| Pool II | Pool III | Pool IV | ||
| ΔDi-0Sa | 16.5 | 14.0 | 6.6 | 11.5 |
| ΔDi-6S | 61.2 | 61.9 | 57.8 | 65.6 |
| ΔDi-4S | 21.1 | 23.2 | 34.4 | 21.8 |
| ΔDi-diSD | 1.1 | 0.8 | 1.0 | 1.1 |
| ΔDi-diSE | 0.1 | 0.1 | 0.2 | N.D. |
| ΔDi-triS | N.D.b | N.D. | N.D. | N.D. |
Following digestion of GAGs with chondroitin ABC lyase unsaturated disaccharides were analyzed by HPLC on a YMC-Pack Polyamine II column. The percentage of each detected unsaturated disaccharide to total unsaturated disaccharides was calculated. PG, proteoglycan. Pools II, III, and IV were obtained in CL-4B chromatography (see Fig. 1). aΔDi-0S, ΔGlcUAβ1–3GalNAc; ΔDi-4S, ΔGlcUAβ1–3GalNAc(4S); ΔDi-6S, ΔGlcUAβ1–3GalNAc(6S); ΔDi-diSD, ΔGlcUA(2S)β1–3GalNAc(6S); 2S, 4S, and 6S, represent 2-O-sulfate, 4-O-sulfate, and 6-O-sulfate. bNot detected.
Immunological analysis of purified PG from hot water extract of salmon cartilage.
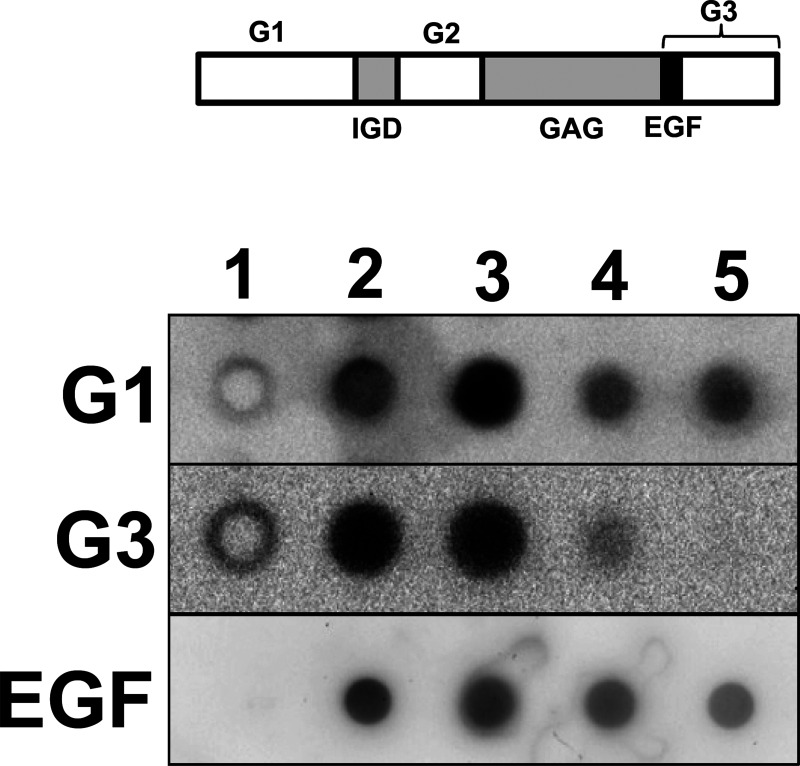
Pools II and III from hot water extract of salmon cartilage PG were shown to have G1, and G3 domains and an EGF-like module by dot blot analysis using antibodies against them (Fig. 3). However, signal intensities of pool III were weaker than those of pool II and PG from 4 M GdnHCl-extract, which had similar signal intensity. In pool IV there was no signal responding to the antibody against the internal region of the G3 domain. However pool IV still had the reactivity to the antibody against G1 domain at the N-terminus and the EGF-like module standing at the N-terminal region of the G3 domain. These results suggested that pool IV contained fragmented PGs that have a G1 domain and/or EGF-like module but do not have an intact G3 domain.
Fig. 3. Immunological analysis of PGs in hot water extract of salmon cartilage.
Reduced-alkylated proteins (0.2 μg each) were dot-blotted and probed with antibodies to aggrecan G1 domain, G3 domain, or salmon aggrecan EGF-like module. 1, H2O; 2, PG purified from 4 M GdnHCl-extract of salmon nasal cartilage. 3, 4, 5: PG purified from hot water extract of salmon cartilage (pools II, III, IV in Fig. 1B, respectively). Domain structure of salmon cartilage aggrecan core protein (GenBank ID, BAJ61837) is shown in upper bar. G1, globular domain 1; IGD, interglobular domain; G2, globular domain 2; GAG, glycosaminoglycan attachment domain; EGF, EGF-like module; G3, globular domain 3.
Molecular images of PGs.
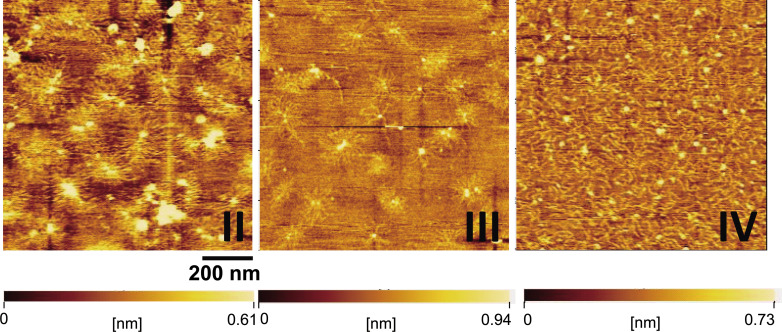
We found that molecular images of PG monomers in pools II and III from hot water extract by AFM were similar to those of PG monomers from 4 M GdnHCl-extract considered as native PG that we previously studied (comparative data can be found in our previous reports5),32)) (Fig. 4). The AFM images demonstrated that not only PG monomers in pool II but also those in pool III retained intact structure as we expected from size analysis and immunological analysis. In the AFM image of pool IV, what appear to be disassembled strings are found diffusely throughout the field. These are fragmented PGs with incomplete core protein but mostly intact GAG chains. This observation supports the results of the gel filtration HPLC analysis (Table 1) and the immunological analysis (Fig. 3). Together with the biochemical analytical data, the AFM images supported the idea that PGs in pools II and III from hot water extract have nearly the same quality as native PG.
Fig. 4. AFM images of PGs purified from hot water extract of salmon cartilage.
II, III, IV: PG in pools II, III, and IV (Fig. 1B), respectively. Magnification bar represents 200 nm. The color bars indicate ranges of height and error signal of amplitude change in the image area.
Content and size distribution of HA in hot water extract of salmon cartilage.
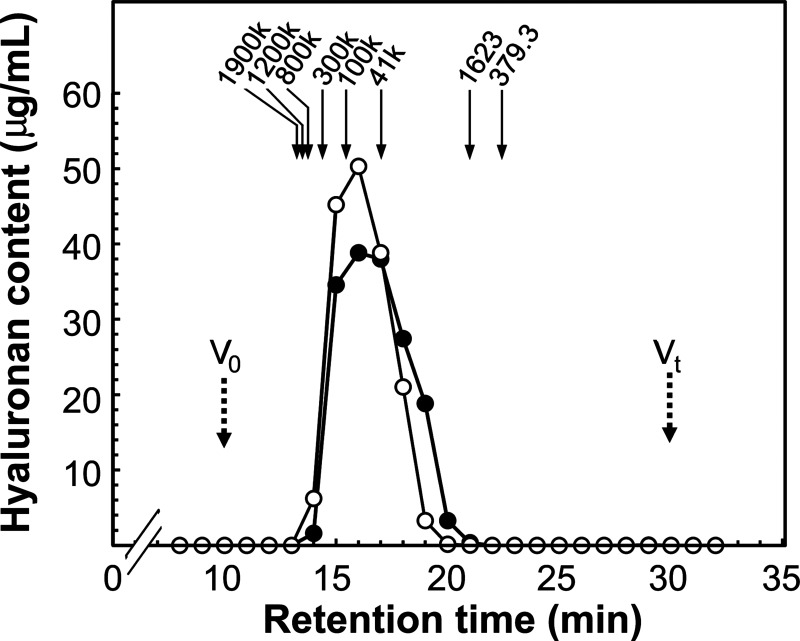
In extracellular matrices PGs function with interacting HA or other molecules, rather than PG monomers individually.3),33),34) Materials containing HA and related molecules together with PG, would be therefore, more valuable as materials for future applications in various fields including foods and cosmetics. We determined the HA amount in hot water extract of salmon cartilage. HA was purified as pool X by DEAE chromatography (Fig. 1A) and the amount was measured by ELISA like assay. The total HA content in pool X of DEAE chromatography (Fig. 1A) was 13.7 mg, and the HA content in cartilage hot water extract was calculated to be 96.2 μg (by ELISA like assay) per 1 mg total uronic acid (by the carbazole sulfuric acid method). Size distribution of HA in the hot water extract of salmon cartilage was found to be broad, from 3,400 to 550,000 < with the peak at 65,000 (Fig. 5). This distribution was similar to that of HA in 4 M GdnHCl-extract of salmon, from 7,300 to 550,000 < with the peak at 65,000. Therefore, hot water extract is likely to be an extremely useful material containing high molecular weight HA.
Fig. 5. Gel filtration HPLC of HA contained in hot water extract of salmon cartilage.
Pool X of DEAE chromatogram (Fig. 1A) was concentrated and fractionated by Shodex OHpak SB-804 HQ gel filtration column. Fractions were collected and HA content in each fraction was measured by ELISA like assay using HABP. Solid circles, HA fraction from DEAE chromatography of hot water extract of salmon nasal cartilage (pool X); open circles, HA fraction from DEAE chromatography of 4 M GdnHCl-extract of salmon nasal cartilage as a control. Arrows indicate the elution positions of size standards of HA. Dashed arrows indicate the void volume (V0) and total column volume (Vt).
In conclusion, hot water extract of salmon cartilage did contain a high proportion of intact PG with complete protein domain structure as well as HA with high molecular weight. Heating biological samples to high temperatures has been thought to cause protein denaturation or degradation. However, it was demonstrated that optimal conditions of hot water extraction did not affect the quality of PG and HA. The condition is considered to be preferable in that probably proteases are inactive under high temperature. This simple extraction method requiring only water with no toxic compounds will contribute to the food and cosmetic research and industry.
ACKNOWLEDGMENTS
This work was supported by the Regional Innovation Strategy Support Program (City Area Type) and Grants-in-Aid for Scientific Research (JSPS KAKENHI Grant Nos. JP26462470 for I. K., and JP23650253, JP15H03940 for T. M.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1).Watanabe H., Yamada Y., and Kimata K.: Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J. Biochem., 124, 687–693 (1998). [DOI] [PubMed] [Google Scholar]
- 2).Kakizaki I., Tatara Y., Majima M., Kato Y., and Endo M.: Identification of proteoglycan from salmon nasal cartilage. Arch. Biochem. Biophys., 506, 58–65 (2011). [DOI] [PubMed] [Google Scholar]
- 3).Kjellén L. and Lindahl U.: Proteoglycans: structures and interactions. Annu. Rev. Biochem., 60, 443–475 (1991). [DOI] [PubMed] [Google Scholar]
- 4).Kiani C., Chen L., Wu Y.J., Yee A.J., and Yang B.B.: Structure and function of aggrecan. Cell Res., 12, 19–32 (2002). [DOI] [PubMed] [Google Scholar]
- 5).Kakizaki I., Mineta T., Sasaki M., Tatara Y., Makino E., and Kato Y.: Biochemical and atomic force microscopic characterization of salmon nasal cartilage proteoglycan. Carbohydr. Polym., 103, 538–549 (2014). [DOI] [PubMed] [Google Scholar]
- 6).Sashinami H., Takagaki K., and Nakane A.: Salmon cartilage proteoglycan modulates cytokine responses to Escherichia coli in mouse macrophages. Biochem. Biophys. Res. Commun., 351, 1005–1010 (2006). [DOI] [PubMed] [Google Scholar]
- 7).Kashiwakura I., Takahashi K., and Takagaki K.: Application of proteoglycan extracted from the nasal cartilage of salmon heads for ex vivo expansion of hematopoietic progenitor cells derived from human umbilical cord blood. Glycoconj. J., 24, 251–258 (2007). [DOI] [PubMed] [Google Scholar]
- 8).Kashiwakura I., Takahashi K., Monzen S., Nakamura T., and Takagaki K.: Ex vivo expansions of megakaryocytopoiesis from placental and umbilical cord blood CD34+ cells in serum-free culture supplemented with proteoglycans extracted from the nasal cartilage of salmon heads and the nasal septum cartilage of whale. Life Sci., 82, 1023–1031 (2008). [DOI] [PubMed] [Google Scholar]
- 9).Yoshino H., Takahashi K., Monzen S., and Kashiwakura I.: Effects of proteoglycan extracted from nasal cartilage of salmon heads on maturation of dendritic cells derived from human peripheral blood monocytes. Biol. Pharm. Bull., 33, 311–315 (2010). [DOI] [PubMed] [Google Scholar]
- 10).Fukuyama A., Tanaka K., Kakizaki I., Kasai K., Chiba M., Nakamura T., and Mizunuma H.: . Anti-inflammatory effect of proteoglycan and progesterone on human uterine cervical fibroblasts. Life Sci., 90, 484–488 (2012). [DOI] [PubMed] [Google Scholar]
- 11).Ito G., Kobayashi T., Takeda Y., and Sokabe M.: Proteoglycan from salmon nasal cartridge promotes in vitro wound healing of fibroblast monolayers via the CD44 receptor. Biochem. Biophys. Res. Commun., 456, 792–798 (2015). [DOI] [PubMed] [Google Scholar]
- 12).Kobayashi T., Kakizaki I., Nozaka H., and Nakamura T.: . Chondroitin sulfate proteoglycans from salmon nasal cartilage inhibit angiogenesis. Biochem. Biophys. Rep., 9, 72–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ota S., Yoshihara S., Ishido K., Tanaka M., Takagaki K., and Sasaki M.: . Effects of proteoglycan on dextran sulfate sodium-induced experimental colitis in rats. Dig. Dis. Sci., 53, 3176–3183 (2008). [DOI] [PubMed] [Google Scholar]
- 14).Mitsui T., Sashinami H., Sato F., Kijima H., Ishiguro Y., Fukuda S., Yoshihara S., Hakamada K., and Nakane A.: Salmon cartilage proteoglycan suppresses mouse experimental colitis through induction of Foxp3+ regulatory T cells. Biochem. Biophys. Res. Commun., 402, 209–215 (2010). [DOI] [PubMed] [Google Scholar]
- 15).Goto M., Ito S., Kato Y., Yamazaki S., Yamamoto K., and Katagata Y.: Anti-aging effects of extracts prepared from salmon nasal cartilage in hairless mice. Mol. Med. Rep., 4, 779–784 (2011). [DOI] [PubMed] [Google Scholar]
- 16).Goto M., Yamazaki S., Kato Y., Yamamoto K., and Katagata Y.: . Anti-aging effects of high molecular weight proteoglycan from salmon nasal cartilage in hairless mice. Int. J. Mol. Med., 29, 761–768 (2012). [DOI] [PubMed] [Google Scholar]
- 17).Sashinami H., Asano K., Yoshimura S., Mori F., Wakabayashi K., and Nakane A.: . Salmon proteoglycan suppresses progression of mouse experimental autoimmune encephalomyelitis via regulation of Th17 and Foxp3+ regulatory T cells. Life Sci., 91, 1263–1269 (2012). [DOI] [PubMed] [Google Scholar]
- 18).Asano K., Yoshimura S., and Nakane A.: . Alteration of intestinal microbiota in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. PLoS One, 8, e75008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Yoshimura S., Asano K., and Nakane A.: . Attenuation of collagen-induced arthritis in mice by salmon proteoglycan. Biomed. Res. Int., 2014, 406453–406461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hirose S., Asano K., and Nakane A.: . Attenuation of obesity-induced inflammation in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. Biochem. Biophys. Res. Commun., 484, 480–485 (2017). [DOI] [PubMed] [Google Scholar]
- 21).Hirosaki University and Sunstar Inc., Kato Y.: Method for Mass Preparation of Proteoglycan, Japan Patent ; 6071558, (2017). [Google Scholar]
- 22).Suekawa Y., Goto M., Yamamoto K., and Kato Y.: Development of hyaluronic acid-collagen-proteoglycan complex (Hyaluco PG) by hot water extraction from salmon nasal cartilage. J. Appl. Glycosci., 62, 101–106 (2015). [Google Scholar]
- 23).Bitter T. and Muir H.M.: . A modified uronic acid carbazole reaction. Anal. Biochem., 4, 330–334 (1962). [DOI] [PubMed] [Google Scholar]
- 24).Bradford M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- 25).Iwafune M., Kakizaki I., Nakazawa H., Nukatsuka I., Endo M., and Takagaki K.: . A glycomic approach to proteoglycan with a two-dimensional polysaccharide chain map. Anal. Biochem., 325, 35–40 (2004). [DOI] [PubMed] [Google Scholar]
- 26).Takagaki K., Iwafune M., Kakizaki I., Ishido K., Kato Y., and Endo M.: . Cleavage of the xylosyl serine linkage between a core peptide and a glycosaminoglycan chain by cellulases. J. Biol. Chem., 277, 18397–18403 (2002). [DOI] [PubMed] [Google Scholar]
- 27).Sugahara K., Okumura Y., and Yamashina I.: The Engelbreth-Holm-Swarm mouse tumor produces undersulfated heparan sulfate and oversulfated galactosaminoglycans. Biochem. Biophys. Res. Commun., 162, 189–197 (1989). [DOI] [PubMed] [Google Scholar]
- 28).Yoshida K., Miyauchi S., Kikuchi H., Tawada A., and Tokuyasu K.: . Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid chromatography. Anal. Biochem., 177, 327–332 (1989). [DOI] [PubMed] [Google Scholar]
- 29).Kakizaki I., Ibori N., Kojima K., Yamaguchi M., and Endo M.: Mechanism for the hydrolysis of hyaluronan oligosaccharides by bovine testicular hyaluronidase. FEBS J., 277, 1776–1786 (2010). [DOI] [PubMed] [Google Scholar]
- 30).Yeh M.L. and Luo Z.P.: The structure of proteoglycan aggregate determined by atomic force microscopy. Scanning 26, 273–276 (2004). [DOI] [PubMed] [Google Scholar]
- 31).Ng L., Grodzinsky A.J., Patwari P., Sandy J., Plaas A., and Ortiz C.: . Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J. Struct. Biol., 143, 242–257 (2003). [DOI] [PubMed] [Google Scholar]
- 32).Kakizaki I., Miura A., Ito S., Mineta T., Jin Seo H., and Kato Y.: . Characterization of proteoglycan and hyaluronan in water-based delipidated powder of salmon cartilage. J. Appl. Glycosci., 62, 115–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Lindahl U. and Höök M.: Glycosaminoglycans and their binding to biological macromolecules. Annu. Rev. Biochem., 47, 385–417 (1978). [DOI] [PubMed] [Google Scholar]
- 34).Aspberg A.: . The different roles of aggrecan interaction domains. J. Histochem. Cytochem., 60, 987–996 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]