Abstract
Objectives
Multi-system inflammatory syndrome in children (MIS-C) is a post-viral inflammatory vasculopathy of children and adolescents following Covid-19 infection. Since the incidence of SARS-CoV-infections has been increasing in Germany since October 2020, we observe an increasing number of children presenting with MIS-C.
Design
We present detailed clinical characteristics of a cohort of nine children with MIS-C admitted to a tertiary PICU at the University Hospital of Cologne between March 2020 and February 2021.
Results
The clinical sings and symptoms are largely in line with recent reports. All but one patient had positive SARS-CoV-2 antibodies. Latency form infection to MIS-C was 4–6 weeks. Two children presented with unusual findings: A girl had encephalomyelitis and a boy developed MIS-C side to side with acute leukemia.
Conclusion
MIS-C has been increasing in Germany paralell to SARS-CoV-2 infections. Rarely, unuasual findings may be associated with MIS-C.
Keywords: MIS-C, PIMS, SARS-CoV-2 infection, Children, COVID-19
Multi-system inflammatory syndrome in children (MIS-C) is a post-viral inflammatory vasculopathy of children and adolescents following coronavirus disease 2019 (COVID-19). Since its first description in May 2020 (Verdoni et al., 2020), specific diagnostic criteria have been issued by the Centers for Disease Control and Prevention (2020) and the World Health Organization (2020). Recently, case series have been reported from the UK (Davies et al., 2020) and Spain (Garcia-Salido et al., 2020), and a systematic review has summarized numerous cases of MIS-C from European, American and Asian countries (Radia et al., 2020). In Germany, the incidence of COVID-19 in Spring 2020 was low, with a peak of 6500 infections on 2 April 2020. Furthermore, the numbers were decreasing rapidly, with <2000 infections on 24 April 2020 (https://corona.rki.de). Consequently, patients with MIS-C were rare in Germany during the first wave of the pandemic. However, since then, there has been a marked increase in the number of children hospitalized with COVID-19 [1056 children up to February 2021 (https://dgpi.de/covid-19-survey-update-2021)], and paediatric intensive care units (PICUs) in Germany have been confronted with increasing numbers of children with MIS-C (137 cases up to February 2021) (https://dgpi.de/pims-survey-07-2020).
We present detailed clinical characteristics of a cohort of nine children with MIS-C admitted to a single tertiary PICU at the University Hospital of Cologne, Germany between March 2020 and February 2021. Of note, only a single case of MIS-C occurred prior to September 2020. To rule out the possibility that previous patients with MIS-C may have gone unrecognized, PICU admissions from January to September 2020 were assessed retrospectively but no additional patients with MIS-C were identified. This is in line with a recent systematic review (Radia et al., 2020) which included numerous patients from the USA, France and the UK, but only one patient from Germany. Moreover, in a nationwide MIS-C survey in Germany and Austria, only 43 children with MIS-C were reported within the first 23 weeks of 2020, compared with 94 children within the following 11 weeks. Compared with 74 cases from Spain between 1 March and 15 June 2020 and 78 cases from the UK between 1 April and 10 May, the numbers from Germany were considerably lower.
Interestingly, all but one child came from families that had immigrated from Turkey, Syria, Iran, India and Eastern Europe. Similar findings were reported previously in a small case series of three teenagers with MIS-C (Ng et al., 2020). Furthermore, a recent study from Norway demonstrated higher risk for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in immigrants, presumably caused by social disparity (Indseth et al., 2021).
Clinical signs and symptoms as well as laboratory findings for the patients in our case series are largely in line with recent reports (Table 1 ). All but one child had positive SARS-CoV-2 IgA and IgG antibodies, and three children had positive polymerase chain reaction tests. Of note, in our case series, latency between COVID-19 and MIS-C was 4–6 weeks in most patients. All patients had persistent high fever and signs of hyperinflammation, with markedly elevated levels of C-reactive protein and ferritin but less pronounced levels of procalcitonin. Gastrointestinal symptoms were common and were the cause of hospital admission in three patients. Additionally, cardiac involvement was observed frequently, indicated by increased levels of troponin T and abnormal echocardiographic findings, such as pericardial effusion, impaired fractional shortening or coronary anomalies.
Table 1.
Characteristics of children with multi-system inflammatory syndrome.
| Hospital admission | Mar 2020 | Jan 2021 | Nov 2020 | Dec 2020 | Dec 2020 | Sep 2020 | Dec 2020 | Nov 2020 | Feb 2021 |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | Male | Male | Female | Male | Male |
| Age (years) | 1 | 14 | 6 | 14 | 15 | 15 | 12 | 16 | 16 |
| Comorbidities | None | None | None | Obesity | None | Leukaemia | None | SLE | None |
| Documented SARS-CoV-2 infection | No | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Latency SARS-CoV-2 infection – onset of symptoms | Unknown | Unknown | 4 weeks | 6 weeks | 6 weeks | 6 weeks | Unknown | 1 week | 5 weeks |
| SARS-CoV-2 PCR (nasopharyngeal swab) | Negative | Positive | Negative | Negative | Negative | Positivee | Negative | Positive | Negative |
| SARS-CoV-2 spike protein antibody | IgA pos, IgG pos | ND | IgA pos, IgG pos2 | IgA pos, IgG pos | IgA pos, IgG pos | IgA pos, IgG pos | IgA pos, IgG pos | IgA pos, IgG pos | IgA pos, IgG pos |
| Suspected diagnosis on admission | Encephalo-myelitis | Thrombosis | Gastro-enteritis | Gastro-enteritis | Septicaemia | Septicaemia | Gastro-enteritis | Nephrotic syndrome | Fever of unknown origin |
| Symptoms at hospitalization | |||||||||
| Fever | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Gastrointestinala | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes |
| Fatigue | No | No | Yes | Yes | Yes | No | Yes | No | No |
| Hypotension | No | No | Yes | Yes | Yes | No | Yes | No | No |
| Respiratory | No | No | Dyspnoea | No | No | No | No | Dyspnoea | No |
| Conjunctivitis | No | No | No | Yes | Yes | No | Yes | No | Yes |
| Rash | No | No | No | Yes | No | No | No | Yes | No |
| Other | Coma, cardiac arrest | Back pain | No | No | Headache | Sore throat, hip pain | Seizure | Oedema | Scrotal pain |
| Laboratory and imaging results | |||||||||
| Neutrophil count x1E9/L (admission, max) | 24.5 (27.0) | 17.3 (17.3) | 8.5 (24.3) | 16.3 (27.1) | 15.8 (18.3) | 8.8 (19.5) | 16.0 (16.0) | 17.5 (21.2) | 8.2 (10.8) |
| Platelet count x1E9/L (admission, min) | 254 (42) | 184 (142) | 150 (73) | 236 (95) | 134 (111) | 59 (3) | 210 (210) | 297 (160) | 210 (210) |
| CRP mg/L (admission, max) | <0.6 (217) | 42 (165) | 62 (173) | 328 (333) | 128 (128) | 44 (376) | 181 (181) | 0.8 (185) | 206 (206) |
| PCT μg/L (admission, max) | 0.1 (96) | 0.1 (0.2) | 2.1 (2.6) | 2.3 (2.3) | 5.6 (6.9) | 5.0 (17.8) | 1.8 (1.8) | 0.1 (16) | 0.5 (0.5) |
| LDH (U/L) (admission, max) | 964 (2714) | 226 (317) | 300 (449) | 311 (311) | 254 (263) | 830 (4068) | 193 (218) | 281 (455) | 410 (438) |
| Ferritin μg/L (admission, max) | ND | 525 (525) | 364 (364) | 1281 (1341) | 680 (680) | 789 (32792) | 654 (654) | ND (1014) | ND (110) |
| D-dimer mg/L (admission,max) | 4.30 (4.30) | 5.72 (5.72) | 4.90 (10.20) | 6.23 (6.23) | 2.24 (2.24) | 6.77 (7.64) | 1.22 (2.27) | ND (8.40) | ND (1.72) |
| Troponin T μg/L (admission, max) | 4.400 (10.900) | <0.003 (0.052) | ND (0.025) | 0.129 (0.129) | 0.095 (0.354) | ND | 0.350 (0.410) | ND | ND (0.179) |
| Echocardiography | Pericardial effusion, FS 35% | Increased coronary flares | Normal | Pericardial effusion, increased coronary flares | Coronary aneurysm, FS 28% | Normal | Pericardial effusion, FS 15% | Pericardial effusion | |
| Chest X-ray | Pulmonary oedema | ND | ARDS | ARDS | Normal | ARDS | ND | Atelectasis | |
| CT | ND | Pulmonary embolism | ARDS | ND | ND | ARDS | ND | ARDS | |
| Drug therapy | |||||||||
| Intravenous immunoglobin | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Steroids | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Aspirin | No | No | No | Yes | Yes | No | Yes | No | No |
| Anticoagulation | No | Yes therapeutic | Yes prophylactic | Yes prophylactic | Yes prophylactic | No | Yes prophylactic | Yes prophylactic | Yes prophylactic |
| Respiratory support | |||||||||
| HFNC | No | No | Yes | Yes | Yes | Yes | No | Yes | No |
| MV | Yes | No | Yes | No | No | Yes | No | Yes | No |
| ECMO | No | No | No | No | No | Yes (V-V) | No | No | No |
| Latency to transfer to PICU | Directly from ER | 1 day | 5 days | Directly from ER | Directly from ER | 7 days | Directly from ER | 17 days | No PICU |
| Infection with non-SARS-CoV-2 pathogen | Yes adenovirus | No | No | No | No | Yes candidaemia, aspergillosis | No | Yes CMV | No |
| Discharged healthy | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| Discharged with sequelae | Yes (minimal conscious state) | No | Yes | No | No | No | No | Yes, fatigue | No |
| Died | Yes, 4 months after discharge (cause?) | No | Aneurysma left coronary | No | No | Yes aspergillosis | No | No | No |
ARDS, acute respiratory distress syndrome; CMV, cytomegalovirus; CRP, C-reactive protein; CT, computer tomography; ECMO, extracorporeal membrane oxygenation; ER, emergency room; HFNC, high flow nasal cannula; Ig, immunoglobulin; LDH, lactate dehydrogenase; MV, mechanical ventilation; ND, not done; PCR, polymerase chain reaction; PCT, procalcitonin; PICU, paediatric intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; V-V, veno-venous ECMO.
Diarrhoea, vomiting, abdominal pain.
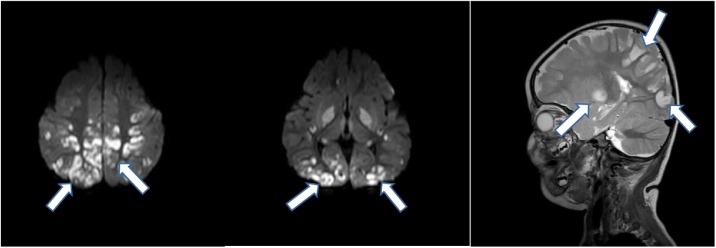
Two children presented with unusual findings. A girl aged 11 months was admitted after cardiac arrest at home. She had no evidence of any previous illness except for self-limited diarrhoea a few days ago. A magnetic resonance imaging (MRI) scan of her brain showed distinct signs of encephalomyelitis (Figure 1 ), but cerebrospinal fluid analysis was unremarkable and failed to identify a pathogen. She was discharged in a minimal conscious state and passed away a few months later. Apart from the unusual pattern of her brain MRI scan, she had frequent episodes of generalized flush that were unresponsive to any medication but were self-limiting after 10–30 min. Neurological symptoms in MIS-C are wide-ranging (Lin et al., 2021) but mainly non-specific, such as headache and fatigue. Severe neurological complications such as coma, encephalopathy, ataxia and peripheral neuropathy were described in individual cases, but we did not find any reports highlighting MRI abnormalities comparable with our case. Although the majority of neurological symptoms in children were reported to improve, data on long-term neurodevelopment are elusive. Our case probably indicates that MIS-C may be associated with critical neurological sequelae in a small number of cases.
Figure 1.
Cranial magnetic resonance imaging scan of Patient #1 diagnosed in March 2020 [diffusion weighted images (DWI) and T2-weighted image]. Arrows indicate multiple patchy lesions in the occipital, parietal and temporal lobes indicating an inflammatory process (lesions appear ‘bright’ in DWI and T2-weighted images).
The second case was a 15-year-old boy who developed severe MIS-C while receiving chemotherapy for newly diagnosed acute myeloid leukaemia. Intriguingly, leukaemia was diagnosed only a few weeks after oligosymptomatic SARS-CoV-2 infection. After 2 days of induction chemotherapy with cytarabine, he developed sudden dyspnoea and was admitted to the PICU where he was treated with broad-spectrum antibiotics for suspected septicaemia. No pathogen was identified but his respiratory function deteriorated, and he required mechanical ventilation for acute respiratory distress syndrome (ARDS). Following a steroid pulse, he was successfully weaned off mechanical ventilation after 3 days, but his pulmonary function deteriorated again after a few days of chemotherapy. This time he progressed rapidly to severe ARDS with haemorrhagic alveolitis. He was mechanically ventilated and started on rescue extracorporeal membrane oxygenation therapy for 25 days, and he continued on mechanical ventilation after tracheostomy. Although his respiratory function improved, he developed both cerebral and pulmonary aspergillosis treated with neurosurgery and a triple antifungal medication. Despite these efforts, he passed away after 2 months of intensive care treatment. While there is currently no evidence that infection with SARS-CoV-2 could trigger leukaemic malignancies, the observation that only short cytotoxic treatment was followed by severe MIS-C-associated organ failure is intriguing. Clinically, it appeared that MIS-C was triggered by chemotherapy, raising the question of whether the cytotoxic dose may need to be reconsidered carefully in patients with recent COVID-19.
Ethical approval
Not required.
Funding
None.
Conflict of interests
None declared.
References
- Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. Information for pediatric healthcare providers. Coronavirus. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html [Accessed 16 April 2021] [Google Scholar]
- Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Salido A., de Carlos Vicente J.C., Belda Hofheinz S., Balcells Ramirez J., Slocker Barrio M., Leoz Gordillo I. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. 2020;24:666. doi: 10.1186/s13054-020-03332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indseth T., Grosland M., Arnesen T., Skyrud K., Klovstad H., Lamprini V. COVID-19 among immigrants in Norway, notified infections, related hospitalizations and associated mortality: a register-based study. Scand J Public Health. 2021;49:48–56. doi: 10.1177/1403494820984026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.E., Asfour A., Sewell T.B., Hooe B., Pryce P., Earley C. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743 doi: 10.1016/j.neulet.2020.135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.F., Kothari T., Bandi S., Bird P.W., Goyal K., Zoha M. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J Med Virol. 2020;92:2880–2886. doi: 10.1002/jmv.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radia T., Williams N., Agrawal P., Harman K., Weale J., Cook J. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2020 doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Multisystem inflammatory syndrome in children and adolescents with COVID-19: scientific brief. Available at: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 [Accessed 16 April 2020] [Google Scholar]