Abstract
Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). We succeeded in preparing disinfectant cellulose-based wipes treated with antimicrobial and antiviral silver nanoparticles to be used for prevention of contamination and transmission of several pathogenic viruses and microbes to human in critical areas such as hospitals and healthcare centers especially coronavirus. In this work, the antimicrobial and antiviral activities of silver nanoparticles (AgNPs) prepared with four different techniques were investigated for the utilization as a disinfectant for cellulose-based wipes. These four methods are namely; 1) trisodium citrate with cotton yarn as a reducing agent, 2) preparing AgNP's using aqueous solution of PVA in the presence of glucose, 3) trisodium citrate with cotton fabric as a reducing agent, and 4) photochemical reaction of polyacrylic acid and silver nitrate solution. Polyester/viscose blended spunlace nonwoven fabrics as cellulose based fabrics were treated with the prepared silver nanoparticles to be used as surfaces disinfection wipes. The properties of the nonwoven fabrics were examined including thickness, tensile strength in dry and wet conditions in both machine direction (MD) and cross-machine direction (CMD), bursting strength, air permeability, water permeability and surface wettability. Characterization of the AgNPs was carried out in terms of UV-VIS spectroscopy, TEM, SEM, and Zeta potential analysis. The assessment of AgNPs active solutions for antimicrobial and antiviral activities was evaluated. The results obtained from the analyses of the AgNPs samples prepared with different techniques showed good uniformity and stability of the particles, as well uniform coating of the AgNPs on the fibers. Additionally, there is a significant effect of the AgNPs preparation method on their disinfectant performance that proved its effectiveness against coronavirus (MERS-CoV), S. aureus and B. subtilis as Gram-positive bacteria, E. coli and P. mirabilis as Gram-negative bacteria, A. niger and C. albicans fungi.
Keywords: MERS-CoV, Silver nanoparticles, Wet cellulosic based wipes, Surface disinfection, Antiviral activity, Antibacterial activity
1. Introduction
With the recent outbreak of COVID-19 pandemic worldwide, the fear of infection has increased dramatically, and therefore the demands for medical nonwoven masks and disinfection wipes have rapidly increased too [1]. The severity of coronavirus infection can cause pneumonia, sever acute respiratory syndrome (SARS), and even can leads to death. So, this pandemic had resulted in a crucial socio-economic impact globally [2]. It was stated that in 2019, the global medical nonwovens market value will reach 1.28 billion dollars and will continue to grow at an annual rate of 1.28 billion dollars and will continue to grow at annual rate of 4.6% and it is estimated to reach a market size of 1.61 size of billion dollars by 2024 [1]. Disinfection wipes known also as pre-impregnated disinfecting wipes, they are widely used for various applications particularly in critical areas in hospitals and healthcare centers for the decontamination of medical devices and high-touch environmental surfaces due to their effective performance [3]. Surfaces can be contaminated by hands, objects, settling of virus containing aerosols or contaminated fluids, and therefore leads to the rapid transmission of pathogens between human [4]. Surfaces disinfection wipes play an essential role in infection control, since their usage assists in removing the organic debris and microorganisms through the mechanical action of wiping along with the microbicidal action resulted from the disinfectant solution released by the wipes on the hard surface [5,6].
The disinfection process is influenced by several factors such as the wipes inherent properties including fiber types and fabric structure, the applied pressure force, the target microorganism, the disinfectant type, concentration and bactericidal activity, the application method and the interaction between the wipe substrate and the disinfectant active ingredients. Also, the amount of the released disinfectant solution highly depends on the wipes absorption property [6,7]. The disinfectant protection activity performs in two ways: growth inhabitation of bacteria and fungi and the fatal action (sporicidal, bactericidal, fungicidal, and virucidal effects). The nonwoven wipes used in disinfection purposes are made of cellulosic fibers such as cotton, viscose, lyocell, wood pulp fibers, and polyolefin fibers such as polyester and polypropylene. Most of the disinfection wipes in the market are produced from polyester and viscose/wood pulp fibers blends [6]. The cellulosic fibers are used to afford high water retention and storage capacities, while the polyolefin fibers are responsible for the high tensile strength, abrasion and solvent resistance [8]. The nonwoven fabric for wipes is manufactured using spunlace processing technology, where the produced fabric exhibits sufficient softness, flexibility, conformability making it extensively used in wet wipes and medical field compared to the traditional fabrics [9,10].
The utilization of metals nanoparticles to impart antiviral properties in textiles is one of the most promising approaches. Metals such as silver, copper, zinc nanoparticles are widely used in various textile applications exhibiting new functional properties. Silver nanoparticles have gained much interest due to their high antibacterial and antifungal activities than normal silver metal. This behavior is attributed to the releasing of silver ions (Ag+) that inhibit the respiratory enzymes [[11], [12], [13]]. Silver nanoparticles (AgNPs) have a broad-spectrum antibacterial activity against Gram positive and Gram-negative bacteria such as S. aureus and E. coli [[14], [15], [16], [17]]. Silver ions are linked to the bacterial cell wall and interact with the thiol groups in enzymes and proteins, causing inhibition of bacterial growth and deposited in the vacuole and cell wall as granules which leads to bacterial cell death [18,19]. Recently, the antimicrobial activity of silver nanoparticles against numerous viruses such as HIV-1, hepatitis, respiratory syncytial, herpes simplex, monkeypox and H1N1 influenza A have been investigated. AgNPs antiviral performance is based on the physical inhibition of binding among the virus and the host cell [11,[20], [21], [22], [23]]. The effect of AgNPs size on their antiviral behavior was studied for the pervious mentioned viruses. Silver nanoparticles size smaller than 10 nm had inhibited the infection by HIV-1 [20,24,25]. This property increases the potential of using AgNPs-based antimicrobial materials that will be effective against several types of bacteria, fungi and viruses [11,13,26,27].
The incorporation of metal nanoparticles in polymer composites has been investigated in several studies because of their distinctive properties. Glucose is utilized in reducing silver nitrate into silver nanoparticles in the presence of polyvinyl alcohol (PVA), which reacts as a good host material for metal and semiconductor due to its good thermal stability and chemical resistance [18,28]. PVA is a green and ecofriendly biopolymer because of its water solubility, biocompatibility and non-toxicity, so it is widely used in various biomedical applications [[29], [30], [31], [32], [33]]. Mehrbod et al. [34,35] studied the antiviral behavior of AgNPs against influenza virus, and had demonstrated that AgNPs showed a destructive effect on the virus membrane glycoprotein knobs and the cells. Shigita et al. [36] registered a US patent (2007/016278 A1) based on the utilization of metallic ions such as Ag, Cu, Zn, Al, Mg and Ca to textiles to obtain an antiviral effect. Seino et al. [37] revealed that the addition of AgNPs synthesized by radiochemical process to a cotton textile showed its antiviral effect against influenza A and feline calicivirus. Lara et al. [38] found that the preparation of PVP-coated AgNPs homogenized in Replens gel rapidly inhibited the transmission of HIV-1 and provides long-lasting protection of the cervical tissue from infection for 48 h with no evidence of cytotoxicity detected. Sun et al. [39] found that AgNPs capped with poly(N-vinyl-2-pyrrolidone) (PVP), bovine serum albumin (BSA) and a recombinant F protein from RSV (RF 412) had inhibited the respiratory syncytial virus infection. Mori et al. [11] reported that the Ag-chitosan composites antiviral activity against H1N1 influenza A increased as the concentration of AgNPs increased. Balagna et al. [40] had studied coating FFB3 masks with Ag-silica composite nanoparticles by sputtered coating technique and assessed its antiviral effect towards SARS-CoV-2. The results demonstrated that Ag nanocluster/silica composite sputtered coated had possessed virucidal effect [40].
So, this study aims to investigate the antimicrobial and antiviral activities of silver nanoparticles (AgNPs) prepared with four different techniques to be utilized as a disinfectant for polyester/viscose spunlace wipes for using in surface disinfection. Two techniques of AgNPs preparation were based on using cotton material (yarns and fabrics as technique 1 and 3) as a source of cellulose to be act as a reducing agent. As well, eco-friendly AgNPs have been prepared by using polyvinyl alcohol as reducing agent in the presence of glucose (technique 2). Synthesis of AgNPs with low molecular weight of sodium salt of poly (acrylic acid) (PAA) as stabilizing agent by photochemical reduction method was also carried out (technique 4). Characterization of the prepared AgNPs, mechanical and physical analysis of the wipes along with cytotoxicity, antimicrobial and antiviral activities of the AgNPs were investigated.
2. Materials and methods
2.1. Materials
2.1.1. Textile materials
Two types of spunlace nonwoven fabric (A&B) made of blended polyester/viscose fibers with blending ratio of (80/20%) were used in the study. The basis weight of the nonwoven fabrics' types (A&B) was 42 g/m2 and 38 g/m2 respectively. The nonwoven fabric type A has a pearl pattern and the fabric type B has a plain pattern. As well, two types of cotton materials were used (yarn and fabric); 500 g of cotton yarn with count of 30/1 Ne and 250 g of weft-knitted single jersey fabric.
2.1.2. Chemicals
Trisodium citrate was purchased from Aldrich Chemical. Silver nitrate was purchased from Fisher Scientific. Polyvinyl alcohol (M.W. 115,000, polymerization 1700–1800, viscosity 25–32 cP and hydrolysis 98–99 mol%) was purchased from Alpha chemika, India. Glucose was purchased from Aldrich Chemical. Poly (acrylic acid, sodium salt), 45% solution in water from Aldrich Chemical. The other chemicals are used at analytical grade and without further purification.
2.2. Methods
2.2.1. Preparation of the silver nanoparticles (AgNPs)
Four different techniques were used to prepare the silver nanoparticles in order to investigate the effect of AgNPs preparation method on their antimicrobial and antiviral activities. The prepared AgNPs solution was later used to treat the spunlace nonwoven fabric to be used as disinfectant wet wipes. It is worth mentioning that the purpose of using the cotton material in the two preparation methods 1 and 3 is to carry out the in-situ production of silver nanoparticles on cotton material. The other purpose is to obtain an antimicrobial and antiviral cotton yarns and fabric to be later used in producing winter sweaters, gloves, scarfs, T-shirts, cloth face masks, etc.
2.2.1.1. Trisodium citrate/AgNPs method
In this technique, two different cotton forms were used. The first trial was conducted using 500 g of cotton yarn and this sample will be coded with S1. The cotton yarn was wounded around a perforated dyeing tube to allow the solution to flow from inside to outside to ensure the homogeneous treatment of the cotton yarn. As the treatment was carried out inside a lab scale yarn dyeing unit (Fig. 1 ). The other trial of silver nanoparticles preparation was carried out using 250 g of weft-knitted single jersey cotton fabric and this sample will be coded with S3. This preparation method was carried out in a lab scale fabric dyeing machine (Fig. 1).
Fig. 1.
Yarns and fabrics lab scale dyeing and finishing units.
In the preparation procedure of sample S1, AgNPs were synthesized according to literature [25], in a typical procedure; 500 g of cotton yarn were immersed in 15 L of 0.4 g/L silver nitrate solution (400 ppm) at room temp. Using liquor ratio 1:50 LR, the temperature then was raised to 100 °C until boiling. A 3 g/L of an aqueous solution of trisodium citrate (45 g) was added drop wise to the mixture. The temperature was kept at 100 °C for 45 min, then the cotton yarn was rinsed several times with cold tap water, and then the yarn was centrifuged and hot air dried. For the second trial to prepare sample S3, 250 g of cotton single jersey knitted fabric was immersed in 15 L of 0.2 g/L silver nitrate solution (100 ppm) at room temp. Using liquor ratio 1/50, the temperature then was raised to 100 °C until boiling. A 1.5 g/L of an aqueous solution of trisodium citrate (22.5 g) was added drop wise to the mixture. The temperature was kept at 100 °C for 45 min, then the cotton fabric was rinsed and hot air dried [41].
2.2.1.2. PVA/AgNPs method
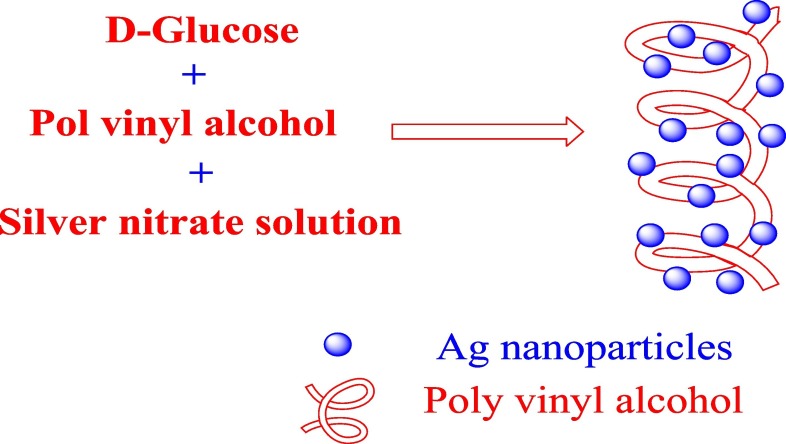
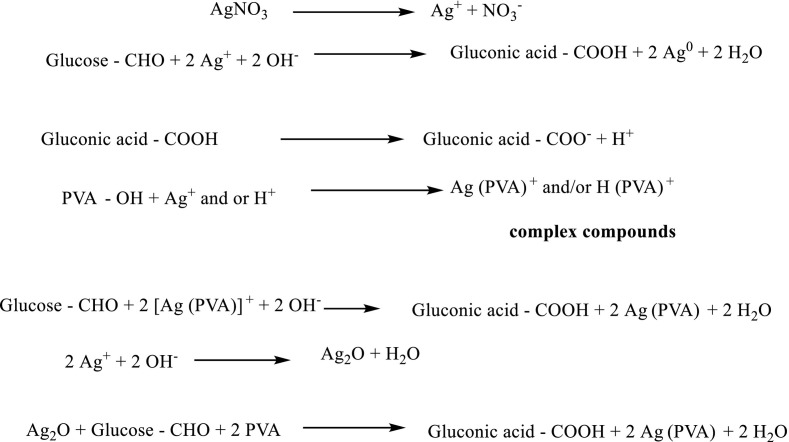
Silver nitrate (AgNO3) was dissolved in an aqueous (355 ppm) solution of PVA (3 g/100 ml) in the presence of glucose (0.15 g/100 ml) to overcome the oxidation of silver nitrate into silver oxide. The reduction time was 72 h. at room temperature [18]. The prepared AgNPs sample is coded with S2. This preparation of silver nanoparticles by using this method can be represented in Scheme 1 .
Scheme 1.
Preparation of silver nanoparticles via reduction of silver nitrate by poly vinyl alcohol in the presence of glucose.
2.2.1.3. Polyacrylic acid/AgNPs
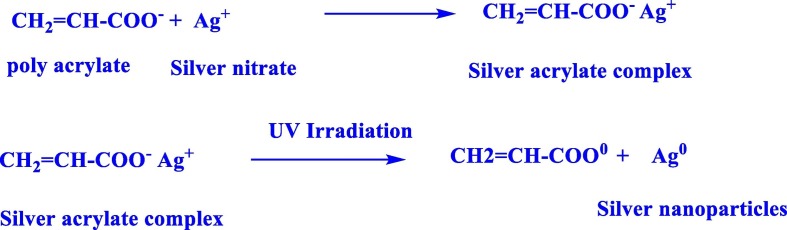
Silver nitrate (AgNO3) was dissolved (250–300 ppm) in distilled water with 5 wt% of poly (acrylic acid (PAA), sodium salt), 45% solution in Water-Aldrich. 1000 ml of this solution was poured in a Beaker glass then exposed to a broad-band UV lamp (Original Philips UVA) (Fig. 2 ) at a distance of 10 cm and for 10 min. The initiation of AgNPs formation by UV irradiation depends on the reactivity between the silver ions and PAA molecules and on the molecular weight of PAA. The mechanism of AgNPs formation under UV irradiation in aqueous solutions of PAA carried out through reduction of silver ions by reductive (reactive) species i.e., reducing agents which result from the photochemical reaction of Na PAA [42]. The prepared AgNPs sample is coded with S4.
Fig. 2.
Preparation of silver nanoparticles with broad-band UV lamp.
Fig. 3 shows the prepared silver nanoparticles samples with different techniques, their color ranges from brown, light brown, yellow and blue. The color differences of the prepared silver nanoparticles solutions are attributed to the preparation method of each sample and concentration of the AgNPs.
Fig. 3.
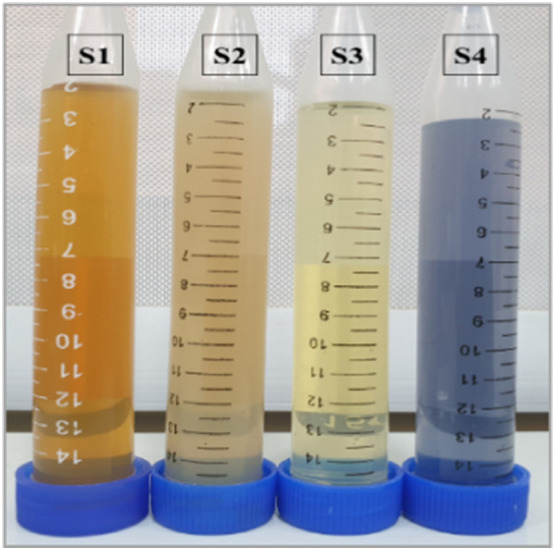
The prepared silver nanoparticles samples with different colors.
2.2.2. Preparation of the cellulose-based wipes loaded with AgNPs
The prepared AgNPs solution was loaded to a wet wipe making machine, and the AgNPs were sprayed at a ratio of 3 ml per wipe. The dry spunlace wipes fabric was in roll form with 14 cm width. After the AgNPs solution was sprayed into the dry wipes, the machine cut the wipes in 18 cm length and the wet wipes are folded and packed into a sealed plastic package. Fig. 4 shows the produced wet wipes in individual packages.
Fig. 4.

Individual packages of the wet wipes loaded with the silver nanoparticles.
2.3. Testing and analysis
2.3.1. Characterization of the nonwoven wipes
The physical and mechanical properties of the spunlace nonwoven fabrics were characterized before treatment with the AgNPs as follows: Fabric thickness was measured according to ASTM-D1777-02. Tensile strength in machine direction (MD) and cross-machine direction (CMD) in dry and wet conditions according to ISO 9073-3. Bursting strength was determined according to ASTM-D 3786-01. Air permeability was determined according to ASTM-D737-96. Water permeability was determined according to ASTM D4491. Surface wettability-spray test according to AATCC Test Method 22-2005.
2.3.2. Ultraviolet-visible (UV–vis) absorption spectroscopy
The UV–vis spectra are used to confirm the formation of silver colloids because the AgNPs exhibit an intense absorption peak due to the surface plasmon excitation, which describes the collective excitation of conductive electrons in a metal. The spectra were collected over a range of 200–800 nm.
2.3.3. Transmission electron microscopy (TEM)
The shape and size of AgNPs were obtained by using transmission electron microscopy (TEM) JEOL-JEM-1200. The specimens were prepared by casting a drop of the colloidal solution of AgNPs on 400 mesh carbon-coated copper grids, and evaporating the solvent in air at room temperature. The average diameter of the prepared AgNPs was determined from the diameter of 200 nanoparticles found in several arbitrarily chosen areas in enlarged microphotographs.
2.3.4. Scanning electron microscopy (SEM)
The surface morphology of the wipes loaded with the prepared silver nanoparticles was characterized using TESCAN-VEGA 3 scanning electron microscope (SEM) with 30 kV scanning voltages.
2.3.5. Zeta potential analysis of AgNPs
Zeta potential analysis is used to determine the surface charge of the nanoparticles in solution (colloids). Results of zeta potential give an indication of the potential stability of the prepared AgNPs. It should be taken in consideration that the particles with values more than +30 mV or more than −30 mV are considered stable [43].
2.3.6. MTT cytotoxicity assay (TC50)
The prepared AgNPs samples were diluted with Dulbecco's Modified Eagle's Medium (DMEM). Stock solutions of the test compounds were prepared in 10% DMSO in bi distilled H2O. The cytotoxic effect of the extracts was tested in Vero E6 cells by using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) according to Mossman method [44] with a minor modification. Briefly, the cells were seeded in 96 well-plates (100 μl/well at a density of 3 × 105 cells/ml) and incubated for 24 h at 37 °C in 5% CO2. After 24 h, the cells were treated with various concentrations of the tested AgNPs compounds in triplicates. After further 24 h, the supernatant was discarded and cell monolayers were washed with sterile phosphate buffer saline (PBS) 3 times, then MTT solution (20 μl of 5 mg/ml stock solution) was added to each well and incubated at 37 °C for 4 h followed by medium aspiration. In each well, the formed formazan crystals were dissolved with 200 μl of acidified isopropanol (0.04 M HCL in absolute isopropanol = 0.073 ml HCL in 50 ml isopropanol). Absorbance of formazan solutions was measured at λmax 540 nm with 620 nm as a reference wavelength using a multi-well plate reader. The percentage of cytotoxicity compared to the untreated cells was determined according to the following equation:
The plot of cytotoxicity % versus sample concentration was used to calculate the concentration which exhibited 50% cytotoxicity (TC50).
2.3.7. Plaque reduction assay
The antiviral activity of the prepared AgNPs was investigated using the plaque reduction assay. It was carried out according to the method described by Hayden et al. [45] in a six well plate where Vero E6 cells (105 cells/ml) were cultivated for 24 h at 37 °C. Middle East Respiratory Syndrome Related Coronavirus Isolate (MERS-CoV) NRCE-HKU270 (accession number: KJ477103.2) virus was diluted to give 103 PFU/well and mixed with the safe concentration of the tested compounds and incubated for 1 h at 37 °C before being added to the cells. The growth medium was removed from the cell culture plates, and the cells were inoculated with (100 μl/well) virus with the tested compounds, after 1-h contact time for virus adsorption, 3 ml of DMEM supplemented with 2% agarose and the tested compounds was added onto the cell monolayer, plates were left to solidify and incubated at 37 °C till formation of viral plaques (3 to 4 days). Formalin (10%) was added for 2 h then plates were stained with 0.1% crystal violet in distilled water. Control wells were included where untreated virus was incubated with Vero E6 cells and finally plaques were counted and percentage reduction in plaques formation in comparison to control wells was recorded as follows:
2.3.8. Evaluation of the antimicrobial activity in vitro
The antibacterial and antifungal activities of the prepared AgNPs treated samples were evaluated against four bacterial strains which are; Staphylococcus aureus (S. aureus, ATCC 6538) and Bacillus subtilis (B. subtilis, ATCC 6633) as Gram-positive bacteria and Escherichia coli (E. coli, ATCC 11229) and Proteus mirabilis (ATCC 33420) as Gram-negative bacteria. Antifungal activity was carried out against Aspergillus niger (ATCC 13497) and Candida albicans (ATCC 10231). Ciprofloxacin was used as standard drug. These bacterial and fungal strains were selected as test cells because they are the most frequent microbes. Fresh inoculants for antibacterial assessment were prepared on nutrient broth at 37 °C for 24 h.
2.3.8.1. Test method
The antibacterial and antifungal activities of the prepared AgNPs samples were evaluated using the disk diffusion method on an agar plate [46]. Briefly, 1 cm diameter of each fabric sample was cut and put into 10 ml of nutrient agar, to which 10 μl of microbe culture was inoculated, after the solidification. The plates were incubated at 37 °C for 24 h, after which the diameter of the inhibition zone around samples was measured and recorded.
3. Results and discussion
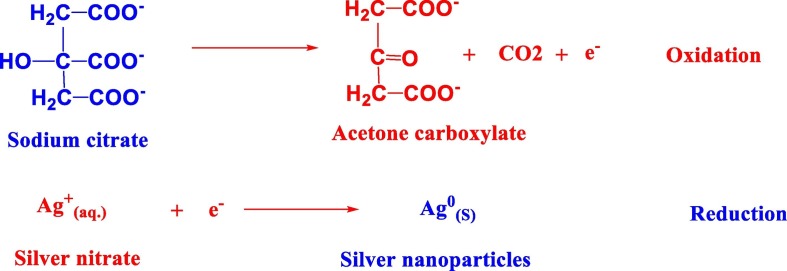
Generally silver nanoparticles can be formed through reduction of silver nitrate in the presence of suitable reducing agent. Sodium citrate has been used to reduce silver nitrate to silver nanoparticles (S1 and S3) as shown in the following reaction:
Poly vinyl alcohol/glucose can be used to reduce silver nitrate into silver nanoparticles (S2) as follows:
In addition, silver nitrate can be reduced to silver nanoparticles by acrylic acid in sodium form under exposure to ultra-violet irradiation as follows:
3.1. Nonwoven wipes physical and mechanical properties
The physical and mechanical properties of the spunlace nonwoven fabric samples are presented in Table 1 .
Table 1.
Properties of the nonwoven samples.
| Fabric properties | Nonwoven fabrics |
|
|---|---|---|
| Type A | Type B | |
| Thickness (mm) | 0.30 | 0.27 |
| Tensile strength MD (dry) (N) | 70.23 ± 2.83 | 56.9 ± 6.57 |
| Tensile strength MD (wet) (N) | 56.25 ± 7.19 | 55.13 ± 7.35 |
| Tensile strength CMD (dry) (N) | 19.6 ± 0.0 | 18.62 ± 2.19 |
| Tensile strength CMD (wet) (N) | 14.7 ± 0.0 | 15.93 ± 4.69 |
| Bursting strength (kPa) | 187.5 | 190 |
| Air permeability (cm3/cm2/s) | 398 | 366.53 |
| Water permeability (l/m2·s) | 1.1 | 1.15 |
| Surface wettability (%) | 100% | 100% |
It can be observed from the results in Table 1 that the thickness of the nonwoven sample A is higher than the sample B due to the presence of pearl pattern on its surface. The rate of water permeability and surface wettability of the nonwoven samples (A&B) are almost the same. This could be related to their composition of the same materials. In nonwoven fabrics production, the machine direction (MD) refers to the lengthwise direction of the produced fabric, while cross machine direction (CMD) refers to the widthwise of the machine. Both nonwoven samples showed high tensile strength in (MD) compared to their strength in (CMD) in the dry and wet conditions due to the increased ratio of the parallel webs of fibers and their entanglement in the machine direction which in turn increased the fabric strength. Sample A showed high tensile strength in (MD) and (CMD) in the dry and wet conditions compared to sample B. The wet tensile strength of sample A decreased by 19.91% in (MD) and by 24.87% in (CMD) compared to its dry strength, while for sample B the wet tensile strength decreased by 3.11% in (MD) and by 14.45% in (CMD) compared to its dry strength. The decrease in fabrics strength in wet conditions may be attributed to the low friction between the wet fibers and their displacement during exposure to tensile load which leads to decrease their entanglement and reduce their strength [46]. Also, it was found that the bursting strength of sample B is higher to that of sample A. Sample A showed high air permeability value compared to sample B, and this could be attributed to the pearl pattern distribution on the sample's surface that increased the pore size of sample A.
3.2. Ultraviolet-visible (UV–vis) spectra
Silver nanoparticles colloidal solution has a characteristic peak in the range of 400–450 nm using UV–vis Spectrophotometer, the intensity of peaks is related to the concentration of the AgNPs in the solution. Fig. 5 illustrates the UV–vis absorption spectra of the colloidal silver nanoparticles that formed from the four different methods. The figure shows a homogeneous narrow absorption band around 420–428 nm, which confirms the presence of AgNPs in the colloidal solution with semispherical shape except for sample S4 that have polymorphism shape with absorption band peaks at 540–550 nm [47].
Fig. 5.
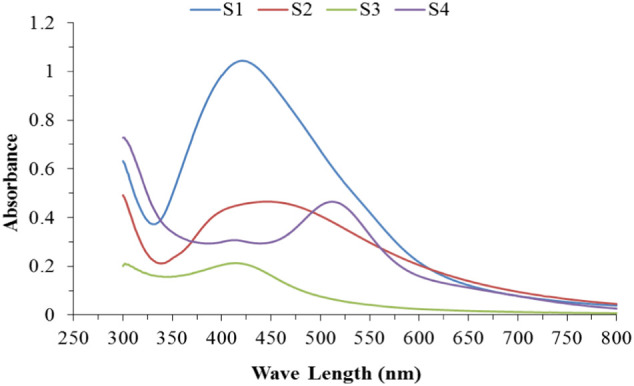
UV–vis spectra of the silver nanoparticles samples.
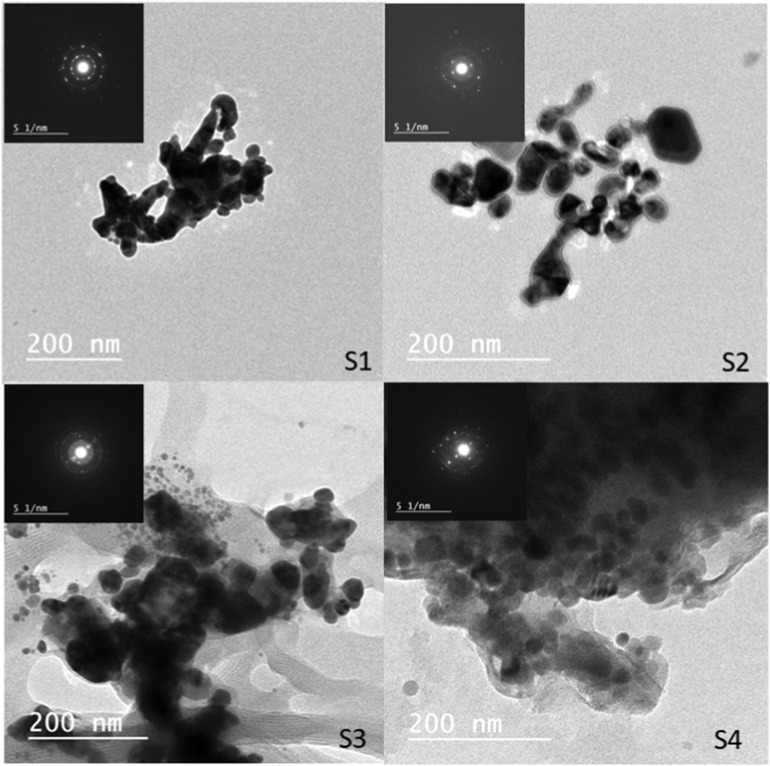
3.3. Transmission electron microscopy (TEM)
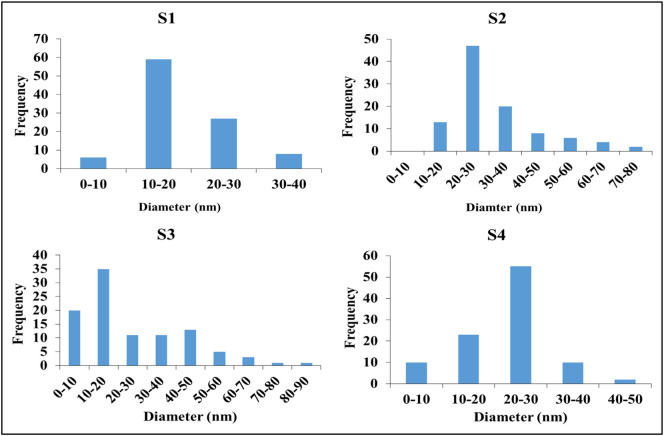
Transmission electron microscopy (TEM) images show the shape and size of the silver nanoparticles four samples in Fig. 6 . The size distribution histograms of the AgNPs samples are shown in Fig. 7 . It was observed that the AgNPs are polydisperse in the solutions. The shape of the majority of nanoparticles is spherical with a small range of semi-spherical and elongated particles in the four samples. From the histograms in Fig. 7, it was indicated that S1 particles size ranged from 6.32 to 35.88 nm with a major diameter range 10–20 nm, S2 particles size ranged from 10.11 to 79.94 nm with a major diameter range from 20 to 30 nm, S3 particles size ranged from 5.67 to 87.31 nm with a major diameter range from 10 to 20 nm, and S4 particles size ranged from 6.27 to 43.55 nm with major diameter range from 20 to 30 nm. So, sample S1 exhibited the highest ratio of small nanoparticles' size with ratio of 60% which may attribute to the preparation technique. These results data agreed with the UV–vis spectral data. Therefore, the size distribution of AgNPs was controlled by adjustment of the concentrations of AgNO3.
Fig. 6.
TEM images of the AgNPs samples.
Fig. 7.
AgNPs samples size distribution.
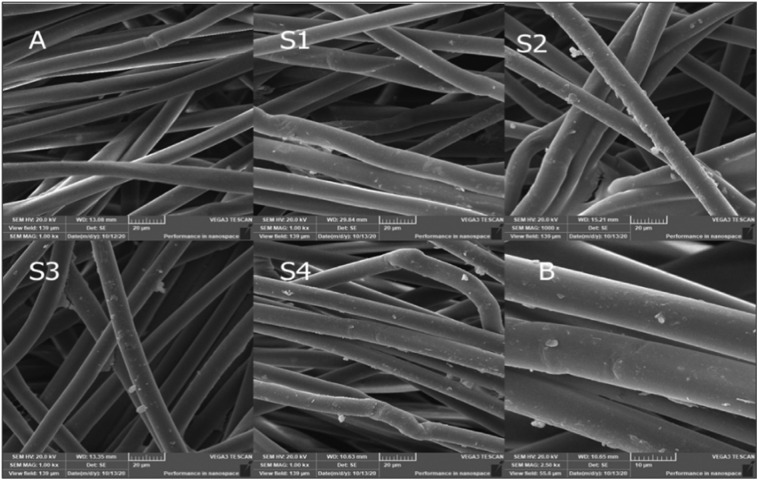
3.4. Scanning electron microscopy (SEM)
Fig. 8 shows the SEM images of the cellulose-based wipes treated nonwoven fabric samples with the silver nanoparticles solutions prepared with the different techniques. Fig. 8(A) shows the blank nonwoven fabric before treatment. It can be seen from Fig. 7(S1–S4) that the fibers in each treated sample are coated with the silver nanoparticles. Fig. 8(B) shows 8× magnification image of sample S4, and it is clear that the spraying technique was able to cover the fiber with the silver nanoparticles to the maximum effect.
Fig. 8.
SEM images of the wipes nonwoven fabric, A) blank spunlace wipe fabric, the treated wipe fabric samples S1, S2, S3& S4, and B) sample S4 at 8000×.
3.5. Zeta potential analysis
The prepared silver nanoparticles solutions were characterized for their polydispersity and zeta potential and the obtained results are presented in Table 2 .
Table 2.
Particle size, polydispersity and zeta potential results.
| Samples | Polydispersity index | Zeta potential (mV) |
|---|---|---|
| S1 | 0.249 | −12.73 |
| S2 | 0.247 | −1.99 |
| S3 | 0.237 | −9.17 |
| S4 | 0.095 | −13.37 |
The results show that the polydispersity index showing homogenous particle size distribution of the prepared silver nanoparticles (AgNPs) within the solution. The Zeta potential values varied in the range of −1.99 mV to −13.37 mV. The zeta potential value in sample S2 was very low showing that the solution maybe doesn't have high stability as nanoparticles and most probably, it starts to aggregate. Sample S4 showed the highest zeta potential value among all samples and it can be indicated that it is the most stable of all tackled solutions.
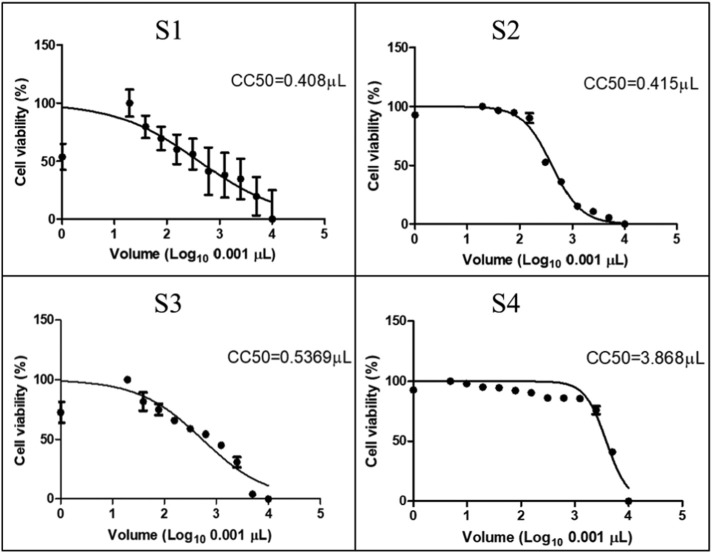
3.6. MTT cytotoxicity assay (TC50)
Fig. 9 shows the cell viability using the tested silver nanoparticles samples, which was carried out by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric assay to determine the sample concentration that inhibits cell growth by 50% cytotoxic concentration 50-TC 50. The results revealed that sample S4 had the highest TC 50 = 3.868 μL, followed by sample S3 TC 50 = 0.5369 μL then sample S2 TC 50 = 0.415 μL and sample S1 TC 50 = 0.408 μL. Thus, sample S4 is the safest as higher concentration is required to kill 50% of viable cells. This may be attributed to the AgNPs' preparation method that has been used to prepare S4, as the technique used to prepare S4 is the safest method with low toxicity that may harm the cell at high TC 50. In this method, no reducing agent was used, as the polyacrylic acid and the sodium salt produce the reducing agent when exposed to UV lamp and photochemical reaction is occurred. Nevertheless, the remaining polyacrylic acid acts as a stabilizing agent [32]. Therefore, this technique is considered safe with high volume of TC 50.
Fig. 9.
MTT cytotoxicity assay of the prepared AgNPs samples in the form of growth inhibition curves.
3.7. Antiviral activity
Assessment of antiviral activity of the silver nanoparticles samples based on Plaque forming assay is presented in Table 3 . Evaluating the antiviral activity of the different samples was carried out against (MERS-CoV), and the number of Plaque forming Units (PFUs) was examined in sample treated cells compared to PFUs in the untreated virus control cells (2.93 × 10−4) to determine the percentage of viral inhibition. The lower the sample concentration the lesser the antiviral activity. Sample S3 has the highest antiviral activity at a concentration less the 1/10 of TC 50 with viral inhibition 51.7%, while Sample S4 at a concentration less the 1/60 of TC 50 gave viral inhibition 48.3%. Sample S2 didn't show any antiviral activity at the tested concentrations, this may be related to the formation of a polyvinyl alcohol (PVA) membrane coating the silver nanoparticles which negatively affect its antiviral activity.
Table 3.
The viral activity for Middle East Respiratory Syndrome (MERS-CoV) measured using Plaque assay.
| Samples | Volume (μl) | Viral count after treatment (PFU/ml) | Virus control (PFU/ml) | Viral Inhibition (%) |
|---|---|---|---|---|
| S1 | 0.5 | 1.9 × 10−4 | 3.0 × 10−4 | 36.7 |
| 0.25 | 1.9 × 10−4 | 36.7 | ||
| 0.125 | 2.5 × 10−4 | 16.7 | ||
| 0.0625 | 3.1 × 10−4 | 0.0 | ||
| S2 | 0.007813 | 3.2 × 10−4 | 3.0 × 10−4 | 0.0 |
| 0.003906 | 3.7 × 10−4 | 0.0 | ||
| 0.001953 | 4.0 × 10−4 | 0.0 | ||
| 0.000977 | 4.2 × 10−4 | 0.0 | ||
| S3 | 0.0625 | 1.4 × 10−4 | 2.9 × 10−4 | 51.7 |
| 0.03125 | 1.5 × 10−4 | 48.3 | ||
| 0.015625 | 2.4 × 10−4 | 17.2 | ||
| 0.007813 | 2.5 × 10−4 | 13.8 | ||
| S4 | 0.0625 | 1.5 × 10−4 | 2.9 × 10−4 | 48.3 |
| 0.03125 | 1.8 × 10−4 | 37.9 | ||
| 0.015625 | 2.0 × 10−4 | 31.0 | ||
| 0.007813 | 2.0 × 10−4 | 31.0 |
3.8. Antimicrobial activity
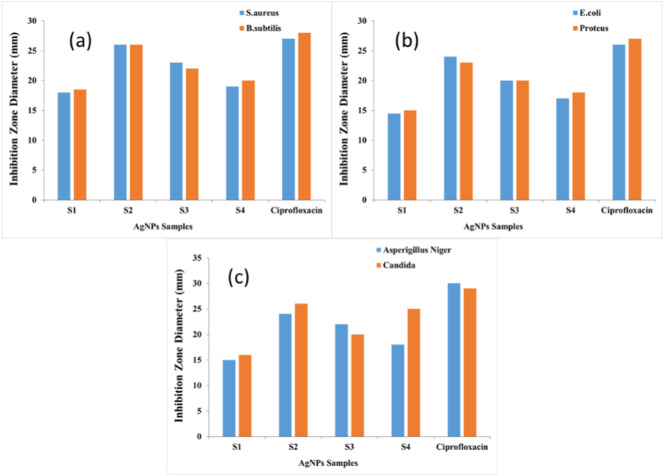
The antimicrobial activity of silver nanoparticles samples was evaluated using the disk diffusion method against four bacterial species and two fungal species. Fig. 10 shows the antimicrobial activity expressed by the diameter of the inhibition zone. It was found that samples S2 showed the highest antibacterial activity among the other samples followed by sample S3. This could be attributed to its cavitation with polyvinyl alcohol which helped it to attach the bacterial cell membrane causing the bacterial cell death. In addition, Fig. 10 shows that all the AgNPs have antimicrobial activity towards the tested microbes, although other cationic antimicrobial materials such as chitosan and its derivatives higher antibacterial activity towards Gram positive bacteria than Gram negative one. All the AgNPs samples showed higher antibacterial activity against both Gram positive, Gram negative and fungi with the same trend due to nanostructure of Ag that make it able to penetrate the microbial cell.
Fig. 10.
Antimicrobial activity expressed in inhibition zone of silver nanoparticles prepared via different method (S1, S2, S3, S4) against Gram positive bacteria (a), Gram negative bacteria (b) and fungi (c).
4. Application and utilization of treated cotton yarns with AgNPs
The treated cotton yarns with silver nanoparticles produced from procedure 1 (S1) was utilized in manufacturing of an antimicrobial and antiviral winter sweater as shown in Fig. 11 . This kind of cost-effective winter garments with high protection efficiency against microbes is suggested to be worn especially in the critical times of pandemics which can assist in mitigating the risk of infection along with wearing face masks and eye protection devices.
Fig. 11.

Antimicrobial and antiviral winter sweater made of cotton yarns treated with AgNPs.
5. Conclusion
The usage of disinfectant wet wipes is rapidly increasing nowadays to meet the daily needs and reduce risk of infection from high-touch environmental surfaces, which become a challenging issue especially with the outbreak of coronavirus pandemic. In this work, the antimicrobial and antiviral activities of silver nanoparticles prepared with four different techniques was investigated to be utilized as a disinfectant for polyester/viscose spunlace wipes for using in surface disinfection. The results showed that the spunlace nonwoven fabrics had adequate physical and mechanical properties. The AgNPs prepared with different techniques showed good uniformity and stability of the particles, and their size distribution was controlled by adjustment of the concentration of AgNO3. The MTT cytotoxicity assay revealed that the AgNPs prepared with the Polyacrylic Acid by photochemical reduction method (S4) is the safest, as higher concentration is required to kill 50% of the viable cells. Also, it exhibited a high antiviral activity against MERS-CoV with 48.3% viral inhibition at 0.0625 μL.
On the other hand, the AgNPs (S3) prepared with using cotton fabric as a reducing agent showed the highest antiviral activity among all samples with 51.7% viral inhibition at 0.0625 μL with moderate cytotoxicity activity. Although the AgNPs (S2) prepared by using glucose and PVA as a reducing matrix didn't show any antiviral activity. Furthermore, all the prepared AgNPs samples showed high antibacterial effects against Gram positive and Gram-negative bacteria, and antifungal effect. Thus, the produced surface disinfectant wet wipes treated with silver nanoparticles are effective in using in critical areas like hospitals, healthcare centers, and crowded places and are promising to reduce the risk of coronavirus infection.
CRediT authorship contribution statement
Conception and design of study: All authors
Acquisition of data: All authors contributed equally in this article
Analysis and/or interpretation of data: All authors contributed equally in this article
Drafting the manuscript: All authors contributed equally in this article
Revising the manuscript critically for important intellectual content: All authors contributed equally in this article
Approval of the version of the manuscript to be published: All authors contributed equally in this article.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgment
The authors would like to thank Dr. Mohamed Ahmed Ali Professor of Virology and Director of the Centre for Scientific Excellence for Influenza Viruses at National Research Center, and his team for their assistance in the antiviral testing against coronavirus (MERS-CoV). This work was carried out by the Textile Alliance Project (Innovative Textile Technology Centre, ITTC) funded by the Academy of Scientific Research and Technology. The ITTC is located in the National Research Center, Egypt.
Funding
This study was funded by Academy of Scientific Research and Technology (ASRT) under the alliance project.
References
- 1.Yun T., Cheng P., Qian F., Cheng Y., Lu J., Lv Y., Wang H. Balancing the decomposable behavior and wet tensile mechanical property of cellulose-based wet wipe substrates by the aqueous adhesive. Int. J. Biol. Macromol. 2020;164:1898–1907. doi: 10.1016/j.ijbiomac.2020.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keni R., Alexander A., Nayak P.G., Mudgal J., Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Front. Public Health. 2020;8:216. doi: 10.3389/fpubh.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández A., Carrasco M., Ausina V. Mycobactericidal activity of chlorine dioxide wipes in a modified prEN 14563 test. J. Hosp. Infect. 2008;69(4):384–388. doi: 10.1016/j.jhin.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Becker B., Henningsen L., Paulmann D., Bischoff B., Todt D., Steinmann E., Steinmann J., Brill F.H.H., Steinmann J. Evaluation of the virucidal efficacy of disinfectant wipes with a test method simulating practical conditions. Antimicrobial Resistance & Infection Control. 2019;8(1):121. doi: 10.1186/s13756-019-0569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrobial Resistance & Infection Control. 2016;5(1) doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song X., Vossebein L., Zille A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: a review. Antimicrobial Resistance & Infection Control. 2019;8(1) doi: 10.1186/s13756-019-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards N.W.M., Best E.L., Connell S.D., Goswami P., Carr C.M., Wilcox M.H., Russell S.J. Role of surface energy and nano-roughness in the removal efficiency of bacterial contamination by nonwoven wipes from frequently touched surfaces. Sci. Technol. Adv. Mater. 2017;18(1):197–209. doi: 10.1080/14686996.2017.1288543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rengasamy R.S. In: Composite Non-woven Materials. Das D., Pourdeyhimi B., editors. Woodhead Publishing; 2014. 6 - composite nonwovens in wipes; pp. 89–119. [Google Scholar]
- 9.Zhang Y., Deng C., Qu B., Zhan Q., Jin X. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. A study on wet and dry tensile properties of wood pulp/lyocell wetlace nonwovens; p. 012013. [Google Scholar]
- 10.Zhang G., Wang D., Xiao Y., Dai J., Zhang W., Zhang Y. Fabrication of Ag Np-coated wetlace nonwoven fabric based on amino-terminated hyperbranched polymer. J Nanotechnology Reviews. 2019;8(1):100–106. [Google Scholar]
- 11.Mori Y., Ono T., Miyahira Y., Nguyen V.Q., Matsui T., Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Research Letters. 2013;8(1):1–6. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dung T.T.N., Nam V.N., Nhan T.T., Ngoc T.T.B., Minh L.Q., Nga B.T.T., Quang D.V. Silver nanoparticles as potential antiviral agents against African swine fever virus. Materials Research Express. 2020;6(12) [Google Scholar]
- 13.Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16(10):8894–8918. doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim N., Kadry G., Eid B., Ibrahim H. Enhanced antibacterial properties of polyester and polyacrylonitrile fabrics using Ag-Np dispersion/microwave treatment. AATCC Journal of Research. 2014;1(2):13–19. [Google Scholar]
- 15.Franci G., Falanga A., Galdiero S., Palomba L., Rai M., Morelli G., Galdiero M.J.M. Silver nanoparticles as potential antibacterial agents. 2015;20(5):8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farag S., Ibrahim H.M., Asker M.S., Amr A., El-Shafaee A. Impregnation of silver nanoparticles into bacterial cellulose: green synthesis and cytotoxicity. Int. J. ChemTech Res. 2015;8(12):651–661. [Google Scholar]
- 17.Eid B.M., El-Sayed G.M., Ibrahim H.M., Habib N.H. Durable antibacterial functionality of cotton/polyester blended fabrics using antibiotic/MONPs composite. Fibers and Polymers. 2019;20(11):2297–2309. [Google Scholar]
- 18.Ibrahim H.M., Zaghloul S., Hashem M., El-Shafei A. A green approach to improve the antibacterial properties of cellulose based fabrics using Moringa oleifera extract in presence of silver nanoparticles. Cellulose. 2021;28(1):549–564. [Google Scholar]
- 19.Potera C. Understanding the germicidal effects of silver nanoparticles. Environ. Health Perspect. 2012;120(10):a386. doi: 10.1289/ehp.120-a386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. Journal of Nanobiotechnology. 2010;8(1):1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori Y., Ono T., Miyahira Y., Nguyen V.Q., Matsui T., Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013;8(1):93. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huy T.Q., Hien Thanh N.T., Thuy N.T., Chung P.V., Hung P.N., Le A.-T., Hong Hanh N.T. Cytotoxicity and antiviral activity of electrochemical – synthesized silver nanoparticles against poliovirus. J. Virol. Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Haggag E., Elshamy A., Rabeh M., Gabr N., Salem M., Youssif K., Samir A., Muhsinah A. Bin, Alsayari A., Abdelmohsen U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomedicine. 2019;14:6217–6229. doi: 10.2147/IJN.S214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris D., Ansar M., Speshock J., Ivanciuc T., Qu Y., Casola A., Garofalo R. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses. 2019;11(8):732. doi: 10.3390/v11080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Yacaman M.J. Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology. 2005;3(1):1–10. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orlowski P., Tomaszewska E., Gniadek M., Baska P., Nowakowska J., Sokolowska J., Nowak Z., Donten M., Celichowski G., Grobelny J., Krzyzowska M. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orłowski P., Kowalczyk A., Tomaszewska E., Ranoszek-Soliwoda K., Węgrzyn A., Grzesiak J., Celichowski G., Grobelny J., Eriksson K., Krzyzowska M. Antiviral activity of tannic acid modified silver nanoparticles: potential to activate immune response in herpes genitalis. Viruses. 2018;10(10):524. doi: 10.3390/v10100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenashen M.A., El-Safty S.A., Elshehy E.A. Characterization, synthesis, morphological control, and properties of silver nanoparticles in potential applications. Particle & Particle Systems Characterization. 2014;31(3):293–316. [Google Scholar]
- 29.Ibrahim H.M., Dakrory A., Klingner A., El-Masry A.M.A. Carboxymethyl chitosan electrospun nanofibers: preparation and its antibacterial activity, Journal of Textile and Apparel. Technology and Management. 2015;9(2) [Google Scholar]
- 30.Ibrahim H.M., El-Zairy E.M.R. Carboxymethylchitosan nanofibers containing silver nanoparticles: preparation, characterization and antibacterial activity. Journal of Applied Pharmaceutical Science. 2016;6(7):43–48. [Google Scholar]
- 31.Ibrahim H.M., Reda M.M., Klingner A. Preparation and characterization of green carboxymethylchitosan (CMCS) – polyvinyl alcohol (PVA) electrospun nanofibers containing gold nanoparticles (AuNPs) and its potential use as biomaterials. Int. J. Biol. Macromol. 2020;151:821–829. doi: 10.1016/j.ijbiomac.2020.02.174. [DOI] [PubMed] [Google Scholar]
- 32.Gaaz T.S., Sulong A.B., Akhtar M.N., Kadhum A.A.H., Mohamad A.B., Al-Amiery A.A.J.M. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. 2015;20(12):22833–22847. doi: 10.3390/molecules201219884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Díaz-Cruz C., Nuñez G.A., Espinoza-Gómez H., Flores-López L.Z. Effect of molecular weight of PEG or PVA as reducing-stabilizing agent in the green synthesis of silver-nanoparticles. European Polymer Journal. 2016;83:265–277. [Google Scholar]
- 34.Mehrbod P., Motamed N., Tabatabaeian M., Soleymani E.R., Amini E., Shahidi M., Kheyri M. In vitro antiviral effect of “nanosilver” on influenza virus. Archive of SID. 2009;17(2):88–93. [Google Scholar]
- 35.Lara H.H., Garza-Treviño E.N., Ixtepan-Turrent L., Singh D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. Journal of Nanobiotechnology. 2011;9(1):1–8. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shigita S., Tsurumi H., Naka H. 2007. Anti-viral fiber, process for producing the fiber, and textile product comprising the fiber, Google Patents. [Google Scholar]
- 37.Seino S., Imoto Y., Kosaka T., Nishida T., Nakagawa T., Yamamoto T.A. Antiviral activity of silver nanoparticles immobilized onto textile fabrics synthesized by radiochemical process. MRS Advances. 2016;1(11):705–710. [Google Scholar]
- 38.Lara H.H., Ixtepan-Turrent L., Garza-Treviño E.N., Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. Journal of Nanobiotechnology. 2010;8(1):1–11. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L., Singh A.K., Vig K., Pillai S.R., Singh S.R. Silver nanoparticles inhibit replication of respiratory syncytial virus. Journal of Biomedical Nanotechnology. 2008;4(2):149–158. [Google Scholar]
- 40.Balagna C., Perero S., Percivalle E., Nepita E.V., Ferraris M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics. 2020;1:100006. [Google Scholar]
- 41.Rehan M., Mashaly H.M., Mowafi S., El-Kheir A. Abou, Emam H.E. Multi-functional textile design using in-situ Ag NPs incorporation into natural fabric matrix. Dyes and Pigments. 2015;118:9–17. [Google Scholar]
- 42.Xu G.-n., Qiao X.-l., Qiu X.-l., Chen J.-g. Preparation and characterization of stable monodisperse silver nanoparticles via photoreduction. Colloids Surf. A Physicochem. Eng. Asp. 2008;320(1):222–226. [Google Scholar]
- 43.Meléndrez M., Cárdenas G., Arbiol J. Synthesis and characterization of gallium colloidal nanoparticles. Journal of Colloid and Interface Science. 2010;346(2):279–287. doi: 10.1016/j.jcis.2009.11.069. [DOI] [PubMed] [Google Scholar]
- 44.Mosdam T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assay. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 45.Hayden F.G., Cote K., Douglas R.G. Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrobial Agents and Chemotherapy. 1980;17(5):865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim H., Dakrory A., Klingner A., El-Masry A. Carboxymethyl chitosan electrospun nanofibers: preparation and its antibacterial activity. Journal of Textile & Apparel Technology & Management (JTATM) 2015;9(2) [Google Scholar]
- 47.Tang B., Wang J., Xu S., Afrin T., Tao J., Xu W., Sun L., Wang X. Function improvement of wool fabric based on surface assembly of silica and silver nanoparticles. Chem. Eng. J. 2012;185-186:366–373. [Google Scholar]